Abstract
γ-Glutamyltransferase (GGT) has been associated with hypertension (HTN); however, the nature of this association remains unclear. GGT is a marker of alcohol consumption, but it is also related to the infiltration of fat in the liver (fatty liver). The association between GGT and HTN was examined in a 6-year longitudinal investigation among 1455 men and women who returned for the follow-up visit. Baseline variables included serum GGT, blood pressure, and anthropometric measures. Incident HTN was defined as blood pressure ≥140/90 or on antihypertensive medication at the follow-up visit. To eliminate individuals with potential liver pathology, analyses focused only on individuals with GGT within its normal range (n=897). Participants were divided in quintiles (Q) based on their baseline GGT levels. Multiple logistic regression analyses [odds ratio (95% confidence intervals)] revealed a significant association of GGT with incident hypertension [2.1 (1.1 to 4.0) Q5 versus Q1]. In subgroup analyses, GGT and HTN were significantly associated among both noncurrent and current drinkers, but only for participants above the median of anthropometric measures [eg, body mass index >26.4, 2.3 (0.9 to 5.7), waist circumference >86.1 cm, 3.7 (1.4 to 9.9), and abdominal height >19.8 cm, 3.1 (1.2 to 8.5), for Q5 versus Q1, in fully adjusted models]. These findings suggest that the association between GGT and hypertension is not caused solely by alcohol consumption and indicate that serum GGT, within its normal range, may predict hypertension among individuals with increased central fat distribution, suggesting that fatty liver may represent an important underlying mechanism for this association.
Keywords: adipose tissue, blood pressure, epidemiology, hypertension, liver
Recent epidemiologic and clinical studies have reported a strong association between γ-glutamyltransferase (GGT), a commonly used biochemical liver test, and several cardiovascular risk factors and diseases including hypertension.1–15 The mechanism underlying this association is still not well understood. Specifically, it is unclear whether these observations are not confounded by use of alcohol or obesity, especially central (visceral) fat, and may reflect an underlying condition, such as hepatic insulin resistance or nonalcoholic fatty liver (NAFL). It is known that GGT has a protective function in maintaining appropriate hepatic glutathione levels, which are crucial in antioxidant defenses.16 In addition, GGT has been widely used as a biological marker of alcohol consumption.17,18 Recent findings have shown as well that regional body fat distribution, with abdominal accumulation, may represent a stronger predictor of elevated liver enzymes including GGT than relative weight, as assessed by body mass index (BMI).19,20 Furthermore, central adiposity can be an independent predictor of NAFL.21–23 This common clinical and histological condition has been recently related to insulin resistance and has been suggested as an additional feature of the metabolic syndrome.24–26 There is evidence that both fatty liver and central obesity are associated with free radical generation thus enhancing oxidative stress.16,27,28 Therefore, it is possible that NAFL may represent the link in the association of GGT with hypertension and other components of the metabolic syndrome.
We examined this question in a 6-year longitudinal investigation of the Western New York Study, a population-based study of diabetes and cardiovascular risk factors among residents of Erie and Niagara Counties, New York.
Methods
Study Population
Participants in this report were originally enrolled as healthy control participants in the Western New York Health Study, an epidemiologic case-control investigation of patterns of alcohol intake and coronary heart disease in Erie and Niagara Counties, New York, conducted from 1986 to 2001 (59.5% initial response rate). The details of the methodology have been previously described.29 The initial cohort was selected from drivers’ license lists and Health Care Finance Administration lists. Eligible participants for the current study were men and women aged 39 to 79 years selected from the baseline examination without known clinical cardiovascular disease (self-report) or type 2 diabetes (fasting plasma glucose >125 mg/dL or self-report) and who were capable of completing the current study protocol (n=2652). Exclusion criteria included self-report of any medical condition that would prohibit participation (eg, all cancers except skin cancer, type 1 diabetes, physical or mental impairment, permanent change in residence out-of-state, deceased, or inability to contact and determine eligibility). This left 2139 persons eligible, of whom 1455 completed the full clinic examination (68.0%) at the follow-up visit (6.0 years ±0.8). Participants with prevalent hypertension (blood pressure ≥140/90 or on antihypertensive treatment) at baseline were further excluded (n=448). Finally, to eliminate individuals with potential liver pathology, we excluded 110 individuals with GGT values above the normal reference range of the laboratory (5 to 55 U/L). The remaining 897 participants are included in this analysis.
The protocol was approved by the University at Buffalo Health Science institutional review board and all participants provided written informed consent before participation.
Compared with those who refused, participants in the current report were less likely to be smokers at the baseline and somewhat more educated (14.4 years versus 13.1 years of formal education; P<0.001). There were no significant differences in race, sex ratio, BMI, fasting glucose concentration or blood pressure values between participants and refusers.
Study Protocol
All participants received a clinical examination that included resting blood pressure, measures of height, weight, waist circumference, and abdominal height. In addition, several questionnaires that were first administered at the baseline examination were re-administered. These assessed lifestyle and health habit information including: cigarette use, physical activity, alcohol use, general health and well-being, personal and family health history, medication use, and socioeconomic status.
Anthropometric measurements were determined by trained and certified interviewers on participants who wore light clothing and no shoes. Waist circumference was determined with participants standing erect with the abdomen relaxed, arms at the side, and feet together. The tape was horizontally placed between the bottom of the rib cage and the top of the iliac crest (hip bones) around the smallest circumference between these 2 reference points. The measurement was taken at the end of a normal expiration, without the tape compressing the skin, to the nearest 0.1 cm. Abdominal height was measured using the Holtain-Kahn abdominal caliper.30 Three separate measurements were made to the nearest 0.1 cm of the sagittal (eg, antero-posterior) abdominal diameter. If the 3 readings were not within 0.5 cm of each other, the 3 readings were repeated until they were all within 0.5 cm of each other. The mean of the 3 readings were used in these analyses. During the study we examined the intra-and inter-observer variability of the abdominal height measurement. The intra-observer variability, evaluated by the intra-class correlation (ICC) coefficient, was 0.99. The inter-observer variability was 0.99. Both waist circumference and abdominal height have been shown to be highly correlated with the volume of visceral fat as determined by multi-scan tomography.31–33 Height was measured on a permanently mounted vertical board (Perspective Enterprises, Kalamazoo, Mich), according to a standardized protocol. Weight was measured to the nearest tenth of a pound on a calibrated balance beam scale (Detecto, Inc, Webb City, Mo). BMI was calculated as weight (kg) divided by height in meters2.
At both examinations, blood pressure was measured 3 times using a standard mercury manometer by trained and certified technicians. The onset of the first phase (systolic) and fifth phase (diastolic) Korotkoff sounds were recorded. The mean of the second and third measures were used in the analyses. At both examinations, hypertension was defined as blood pressure ≥140/90 or use of antihypertensive medications.34
At the baseline, a blood sample was obtained for determination of routine chemistry between 7:30 and 9:30 AM after a fasting for 8 to 12 hours. Immediately after phlebotomy, tubes were wrapped in aluminum foil to protect them from light and kept at room temperature for 30 minutes and allowed to clot. Blood tubes were centrifuged at 3000g for 10 minutes and 1.5 mL of serum was transferred to polypropylene screw cap vials and placed in a cooler with a cold pack. Samples were delivered by courier to Millard Fillmore Center for Laboratory Medicine (Amherst, NY) for analysis the same day. Hepatic enzymes alanine amino transferase (ALT), aspartate aminotransferase (AST), serum γ-glutamyl transferase (SGGT), and alkaline phosphatase (ALP) were measured by kinetic enzyme assays as part of a chemistry profile on a Paramax Automated Chemistry System.35,36
Statistical Analysis
All analyses were conducted using the Statistical Package for Social Sciences (SPSS-12.0; SPSS Inc, Chicago, Ill). Differences in baseline characteristics between participants who remained normotensive and those who became hypertensive at the follow-up visit were evaluated using independent sample t tests for continuous variables and χ2 test for categorical variables. Participants were divided into quintiles (Q) of GGT concentration according to the baseline distribution. Differences in baseline characteristics were also evaluated across GGT quintiles. Tests for interaction between GGT and gender were not significant; therefore, all analyses were conducted without stratifying for gender.
Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CI) of incident hypertension across baseline GGT quintiles. The lowest quintile was used as the reference category. Covariates included: the baseline values of age, gender, race, average amount of alcohol, smoking status, BMI, physical activity, and systolic blood pressure. Subgroup analyses were performed to assess the association between GGT and incident hypertension across baseline categories of drinking status (nondrinkers and current drinkers) and anthropometric measures, including BMI, waist circumference, and abdominal height, categorized by the median values.
Results
Table 1 shows the baseline characteristics of the study participants according to the subsequent development of hypertension. Mean values of age, anthropometric measures, concentrations of total cholesterol and triglycerides, blood pressure, and GGT were significantly higher among participants who became hypertensive than among those who remained normotensive, whereas no significant difference between the 2 groups was found in mean values of physical activity and alcohol consumption. Participants who became hypertensive were also significantly less educated and characterized at baseline by significantly lower percentage of women and higher percentage of smokers (both former and current), whereas no significant difference between the 2 groups was found in the baseline distribution of race and drinking status.
TABLE 1.
Baseline Characteristics of Participants According to the Subsequent Development of Hypertension*: The Western New York Study, 1995–2001
| Variable | Normotensive (n=702) Mean (SD) | Hypertensive (n=195) Mean (SD) | P† |
|---|---|---|---|
| Age (years) | 53.2 (11.0) | 58.3 (10.5) | <0.0001 |
| Education (years) | 14.3 (2.5) | 13.6 (2.3) | <0.0001 |
| BMI (kg/m2) | 26.5 (4.7) | 28.1 (5.0) | <0.0001 |
| Waist circumference (cm) | 85.8 (12.0) | 89.9 (14.6) | 0.001 |
| Abdominal height (cm) | 19.8 (3.1) | 20.9 (3.5) | <0.0001 |
| Physical activity (metabolic equivalent unit · h) | 262.4 (47.4) | 263.4 (49.1) | 0.804 |
| Drinks per day | 0.4 (0.8) | 0.5 (1.0) | 0.378 |
| Total cholesterol (mg/dL) | 211.3 (37.8) | 222.4 (38.2) | <0.0001 |
| Triglycerides (mg/dL) | 119.2 (80.0) | 132.9 (74.2) | 0.030 |
| Systolic blood pressure (mm Hg) | 111.3 (10.9) | 122.5 (9.5) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 68.9 (7.9) | 74.3 (8.0) | <0.0001 |
| GGT (U/L) | 21.6 (10.3) | 25.4 (10.9) | <0.0001 |
|
| |||
| % | % | ||
|
| |||
| Women | 67.3 | 59.0 | 0.036 |
| White | 96.6 | 94.7 | 0.222 |
| Smoking status | |||
| Never-smokers | 54.1 | 42.2 | |
| Former-smokers | 35.1 | 45.5 | |
| Current smokers | 10.8 | 12.3 | 0.014 |
| Drinking status | |||
| Lifetime abstainers | 9.7 | 8.0 | |
| Former drinkers | 18.9 | 23.0 | |
| Current drinkers | 71.4 | 69.0 | 0.412 |
Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or on medication for hypertension.
P values for comparison between normotensive and hypertensive participants at the follow-up visit.
BMI indicates body mass index; GGT, γ-glutamyltransferase; SD, standard deviation.
The mean values of the continuous characteristics at baseline by GGT quintiles are shown in Table 2. For all but education and physical activity, a significant linear trend was found across quintiles of GGT.
TABLE 2.
Mean (SD) of Selected Covariates According to GGT Quintiles at Baseline: The Western New York Study,1995–2001
| GGT at Baseline
|
||||||
|---|---|---|---|---|---|---|
| U/L | ≤14 | 15–19 | 20 –25 | 26 –38 | 39 –55 | P* for Trend |
| No. | 220 | 209 | 178 | 196 | 94 | |
| Variable | ||||||
| Age (years) | 51.7 (10.8) | 53.4 (10.3) | 54.8 (11.1) | 55.3 (11.2) | 55.1 (10.5) | 0.006 |
| Education (years) | 14.4 (2.5) | 14.2 (2.4) | 14.3 (2.4) | 14.0 (2.4) | 13.8 (2.5) | 0.268 |
| Body mass index (kg/m2) | 25.5 (4.4) | 26.6 (4.8) | 26.8 (4.9) | 27.7 (4.6) | 29.1 (5.3) | <0.0001 |
| Waist circumference (cm) | 80.4 (11.9) | 84.6 (12.1) | 87.8 (12.1) | 91.1 (13.9) | 93.8 (14.5) | <0.0001 |
| Abdominal height (cm) | 18.6 (2.8) | 19.7 (3.0) | 20.1 (3.0) | 20.8 (3.1) | 22.1 (3.8) | <0.0001 |
| Physical activity (metabolic equivalent unit · h) | 257.1 (36.9) | 262.4 (47.0) | 265.8 (49.2) | 264.0 (48.5) | 274.3 (68.4) | 0.068 |
| Drinks per day | 0.3 (0.6) | 0.4 (0.8) | 0.5 (0.8) | 0.6 (1.1) | 0.6 (0.9) | 0.048 |
| Total cholesterol (mg/dL) | 205.0 (34.5) | 214.0 (39.4) | 213.8 (40.6) | 218.5 (40.3) | 220.8 (32.0) | 0.001 |
| Triglycerides (mg/dL) | 102.0 (58.3) | 111.6 (69.2) | 125.2 (71.7) | 137.8 (95.7) | 150.8 (92.6) | <0.0001 |
| Systolic blood pressure (mm Hg) | 110.0.9 (11.6) | 111.8 (10.7) | 113.7 (11.7) | 116.7 (11.7) | 117.2 (10.7) | <0.0001 |
| Diastolic blood pressure (mm Hg) | 69.3 (7.7) | 69.0 (7.8) | 70.2 (9.0) | 70.9 (7.7) | 72.4 (8.8) | 0.005 |
P values for linear trend.
Table 3 displays the ORs of incident hypertension across baseline GGT quintiles. Model 1 is adjusted for age, gender, and race. Compared with the bottom quintile, the ORs of incident hypertension increased monotonically from quintile 2 through quintile 5:0.8, 1.6, 1.8, and 2.7 (P for trend <0.0001). After further adjustment for baseline average amount of alcohol, smoking status, BMI, and physical activity (model 2), these risk estimates were only slightly attenuated: 0.8, 1.6, 1.8, and 2.1 (P for trend <0.0001). Model 3 is adjusted further for baseline systolic blood pressure with little notable change: 0.9, 1.7, 2.0, and 2.1 (P for trend 0.002).
TABLE 3.
Odds Ratio (95% CI) of Incident Hypertension* by GGT Quintiles at Baseline: The Western New York Study,1995–2001
| GGT at Baseline | P for Trend | |||||
|---|---|---|---|---|---|---|
| U/L | ≤14 | 15–19 | 20–25 | 26–38 | 39–55 | |
| N | 220 | 209 | 178 | 196 | 94 | |
| Model 1† | 1.0 | 0.8 (0.5–1.4) | 1.6 (0.9–2.8) | 1.8 (1.1–3.1) | 2.7 (1.5–4.9) | <0.0001 |
| Model 2‡ | 1.0 | 0.8 (0.4–1.4) | 1.6 (0.9–2.7) | 1.8 (1.0–3.0) | 2.1 (1.1–4.0) | <0.0001 |
| Model 3§ | 1.0 | 0.9 (0.5–1.6) | 1.7 (0.9–3.0) | 2.0 (1.1–3.4) | 2.1 (1.1–4.0) | 0.002 |
| By drinking status | ||||||
| Nondrinkers | ||||||
| N | 77 | 64 | 39 | 56 | 31 | |
| Model 1 | 1.0 | 0.8 (0.3–2.0) | 1.0 (0.3–2.9) | 1.6 (0.6–3.8) | 3.9 (1.4–10.4) | 0.006 |
| Model 2 | 1.0 | 0.7 (0.3–1.9) | 1.0 (0.3–2.9) | 1.5 (0.6–3.8) | 3.4 (1.2–9.4) | 0.011 |
| Model 3 | 1.0 | 0.8 (0.3–2.2) | 1.0 (0.3–3.0) | 1.8 (0.7–4.8) | 3.5 (1.2–10.0) | 0.010 |
| Current drinkers | ||||||
| N | 143 | 145 | 139 | 140 | 63 | |
| Model 1 | 1.0 | 0.8 (0.4–1.7) | 1.8 (0.9–3.5) | 2.0 (1.0–3.8) | 1.9 (0.9–4.3) | 0.008 |
| Model 2 | 1.0 | 0.8 (0.4–1.7) | 1.8 (0.9–3.6) | 2.0 (1.0–3.9) | 1.5 (0.6–3.4) | 0.035 |
| Model 3 | 1.0 | 0.9 (0.4–2.0) | 2.1 (1.0–4.4) | 2.2 (1.0–4.5) | 1.4 (0.6–3.4) | 0.057 |
Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or on medication for hypertension.
Adjusted for age, gender, and race.
Adjusted as above plus average amount of alcohol (except among categories of drinking status), smoking status, body mass index, and physical activity.
Adjusted as above plus systolic blood pressure.
To examine any confounding by drinking status, we stratified the results by baseline drinking status. A significant linear relationship between GGT quintiles and incident hypertension was found among both nondrinkers (including lifetime abstainers and former drinkers) and current drinkers; however, the association was stronger among nondrinkers than among current drinkers with an OR of 3.5 (1.2 to 10.0) comparing Q5 versus Q1 in the fully adjusted model.
We also examined the impact of obesity and visceral fat on the results (Table 4). After stratification by the median values of baseline anthropometric measures, such as BMI, waist circumference, and abdominal height, GGT and incident HTN were significantly associated only among participants above the median of all anthropometric measures [eg, for BMI >26.4, 2.3 (0.9 to 5.7), for waist circumference >86.1 cm, 3.7 (1.4 to 9.9), and for abdominal height >19.8 cm, 3.1 (1.2 to 8.5), comparing Q5 versus Q1, in fully adjusted models]. However, it should be noted that the association between GGT and HTN, generally, appeared to be stronger among participants who were above the median of waist circumference and abdominal height compared with participants who were above the median of BMI in the fully adjusted model (model 3). These findings suggest that GGT may be differentially related to these anthropometric measures. To further examine this, we cross-classified tertiles of waist circumference and BMI. A direct and statistically significant relation between the age-adjusted mean values of GGT and waist circumference persisted within each tertile of BMI (Figure 1). By contrast, no significant association was found between GGT and BMI across tertiles of waist circumference (Figure 2). Thus, these figures indicate that mean values of GGT vary as a function of waist circumference, independently of BMI.
TABLE 4.
Odds Ratio (95% CI) of Incident Hypertension* by GGT Quintiles at Baseline: The Western New York Study, 1995–2001
| GGT at Baseline | P for Trend | |||||
|---|---|---|---|---|---|---|
| U/L | ≤14 | 15–19 | 20–25 | 26–38 | 39–55 | |
| By median of BMI | ||||||
| ≤26.4 | ||||||
| N | 143 | 115 | 90 | 78 | 33 | |
| Model 1† | 1.0 | 0.8 (0.4–1.8) | 1.3 (0.6–2.8) | 1.3 (0.6–2.9) | 1.4 (0.5–4.1) | 0.342 |
| Model 2‡ | 1.0 | 0.8 (0.4–1.7) | 1.1 (0.5–2.5) | 1.3 (0.6–2.9) | 1.2 (0.4–3.8) | 0.457 |
| Model 3§ | 1.0 | 1.0 (0.5–2.2) | 1.2 (0.5–2.8) | 1.5 (0.6–3.5) | 1.6 (0.5–5.4) | 0.243 |
| >26.4 | ||||||
| N | 77 | 94 | 88 | 118 | 61 | |
| Model 1 | 1.0 | 0.8 (0.3–1.9) | 2.1 (0.9–4.5) | 2.2 (1.0–4.7) | 3.3 (1.4–7.6) | <0.0001 |
| Model 2 | 1.0 | 0.8 (0.3–1.8) | 2.0 (0.9–4.4) | 2.1 (1.0–4.6) | 3.0 (1.3–6.9) | 0.001 |
| Model 3 | 1.0 | 0.8 (0.3–1.9) | 2.1 (0.9–4.8) | 2.2 (1.0–4.9) | 2.3 (0.9–5.7) | 0.006 |
| By median of waist circumference | ||||||
| ≤86.1 (cm) | ||||||
| N | 159 | 122 | 79 | 78 | 31 | |
| Model 1 | 1.0 | 0.9 (0.4–1.7) | 1.1 (0.5–2.3) | 1.3 (0.6–2.7) | 1.4 (0.5–3.9) | 0.349 |
| Model 2 | 1.0 | 0.9 (0.4–1.7) | 1.0 (0.5–2.3) | 1.3 (0.6–2.8) | 1.2 (0.4–3.5) | 0.426 |
| Model 3 | 1.0 | 1.1 (0.5–2.3) | 1.1 (0.5–2.7) | 1.5 (0.7–3.4) | 1.1 (0.4–3.3) | 0.434 |
| >86.1 (cm) | ||||||
| N | 61 | 87 | 99 | 118 | 63 | |
| Model 1 | 1.0 | 0.9 (0.3–2.5) | 2.8 (1.1–6.8) | 2.9 (1.2–6.9) | 4.4 (1.7–11.1) | <0.0001 |
| Model 2 | 1.0 | 0.9 (0.3–2.4) | 2.7 (1.1–6.5) | 2.9 (1.2–7.0) | 4.0 (1.5 −10.2) | <0.0001 |
| Model 3 | 1.0 | 1.0 (0.3–2.7) | 2.8 (1.1–7.1) | 3.1 (1.2–7.6) | 3.7 (1.4–9.9) | <0.0001 |
| By median of abdominal height | ||||||
| ≤19.8 (cm) | ||||||
| N | 164 | 117 | 85 | 75 | 27 | |
| Model 1 | 1.0 | 1.0 (0.5–2.0) | 1.2 (0.6–2.6) | 1.6 (0.8–3.4) | 0.8 (0.2–2.9) | 0.450 |
| Model 2 | 1.0 | 1.0 (0.5–2.0) | 1.1 (0.5–2.5) | 1.7 (0.8–3.5) | 0.6 (0.1–2.5) | 0.521 |
| Model 3 | 1.0 | 1.3 (0.6–2.6) | 1.3 (0.6–2.9) | 1.8 (0.8–3.9) | 0.6 (0.1–2.9) | 0.459 |
| >19.8 (cm) | ||||||
| N | 56 | 92 | 93 | 121 | 67 | |
| Model 1 | 1.0 | 0.8 (0.3–2.1) | 2.3 (0.9–5.8) | 2.0 (0.8–5.1) | 3.7 (1.4–9.4) | <0.001 |
| Model 2 | 1.0 | 0.8 (0.3–2.1) | 2.3 (0.9–5.8) | 2.1 (0.9–5.3) | 3.4 (1.3–8.9) | 0.001 |
| Model 3 | 1.0 | 0.8 (0.3–2.4) | 2.6 (1.0–6.8) | 2.6 (1.0–6.7) | 3.1 (1.2–8.5) | 0.002 |
Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or on medication for hypertension.
Adjusted for age, gender, and race.
Adjusted as above plus average amount of alcohol, smoking status, and physical activity.
Adjusted as above plus systolic blood pressure.
Figure 1.
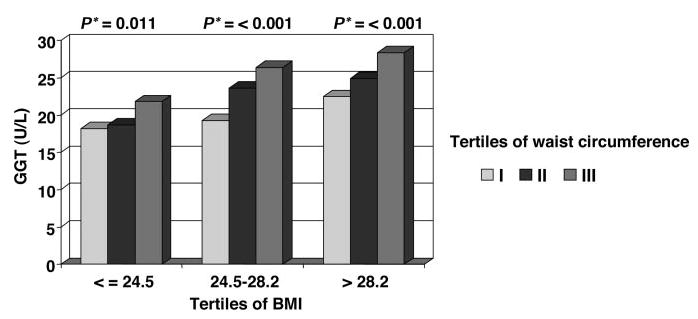
Age-adjusted GGT mean values across tertiles of waist circumference within each tertile of BMI. The Western New York Study, 1995 to 2001. *P values for linear trend across tertiles of waist circumference within each tertile of BMI.
Figure 2.
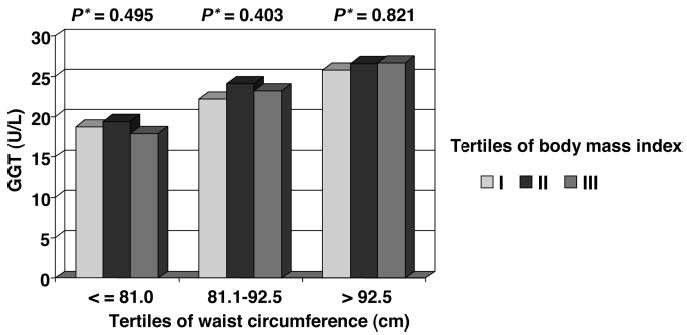
Age-adjusted GGT mean values across tertiles of BMI within each tertile of waist circumference. The Western New York Study, 1995 to 2001. *P values for linear trend across tertiles of BMI within each tertile of waist circumference.
Discussion
In this prospective population-based study GGT, within the physiological range, was a strong predictor of incident hypertension during 6 years of follow-up in a dose-response relationship. This association was independent of the effects of alcohol consumption and was present in both nondrinkers and drinkers; however, it appeared stronger among nondrinkers than among drinkers. When we evaluated the association between GGT and incident hypertension according to anthropometric measures of either relative weight, ie, BMI, or body fat distribution, ie, waist circumference and abdominal height, GGT was a significant predictor of incident hypertension only among the overweight and especially among persons with increased central fat distribution. The latter is a novel finding and is consistent with the hypothesis that fatty liver may represent an important underlying mechanism for the observed associations between GGT and hypertension.
Over the past 20 years, many cross-sectional studies and fewer longitudinal investigations have reported a positive association of GGT with blood pressure and risk of hypertension.2–7,12,13 This association has been shown to be independent of alcohol consumption and to be present among both drinkers and nondrinkers.2,7,13 Our findings are consistent with previous work, further supporting the conclusion that the association between GGT and blood pressure is not mediated by alcohol consumption. Unfortunately, we were not able to assess this association separately in lifetime abstainers and former drinkers, because our sample size precluded us from performing meaningful comparisons within these subsets of drinkers. However, other studies have shown that GGT is associated with blood pressure even among lifetime abstainers.2
By contrast, the association of GGT with blood pressure has been shown to be affected by variation in body fat distribution and parameters of insulin resistance. For example, in a study of 38-year-old Dutch men GGT was not associated with either systolic or diastolic blood pressure in multiple regression analysis including waist-to-hip circumference ratio, as a measure of body fat distribution, whereas the letter was significantly associated with diastolic blood pressure.1 Similarly, in a large population-based Italian study the significant univariate correlations between GGT and both systolic and diastolic blood pressures were no longer significant in multiple regression analysis including blood lipids.9 A study of Japanese male workers showed that blood pressure was more strongly related to plasma insulin levels after a glucose tolerance test than to GGT, and that GGT was no longer significantly associated with blood pressure after adjustment for insulin levels.6 Our findings extend previous work and indicate that the association of GGT with hypertension risk is strongly affected by variation in relative weight and, above all, body fat distribution. Specifically, we found that GGT was a significant predictor of incident hypertension only among overweight or individuals with increased central fat distribution. In addition, the association between GGT and HTN appeared to be stronger among participants who were above the median of waist circumference and abdominal height than among those who were above the median of BMI. Our results also indicate that mean values of GGT vary as a function of waist circumference independent of BMI, supporting the notion that central adiposity may represent a stronger predictor of elevated liver enzymes including GGT than relative weight, as assessed by BMI.19,20 Because central adiposity can correlate with the development of fatty liver,21–23 our findings further support the hypothesis that NAFL may represent an important underlying mechanism for the observed associations between GGT and hypertension. Moreover, the association between hepatic insulin resistance and fatty liver has been shown in several clinical studies and some authors have suggested that fatty liver should be considered part of the metabolic syndrome.24–26 In addition, there is evidence that both fatty liver and central obesity are associated with increased free radical generation.27,28 It is known that GGT has a protective function in maintaining appropriate hepatic glutathione levels, which are crucial in antioxidant defenses.16 Therefore, it is possible that the generation of free radicals, which can occur in fatty liver and central obesity, may deplete intracellular glutathione and thus induce the activity of GGT to enhance glutathione levels. The increase in GGT at the sinusoidal membrane of hepatocytes can lead to an increased release of GGT into the circulation. Unfortunately, in our study we did not assess at baseline plasma insulin levels and could not further investigate the association between GGT and parameters of insulin resistance.
Consistently with previous work,13 in our study no association was found between hypertension risk and other hepatic enzymes including ALT, AST, and ALP (data not shown). The lack of association between hypertension risk and more specific enzymes of liver damage (ALT and AST) further suggests that the association of GGT, within its normal range, and hypertension may be caused by an increased condition of oxidative stress produced by either central adiposity or fatty liver rather than to merely liver damage. Additionally, there is evidence that GGT can act as a pro-oxidant and lead to formation of free radicals and lipid peroxidation,16,37 which are pathologic mechanisms commonly associated with hypertension and other cardiovascular risk factors.38
When we performed analyses including participants with elevated GGT (>55 U/L), the point estimates of hypertension risk among these participants were somewhat attenuated and not significant (data not shown). Although these findings indicate that the predictive value of GGT for hypertension may decrease in persons with potential liver damage, they further support the hypothesis that GGT, within its normal range, may represent an early and sensitive biomarker for the development of hypertension as well as of other components of the metabolic syndrome.10,13,14
Several limitations of this study deserve mention. First, the suboptimal initial participation rate (59.5%) and reexamination rate (68.0%) may leave the possibility for selection bias and restrict the generalization of our findings to the general public. However, this would not affect the internal validity of our results. Second, we cannot rule out the presence of additional unknown confounding variables that we were unable to control for in our analyses, and the potential of residual confounding that, in the absence of a known physiological link, may have contributed to our findings. The strengths of this study include the very detailed information elicited on several covariates known to be related to either GGT or blood pressure elevation including alcohol consumption and several measures of body fatness. A further strength is that we enrolled participants randomly selected from a community-wide population.
Perspectives
Our study adds new and important information to the current body of evidence about the association of GGT with hypertension and other components of the metabolic syndrome. Our findings indicate that the association between GGT and hypertension is not caused solely by alcohol consumption; in addition, they further support the hypothesis that NAFL, and its metabolic consequences (eg, insulin resistance), may represent an important link between GGT and components of the metabolic syndrome. These findings may have both clinical and public health implications if we consider that fatty liver is the most common cause of liver injury in the United States.39 Population-based studies are necessary to further investigate the association between fatty liver and hepatic insulin resistance. Moreover, experimental studies are needed also to better understand the physiological functions of GGT with respect to oxidative stress and to support the epidemiologic and clinical evidence regarding the association between metabolic abnormalities and fatty liver.
Acknowledgments
This study was supported in part by grant R01 DK60587 to Dr Donahue. We acknowledge the assistance of Mya Swanson in data management and file preparation.
References
- 1.van Barneveld T, Seidell JC, Traag N, Hautvast JG. Fat distribution and gamma-glutamyl transferase in relation to serum lipids and blood pressure in 38-year old Dutch males. Eur J Clin Nutr. 1989;43:809–818. [PubMed] [Google Scholar]
- 2.Nilssen O, Forde OH, Brenn T. The Tromso Study. Distribution and population determinants of γ-glutamyltransferase. Am J Epidemiol. 1990;132:318–326. doi: 10.1093/oxfordjournals.aje.a115661. [DOI] [PubMed] [Google Scholar]
- 3.Yamada Y, Ishizaki M, Kido T, Honda R, Tsuritani I, Ikai E, Yamaya H. Alcohol, high blood pressure, and serum gamma-glutamyl transpeptidase level. Hypertension. 1991;18:819–826. doi: 10.1161/01.hyp.18.6.819. [DOI] [PubMed] [Google Scholar]
- 4.Ikai E, Honda R, Yamada Y. Serum gamma-glutamyl transpeptidase level and blood pressure in nondrinkers: a possible pathogenetic role of fatty liver in obesity-related hypertension. J Hum Hypertens. 1994;8:95–100. [PubMed] [Google Scholar]
- 5.Miura K, Nakagawa H, Nakamura H, Tabata M, Nagase H, Yoshida M, Kawano S. Serum γ-glutamyl transferase level in predicting hypertension among male drinkers. J Hum Hypertens. 1994;8:445–449. [PubMed] [Google Scholar]
- 6.Ikai E, Ishizaki M, Suzuki Y, Ishida M, Noborizaka Y, Yamada Y. Association between hepatic steatosis, insulin resistance and hyperinsulinaemia as related to hypertension in alcohol consumers and obese people. J Hum Hypertens. 1995;9:101–105. [PubMed] [Google Scholar]
- 7.Yamada Y, Ikai E, Tsuritani I, Ishizaki M, Honda R, Ishida M. The relationship between serum gamma-glutamyl transpeptidase levels and hypertension: common in drinkers and nondrinkers. Hypertens Res. 1995;18:295–301. doi: 10.1291/hypres.18.295. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee G, Ebrahim S, Shaper AG. γ-Glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 9.Pintus F, Mascia P. Distribution and population determinants of gamma-glutamyltransferase in a random sample of Sardinian inhabitants. ‘ATS-SARDEGNA’ Research Group. Eur J Epidemiol. 1996;12:71–76. doi: 10.1007/BF00144431. [DOI] [PubMed] [Google Scholar]
- 10.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum γ-glutamyltransferase and risk of NIDDM. Diabetes Care. 1998;21:732–737. doi: 10.2337/diacare.21.5.732. [DOI] [PubMed] [Google Scholar]
- 11.Jousilahti P, Rastenyte D, Tuomilehto J. Serum γ-glutamyl transferase, self-reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851–1855. doi: 10.1161/01.str.31.8.1851. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Ha MH, Kim JR, Gross M, Jacobs DR. γ-Glutamyltransferase, alcohol, and blood pressure: a four year follow-up study. Ann Epidemiol. 2002;12:90–96. doi: 10.1016/s1047-2797(01)00252-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Jacobs DR, Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, Steffes M. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–1366. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Silventoinen K, Jacobs DR, Jr, Jousilahti P, Tuomileto J. γ-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004;89:5410–5414. doi: 10.1210/jc.2004-0505. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Sattar N, Smith GD, Ebrahim S. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am J Epidemiol. 2005;161:1081–1088. doi: 10.1093/aje/kwi125. [DOI] [PubMed] [Google Scholar]
- 16.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 17.Shaper AG, Pocock SJ, Ashby D, Walker M, Whitehead TP. Biochemical and haematological response to alcohol intake. Ann Clin Biochem. 1985;22:50–61. doi: 10.1177/000456328502200104. [DOI] [PubMed] [Google Scholar]
- 18.Sillanaukee P, Massot N, Jousilahti P, Vartiainen E, Sundvall J, Olsson U, Poikolainen K, Ponnio M, Allen JP, Alho H. Dose response of laboratory markers to alcohol consumption in a general population. Am J Epidemiol. 2000;152:747–751. doi: 10.1093/aje/152.8.747. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 20.Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754–763. doi: 10.1002/hep.20149. [DOI] [PubMed] [Google Scholar]
- 21.Kral JG, Schaffner F, Pierson RN, Jr, Wang J. Body fat topography as an independent predictor of fatty liver. Metabolism. 1993;42:548–551. doi: 10.1016/0026-0495(93)90210-f. [DOI] [PubMed] [Google Scholar]
- 22.Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19:846–850. [PubMed] [Google Scholar]
- 23.Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K, Murase K, Kadota T, Murata I, Kohno S. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098–1105. doi: 10.1046/j.1440-1746.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 24.Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver–an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73–79. doi: 10.1093/qjmed/92.2.73. [DOI] [PubMed] [Google Scholar]
- 25.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Non-alcoholic fatty liver disease. A feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 26.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, Weltman M, George J. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 27.Blazovics A, Feher E, Feher J. Role of free radical reactions in experimental hyperlipidaemia in the pathomechanism of fatty liver. In: Csomos G, Feher J, editors. Free Radicals and the Liver. Berlin: Springer-Verlag; 1992. pp. 96–123. [Google Scholar]
- 28.Bakker SJ, IJzerman RG, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000;148:17–21. doi: 10.1016/s0021-9150(99)00329-9. [DOI] [PubMed] [Google Scholar]
- 29.Stranges S, Wu T, Dorn JM, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, Trevisan M. Relationship of alcohol drinking pattern to the risk of hypertension: a population-based study. Hypertension. 2004;44:813–819. doi: 10.1161/01.HYP.0000146537.03103.f2. [DOI] [PubMed] [Google Scholar]
- 30.Kahn HS. Choosing an index for abdominal obesity: an opportunity for epidemiologic clarification. J Clin Epidemiol. 1993;46:491–494. doi: 10.1016/0895-4356(93)90027-x. [DOI] [PubMed] [Google Scholar]
- 31.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 32.van der Kooy K, Leenen R, Seidell JC, Deurenberg P, Visser M. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Brit J Nutr. 1993;70:47–58. doi: 10.1079/bjn19930104. [DOI] [PubMed] [Google Scholar]
- 33.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric index of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 34.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Evaluation, and Detection Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 35.Moss DW, Henderson AR. Enzymes. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 2nd edition. WB Saunders; 1994. pp. 735–890. [Google Scholar]
- 36.Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. Recommended method for the determination of gamma-glutamyl transferase in blood. Scand J Clin Lab Invest. 1976;36:119–125. doi: 10.1080/00365517609055236. [DOI] [PubMed] [Google Scholar]
- 37.Stark AA. Oxidative metabolism of glutathione by gamma-glutamyl transpeptidase and peroxisome proliferation: the relevance to hepatocarcinogenesis. A hypothesis. Mutagenesis. 1991;6:241–245. doi: 10.1093/mutage/6.4.241. [DOI] [PubMed] [Google Scholar]
- 38.Orie NN, Zidek W, Tepel M. Reactive oxygen species in essential hypertension and non-insulin-dependent diabetes mellitus. Am J Hypertens. 1999;12:1169–1174. doi: 10.1016/s0895-7061(99)00129-6. [DOI] [PubMed] [Google Scholar]
- 39.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]


