Abstract
Chloroplasts must import a large number of proteins from the cytosol. It generally is assumed that this import proceeds for all stromal and thylakoid proteins in an identical manner and is caused by the operation of two distinctive protein import machineries in the outer and inner plastid envelope, which form the general import site. Here we show that there is a second site of protein translocation into chloroplasts of barley, tobacco, Arabidopsis thaliana, and five other tested monocotyledonous and dicotyledonous plant species. This import site is specific for the cytosolic precursor of the NADPH:protochlorophyllide (Pchlide) oxidoreductase A, pPORA. It couples Pchlide synthesis to pPORA import and thereby reduces the actual level of free Pchlide, which, because of its photodynamic properties, would be destructive to the plastids. Consequently, photoprotection is conferred onto the plant.
Chloroplasts are semiautonomous cell organelles that contain only a limited coding information for their constituents. Whereas the major part of chloroplast proteins is of cytosolic origin, only a minor fraction of the enzyme and protein complement is encoded in plastid DNA. Chloroplasts hence must import the overwhelming part of their polypeptides from the cytosol. This import occurs posttranslationally (see refs. 1 and 2 for review).
Chloroplast protein import proceeds for all of the different cytosolic precursors in three principal steps: (i) the transit sequence-dependent binding of the precursors to proteinaceous receptor components at the outer plastid surface, (ii) the translocation of the precursors through the import machinery of the outer and inner envelope, and (iii) the intraorganellar routing of imported proteins to their final destinations (1, 2). Import is energy-dependent (3–10) and was thus far considered to proceed in an identical manner regardless of what chloroplast precursor protein was actually present (11–15).
Doubts on this dogma of chloroplast protein translocation recently have come up, based on studies of the posttranslational import pathways of the two NADPH:protochlorophyllide (Pchlide) oxidoreductase precursors of barley, termed pPORA and pPORB (16). These precursors were differentially imported into the plastids (17, 18). In vitro studies showed that import of the pPORA depends on Pchlide, which is one of the enzyme's two substrates (17). Chloroplasts, which normally do not contain spectroscopically detectable levels of Pchlide, were unable to import the pPORA. However, when fed the Pchlide precursor 5-aminolevulinic acid (5-ALA), their capability to import the pPORA could be restored (17). By contrast, import of the pPORB was possible both into Pchlide-containing and Pchlide-free chloroplasts (18). This suggested to us that pPORA and pPORB entered the plastids through different sites.
In the present study, we consequently asked three different questions. First, are pPORA and pPORB imported through the general import site comprising the translocon of the outer chloroplast envelope (TOC) and translocon of the inner plastid envelope (see refs. 19 and 20 for review)? Second, is there, maybe, a distinctive import site that specifically sequesters the pPORA? Third, is the operation of the Pchlide-dependent import pathway of the pPORA confined to barley, or may it also occur in other monocotyledonous and dicotyledonous plant species?
Materials and Methods
Plastid Isolation and Protein Import.
Seeds of barley (Hordeum vulgare cv. Carina), oat (Secale cereale), wheat (Triticum aestivum), pea (Pisum sativum Feltham First), bean (Phaseolus vulgaris Daisy), tobacco (Nicotiana tabacum), and spinach (Spinacia oleracea) were germinated on moist vermiculite at 25°C and grown under continuous white-light illumination provided by fluorescent bulbs (30 W/m2). Similarly, seeds of Arabidopsis thaliana, ecotype Columbia, kindly provided by E. Grill, The University of Munich, Germany, were germinated on agar medium before being tranferred onto soil and grown to maturity. Chloroplasts were isolated from surface-sterilized leaves of the various plant species by differential centrifugation, followed by Percoll (Amersham Pharmacia) density gradient centrifugation (17). Chloroplasts subsequently were incubated with 5-ALA dissolved in phosphate buffer or phosphate buffer alone for 15 min in the dark and repurified on Percoll (17). Chloroplasts containing the exogenous 5-ALA-derived Pchlide or lacking Pchlide finally were resuspended in the import buffer described in ref. 17 but lacking ATP, and added to radiolabeled pPORA, transA-dihydrofolate reductase (DHFR), pPORB, transB-DHFR or transPC-DHFR precursor molecules that had been synthesized by coupled in vitro transcription/translation of respective cDNA clones (21, 22), concentrated by ammonium sulfate precipitation as described (22), and adjusted to the desired concentration with mock-incubated wheat germ extract. Final 50-μl import mixtures consisted of 25 μl of the doubly concentrated import buffer (see above), 10 μl of the chloroplast suspension containing 5⋅107 plastids, 5 μl of the different urea-denatured, radiolabeled precursors, and 2.5 μl of 2 mM Mg-ATP, as well as doubly distilled water to adjust the final reaction volume. After a 15-min incubation, the plastids were sedimented by centrifugation (17), if not stated otherwise treated with thermolysin (23), and repurified on Percoll. Plastid protein was recovered from lysed plastids by precipitation with trichloroacetic acid [5% (wt/vol) final concentration] and separated by denaturing SDS/PAGE on 11–20% (wt/vol) polyacrylamide gradients (17). Proteins present in the supernatant fraction obtained after centrifugation of the import mixtures were prepared identically and detected by autoradiography (21).
Quantitative Receptor Binding Studies.
The methodology used was that described in ref. 24. Chloroplasts were isolated as described, treated with 5 mM Mg-ATP plus 5-ALA or phosphate buffer instead of 5-ALA, and subsequently depleted of ATP, as described (17). Chloroplasts containing or lacking Pchlide produced by 5-ALA pretreatment then were incubated at 4°C with 0.1 mM Mg-ATP and increasing concentrations of the radiolabeled pPORA or pPORB. After a 15-min incubation in the dark and a subsequent step of centrifugation, the numbers of plastid-bound pPORA and pPORB molecules recovered in the sediment fractions and of nonbound precursor molecules present in the supernatant fractions were determined (24) and plotted as a function of the total precursor concentrations in the assays (25). To correct for nonspecific binding, chloroplasts were incubated with radiolabeled mature PORA and PORB polypeptides generated by a PCR-based approach (21) and analyzed identically.
Competition and Antibody Blocking Experiments.
Competitive receptor binding and translocation studies were performed with the indicated radiolabeled precursors and increasing concentrations of small subunit ribulose-1,5-bisphosphate carboxylase/oxygenase (SSU) and ferredoxin (Fd), produced in Escherichia coli and purified from inclusion bodies as described (10, 26). For the experiments described in Figs. 2 and 3, pSSU and pFd were added to the incubation mixtures to 2.5 μM final concentrations. This represented a 100-fold excess relative to the radiolabeled precursors, as determined according to ref. 21.
Figure 2.
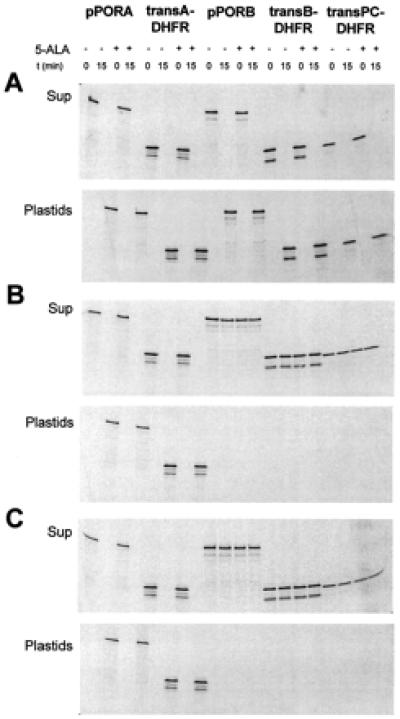
pSSU and pFd compete for binding of pPORB, transB-DHFR, and transPC-DHFR, but not pPORA and transA-DHFR, to barley chloroplasts. (A–C) Quantitative receptor binding studies analogous to those described in Fig. 1 A and C, but with three additional radiolabeled precursors, were performed either in the absence (A) or presence of 2.5 μM nonradioactively labeled pSSU (B) or pFd (C). The autoradiograms show the levels of free (Sup) and plastid-bound (Plastids) precursors before (0 min) and after a 15-min incubation with chloroplasts containing (+ 5-ALA) or lacking (− 5-ALA) 5-ALA-derived Pchlide.
Figure 3.
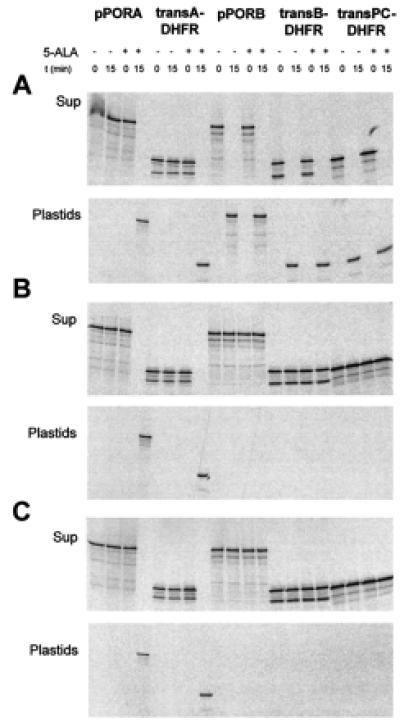
pSSU and pFd block import of pPORB, transB-DHFR, and transPC-DHFR, but not pPORA and transA-DHFR, into Pchlide-containing barley chloroplasts. (A–C) Import of the indicated radiolabeled precursors was determined either in the absence (A) or presence of 2.5 μM pSSU (B) or 2.5 μM pFd (C) as described in Fig. 1 E and F, except that the recovered plastids were treated with thermolysin and repurified on Percoll before final analysis. The autoradiograms show precursor (Sup) and mature protein (Plastids) levels before (0 min) and after a 15-min incubation with chloroplasts containing (+ 5-ALA) or lacking (− 5-ALA) Pchlide produced by 5-ALA pretreatment.
For antibody blocking experiments, Fab fragments were prepared from respective antisera and bound to chloroplasts as described (27, 28). After two subsequent steps of centrifugation and repurification, chloroplasts bearing the anti-Toc75 and anti-Toc86 Fab fragments were added to incubation mixtures containing 2 mM Mg-ATP and the radiolabeled transA-DHFR, transB-DHFR and transPC-DHFR precursors. Precursor translocation was determined as described above.
Results
Monocotyledonous and Dicotyledonous Plant Species Import the pPORA, but Not the pPORB, in a Pchlide-Dependent Manner.
Chloroplasts were isolated from light-grown seedlings of barley, A. thaliana, and six other monocotyledonous and dicotyledonous plant species, as described in Materials and Methods, and depleted of endogenous ATP by keeping them on ice for 1 h (8). When incubated in the presence of exogenously added Mg-ATP (2 mM final concentration) at 23°C in the dark, these chloroplasts could import the pPORB but not the pPORA (Table 1, −Pchlide). Because import of pPORA requires Pchlide not present in a transport-active form in chloroplasts (17), no uptake of the radiolabeled precursor was observed despite the presence of import-saturating ATP concentrations. However, when the pool of transport-active Pchlide was repleinished by pretreating isolated chloroplasts with the porphyrin precursor 5-ALA in the presence of 5 mM Mg-ATP (17), import of the pPORA could be restored (Table 1, +Pchlide).
Table 1.
Pchlide-dependent import of pPORA into chloroplasts of monocotyledonous and dicotyledonous plant species
| Plant species | Import, % of added precursor
|
|||
|---|---|---|---|---|
| pPORA
|
pPORB
|
|||
| −Pchlide | +Pchlide | −Pchlide | +Pchlide | |
| Monocotyledonous | ||||
| Barley | n.d. | 62 | 58 | 56 |
| Oat | n.d. | 48 | 52 | 54 |
| Wheat | n.d. | 65 | 56 | 54 |
| Dicotyledonous | ||||
| Pea | n.d. | 40 | 34 | 38 |
| Bean | n.d. | 42 | 36 | 32 |
| Arabidopsis | n.d. | 38 | 32 | 32 |
| Tobacco | n.d. | 32 | 30 | 28 |
| Spinach | n.d. | 34 | 28 | 26 |
Import reactions were carried out at 23°C in the dark with 2 mM Mg-ATP and chloroplasts containing (+Pchlide) or lacking (−Pchlide) Pchlide produced by 5-ALA pretreatment. Percentages refer to the levels of imported, thermolysin-resistant mature PORA and PORB, relative to the levels of added precursors per assay, after a 15-min import reaction. n.d., not detectable.
Barley Chloroplasts Contain Different Numbers of Receptor Sites for the pPORA and pPORB.
The strikingly different Pchlide requirements of import suggested that pPORA and pPORB entered the chloroplast through different sites. As a first step to test this idea, we determined the actual numbers of receptors for the pPORA and pPORB on the surface of barley chloroplasts. As shown by Kouranov and Schnell (29), binding of precursors to the plastids initially is reversible and occurs in the absence of nucleoside triphosphates. Low (<0.1 mM) ATP concentrations, however, favor partial integration of the receptor-bound precursors into the import machinery (29). This step, which previously has been referred to as binding (3–6), is stimulated by GTP (28–30). The precursors then insert across the outer envelope membrane and also interact with components of the inner envelope (29). As a result, early import intermediates are formed (10). The precursor concentrations that are necessary to saturate the sites for establishing early import intermediates were found to be nearly identical to those seen for energy-independent binding (10, 29). This allows extrapolating the number of energy-independent preprotein-binding sites and suggests that they may limit the number of preproteins that associate with the outer envelope membrane (29). If high (e.g., 2 mM) ATP concentrations are present, the precursors ultimately translocate across the inner envelope (7–10, 29).
Taking into account these findings, chloroplasts were isolated as described, treated with 5-ALA dissolved in phosphate buffer or phosphate buffer alone, repurified on Percoll cushions, and subsequently depleted of ATP (8, 17). Then, various amounts of the radiolabeled pPORA and pPORB were added to incubation mixtures containing 0.1 mM Mg-ATP. Before use, all precursors were concentrated by ammonium sulfate precipitation and supplemented with mock-incubated, concentrated wheat germ extract, to obtain identical reaction mixtures.
After a 15-min incubation at 4°C in the dark, the plastids were sedimented by centrifugation and repurified, and the amounts of bound pPORA and pPORB were determined (17). pPORA and pPORB molecules not bound to the chloroplasts were recovered from the supernatant fractions obtained after sedimentation of the plastids by precipitation with trichloroacetic acid (17) and quantified as described (21, 24).
When the numbers of pPORA and pPORB molecules bound to barley chloroplasts were determined and plotted as a function of the precursor concentrations, striking differences were observed (Fig. 1). At saturating precursor concentrations, each barley chloroplast bound approximately 2,250 pPORB molecules but only 380 pPORA molecules (Fig. 1 C and A, respectively, dashed lines). Pretreatment of the chloroplasts with 5-ALA, giving rise to intraplastidic Pchlide formation (17), did not affect subsequent binding of either pPORA or pPORB to the plastids: the 5-ALA-pretreated chloroplasts bound approximately 2,200 pPORB and 400 pPORA molecules, respectively (Fig. 1 C and A, respectively, solid lines). Scatchard analyses (25) of the binding data (Fig. 1 B and D) revealed that the KD values were almost indistinguishable for the 5-ALA-pretreated and the non-5-ALA-pretreated chloroplasts and either precursor, all KD values lying in the range of 8.5 to 8.6 nM. These KD values were similar to those determined by Friedman and Keegstra (24) in their pioneering work on binding of the small subunit precursor of SSU to chloroplasts. But they were at variance with that determined by Schnell and Blobel (10) who found a ca. 5-fold higher KD value for a chimeric precursor (pSSU-protein A) in which the entire SSU precursor had been fused to protein A. The reasons for these discrepancies are unknown but one may speculate at least that, because of the relatively high concentration of wheat germ extract in the incubation mixtures, some lowering in the binding affinity of the precursor was observed in our experiments. This would not be observed with bacterially produced precursors such as pSSU-protein A.
Figure 1.
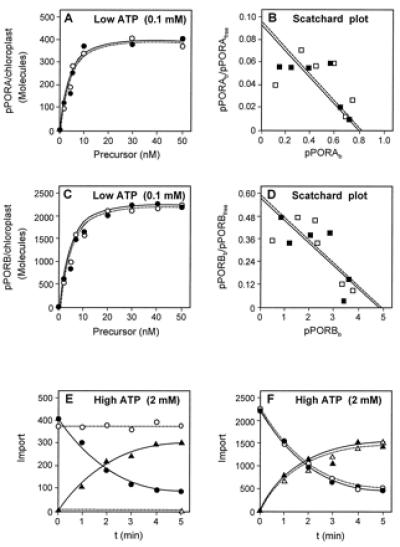
Quantitative receptor binding studies reveal different numbers of receptor sites for the pPORA and pPORB on the outer envelope of barley chloroplasts. (A and C) Binding of pPORA (A) and pPORB (C) to chloroplasts containing (solid lines) or lacking (dashed lines) Pchlide produced by 5-ALA pretreatment. The incubations were performed at 4°C in the dark in the presence of 0.1 mM Mg-ATP. (B and D) Scatchard analysis of the binding data shown in A and C, respectively, to determine the numbers of receptor sites for the pPORA and pPORB per plastid, as well as the KD values of their binding to barley chloroplasts containing (solid lines) or lacking (dashed lines) the exogenous 5-ALA-derived Pchlide. (E and F) Translocation competence of the envelope-bound pPORA (E) and pPORB (F) determined at 23°C after readdition of Mg-ATP to a 2 mM final assay concentration. The different graphs show time courses of precursor (circles) and mature protein (triangles) levels with chloroplasts containing (solid lines) or lacking (dashed lines) Pchlide produced by 5-ALA pretreatment. Note that pPORA is imported only into Pchlide-containing chloroplasts.
In our experiments, readdition of Mg-ATP to a 2 mM final assay concentration caused import of the envelope-bound pPORB into both chloroplasts that contained or lacked the exogenous 5-ALA-derived Pchlide (Fig. 1F, solid line and dashed line, respectively). Import was completed within the first 5 min of incubation in either case. Remarkably, a maximum of 1,500 pPORB molecules could be recovered in the mature form. The remainder (ca. 500 pPORB molecules) was translocation-incompetent. A similar proportion was found for the envelope-bound pPORA and Pchlide-containing chloroplasts (Fig. 1E, solid line). Chloroplasts lacking the 5-ALA-derived Pchlide were unable to import the envelope-bound pPORA (Fig. 1E, dashed line), consistent with our previous findings (17, 18, 21).
Competition Experiments Suggest the Operation of Two Distinctive Protein Import Pathways into Chloroplasts.
The observed large difference in the number of binding sites for the pPORA and pPORB per barley chloroplast suggested the existence of two different surface-exposed receptors. This idea was reinforced by findings on the import of cytosolic precursors into mitochondria of yeast. In this case, two pairs of receptor subunits (31) sequentially act during binding of a specific subset of precursors (32). These mitochondrial precursors normally need MSF, a mitochondrial import stimulating factor (33, 34), for binding. They first interact with MSF, then with TOM37 and TOM72 (32). Upon ATP-dependent release of MSF, the precursors then bind TOM19/TOM22, the second pair of receptor subunits, and subsequently are delivered into the import channel of the outer mitochondrial membrane (32). Import in the absence of MSF bypasses TOM37/TOM72 (32).
Taking into account these findings, we speculated that there might be a specific receptor subcomplex in the envelope of barley chloroplasts that bound the pPORA. Its activity in terms of transferring the precursor to TOC86, the previously identified, surface-exposed receptor component of the outer plastid envelope membrane (27, 30, 35), could be regulated by Pchlide synthesized in the plastid envelope (36). In the absence of Pchlide, pPORA would bind to this receptor subcomplex but because of the lack of transfer to TOC86 would not be imported. In the presence of Pchlide, however, pPORA would be transferred to TOC86 from which it could be delivered to TOC75, the translocation channel component of the outer envelope (27, 30, 35). pPORB, by contrast, would directly bind TOC86 and hence would immediately enter a productive import pathway. This would explain why pPORB was imported into the plastids in a Pchlide-independent, unregulated manner. We assumed that, if this model would be correct, it should be possible to block the import of pPORA into Pchlide-containing chloroplasts by an excess of pPORB or other precursors that use TOC86 as a common receptor.
Two different types of experiments were performed to test this model. In the first type of experiment, competitive receptor binding studies were performed with bacterially expressed precursors to the small subunit of SSU of pea (10) and Fd of Silene pratensis (26). These precursors previously had been demonstrated to enter the chloroplasts through the general import site, including TOC86 (11, 12).
In addition to the authentic pPORA and pPORB, three different chimeric precursor proteins were used. The first precursor, transA-DHFR, consisted of the transit sequence of pPORA (transA) and a cytosolic DHFR of mouse (21). As shown previously (21), import of this chimeric precursor is strictly Pchlide-dependent. The second precursor, transB-DHFR, consisted of the transit peptide of pPORB (transB) and the DHFR (21). Reflecting the different primary structure and incapability of transB, compared with transA, to bind Pchlide, import of this precursor had been shown not to require Pchlide (21). For the same reasons, we used transPC-DHFR, carrying the DHFR behind the transit peptide of plastocyanin of S. pratensis (37), as a constitutively imported precursor (17, 21).
When pSSU and pFd were added to 2.5 μM final concentrations to incubation mixtures containing 0.1 mM Mg-ATP and the radiolabeled pPORA or its transA-DHFR derivative, no inhibition of receptor binding was observed (Fig. 2 B and C). As found for assays not supplemented with the competitors (see Fig. 2A), either radiolabeled precursor likewise bound to chloroplasts that contained or lacked Pchlide produced by 5-ALA pretreatment. Because the incubations were performed at 4°C, this binding led to the quantitative shift of the precursors from the supernatant to the plastid fractions, respectively (Fig. 2 B and C, compare the precursor levels before and after 15 min). However, when pSSU and pFd were added to the radiolabeled transB-DHFR and transPC-DHFR, no receptor binding was observed: the radiolabeled precursors were left in the supernatant fraction (Fig. 2 B and C, respectively, versus A).
In the second type of experiment, both the initial binding of the precursors as well as their subsequent translocation were allowed to proceed; the different radiolabeled precursors were incubated with Pchlide-containing or Pchlide-free chloroplasts at 23°C in the dark in the presence of 2 mM Mg-ATP. When import was analyzed in the absence of added competitors, no difference was observed for pPORB, transB-DHFR, transPC-DHFR, pPORA, transA-DHFR, and chloroplasts containing the exogenous 5-ALA-derived Pchlide. All radiolabeled precursors were imported and processed to their mature size (Fig. 3A, +5-ALA). This import was sensitive to excess pSSU and pFd in the case of pPORB, transB-DHFR, and transPC-DHFR (Fig. 3 B and C, see the constant levels of the precursors in the supernatant fractions at 0 and 15 min). But it turned out to be insensitive to the competitors in case of pPORA and transA-DHFR (Fig. 3 B and C, see the appearance of the mature PORA and DHFR, respectively, in the plastid fraction). Increasing the levels of pSSU and pFd to 25 μM did not affect subsequent import of pPORA into Pchlide-containing chloroplasts (data not shown). Import of pPORB, by contrast, was already drastically diminished at 0.25 μM pSSU or 0.25 μM pFd (data not shown).
When import was analyzed into chloroplasts lacking the 5-ALA-derived Pchlide, exactly the same results were obtained: pPORB, transB-DHFR, and transPC-DHFR import occurred in a pSSU- and pFd-sensitive manner (Fig. 3 B and C, −5-ALA); however, no import was seen for pPORA and transA-DHFR regardless of whether the incubation mixtures contained or lacked pSSU and pFd, respectively (Fig. 3).
Antibodies Against Previously Characterized Receptor and Translocation Channel Components of the TOC Machinery Do Not Inhibit Import of the pPORA.
The results presented thus far suggested the operation of two distinctive protein import pathways into chloroplasts: one for pPORB, transB-DHFR and transPC-DHFR, as well as pSSU and pFd, and the other for pPORA and transA-DHFR. We hypothesized that these two pathways may not share TOC86 and TOC75 as common receptor and translocation channel components, respectively. To test this idea, Fab fragments were prepared from respective antisera (27, 28) and bound to Pchlide-free or Pchlide-containing chloroplasts during a preincubation. When the import of transA-DHFR, transB-DHFR, and transPC-DHFR was determined at 23°C in the dark in assay mixtures supplemented with 2 mM Mg-ATP, striking differences were seen. Neither anti-TOC86 Fab-pretreated nor anti-TOC75 Fab-pretreated, but Pchlide-containing chloroplasts could efficiently import transB-DHFR and transPC-DHFR (Table 2, +Pchlide). However, these different chloroplasts imported transA-DHFR well. With Pchlide-free chloroplasts, more or less the same results were obtained, except for the fact that transA-DHFR could not be imported because of the lack of transport-active Pchlide (Table 2, −Pchlide).
Table 2.
Blocking TOC86 or TOC75 does not inhibit import of pPORA into Pchlide-containing barley chloroplasts
| Precursor | Import, % of added precursor
|
|||||
|---|---|---|---|---|---|---|
| transA-DHFR
|
transB-DHFR
|
transPC-DHFR
|
||||
| −Pchlide | +Pchlide | −Pchlide | +Pchlide | −Pchlide | +Pchlide | |
| anti-TOC86 | n.d. | 64 | 2 | 4 | 5 | 3 |
| anti-TOC75 | n.d. | 62 | 8 | 6 | 4 | 6 |
Protochlorophyllide-containing (+Pchlide) and Pchlide-free (−Pchlide) chloroplasts bearing anti-TOC86 or anti-TOC75 Fab fragments were incubated with the indicated radiolabeled precursors at 23°C in the dark in the presence of 2 mM Mg-ATP. Quantification of the import data was performed as described in Table 1, but percentages refer to mature DHFR levels relative to added precursor levels, respectively, per assay. n.d., not detectable.
Blocking the Import Site for the pPORA Does Not Impair Subsequent Import of Other Precursors.
We next asked what would happen if the import site sequestering the pPORA would be blocked. Etiolated barley seedlings were exposed to light for 8 h, which previously has been shown to lead to an accumulation of pPORA in vivo (38). Under these conditions, the light-induced decline in the intraplastidic Pchlide level ultimately limits import of the precursor (38). Using such chloroplasts, two different experiments were performed.
When chloroplasts bearing the arrested pPORA (data not shown, but see ref. 38) were incubated with the different radiolabeled chimeric precursors under binding conditions (4°C, 0.1 mM Mg-ATP), a differential effect on transA-DHFR could be seen. With chloroplasts lacking the exogenous 5-ALA-derived Pchlide, the precursor remained in the supernatant obtained after sedimentation of the assay mixtures and no radioactivity was recovered in the plastid fraction (Table 3, Binding, CP+pPORA:−5-ALA). With Pchlide-containing chloroplasts, no inhibition of binding was detectable (Table 3, Binding; CP+pPORA:+5-ALA). In the case of transPC-DHFR and transB-DHFR, no such differences were observed; the precursors almost quantitatively bound to chloroplasts that contained or lacked Pchlide produced by 5-ALA pretreatment (Table 3, CP+pPORA: compare +5-ALA versus −5-ALA). Controls with respective chloroplasts lacking the arrested pPORA were positive in all cases; the precursors similarly bound chloroplasts containing or lacking Pchlide produced by 5-ALA pretreatment (Table 3, CP−pPORA).
Table 3.
Authentic pPORA, accumulating in vivo, does not inhibit either binding or import of transB-DHFR and transPC-DHFR into Pchlide-containing chloroplasts
| Precursor | Binding/import, % of added precursor
|
|||||
|---|---|---|---|---|---|---|
| transA-DHFR
|
transB-DHFR
|
transPC-DHFR
|
||||
| −5-ALA | +5-ALA | −5-ALA | +5-ALA | −5-ALA | +5-ALA | |
| Binding | ||||||
| CP+pPORA | n.d. | 91 | 93 | 95 | 93 | 94 |
| CP−pPORA | 95 | 93 | 96 | 91 | 93 | 93 |
| Import | ||||||
| CP+pPORA | n.d. | 24 | 56 | 58 | 51 | 50 |
| CP-pPORA | n.d. | 58 | 55 | 60 | 47 | 52 |
Chloroplasts bearing the authentic pPORA (CP+pPORA) at their outer envelope were prepared from etiolated barley seedlings that had been exposed to light for 8 h, as described in ref. 38. By analogy, chloroplasts lacking the pPORA (CP-pPORA) were prepared from light-grown plants. Chloroplasts to be used for binding assays were preincubated at 23°C in the dark with 2 mM Mg-ATP plus 5-ALA (+5-ALA) or Mg-ATP plus phosphate buffer instead of 5-ALA (−5-ALA) and repurified, whereas chloroplasts to be used for studying import were supplemented with these compounds just during their incubation with the different radiolabeled precursors. Quantification of binding and import data was done as described in Fig. 1 and Table 2, respectively. n.d., not detectable.
We next incubated chloroplasts that had not been pretreated with 5-ALA and thus still bore the arrested pPORA at their outer envelope, under import conditions (23°C, 2 mM Mg-ATP). Parallel assays were run either in the presence or absence of 5-ALA. As shown in Table 3 (Import, CP+pPORA:+5-ALA), only chloroplasts producing Pchlide from the exogenous 5-ALA were able to transport transA-DHFR. In the absence of the pigment, no import was detectable (Table 3, CP+pPORA:−5-ALA). That transA-DHFR import into such Pchlide-containing chloroplasts did not reach saturation during the tested time period was not unexpected, given the fact that first the transport-competent part of the envelope-bound authentic pPORA had to be chased into the chloroplasts before transA-DHFR could follow. With transB-DHFR and transPC-DHFR, no inhibition of translocation caused by arrested pPORA molecules was seen, regardless of whether the assay mixtures contained or lacked 5-ALA (Table 3, CP+pPORA: compare +5-ALA versus −5-ALA). Similarly, import of these precursors was independent of the 5-ALA-derived Pchlide in the case of chloroplasts lacking the endogenous pPORA (Table 3, CP−pPORA:+5-ALA versus −5-ALA). With such chloroplasts, transA-DHFR import was not possible unless Pchlide synthesis was allowed to proceed in the presence of 5-ALA (Table 3, CP−pPORA, compare −5-ALA versus +5-ALA).
Discussion
The results shown in this study provide strong evidence that two distinctive protein import pathways operate in chloroplasts. The first pathway is presumed to proceed through the general import site, comprising the previously characterized import machinery of the outer (TOC) and inner plastid envelope membranes (see refs. 19 and 20 for review). It sequestered pPORB, transB-DHFR, and transPC-DHFR in a pSSU- and pFd-sensitive, TOC86- and TOC75-mediated manner (Figs. 2 and 3, Table 2).
The second import pathway was found to be specific for the pPORA; its operation could not be inhibited by excess pSSU and pFd (Figs. 2 and 3) or blocking the receptor and translocation channel components of the TOC machinery, TOC86 and TOC75, respectively (Table 2). The actual role of Pchlide in this pathway was to trigger the translocation of the pPORA (21). No evidence was obtained that the porphyrin pigment promoted the docking of the precursor to its receptor (Figs. 1 and 2) or the subsequent transfer into the translocation channel (Figs. 1 and 3).
The pPORA-specific import pathway operates both in monocotyledonous and dicotyledonous plant species (Table 1). In either case, it coupled porphyrin biosynthesis to protein translocation and consequently lowered the actual level of free excited Pchlide molecules, which would photooxidatively damage the chloroplast.
Coupling the sites of porphyrin biosynthesis and protein translocation has another interesting implication. This becomes obvious when studying the compartmentation of the C5 pathway, leading to the biosynthesis of Pchlide and its reduced esterified products, chlorophyll a and chlorophyll b. Whereas Pchlide synthesis has been shown to occur in the plastid envelope (36), the outer surroundings of the plastid, functional PORA-pigment complexes are required inside the organelle (39, 40). This spatial separation necessitates porphyrin transport to take place. By virtue of the Pchlide-dependent import pathway, such a porphyrin transport could have been established. In fact, recent studies have shown that PORA-Pchlide-NADPH complexes formed during the substrate-dependent translocation of the pPORA assemble into higher molecular weight light-harvesting POR-Pchlide complexes of the prolamellar body, termed LHPP, which play a decisive role for seedling de-etiolation (40).
Evidence supporting the operation of the Pchlide-dependent import pathway of the pPORA recently has come from a study of Jarvis et al. (41), who isolated a mutant of Arabidopsis that displayed a defect in TOC33, a twin component of one of the TOC proteins (TOC34) operating in outer envelope membrane translocation (27, 35). The authors noted that the observed drastic delay in greening correlated with a reduction in prolamellar body size and the accumulation of the pPORA in vivo and speculated that TOC33 may operate in the general protein import pathway (41). However, an alternative explanation could be that the incapability in importing the pPORA caused the mutant to establish much less light-harvesting POR-Pchlide complexes of the prolamellar body (LHPP) (as reflected in the reduced prolamellar body size), to display a greater light sensitivity and consequently to exhibit a seeming delay in greening. This would give TOC33 a novel role in pPORA import. Assuming that TOC33 is indeed part of the substrate-dependent import machinery in the outer envelope of chloroplasts, its lack in the TOC33-deficient line presumably would cause more Pchlide to accumulate in a free, photodynamically active form. As a result of the destructive Pchlide effects, the operation of the standard protein import machinery also could be impaired, leading to the observed general depression of protein import (41). Although genetically engineered overexpression of TOC34 or TOC33 could rescue the mutant to wild type, overproduction of the endogenous TOC34 was not seen (41). This points to different regulatory circuits that govern TOC34 and TOC33 expression in planta and seems well consistent with the idea of the operation of separate standard and substrate-dependent protein import machineries.
Acknowledgments
This article is dedicated to Prof. Dr. H. Ziegler on the occasion of his 75th birthday. We are grateful to K. Apel for his generosity in allowing some of the initial experiments described in this study to be performed in his laboratory. For gifts of cDNA clones and antisera, we thank D. J. Schnell, The State University of New Jersey, Rutgers, P. Weisbeek, The University of Utrecht, Utrecht, The Netherlands, and F. Kessler, Institute for Plant Sciences, Zurich, Switzerland. This work was inaugurated at the Institute for Plant Sciences, Swiss Federal Institute of Technology, Zurich, Switzerland, and sponsored by a research project grant of the Swiss National Science Foundation (NF) to S.R. and Dr. K. Apel.
Abbreviations
- Pchlide
protochlorophyllide
- pPOR
precursor protochlorophyllide oxidoreductase
- 5-ALA
5-aminolevulinic acid
- TOC
translocon of the outer chloroplast envelope
- DHFR
dihydrofolate reductase
- SSU
small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase
- Fd
ferredoxin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160242597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160242597
References
- 1.Cline K, Henry R. Annu Rev Cell Dev Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Robinson C, Klösgen R B. Plant Mol Biol. 1994;26:15–24. doi: 10.1007/BF00039516. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer J, Lachmann P, Kloppstech K. Eur J Biochem. 1982;126:143–148. doi: 10.1111/j.1432-1033.1982.tb06758.x. [DOI] [PubMed] [Google Scholar]
- 4.Cline K, Werner-Washburne M, Lubben T, Keegstra K. J Biol Chem. 1985;260:3691–3696. [PubMed] [Google Scholar]
- 5.Olsen L J, Theg S M, Selman B R, Keegstra K. J Biol Chem. 1989;264:6724–6729. [PubMed] [Google Scholar]
- 6.Olsen L J, Keegstra K. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- 7.Flügge U-I, Hinz G. Eur J Biochem. 1986;160:563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- 8.Theg S M, Bauerle C, Olsen L J, Selman B R, Keegstra K. J Biol Chem. 1989;264:6730–6736. [PubMed] [Google Scholar]
- 9.Waegemann K, Soll J. Plant J. 1991;1:149–158. [Google Scholar]
- 10.Schnell D, Blobel G. J Cell Biol. 1993;120:103–115. doi: 10.1083/jcb.120.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry S E, Buvinger W E, Bennett J, Keegstra K. J Biol Chem. 1991;266:11882–11889. [PubMed] [Google Scholar]
- 12.Schnell D, Blobel G, Pain D. J Biol Chem. 1991;266:3335–3342. [PubMed] [Google Scholar]
- 13.Oblong J E, Lamppa G. J Biol Chem. 1992;267:14328–14334. [PubMed] [Google Scholar]
- 14.Theg S M, Geske F J. Biochemistry. 1992;31:5053–5060. doi: 10.1021/bi00136a018. [DOI] [PubMed] [Google Scholar]
- 15.Cline K, Henry R, Li C, Yuan J. EMBO J. 1993;12:4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtorf H, Reinbothe S, Reinbothe C, Bereza B, Apel K. Proc Natl Acad Sci USA. 1995;92:3254–3258. doi: 10.1073/pnas.92.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K. Plant Cell. 1995;7:161–172. doi: 10.1105/tpc.7.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinbothe S, Reinbothe C, Holtorf H, Apel K. Plant Cell. 1995;7:1933–1940. doi: 10.1105/tpc.7.11.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuks B, Schnell D J. Plant Physiol. 1997;114:405–409. doi: 10.1104/pp.114.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heins L, Collinson I, Soll J. Trends Plant Sci. 1998;3:56–61. [Google Scholar]
- 21.Reinbothe C, Apel K, Reinbothe S. Proc Natl Acad Sci USA. 1997;94:8890–8894. doi: 10.1073/pnas.94.16.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinbothe S, Krauspe R, Parthier B. Planta. 1990;181:176–183. doi: 10.1007/BF02411535. [DOI] [PubMed] [Google Scholar]
- 23.Cline K, Werner-Washburne M, Andrews J, Keegstra K. Plant Physiol. 1984;75:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman A L, Keegstra K. Plant Physiol. 1988;89:993–999. doi: 10.1104/pp.89.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scatchard G. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 26.Smeekens S, Geerts D, Bauerle C, Weisbeek P. Mol Gen Genet. 1989;216:178–182. [Google Scholar]
- 27.Schnell D J, Kessler F, Blobel G. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Kouranov A, LaSala S E, Schnell D J. J Cell Biol. 1996;134:315–323. doi: 10.1083/jcb.134.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouranov A, Schnell D J. J Cell Biol. 1997;139:1677–1685. doi: 10.1083/jcb.139.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry S E, Keegstra K. Plant Cell. 1994;6:93–105. doi: 10.1105/tpc.6.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramage L, Junne T, Hahne K, Lithgow T, Schatz G. EMBO J. 1993;12:4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hachiya N, Mihara K, Suda K, Horst M, Schatz G, Lithgow T. Nature (London) 1995;376:705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- 33.Hachiya N, Alam R, Sakasegawa Y, Sakaguchi M, Mihara K, Omura T. EMBO J. 1993;12:1579–1586. doi: 10.1002/j.1460-2075.1993.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachiya N, Komiya T, Alam R, Iwahashi J, Sakaguchi M, Omura T, Mihara K. EMBO J. 1994;13:5146–5154. doi: 10.1002/j.1460-2075.1994.tb06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. Science. 1994;266:1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- 36.Joyard J, Block M, Pineau B, Albrieux C, Douce R. J Biol Chem. 1990;265:21820–21827. [PubMed] [Google Scholar]
- 37.Hageman J, Baecke C, Ebskamp M, Pilon R, Smeekens S, Weisbeek P. Plant Cell. 1990;2:479–494. doi: 10.1105/tpc.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinbothe S, Reinbothe C, Neumann D, Apel K. Proc Natl Acad Sci USA. 1996;93:12026–12030. doi: 10.1073/pnas.93.21.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryberg M, Sundqvist C. In: Chlorophylls. Scheer H, editor. Boca Raton, FL: CRC; 1991. pp. 587–612. [Google Scholar]
- 40.Reinbothe C, Lebedev N, Reinbothe S. Nature (London) 1999;397:80–84. [Google Scholar]
- 41.Jarvis P, Chen L-J, Li H-M, Peto C A, Fankhauser C, Chory J. Science. 1998;282:100–103. doi: 10.1126/science.282.5386.100. [DOI] [PubMed] [Google Scholar]


