Abstract
Fusion proteins comprised of a binding domain and green fluorescent protein (GFP) have the potential to act as one-step binding reagents. In this study, eight single-chain antibodies (scFv) and one single-chain T-cell receptor (scTCR) were secreted as fusions to GFP using a Saccharomyces cerevisiae expression system. Fusion protein secretion levels ranged over 3 orders of magnitude, from 4 μg/liter to 4 mg/liter, and correlated well with the secretion levels of the unfused scFv/scTCR. Three fusion types with various linker lengths and fusion orientations were tested for each scFv/scTCR. Although the fusion protein secretion levels were not significantly affected by the nature of the fusion construct, the properties of the fusion protein were clearly influenced. The fluorescence yield per fusion molecule was increased by separating the scFv/scTCR and GFP with an extended (GGGGS)3 linker, and fusions with scFv/scTCR at the carboxy-terminus were more resistant to degradation. By evaluating leader sequence processing and using GFP fluorescence to track intracellular processing, it was determined that the majority of fusion protein synthesized by the yeast was not secreted and in most cases was accumulating in an immature, although active, endoplasmic-reticulum (ER)-processed form. This contrasted with unfused scFv, which accumulated in both immature ER-processed and mature post-Golgi forms. The results indicated that yeast can be used as an effective host for the secretion of scFv/scTCR-GFP fusion proteins and that as a result of intracellular secretory bottlenecks, there is considerable yeast secretory capacity remaining to be exploited.
Antibodies are widely used for cell immunostaining, flow cytometry, and cellular localization studies. Although many applications make use of a fluorescently labeled secondary antibody for detection purposes, direct conjugation of antibodies with organic fluorophores can also be used for visualization and quantification. Often, the conjugation of the antibody and fluorophore is performed using primary amine-coupling chemistry that results in the attachment of the fluorophore to lysine residues. However, lysine is frequently found within the antigen binding region, and fluorophore coupling can diminish or completely inhibit antigen binding activity (13, 26). In addition, the environmental sensitivities of organic dyes and their susceptibilities to photobleaching are quite variable. Direct fusion of an antibody to green fluorescent protein (GFP) could overcome these disadvantages. Such a one-step immunoreagent would benefit from the fact that GFP is quite stable over a wide range of pH, temperature, and detergent conditions and is resistant to photobleaching (23). In terms of the antibody subunit of a GFP fusion protein, single-chain antibodies (scFvs) consisting of the variable heavy- and light-chain regions of intact antibodies retain binding activity and can be efficiently processed by microbial production hosts. In addition, scFvs are typically used for antibody selection and engineering, in which many scFvs (10 to hundreds) often are simultaneously isolated (7, 11). Thus, fusion to GFP can enable rapid assessment of scFv binding and specificity properties without the requirement for chemical modification and the difficulties associated with losses in activity (2, 8, 18, 20, 21, 29).
The key to an effective scFv-GFP fusion protein platform is the ability to rapidly take an identified scFv or collection of scFvs and produce them efficiently as GFP fusion proteins in an appropriate host. Expression of scFv-GFP fusion proteins using bacteria has resulted in limited success, with yields of 100 to 200 μg/liter, whether periplasmic secretion or inclusion body methods were used (2, 8, 18, 20, 21). In another system requiring cytoplasmic folding of scFv-GFP fusions, it was possible to increase bacterial yields from 30 μg/liter to 15 mg/liter, but this required scFv mutagenesis for optimized expression properties (29). In addition to bacterial systems, relatively efficient secretion of scFv-GFP fusion proteins at up to 50 to 3,000 μg/liter from transiently transfected mammalian cells and insect cells has been achieved (24). Like mammalian and insect hosts, yeast is an effective eukaryotic expression host with protein quality control machinery helping to ensure that secreted protein is active, although this filter is not perfect (16). In fact, yeast was recently shown to be an effective host for GFP and GFP fusion protein secretion (12), and yeast has been demonstrated to be a more robust system than bacteria for cytoplasmic expression of multidomain GFP fusion proteins (3). Although scFv-yellow fluorescent protein (a variant of GFP) fusions have been expressed in yeast as cytoplasmic, nonsecreted proteins (4), secretion of scFv-GFP fusions in yeast has not been investigated.
Yeast possesses a widely varied capacity for secreting heterologous proteins that ranges from no secretion of a single-chain T-cell receptor (scTCR) (15) and moderate expression of single-chain antibodies (20 mg/liter) (31) to extremely high secretion levels of bovine pancreatic trypsin inhibitor (180 mg/liter) (22), indicating that different proteins may undergo different secretory pathway processing and encounter different secretion bottlenecks (31, 32). Of particular relevance to this study, scFv and scTCR secretion in yeast can vary by several orders of magnitude, from micrograms to milligrams per liter (7, 19, 30, 31). Understanding the differences in secretory processing and identifying the location(s) of the intracellular bottleneck(s) might enable the design of strategies to elevate secretion levels. Along these lines, fusion to GFP has been widely used to trace the cellular localization and dynamics of yeast proteins (10). Thus, fusing an scFv to GFP would also enable facile monitoring of the amount and localization of scFv protein in the secretory pathway, provided that fusion to GFP did not alter the trafficking properties.
In this study, we sought to create a yeast expression platform that would allow rapid production of fusions of GFP and scFv or their highly homologous scTCR counterparts. To this end, we employed the yeast Saccharomyces cerevisiae to produce GFP fusion proteins from a total of nine different scFv/scTCR classes. The secretion vectors were also designed to specifically allow direct shuttling of scFv from an existing nonimmune human yeast surface display scFv library so as to be compatible with antibody engineering selections against soluble antigens, cells, and tissues (7, 27, 35). In an attempt to optimize fusion protein expression, each scFv/scTCR was produced in three forms, in which the fusion protein linker length and fusion protein orientation were varied. In addition, GFP fluorescence was used to quantify the amounts and localizations of intracellularly retained fusion proteins to gain insight into the yeast processing of scFv/scTCR-GFP fusions.
MATERIALS AND METHODS
Strains and media.
Saccharomyces cerevisiae strains BJ5464 (α ura3-52 trp1 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL) (Yeast Genetic Stock Center, Berkeley, CA) or BJ5464 harboring a kex2 deletion (kex2::URA3) (this study) were used for all secretion studies except the heavy-chain binding protein (BiP) and protein disulfide isomerase (PDI) overexpression experiment, for which the PDI-overexpressing yeast strain YVH10 harboring the pMR1341 BiP overexpression plasmid was used (9). Escherichia coli strain DH5α (Invitrogen, Carlsbad, CA) was used for plasmid construction and amplification. Yeast was grown in minimal SD-CAA (20.0 g/liter dextrose, 6.7 g/liter yeast nitrogen base, 5.0 g/liter Casamino Acids, 10.19 g/liter Na2HPO4 · 7H2O, 8.56 g/liter NaH2PO4 · H2O) for 3 days to reach a typical cell density of 10 optical density units at 600 nm. In addition to harboring the secretion plasmid, BJ5464 was cotransformed with an empty pRS-314 plasmid that complements the trp1 deficiency. Protein expression was induced for 3 days in SG-CAA medium (with 20.0 g/liter galactose replacing dextrose). To prevent nonspecific adsorption loss of secreted proteins, the SG-CAA media for scFv and fusion protein expression studies were supplemented with bovine serum albumin or ovalbumin, respectively, at 1 mg/ml.
Plasmids.
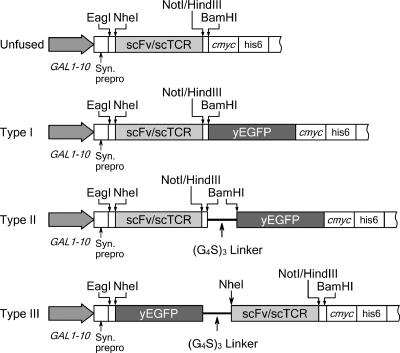
The yEGFP open reading frame (ORF) for the scFv-GFP fusions was obtained from a pADHEGFP plasmid by using standard PCR (5). The codon usage of yEGFP has been optimized for yeast expression, and there are two point mutations in yEGFP (S65G and S72A) that increase fluorescence and stability when expressed in E. coli (5, 6). This yEGFP protein (referred to throughout this paper as GFP) has also been efficiently secreted from yeast (12). The GFP ORF was ligated into the BamHI-single-digested pRS-GALOX26 yeast CEN-based (URA3) secretion plasmid that directs secretion of the unfused anti-transferrin receptor OX26 scFv (9) (Fig. 1). This yielded type I pRS-GALOX26-GFP, which consisted of amino-terminal fusion of OX26 to GFP via a short linker (AAAGS) resulting from the restriction sites present between OX26 and yEGFP (Fig. 1). An oligonucleotide cassette was then used to insert a 15-amino-acid flexible linker (GGGGS)3 between OX26 and yEGFP using the BamHI restriction site to create the type II pRS-GALOX26-GFP plasmid (Fig. 1). The resultant amino acid sequence of the long linker in type II OX26-GFP was AAA(GGGGS)3GS. The LWHI scTCR ORF was obtained from pRS-GALT LWHI (30) by performing standard PCR and was substituted for the OX26 ORF in both type I and II pRS-GALOX26-GFP plasmids through EagI single digestion (NotI has an embedded EagI site) to create type I and II pRS-GALLWHI-GFP plasmids. The same LWHI ORF was then shuttled to NheI/NotI-double-digested pRS-GALOX26 to create pRS-GALLWHI, which encodes the unfused LWHI protein.
FIG. 1.
Schematic of secretion vector expression cassettes for unfused scFv/scTCR and three types of GFP fusion proteins.
To investigate the generality of GFP fusion protein secretion from yeast, six scFv clones were randomly selected from a yeast surface display human single-chain antibody (hAb) library, and the scFv-encoding pPNL6 plasmids (7) were recovered using the Zymo yeast MiniPrep kit (Zymo Research, Orange, CA). To create fusion secretion vectors compatible with the yeast display antibody library, a HindIII restriction site was added between the OX26 and GFP ORFs in both type I and II pRS-GALOX26-GFP plasmids. Then, the six hAb scFvs and scFvA were amplified from pPNL6 plasmids, digested with NheI/HindIII, and ligated to replace OX26 to create type I and II pRS-GALhAb-GFP plasmids. As a consequence of the insertion of the HindIII site, the short linker in type I hAb-GFP (GILEQKLGS) and the long linker in type II hAb-GFP [GILEQKL(GGGGS)3GS] took slightly different forms. The HindIII site was also added to the end of the OX26 ORF in the unfused OX26 scFv secretion vector, pRS-GALOX26, to allow the replacement of the OX26 ORF with the same six human scFvs to create the pRS-GALhAb plasmids, which encode unfused human scFvs.
To create all eight of the type III fusion proteins having the extended (GGGGS)3 linker and reversed orientation with carboxy-terminal fusions of scFv to GFP, standard PCR techniques were used to add the (GGGGS)3 linker to the carboxy terminus of GFP, and NheI sites were added to both ends of the GFP ORF. Subsequently, the modified GFP ORF was inserted into plasmids encoding the unfused scFv/scTCR to create type III pRS-GALGFP-OX26, pRS-GALGFP-LWHI, and six pRS-GALGFP-hAb plasmids at the NheI site (Fig. 1).
The correct sequences and orientations of all of the newly created plasmids were confirmed by DNA sequencing (University of Wisconsin Biotechnology Center). For all three types of fusion proteins, the NotI restriction site exists in the OX26 and LWHI fusion proteins, while the HindIII restriction site exists in the six hAb fusion proteins. For both type I and II fusion proteins, the scFv ORF can be swapped with other ORFs through NheI/NotI or NheI/HindIII double digestion to make new type I or II fusion proteins. For type III, the NheI/NotI or NheI/HindIII subcloning strategy could also be used with NheI partial digestion to make new type III fusion proteins. Any scFvs from the yeast display human scFv library are compatible with the fusion vector system and can be directly shuttled from the library display vector to the fusion vectors as NheI/HindIII cassettes.
Sample preparation and Western blotting.
After 3 days of induction, cell-free supernatant was collected and represented the secreted fraction. Also, 1 × 107 yeast cells were collected for analysis of intracellular protein. For the isolation of intracellular-protein extracts, cells were washed and resuspended in a mixture of 100 μl TCA buffer (20 mM Tris-Cl, pH 7.9, 50 mM ammonium acetate, 2 mM EDTA) and 100 μl 20% (wt/vol) trichloroacetic acid (TCA) supplemented with protease inhibitors (Roche, Mannheim, Germany) and phenylmethylsulfonyl fluoride (ICN Biomedicals, Aurora, OH). The cells were lysed by vigorous vortexing with glass beads. The cell lysate was quantitatively transferred into fresh vials by twice washing the glass beads with 500 μl of a 1:1 mixture of TCA buffer and 20% TCA. Protein was precipitated by centrifugation and solubilized in resuspension buffer (3% SDS, 100 mM Tris base, pH 11, 3 mM dithiothreitol).
For Western blotting, the supernatant or cell lysate was resolved by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 5% stacking gel and a 12.5% separating gel. The proteins were then transferred to a nitrocellulose membrane using an Xcell SureLock Electrophoresis Cell (Invitrogen, Carlsbad, CA). The blots were probed with the anti-c-myc monoclonal antibody, 9E10, as described previously (12). To test for yeast lysis, the supernatant levels of the cytoplasmic enzyme glyceraldehyde 3-phosphate dehydrogenase (G3PDH) were assessed by Western blotting using an anti-yeast G3PDH antibody (Chemicon, Temecula, CA) at a 1:500 dilution. To compare relative protein expression levels, the intensities of the bands on the developed films were measured by using the NIH ImageJ program. The slopes of lines generated by plotting unsaturated band intensities versus exposure time were used for comparisons of relative secretion and intracellular levels. The absolute expression levels of fusion proteins were obtained by comparing their Western blotting signals to those of a known concentration of GFP, which was purified and quantified as described previously (12). Similarly, known quantities of purified OX26 scFv (9) were used as a standard to obtain absolute expression levels of unfused scFvs.
A Storm fluorescence analyzer (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was used to examine and quantify the fluorescences of all three types of secreted hAb3 and scFvA fusion proteins. The fusion proteins were purified using a Ni-nitrilotriacetic acid (NTA) affinity column as described previously (12) and resolved on SDS-PAGE without sample boiling. The fusion protein remained active on this pseudonative gel. The gel was subsequently scanned using the Storm analyzer long-pass filter with excitation of 450 nm and emission of 520 nm.
LWHI fusion protein activity tests.
ImmunoPure agarose beads preimmobilized with protein G (Pierce Biotech, Rockford, IL) were used to test the activities of all three types of LWHI fusion proteins. The protein G beads were first loaded with the 1B2 monoclonal antibody for 2 h and then washed with phosphate-buffered saline (PBS). The beads were then incubated with Ni-NTA-purified type I, II, and III LWHI-GFP fusion proteins (1:4 dilution in PBS) for 2 h, followed by washing them in PBS. Purified type II hAb3-GFP fusion protein was used as the negative control to show the specificity of LWHI binding. The fluorescence of the beads derived from bound LWHI-GFP fusion protein was examined using a fluorescence microscope (Olympus IX70) with excitation of 445 ± 20 nm and emission of 509 ± 24 nm.
For the native isolation of intracellular-protein extracts, cells were washed and resuspended in 100 μl disruption buffer (20 mM Tris-Cl, pH 7.9, 300 mM ammonium sulfate, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 5% glycerol) supplemented with protease inhibitors (Roche, Mannheim, Germany) and phenylmethylsulfonyl fluoride (ICN Biomedicals, Aurora, OH). The cells were lysed by 10 min of vigorous vortexing with 100-μl glass beads. Native dissociation buffer (400 mM Tris, pH 6.8) was added to the cell lysate to a final concentration of 80 mM in fresh vials. The cell lysate was then centrifuged for 30 min at 12,000 × g, and the supernatant was collected as the native extract. The binding activities of all three types of LWHI fusion proteins were tested by using enzyme-linked immunosorbent assays (ELISAs) as described previously (30). An immunosorp plate (Nunc, Rochester, NY) loaded with tetra-His antibody (2.7 μg/ml; QIAGEN, Valencia, CA) was used to capture both secreted and native intracellular LWHI fusion proteins, followed by incubation with biotinylated 1B2 (5 μg/ml; a gift from David Kranz) and then streptavidin-horseradish peroxidase (1:1,000). The samples were developed with a TMB 2-Component Microwell Peroxidase Substrate kit (Kirkegaard and Perry Laboratories), and the absorbance at 450 nm was measured. Absorbance readings versus sample dilution ratios were plotted, and the slopes of the fitted lines were used to obtain the relative binding activity of each sample. The native extract of type II hAb3 fusion protein was used as a negative control. To estimate the percentages of binding activity of intracellular to secreted LWHI fusion proteins, the ELISA signals (slopes) were normalized to their molar concentrations obtained by quantitative Western blotting. The ratio of ELISA signals per mole fusion protein for the intracellular and secreted fractions was used to obtain the percentage of active intracellular protein.
Quantitative fluorescence measurements.
The intracellular-fluorescence levels of the yeast cells expressing fusion proteins were measured using flow cytometry. Yeast cells were harvested after 3 days of induction and washed once with PBS. The fluorescence of the cells was measured using a flow cytometer (BD FACSCalibur) with excitation of 488 nm and emission of 530 ± 15 nm. To estimate the percentage of the total intracellular protein that possessed active GFP, the following measurements were carried out. The relative amounts of total intracellular (I) and secreted proteins (S) were measured by quantitative Western blotting. To minimize experimental errors in protein quantification that might arise from unequal saturation of protein bands, two Western blotting experiments were performed successively for the same proteins. In the second Western blotting experiment, based on their estimated concentrations obtained in the first run, dilution of intracellular protein extracts was performed so that approximately equal amounts of intracellular and secreted proteins were loaded on the same gel side by side.
In parallel, the fluorescences of both the secreted protein (FLS) and cells (FLI) after 3 days of induction were measured using a microplate fluorescence reader (BIO-TEK Instruments; FLx800) with excitation of 485 ± 10 nm and emission of 528 ± 10 nm. Then, assuming that the secreted protein was fully fluorescent, the fraction of the intracellular protein that was fluorescent was determined using the following equation: fraction = (FLI/I)/(FLS/S). The fluorescence yield per molecule for the intracellular fraction of hAb3 and LWHI was then determined as FLI/(fraction × I). The value of FLI was determined both with a plate reader and by flow cytometry, and the resultant fluorescence yields per molecule were very similar. The fluorescence yield per molecule for the secreted fraction was determined as FLS/S, where the secreted fluorescence was measured either with a fluorescence plate reader (LWHI and hAb3) or by Storm fluorescence quantification of the SDS-PAGE gel as described in “Sample preparation and Western blotting” above (hAb3). The levels of fluorescence per secreted hAb3 molecule were very similar using either method. For hAb7 and hAb8, only the fluorescence yield per molecule for intracellular protein was determined because the secreted fluorescence was not measurable at the low supernatant concentrations. Also, the percentages of fluorescent intracellular hAb7 and hAb8 proteins were assumed to be the same as that for hAb3, or 80%.
Flow cytometry analysis of scFvA.
The binding activities of all three types of scFvA fusion proteins were tested by labeling a rat brain endothelium (RBE4) cell line or negative control HEK293 cells in a 24-well plate. The fusion proteins were predimerized by incubating them with the anti-epitope tag antibody 9E10 (molar ratio, 4:1) for 1 h at room temperature. The RBE4 cells were blocked for 30 min with 40% goat serum (diluted in PBS-CM [PBS plus 1 mM CaCl2 plus 0.5 mM MgSO4]) and then incubated with the various dilutions of fusion proteins for 1 h at 4°C. The RBE4 cells were detached by gently scraping the surface of the plate. The GFP fluorescence of propidium iodide-impermeant cells was then measured by using flow cytometry.
Immunocytochemistry and confocal microscopy.
Yeast immunocytochemistry was performed following the protocol described by Pringle and coworkers (25). Cells were fixed for 3 h in 4% methanol and then permeabilized for 30 min at 37°C with beta-mercaptoethanol (0.2% [vol/vol]) and Zymolase (20 μg/ml; Zymo Research, Orange, CA) in solution B (100 mM K2HPO4, 100 mM KH2PO4, and 1.2 M sorbitol). Monoclonal anti-c-myc antibody 9E10 (Covance, Berkeley, CA) at a 1:100 dilution was used as the primary antibody. Anti-mouse Alexa Fluor 555 (Invitrogen, Carlsbad, CA) at a 1:500 dilution was used as the secondary antibody. GFP and Alexa Fluor fluorescences were examined by using a confocal microscope (Bio-Rad MRC-1024) with excitation of 488 nm for GFP and 568 nm for Alexa Fluor 555 and emission of 522 ± 17 nm for GFP and 605 ± 16 nm for Alexa Fluor 555. GFP and Alexa Fluor images were taken sequentially to avoid spectral overlap. The images were then merged using the Confocal Assistant program, and vacuolar localization was assessed by comparison with phase-contrast images. Samples evaluated for GFP fluorescence only were not fixed to preserve the resolution of the GFP fluorescence.
RESULTS
Construction of fusion protein secretion vectors.
Seven scFvs and one scTCR were fused with yeast-enhanced green fluorescent protein (referred to simply as GFP here) to form one-step immunoreagents. One scFv (OX26) (9) and the scTCR (LWHI) (30) were previously characterized as active, secreted yeast products. To test the generality of the fusion protein system, six hAbs were randomly isolated from a yeast surface display human scFv antibody library (7). This particular yeast surface display antibody library has been validated for having antibodies that bind to a variety of soluble antigens, including haptens, peptides, and proteins (7). In the yeast surface display format, the scFvs are tethered to the surface by fusion with the Aga2p protein, and after appropriate scFv selections for antigen binding properties, the unfused scFv proteins can be made as soluble products that retain binding characteristics similar to those seen on the surface of yeast (7, 30). Thus, the only criterion governing the random selection of the six hAbs described here was that they were displayed as full-length products on the yeast cell surface. Each of the scFv/scTCR constructs was then used to create GFP fusion proteins as described below.
Three different constructs (types I, II, and III) (Fig. 1) were created to join the scFv/scTCR and GFP subunits with various linker lengths and fusion orientations. As illustrated in Fig. 1, type I fusion proteins were created by connecting scFv/scTCRs as amino-terminal fusions to GFP, and the two fusion components were separated by only those amino acids resulting from the addition of restriction sites (see Materials and Methods for details). For type II fusions, an additional (Gly4Ser)3 flexible linker was used to further separate the scFv/scTCR and GFP with the intention of minimizing potential folding interference of the two fusion components. Finally, with the idea that the well-expressed and folded GFP protein might enhance productive folding if the scFv/scTCR was instead attached as a carboxy-terminal fusion to GFP, the fusion orientation was switched in type III proteins. In the resultant plasmids, expression of the fusion proteins was under the control of the galactose-inducible GAL1-10 promoter. Secretory processing of the fusion proteins was directed by a synthetic prepro leader sequence. A c-myc epitope and a six-histidine tag were attached to the carboxy termini of the fusion proteins for detection and purification purposes, respectively. The different scFv/scTCRs are referred to as “classes,” and the different fusion constructs are referred to as “types” below.
Comparison of secretion levels of type I, II, and III fusion proteins.
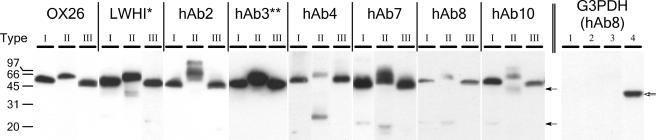
In order to examine the capacity of S. cerevisiae for secretion of the GFP fusion proteins, yeast cells harboring the expression plasmids were induced for 72 h at 20°C. Each of the 24 fusion proteins was successfully secreted into the culture medium as a full-length protein product (Fig. 2). The apparent molecular masses of the type I and III fusion proteins are 57 to 60 kDa (Fig. 2), which are consistent with the calculated molecular masses that range from 57 to 59 kDa. The type II proteins usually resolved at a molecular mass roughly 5 kDa higher than their expected molecular masses. This could possibly be the result of low levels of O-glycosylation as a result of serine introduction into the linker. As demonstrated below, both the binding and GFP domains of type II fusions were active, so the phenomenon was not pursued further. The secreted fusion protein titers ranged from 4 μg/liter to 4 mg/liter (Table 1). For a given scFv/scTCR class, secretion levels of the type I, II, and III fusions were fairly uniform. In contrast, differences in fusion protein secretion levels of up to 3 orders of magnitude were observed between scFv classes (type III hAb3 versus type III hAb8). By comparing the secretion levels of type I and II fusion proteins (Fig. 2, compare lanes 1 and 2 for each species, and Table 1), it was found that the presence of the (G4S)3 linker in type II fusions did not yield a statistically significant effect (P > 0.05) on the secretion level, except for type II hAb3, where secretion was increased threefold versus type I hAb3. In addition, the presence of the (G4S)3 linker in type II fusions resulted in increased levels of degraded product, as was evident from the Western blot signals detected in the 40-kDa range (LWHI and hAb10) or the 25- to 30-kDa range (hAb4, hAb7, and hAb8), with the latter case likely a result of proteolysis within the linker region. Type III proteins, having the fusion orientation changed in the presence of a linker, were not proteolyzed like type II proteins and had secretion levels similar to those observed for type I fusions. To ensure that the supernatant contained proteins that were truly secreted and not a result of yeast cell lysis or leakage, particularly for the low-secretion proteins of the hAb8 and hAb10 classes (Table 1), the amount of the cytoplasm-resident G3PDH protein present in the supernatant was monitored. Comparison of intracellular and extracellular G3PDH levels indicated that the percentages of lysed yeast ranged from undetectable for GFP and hAb8 fusions to 0.1% for OX26 and its fusions (Fig. 2 and data not shown). Thus, general cell lysis was not an appreciable source of supernatant protein, although some contribution cannot be completely ruled out for the very low-secretion hAb8 and hAb10 proteins.
FIG. 2.
Western blots of the 24 fusion proteins secreted from S. cerevisiae. The same volume of each supernatant was evaluated by Western blotting for each fusion type of a given scFv/scTCR class. The same volume of supernatant was loaded for all scFv/scTCR classes except LWHI (*; 4-fold dilution) and hAb3 (**; 10-fold dilution). Table 1 shows quantitative expression level comparisons. Molecular masses are in kDa, and the solid arrows indicate degradation products. The rightmost panel is a Western blot for the intracellular G3PDH protein. Lanes 1 to 3 are triplicate supernatant samples of type III hAb8-secreting yeast, and lane 4 is a cell extract of type III hAb8-secreting yeast to illustrate the intracellular presence of the G3PDH protein (open arrow).
TABLE 1.
Secretion yields of unfused scFv/scTCRs and scFv/scTCR-GFP fusions
| scFv/scTCR class | Secretion level unfused (μg/liter)a | Fusion type | Secretion level fused (μg/liter)a | |
|---|---|---|---|---|
| OX26 | 50 ± 7 | I | 69 ± 44 | |
| II | 48 ± 34 | |||
| III | 60 ± 44 | |||
| LWHI | 2,100 ± 100 | I | 1,200 ± 200 | |
| II | 1,100 ± 200 | |||
| III | 790 ± 260 | |||
| hAb2 | 75 ± 6 | I | 41 ± 5 | |
| II | 35 ± 8 | |||
| III | 51 ± 2 | |||
| hAb3 | 4,800 ± 200 | I | 1,300 ± 500 | |
| II | 4,000 ± 300 | |||
| III | 1,800 ± 100 | |||
| hAb4 | 80 ± 16 | I | 40 ± 8 | |
| II | 20 ± 11 | |||
| III | 55 ± 14 | |||
| hAb7 | 81 ± 12 | I | 270 ± 100 | |
| II | 140 ± 78 | |||
| III | 160 ± 44 | |||
| hAb8 | 23 ± 11 | I | 15 ± 10 | |
| II | 4 ± 3 | |||
| III | 27 ± 14 | |||
| hAb10 | 20 ± 8 | I | 36 ± 8 | |
| II | 13 ± 10 | |||
| III | 20 ± 7 | |||
| scFv A | 4,000 ± 1,100 | I | 1,500 ± 150 | |
| II | 2,900 ± 900 | |||
| III | 2,400 ± 700 | |||
| yEGFP | 3,000 ± 145 | NAb | NA |
Triplicate cultures were analyzed for each construct, and values represent means±standard deviations. The final cell density was nearly the same for each construct, and therefore, comparison by volumetric yields is similar to comparison by per-cell yields.
NA, not applicable.
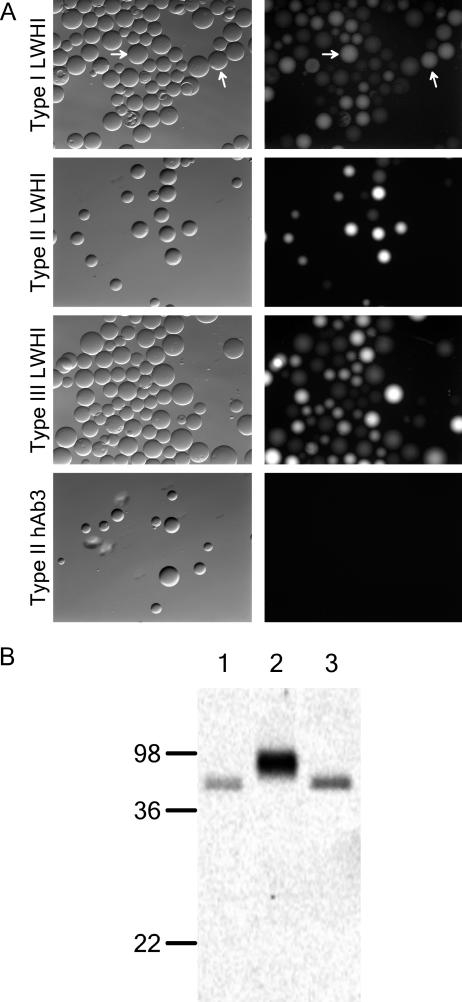
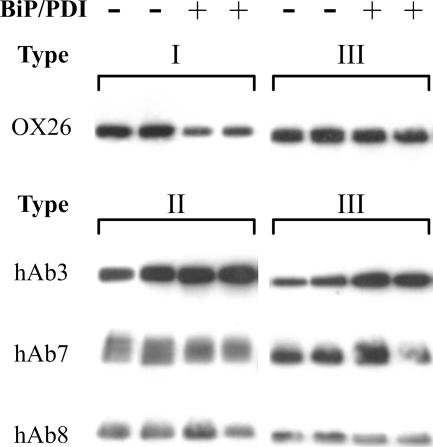
Next, type I, II, and III fusions for the LWHI scTCR were purified using an Ni-NTA affinity column and tested for activity. Since LWHI has a known binding partner, the 1B2 monoclonal antibody that mimics the natural peptide-major histocompatibility complex ligand, the LWHI fusions were used to test both the binding activity and fluorescence of secreted fusions (see Materials and Methods for details). As Fig. 3A indicates, the LWHI fusion component mediated binding to 1B2-coated agarose, while the GFP portion provided a strong fluorescent signal for each fusion type. In contrast, the nonspecific type II hAb3 fusion exhibited no binding to the 1B2-loaded beads. Thus, both fusion partners were properly folded and active for each type of fusion. Since the antigens for the hAb scFv proteins are unknown, as they were randomly selected from a nonimmune human scFv library (7), purified hAb3 fusion proteins could be tested solely for their fluorescence. After resolving the hAb3 fusions on a nonboiling SDS-PAGE gel that preserved GFP fluorescence, the intact gel was excited using 450-nm light and the fluorescence emission at 520 nm was monitored. The results shown in Fig. 3B indicated that the full-length proteins were indeed fluorescent. As discussed below, all of the other fusion proteins were also found to be fluorescent.
FIG. 3.
Fusion protein activity tests. (A) LWHI-GFP binding to 1B2-loaded agarose beads. (Left) Phase-contrast images. (Right) Fluorescence images. A type II hAb3 fusion was used as a negative control to evaluate the specificity of LWHI binding. The arrows indicate two sample agarose beads in the phase-contrast and fluorescence images. (B) Fluorescence scan of SDS-PAGE-resolved hAb3 fusions. Lane 1, type I; lane 2, type II; lane 3, type III. Equal volumes of purified protein were loaded in each lane, and the differences in band intensity are largely the result of differences in the purified yield. (Fluorescence per molecule also varied.) Molecular masses are in kDa. See Materials and Methods for experimental details.
Comparison of fusion protein secretion levels with those of unfused scFv/scTCR.
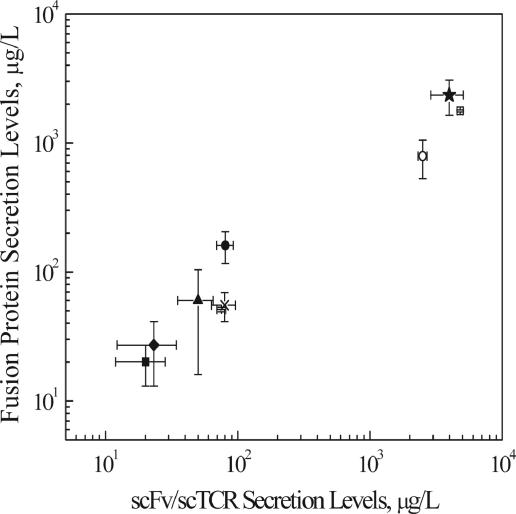
The unfused scFv/scTCR and GFP were secreted using identical expression vectors, yeast strains, and induction conditions (Table 1). Although the unfused GFP was efficiently secreted at 3 mg/liter (Table 1), it did not help to increase the production levels of the fused scFv/scTCR. Rather, the secretion fitness of the unfused scFv appeared to regulate the expression level of the fusion proteins. Type I, II, and III fusion protein secretion levels correlated very well with the unfused scFv/scTCR secretion levels over 3 orders of magnitude regardless of the fusion orientation or linker length (Fig. 4). In addition, the absolute fusion protein secretion levels were on the same order as those for the unfused scFv/scTCR partner (averaged over all types: 0.4 mg fusion protein/mg unfused scFv on a mass basis or 0.2 mol fusion protein/mole unfused scFv on a molar basis). Therefore, using this system, one can predict the approximate expression level of the fusion protein by knowing the secretion characteristics of the protein that is to be fused to GFP.
FIG. 4.
Correlation between type III secretion levels and those of unfused scFv/scTCRs. Secretion data are from Table 1, and the correlation coefficient (r2) for type III was 0.99. Not pictured are type II (r2 = 0.89) and type I (r2 = 0.94). ▴, OX26; ○, scTCR; -, hAb2; ×, hAb4; □, hAb3; •, hAb7; ⧫, hAb8; ▪, hAb10; ★, scFvA (scFvA was not used in determining correlation). Data represent means ± standard deviations (n = 3).
Given that the expression level appeared to be dictated by the secretion properties of the scFv/scTCR fusion partner, the ER folding environment was modulated in a way that had previously been shown to enhance scFv secretion. Yeast strains overexpressing BiP and yeast PDI were used to secrete a subset of the fusion proteins (Fig. 5). Although overexpression of BiP and PDI increases unfused OX26 scFv secretion 10-fold (9), the type I OX26 secretion level was actually decreased (1.6- ± 0.1-fold; P < 0.03) and the type III OX26 secretion level was unchanged. This indicated that although the scFv secretion fitness determines the fusion protein expression, fusion to GFP affects its intracellular processing so that it modifies its interactions with the ER folding environment. Similarly, for other scFvs with low production levels (hAb7 and hAb8), the overexpression of BiP and PDI had no effect on secretion regardless of the fusion orientation. The lone exception was the well-expressed type III hAb3 fusion, which exhibited a modest increase in secretion (1.2- ± 0.04-fold; P < 0.05) upon BiP and PDI overexpression. In addition, overexpression of BiP and PDI had no effect on unfused GFP secretion (data not shown), indicating that fusion to the scFv was responsible for the differential secretion in these engineered strains.
FIG. 5.
Effects of BiP and PDI overexpression on fusion protein secretion levels. The fusion type is indicated above each set of Western blot data. Statistically significant differences in expression are detailed in the text.
Test of fusion platform using cell surface binding scFv.
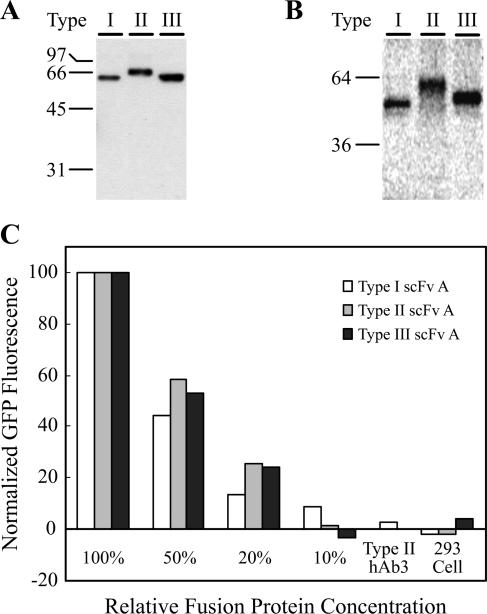
Although the randomly selected hAbs allowed us to establish a working system for the expression of GFP fusions, we also wished to test the platform using an scFv that was isolated from the human nonimmune scFv library based on its binding characteristics. In this way, the fusion protein expression and activity characteristics could be examined in the spirit of a true antibody selection experiment. The scFv, known as scFvA, was isolated from the library in a screen designed to identify scFvs that bound to the plasma membranes of brain endothelial cells (unpublished results). Secreted unfused scFvA protein binds specifically to the surfaces of brain endothelial cells, and scFvA fusions with GFP could therefore be tested for binding activity. ScFvA was subcloned into the fusion protein expression vectors to create type I, II, and III fusions with GFP, and yeast cells harboring the expression plasmids were used to secrete unfused scFvA and each type of fusion protein. The fusion protein secretion levels were similar to those predicted by the correlations shown in Fig. 4, validating the robust nature of fusion protein secretion in yeast (Table 1, Fig. 4, and Fig. 6A). Each of the fusion protein types was not only secreted as a full-length product, but also possessed active GFP fusion moieties (Fig. 6B). Finally, the activity of the scFvA portion of the fusion was tested by labeling the surfaces of live brain endothelial cells and assessing the labeling specificity using flow cytometry. As Fig. 6C indicates, each type of fusion protein specifically labeled the surfaces of the endothelial cells, but not control cells, and the interaction was concentration dependent. Overall, the test with scFvA indicated that active full-length fluorescent fusions could be successfully produced as predicted using the yeast fusion platform.
FIG. 6.
Test of GFP fusion platform using endothelial cell surface binding scFvA. (A) Western blot of equal volumes of supernatant containing scFvA fusion proteins. Molecular masses are expressed in kilodaltons. (B) Fluorescence scan of nonboiling SDS-PAGE of purified scFvA fusions. (C) Flow cytometric analysis of scFvA fusion protein binding to rat brain endothelial (RBE4) cells. Purified fusion protein was used to immunolabel the surfaces of live RBE4 cells or negative control HEK293 cells (at 100% fusion concentration), and the cell surface fluorescence was assessed by flow cytometry. The percent binding was independently normalized for each fusion type to the signal at maximum concentration (100%; ∼250 nM), and the type II hAb3 fusion protein was used at 250 nm as a negative control to indicate the specific nature of the fluorescence signal. The absolute magnitudes of the maximum fluorescent signals for each fusion type were within 20% of each other. The data are representative of triplicate independent flow cytometry experiments.
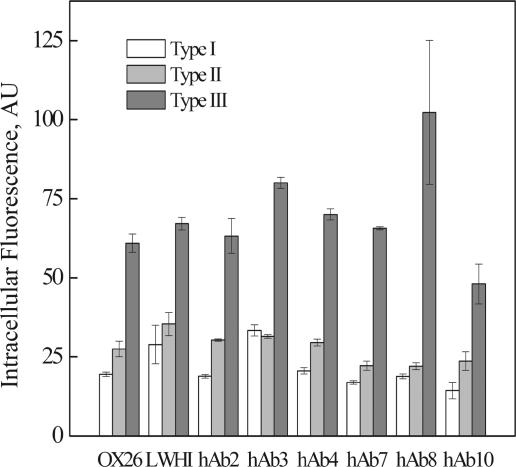
Evaluation of intracellular fusion protein accumulation.
Although secretion levels varied dramatically for fusion proteins paired with different scFv/scTCRs, flow cytometric analysis of the secreting yeast cells indicated that intracellular fluorescence did not differ between the scFv/scTCR classes (Fig. 7). This finding suggested that intracellular retention levels of fluorescent fusion proteins were very similar. In addition, within an scFv/scTCR class, the GFP fluorescence for type III proteins was reproducibly higher than that of types I and II. This could be a result of either more total fusion, more fluorescent fusion, or a higher fluorescence yield per molecule. To determine which hypothesis was most accurate, quantitative Western blotting of intracellular fusion proteins was performed. Four representative fusion classes were analyzed to compare the secretion behaviors of fusions that were secreted at high (hAb3 and LWHI), intermediate (hAb7), and low (hAb8) levels. The levels of total protein retained intracellularly were very similar for each class of fusions (10 to 15 mg/liter), regardless of secretion efficiencies that varied over 3 orders of magnitude (Table 2), and this finding helps explain why the intracellular fluorescences were very similar between the LWHI, hAb3, hAb7, and hAb8 scFv/scTCR classes. Within a class, type II fusions reproducibly exhibited less accumulation.
FIG. 7.
Intracellular fluorescence of yeast cells expressing fusion proteins. The fluorescence intensity was measured after 3 days of induction using flow cytometry. The identities of the scFv/scTCRs are given on the x axis. Triplicate cultures were analyzed for each fusion protein. Data represent means ± standard deviations.
TABLE 2.
Intracellular accumulation of full-length fusion proteins and unfused scFv/scTCR and GFP
| ScFv/scTCR class | Unfused
|
Fused
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Secretion level (μg/liter) | Intracellular (μg/liter)a | Intracellular/ secretion | Fusion type | Secretion level (μg/liter) | Intracellular (μg/liter)a | Intracellular/ secretion | Relative fluorescence/moleculeb
|
||
| Intracellular | Secreted | ||||||||
| hAb3 | 4,800 ± 200 | 7,700 ± 1,600 | 1.6 ± 0.3 | I | 1,300 ± 500 | 15,000 ± 1,000 | 11 ± 0.8 | 1.00 ± 0.02 | 1.00 ± 0.01 |
| II | 4,000 ± 300 | 11,000 ± 2,900 | 2.6 ± 0.7 | 1.35 ± 0.09 | 1.57 ± 0.23 | ||||
| III | 1,800 ± 100 | 15,000 ± 800 | 8.4 ± 0.5 | 2.20 ± 0.04 | 2.19 ± 0.19 | ||||
| hAb7 | 81 ± 12 | 5,400 ± 800 | 67 ± 10 | I | 270 ± 100 | 15,000 ± 2,000 | 55 ± 7 | 1.00 ± 0.02 | ND |
| II | 140 ± 78 | 9,200 ± 200 | 66 ± 14 | 1.89 ± 0.21 | ND | ||||
| III | 160 ± 44 | 12,000 ± 700 | 75 ± 4 | 6.25 ± 0.78 | ND | ||||
| hAb8 | 23 ± 11 | 6,700 ± 1,900 | 290 ± 83 | I | 15 ± 10 | 14,000 ± 4,500 | 940 ± 300 | 1.00 ± 0.08 | ND |
| II | 4 ± 3 | 7,300 ± 2,000 | 1,800 ± 500 | 2.70 ± 0.22 | ND | ||||
| III | 27 ± 14 | 12,000 ± 1,600 | 450 ± 59 | 4.19 ± 0.37 | ND | ||||
| LWHI | 2,100 ± 100 | 1,500 ± 500 | 0.7 ± 0.2 | I | 1,200 ± 200 | 15,000 ± 5,000 | 12.5 ± 4.2 | 1.00 ± 0.02 | 1.00 ± 0.06 |
| II | 1,100 ± 200 | 11,600 ± 2,500 | 10.5 ± 2.3 | 2.11 ± 0.30 | 2.04 ± 0.27 | ||||
| III | 790 ± 260 | 9,900 ± 1,500 | 23.1 ± 1.9 | 6.95 ± 0.47 | 3.61 ± 0.37 | ||||
| YEGFP | 3,000 ± 145 | 11,300 ± 1,900 | 3.8 ± 0.6 | ||||||
Triplicate cultures were analyzed for each condition, and all secretion and intracellular levels were determined by Western blotting. Values represent means±standard deviations.
Relative fluorescence yields were normalized to the type I fusion protein for each species. ND, not determined.
Intracellular fusion protein levels were much higher than those of their secreted counterparts, and ratios of intracellular to secreted fusion proteins ranged from 2.6-fold for type II hAb3 to 1,800-fold for type II hAb8 (Table 2). This trend also held for the unfused hAb3, hAb7, and hAb8 scFvs and LWHI scTCR, as well as for GFP (Table 2). These data suggested that a significant intracellular secretory bottleneck existed that was generalizable for both the unfused partners and each type of fusion protein regardless of secretory fitness. However, fusion proteins clearly inherited from the parent scFv/scTCR differing capacities to pass through the bottleneck, and hence, dramatic differences in the secreted products were observed.
Since the total amounts of intracellular protein do not differ for type I, II, and III proteins but the intracellular fluorescence levels do, either the levels of fluorescent protein differ or the fluorescence yields per molecule differ. To address these possibilities, the fluorescent fraction of intracellular protein for each of the types of hAb3 and LWHI fusions was determined (see Materials and Methods for details). The percentages of fluorescent intracellular protein were calculated to be 83% ± 4% (type I hAb3), 81% ± 4% (type II hAb3), and 84% ± 8% (type III hAb3), and the differences between these values were found to be statistically insignificant. Similar results were observed for LWHI, where all types were retained on average in a 65% fluorescent form (64% ± 3% type I, 68% ± 18% type II, and 61% ± 10% type III). Therefore, the difference in intracellular fluorescence between type I, II, and III fusions was not a result of different amounts of fluorescent protein. To test the second theory, the relative GFP fluorescence levels per molecule of hAb3 protein were determined for both the fluorescent intracellular fusion protein and the secreted fusion protein (Table 2) (see Materials and Methods for details). The fluorescence per molecule was the same whether intracellular or secreted protein was evaluated, with type III hAb3 fusions emitting the most fluorescence per molecule, in 2.2-fold excess of that seen for type I fusions (Table 2). In addition, the type III hAb3 fusions possessed almost the same fluorescence per molecule (70% ± 9%) as unfused GFP. The estimated fluorescence yields for hAb7, hAb8, and LWHI showed similar trends in fluorescence as a function of the linker length and orientation (Table 2). The differences in fluorescence yield, combined with the differences in accumulation of type II protein, fully account for the trends in intracellular fluorescence between fusion types observed in Fig. 7.
Finally, we wished to determine whether the intracellularly retained protein was simply fluorescent or fully active as defined by the binding competence of the scFv/scTCR fusion partner. To address this question, native cell extracts from yeast cells secreting LWHI fusions were prepared and the binding activity of the intracellular protein was quantitatively assessed by ELISA using the 1B2 activity ligand (see Materials and Methods for details). It was determined that a large percentage, 45% on average, of the intracellular fusion protein possessed an active binding domain (45% ± 6% type I, 57% ± 20% type II, and 38% ± 10% type III). Thus, a large amount of fully active LWHI protein (binding and fluorescent) was being retained inside the cell and was subject to a secretory bottleneck.
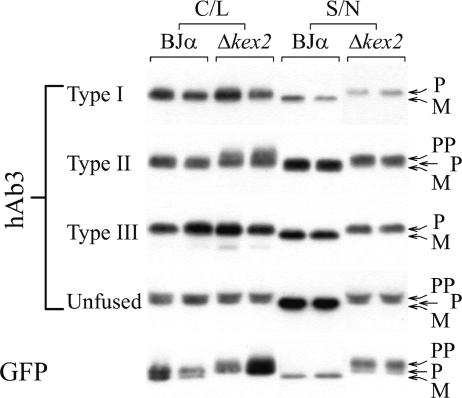
Investigation of the secretory bottleneck.
Secretion of protein began to plateau during day 2 of secretion, and no additional accumulation was seen by day 3 (data not shown), at which time the retained intracellular-protein levels were substantial (Table 2). Therefore, we investigated 3-day-induced yeast to evaluate this terminal bottleneck where all additional secretion was blocked. First, to identify candidate localizations of the secretion bottleneck, the molecular weights of the accumulated intracellular proteins were examined by Western blotting (Fig. 8). As mentioned earlier, the expression system used in this study possesses a prepro leader sequence to direct secretory processing. The pre region supports translocation of the prepro protein (PP) into the ER, at which time it is proteolytically removed. The pro protein (P) retains the pro region through the secretory pathway until the endogenous Kex2p protease removes it in the trans-Golgi to yield mature protein (M). Thus, using a Kex2p deletion strain (Δkex2) that is deficient in pro region processing, it was possible to accurately evaluate whether intracellular or secreted proteins possessed pro regions. In this way, it was deduced that secreted, unfused scFv and scTCR, as well as type I, II, and III fusion proteins, were primarily in the M form, with low levels of P protein (<5%) (Fig. 2 and 8). This indicated that, for the most part, yeast required full processing of the pro region for the unfused and fused proteins to be secreted. In contrast, retained intracellular protein for the unfused scFv/scTCRs (hAb3, hAb7, hAb8, and LWHI) and GFP consisted of all three possible isoforms, PP, P, and M (Fig. 8 and data not shown). The P and M forms were the dominant isoforms, often present in approximately equal amounts. Thus, the unfused proteins were retained as both ER-processed and mature Golgi-processed forms. By comparison, intracellular type I and type III fusions (hAb3, hAb7, and hAb8) were found mainly in the P form, with no detectable M protein, indicating that for these fusions, mature protein is nearly quantitatively exported while the retained protein is in an ER-processed form. Retained type II GFP fusions (hAb3, hAb8, and LWHI) exhibited different behaviors in that the retained protein was equally split between P and M forms. From these data, it was apparent that fusion with GFP affected the intracellular processing of the fusion partner and that different fusion constructs could yield altered intracellular processing. Finally, unlike the type I and III scFv fusions (hAb3, hAb7, and hAb8), which were predominantly retained in the P form, type I and III LWHI fusions were retained in both the P and M forms, indicating that the fusion partner also plays a role in dictating intracellular processing.
FIG. 8.
Analysis of the processing of intracellular and secreted proteins. C/L, cell lysate; S/N, supernatant. Duplicate Western blotting signals are depicted for each condition. The intracellular fractions were diluted substantially prior to being loaded onto the gels to equalize Western blotting signals between intracellular and secreted proteins. Table 2 shows a quantitative comparison.
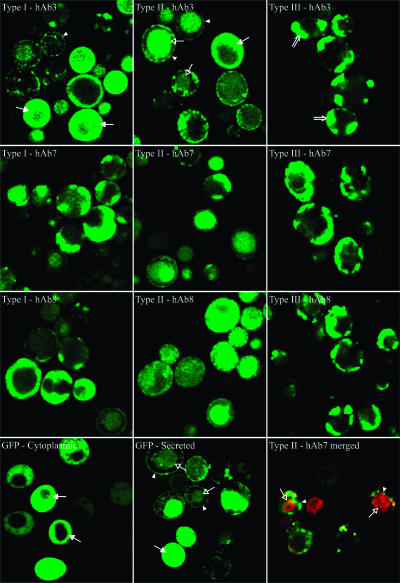
Intracellular proteins detected by Western blotting using the carboxy-terminal c-myc epitope were full length, with no evidence of proteolytic degradation products (data not shown). Thus, the fluorescent properties of the retained fusion proteins were used to examine the general intracellular spatial distribution of full-length protein. As we described in a previous study, unfused GFP was found throughout the secretory pathway and localized in punctate structures at the cell surface (Fig. 9, compare cytoplasmic- and secreted-GFP images) (12). In addition, fluorescent protein was also found in the vacuole. Fluorescent type I hAb3 fusions could also be found distributed near the cell surface, but more cells displayed a putative cytoplasmic localization (Fig. 9). Fluorescent type II hAb3 fusions were distributed in a pattern similar to that of unfused GFP, as suggested by the accumulation of P and M forms for both the GFP and type II hAb3 fusion proteins. However, type II fusions did exhibit more vacuolar localization than unfused GFP or type I or III fusions. Finally, fluorescent type III hAb3 fusions, although similar to type I fusions in sizing experiments, were localized in large, highly fluorescent structures near the cell surface. The same general trends in intracellular localizations of fluorescent fusions were observed for hAb7 and hAb8 fusion proteins (Fig. 9). To examine whether full-length nonfluorescent fusion protein could be detected in the cells, indirect immunofluorescence resulting from labeling of the carboxy-terminal c-myc epitope was compared with the distribution of GFP fluorescence. The fluorescent signals were found to be colocalized for each fusion type of hAb3, hAb7, and hAb8 (Fig. 9 and data not shown). As exemplified by Fig. 9, the only noticeable difference in intracellular localization was that epitope labeling in the absence of GFP fluorescence could be detected in the vacuole. Since no degradation products were apparent on the intracellular Western blots using the same antibody as that employed for the confocal microscopy (data not shown), the vacuolar accumulated product was either very small fragments (<2 kDa) or full-length but nonfluorescent fusion protein.
FIG. 9.
Representative confocal images of cells expressing GFP and fusion proteins. The cells were induced for 3 days prior to microscopy. GFP was expressed in both cytoplasmic and secretion systems for comparison with intracellular distributions of fluorescent fusion proteins. For type II hAb7, total protein was labeled with an anti-c-myc antibody, followed by anti-mouse Alexa Fluor 555 (red), and GFP fluorescence (green) was used to monitor the localization of fluorescent fusion proteins. Alexa Fluor 555 (red) and GFP (green) fluorescences were merged to show colocalization. Typical patterns of GFP fluorescence are indicated as follows: arrowheads, small surface punctate; open arrows, vacuolar localization; solid arrows, cytoplasmic localization; double-line arrows, large surface punctate.
DISCUSSION
The goals of this study were to build a yeast platform for the secretion of a variety of scFv/scTCR GFP fusion proteins and to understand the effects that fusion protein construction can have on intracellular fusion protein processing. A large collection of 27 GFP fusion proteins having scFv/scTCR fusion partners representative of a wide range of secretion fitnesses was analyzed. It was discovered that the fusion secretion levels were governed by scFv/scTCR secretion fitness, rather than by GFP, linker length, or fusion orientation. In addition, type III fusions were the most fluorescent with the least amount of observed degradation, and therefore, they represent the recommended construct for secretion of GFP fusions from yeast. Finally, fusion to GFP clearly affected the intracellular processing of the scFv/scTCR, and in particular helped promote the exit of mature protein from the cell. Finally, large amounts of fully active fusion protein accumulated inside the cell as a result of secretory bottlenecks.
The GFP fusion protein expression vectors were designed to facilitate rapid cloning with any desired secretory partner. In particular, when considering immunoreagents, like those produced in this study, scFvs are often isolated from recombinant antibody libraries. Hence, upon isolating an scFv from a library, fusion to GFP and expression at reasonable yields can facilitate downstream analysis of scFv-antigen interactions. The expression vectors shown in Fig. 1 were designed to be especially compatible with a nonimmune human scFv yeast surface display library, where selected scFvs can be shuttled simply via NheI/HindIII restriction digestion. This system was used to produce fusion proteins secreted at levels ranging from 4 μg/liter to 4 mg/liter. As a comparison to alternative expression systems, scFv-GFP fusion proteins have been expressed in bacteria at a level of 100 to 200 μg/liter (2, 8, 18, 20, 21, 29), and scFvs fused with either GFP or Discosoma red fluorescent protein have been successfully secreted from human 293T cells and SF9 insect cells with yields from 0.1 to 3 mg/liter (24). In this study, it was revealed that the fusion protein secretion level is directly related to that of scFv when expressed alone. This strong correlation indicated that in order to obtain high-level secretion of any type of fusion protein, the secretion level of scFv also needs to be high. The converse is also true, with the implication that an scFv with a high secretion level could be expected to yield high levels of secreted fusion protein. A correlation between the expression levels of unfused scFv and those of scFv-GFP fusions was also observed previously, as directed evolution of the scFv portion for improved expression yielded increased fusion expression in bacteria (29). Although fusion partners can increase protein solubility, stability, and often expression, particularly in bacteria, the well-expressed and soluble GFP did not rescue scFv secretion levels in this study.
Both the scFv and GFP modules of all three types of fusion protein were active, as demonstrated for LWHI and scFvA fusions. The activity of yeast-secreted OX26 has been previously shown (9), and the randomly chosen hAbs were used to test the generality of the secretion system but were not tested for their binding activities, as their antigens were unknown. The hAbs were specifically chosen because they were successfully displayed on the surface of yeast, indicating efficient processing through the secretory pathway. Indeed, properly displayed scFv and scTCR usually yielded secreted protein products that were soluble and active (7, 30). In addition, each hAb fusion type was both full length and fluorescent for every hAb tested. Previously, Waldo and coworkers found that when protein-GFP fusions were expressed in E. coli, GFP could be used as a folding reporter with the ability to correlate proper fusion partner folding with the presence of GFP fluorescence (34). Therefore, it is likely that the fluorescent scFv fusion proteins assembled from hAb chosen in yeast display binding selections would be secreted proteins that retained their binding activities, as demonstrated for the scFvA test antibody. Of course, one cannot rule out the possibility that fusion with GFP could yield an unsecreted product; however, the existence of three types of fusion constructs would likely assist in overcoming such a hurdle. Finally, although the general binding activities of the resultant fusions were demonstrated, the detailed effects of GFP fusion on affinity were not explored, as they are likely antibody dependent.
When designing a fusion protein, it is essential to examine the composition and length of the linker to ensure that the two newly joined proteins can fold independently and retain activity. Too short a linker could lead to folding interference and inactive fusion protein, while excessively long linkers may make fusions more susceptible to proteolysis in the secretory pathway. Numerous linkers have been tested in constructing GFP fusion proteins; the list includes AAA (8), GGGG (2), HHHHHH (20), and (GGGGS)4 (24). In our type I protein constructs, short peptides that arose from restriction site insertion were used to connect the two fusion components (GILEQKLGS for hAbs and AAAGS for OX26 and LWHI). Type II fusion proteins differ only by an extra (GGGGS)3 flexible linker. Although the secretion yields were not substantially different for these two forms, type II fusions demonstrated higher fluorescence per molecule, indicating less interference between the folded protein domains. However, the extended linker in type II proteins yielded substantially more proteolyzed product in the secreted fraction. Degraded scFv-GFP fusions have also been observed from bacterial production systems (18). Placing GFP upstream of the scFv could potentially facilitate the folding and processing of its fusion partner in the secretory pathway, as the efficiently secreted GFP may begin to fold first upon translocation into the ER. As an example, Casey and coworkers fused GFP to an scFv specific for hepatitis B surface antigen at both the amino and carboxy termini and found that the form with GFP at the amino terminus was more stable than the carboxy-terminal GFP fusion, as indicated by increased accumulation of active fusion in the bacterial periplasm (2). Similarly, reversal of fusion partners, with GFP forming the amino terminus in type III fusions, eliminated detectable degradation in the secreted products (Fig. 2). However, reversal of the fusion partners did not increase protein secretion levels. Type III fusions also had the highest relative fluorescence yield per molecule, and GFP fluorescence for the fusions was 70% of that of unfused GFP. The fluorescence yield increased as the linker lengthened between type I and type II proteins. There was then a further increase in fluorescence from type II to type III fusion proteins that could have been the result of the reversed orientation of type III or because the carboxy terminus of GFP extends away from the β-barrel structure, in essence further lengthening the (GGGGS)3 linker of type III fusions. The linker length argument is also supported by the fact that fusions having GFP at the amino terminus but having only a short GGGG linker produced fusions with fluorescence yields that were only 15% of that for unfused GFP (2).
GFP has been widely used as a probe for intracellular dynamics and localization. Given that the secretion efficiencies of both unfused scFv and scFv fusions varied dramatically from one scFv class to another, the possibility of using GFP as a probe for the secretory pathway for heterologous proteins was investigated. First, the BiP and PDI overexpression studies indicated that the fusion to GFP significantly affects the ER folding process, as exemplified by the fact that secretion of unfused OX26 scFv is increased 10-fold in the presence of overexpressed BiP and PDI, while scFv-GFP fusions actually cause BiP and PDI overexpression to decrease secretion (type I) or leave it unchanged (type III). Indeed, fusion to GFP also appeared to affect the intracellular processing based on the differential accumulation of precursor molecules that retain pre or prepro leader sequences. Although both the unfused and fused products were secreted as predominantly mature proteins, the retained intracellular proteins differed in their levels of processing. While unfused scFv/scTCR and GFP could be found significantly accumulated in a mature form that lacked a pro region and was Golgi processed, type I and III scFv fusions were retained only in a pro form that was ER but not Golgi processed. In contrast, type II fusions were retained in both mature and pro forms much more reminiscent of the unfused scFv/scTCR and GFP. These effects were further confirmed by confocal microscopy, which indicated that the different types of fluorescent fusion proteins also possessed distinct intracellular-localization patterns. In addition, although the different fusion types appeared to mediate different trafficking behaviors, the fusion partner also played an important role, as each type of LWHI scTCR fusion, unlike the type I and III scFv fusions, was retained as both mature and pro forms. Another study has also demonstrated that GFP and hexokinase-GFP fusions have different trafficking behaviors and that simply changing secretion leader peptides can route GFP and hexokinase-GFP fusions to different cellular compartments (17). However, GFP can serve as a reasonable secretory probe in certain situations, such as the heterologous production of membrane-localized G-protein-coupled receptors, where the fused and unfused receptors were localized in very similar fashions (1). Taken together, these findings indicate that while fusion to GFP is an enticing approach for monitoring the secretion of heterologous proteins from yeast, one needs to exercise care in interpreting findings, as GFP may play a major role in the regulation of secretion.
Secretion of heterologous proteins is often limited by various intracellular bottlenecks. The endoplasmic reticulum has often been recognized as a bottleneck for foreign-protein secretion in yeast (31, 32). Manipulation of levels of ER chaperones and foldases improves secretion levels for many yeast foreign proteins, including scFv (9, 28, 31, 33), but overexpression of ER chaperones and foldases does not always help foreign-protein secretion (30, 33). Similarly, BiP and PDI did not significantly affect the secretion of fusion proteins in this study, and large amounts of protein were retained intracellularly in a terminal bottleneck. Type I and III fusions accumulated primarily in an ER-processed form that still possessed the pro region, suggesting that the protein had not yet passed through the trans-Golgi. Similarly, approximately 50% of the intracellular protein for the type II fusions, as well as for the unfused scFvs and GFP, was also found to be accumulated with the pro region. However, in contrast with the type I and III fusions, the remaining intracellular product was found to be in a fully trans-Golgi-processed form. Based on these findings, the proposed hypothesis is that unfused scFvs experience both an ER and a post-Golgi bottleneck, while fusion to GFP (types I and III) can render the ER checkpoint dominant. Indeed, intracellular retention of scFv in the ER was observed in previous studies that demonstrated pro-scFv accumulation in yeast (31) and scFv colocalization with the ER-resident chaperone BiP (14). In addition, GFP fusion of a G-protein-coupled receptor (substance P receptor) has been shown processing through the late Golgi, where like the unfused GFP and GFP fusions in this study, it could be found localized in punctate secretory vesicles just below the plasma membrane (1). Finally, the total molar amounts of retained type I and III fusions were essentially the same as those for the unfused scFv/scTCR partner (Table 2), suggesting that processing, and not synthesis or degradation rates, is affected by GFP fusion. In contrast, type II fusions appeared to have less total accumulated protein, with more yeast exhibiting vacuolar accumulation, potentially indicating a higher degradation rate for type II fusions. This theory is also supported by the fact that type II fusions displayed more proteolyzed product in the secreted fraction. Although one cannot completely rule out effects of degradation or transcription/translation effects, it is clear that large amounts of full-length fluorescent (hAb3 and LWHI) and active (LWHI) fusion proteins accumulate inside the yeast cell. Therefore, methods that could help ensure proper folding and/or processing of these proteins could greatly enhance production yields. Furthermore, in addition to studies aimed at optimizing ER folding conditions, effort should also be focused on alleviating the post-Golgi bottleneck that results in accumulation of mature protein to fully optimize yeast for heterologous protein secretion.
Acknowledgments
We thank the members of the University of Wisconsin—Madison W. M. Keck Laboratory for their assistance with confocal microscopy.
This work was funded by a Whitaker Biomedical Engineering Foundation Grant (RG-02-0077) and a National Science Foundation CAREER Award (BES-0238864).
Footnotes
Published ahead of print on 13 October 2006.
REFERENCES
- 1.Butz, J. A., R. T. Niebauer, and A. S. Robinson. 2003. Co-expression of molecular chaperones does not improve the heterologous expression of mammalian G-protein coupled receptor expression in yeast. Biotechnol. Bioeng. 84:292-304. [DOI] [PubMed] [Google Scholar]
- 2.Casey, J. L., A. M. Coley, L. M. Tilley, and M. Foley. 2000. Green fluorescent antibodies: novel in vitro tools. Protein Eng. 13:445-452. [DOI] [PubMed] [Google Scholar]
- 3.Chang, H. C., C. M. Kaiser, F. U. Hartl, and J. M. Barral. 2005. De novo folding of GFP fusion proteins: high efficiency in eukaryotes but not in bacteria. J. Mol. Biol. 353:397-409. [DOI] [PubMed] [Google Scholar]
- 4.Colby, D. W., Y. Chu, J. P. Cassady, M. Duennwald, H. Zazulak, J. M. Webster, A. Messer, S. Lindquist, V. M. Ingram, and K. D. Wittrup. 2004. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody. Proc. Natl. Acad. Sci. USA 101:17616-17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormack, B. P., G. Bertram, M. Egerton, N. A. Gow, S. Falkow, and A. J. Brown. 1997. Yeast-enhanced green fluorescent protein (yEGFP); a reporter of gene expression in Candida albicans. Microbiology 143:303-311. [DOI] [PubMed] [Google Scholar]
- 6.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 7.Feldhaus, M. J., R. W. Siegel, L. K. Opresko, J. R. Coleman, J. M. Feldhaus, Y. A. Yeung, J. R. Cochran, P. Heinzelman, D. Colby, J. Swers, C. Graff, H. S. Wiley, and K. D. Wittrup. 2003. Flow-cytometric isolation of human antibodies from a nonimmune Saccharomyces cerevisiae surface display library. Nat. Biotechnol. 21:163-170. [DOI] [PubMed] [Google Scholar]
- 8.Griep, R. A., C. van Twisk, J. M. van der Wolf, and A. Schots. 1999. Fluobodies: green fluorescent single-chain Fv fusion proteins. J. Immunol. Methods 230:121-130. [DOI] [PubMed] [Google Scholar]
- 9.Hackel, B. J., D. Huang, J. C. Bubolz, X. X. Wang, and E. V. Shusta. 2006. Production of soluble and active transferrin receptor-targeting single-chain antibody using Saccharomyces cerevisiae. Pharm. Res. 23:790-797. [DOI] [PubMed] [Google Scholar]
- 10.Hitchcock, A. L., J. A. Kahana, and P. A. Silver. 2006. The uses of green fluorescent protein in yeasts. Methods Biochem. Anal. 47:179-201. [DOI] [PubMed] [Google Scholar]
- 11.Hoogenboom, H. R. 2005. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 23:1105-1116. [DOI] [PubMed] [Google Scholar]
- 12.Huang, D., and E. V. Shusta. 2005. Secretion and surface display of green fluorescent protein using the yeast Saccharomyces cerevisiae. Biotechnol. Prog. 21:349-357. [DOI] [PubMed] [Google Scholar]
- 13.Kabat, E. A., T. T. Wu, M. Reid-Miller, H. M. Perry, and K. S. Gottesmann. 1991. Sequences of proteins of immunological interests. U.S. Department of Health and Human Services, Washington, D.C.
- 14.Kauffman, K. J., E. M. Pridgen, F. J. Doyle III, P. S. Dhurjati, and A. S. Robinson. 2002. Decreased protein expression and intermittent recoveries in BiP levels result from cellular stress during heterologous protein expression in Saccharomyces cerevisiae. Biotechnol. Prog. 18:942-950. [DOI] [PubMed] [Google Scholar]
- 15.Kieke, M. C., E. V. Shusta, E. T. Boder, L. Teyton, K. D. Wittrup, and D. M. Kranz. 1999. Selection of functional T cell receptor mutants from a yeast surface-display library. Proc. Natl. Acad. Sci. USA 96:5651-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, Y. S., R. Bhandari, J. R. Cochran, J. Kuriyan, and K. D. Wittrup. 2006. Directed evolution of the epidermal growth factor receptor extracellular domain for expression in yeast. Proteins 62:1026-1035. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., H. Xu, W. E. Bentley, and G. Rao. 2002. Impediments to secretion of green fluorescent protein and its fusion from Saccharomyces cerevisiae. Biotechnol. Prog. 18:831-838. [DOI] [PubMed] [Google Scholar]
- 18.Lu, M., X. G. Gong, H. Yu, and J. Y. Li. 2005. Cloning, expression, purification, and characterization of LC-1 ScFv with GFP tag. J. Zhejiang Univ. Sci. B 6:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, K. D., J. Weaver-Feldhaus, S. A. Gray, R. W. Siegel, and M. J. Feldhaus. 2005. Production, purification, and characterization of human scFv antibodies expressed in Saccharomyces cerevisiae, Pichia pastoris, and Escherichia coli. Protein Expr. Purif. 42:255-267. [DOI] [PubMed] [Google Scholar]
- 20.Morino, K., H. Katsumi, Y. Akahori, Y. Iba, M. Shinohara, Y. Ukai, Y. Kohara, and Y. Kurosawa. 2001. Antibody fusions with fluorescent proteins: a versatile reagent for profiling protein expression. J. Immunol. Methods 257:175-184. [DOI] [PubMed] [Google Scholar]
- 21.Oelschlaeger, P., S. Srikant-Iyer, S. Lange, J. Schmitt, and R. D. Schmid. 2002. Fluorophor-linked immunosorbent assay: a time- and cost-saving method for the characterization of antibody fragments using a fusion protein of a single-chain antibody fragment and enhanced green fluorescent protein. Anal. Biochem. 309:27-34. [DOI] [PubMed] [Google Scholar]
- 22.Parekh, R., K. Forrester, and D. Wittrup. 1995. Multicopy overexpression of bovine pancreatic trypsin inhibitor saturates the protein folding and secretory capacity of Saccharomyces cerevisiae. Protein Expr. Purif. 6:537-545. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, G. H., S. M. Knobel, W. D. Sharif, S. R. Kain, and D. W. Piston. 1997. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 73:2782-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peipp, M., D. Saul, K. Barbin, J. Bruenke, S. J. Zunino, M. Niederweis, and G. H. Fey. 2004. Efficient eukaryotic expression of fluorescent scFv fusion proteins directed against CD antigens for FACS applications. J. Immunol. Methods 285:265-280. [DOI] [PubMed] [Google Scholar]
- 25.Pringle, J. R., A. E. Adams, D. G. Drubin, and B. K. Haarer. 1991. Immunofluorescence methods for yeast. Methods Enzymol. 194:565-602. [DOI] [PubMed] [Google Scholar]
- 26.Reimann, K. A., B. C. Waite, D. E. Lee-Parritz, W. Lin, B. Uchanska-Ziegler, M. J. O'Connell, and N. L. Letvin. 1994. Use of human leukocyte-specific monoclonal antibodies for clinically immunophenotyping lymphocytes of rhesus monkeys. Cytometry 17:102-108. [DOI] [PubMed] [Google Scholar]
- 27.Richman, S. A., S. J. Healan, K. S. Weber, D. L. Donermeyer, M. L. Dossett, P. D. Greenberg, P. M. Allen, and D. M. Kranz. 2006. Development of a novel strategy for engineering high-affinity proteins by yeast display. Protein Eng. Des. Sel. 19:255-264. [DOI] [PubMed] [Google Scholar]
- 28.Robinson, A. S., V. Hines, and K. D. Wittrup. 1994. Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Biotechnology 12:381-384. [DOI] [PubMed] [Google Scholar]
- 29.Schwalbach, G., A. P. Sibler, L. Choulier, F. Deryckere, and E. Weiss. 2000. Production of fluorescent single-chain antibody fragments in Escherichia coli. Protein Expr. Purif. 18:121-132. [DOI] [PubMed] [Google Scholar]
- 30.Shusta, E. V., P. D. Holler, M. C. Kieke, D. M. Kranz, and K. D. Wittrup. 2000. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat. Biotechnol. 18:754-759. [DOI] [PubMed] [Google Scholar]
- 31.Shusta, E. V., R. T. Raines, A. Pluckthun, and K. D. Wittrup. 1998. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16:773-777. [DOI] [PubMed] [Google Scholar]
- 32.Smith, J. D., and A. S. Robinson. 2002. Overexpression of an archaeal protein in yeast: secretion bottleneck at the ER. Biotechnol. Bioeng. 79:713-723. [DOI] [PubMed] [Google Scholar]
- 33.Smith, J. D., B. C. Tang, and A. S. Robinson. 2004. Protein disulfide isomerase, but not binding protein, overexpression enhances secretion of a non-disulfide-bonded protein in yeast. Biotechnol. Bioeng. 85:340-350. [DOI] [PubMed] [Google Scholar]
- 34.Waldo, G. S., B. M. Standish, J. Berendzen, and T. C. Terwilliger. 1999. Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 17:691-695. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X. X., and E. V. Shusta. 2005. The use of scFv-displaying yeast in mammalian cell surface selections. J. Immunol. Methods 304:30-42. [DOI] [PubMed] [Google Scholar]