Abstract
The objective of the study was to investigate the prevalence of immunoglobulin G (IgG) antibodies to hepatitis E virus (HEV) infection in a population sample from Catalonia and to analyze the demographic and clinical variables associated with the presence of these antibodies. A total of 1,280 subjects between 15 and 74 years of age were selected randomly from urban and rural areas. Data for sociodemographic and clinical variables were collected by using a questionnaire. IgG antibodies to HEV were determined by an immunoenzymatic method. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for studied variables. Multiple logistic regression analysis was used to determine which variables were independently associated with the prevalence of HEV infection. Anti-HEV antibodies were detected in 96 (7.3%) of the 1,280 samples analyzed. The prevalence of antibodies was greater among males (7.8%) than among women (7%) and increased with age for both sexes, from 3% among subjects 15 to 24 years of age to 12% among subjects ≥65 years of age. Bivariate analysis of the sociodemographic and clinical variables showed an association between the prevalence of hepatitis E virus infection and minor surgery (OR, 1.96; 95% CI, 1.24 to 3.11), abdominal surgery (OR, 1.74; 95% CI, 1.12 to 2.73), and, for women, being uniparous or multiparous (OR, 2.84; 95% CI, 1.19 to 6.79). The multivariate analysis showed an association with minor surgery only (OR, 1.68; 95% CI, 1.03 to 2.70). In conclusion, anti-HEV antibodies were detected in 7.3% of the Catalan population. The seroprevalence of anti-HEV antibodies increased with age and was associated with previous minor surgery.
Hepatitis E virus (HEV) infection is a major cause of epidemic and acute sporadic hepatitis in many nonindustrialized countries, such as Mexico, India, and some parts of Asia and Africa, which are considered areas of endemicity (2, 3, 11, 18). Sporadic cases, among migrant laborers and travelers returning from areas of endemicity, have been observed in developed countries (13).
HEV is an enterically transmitted RNA virus that principally affects young adults and, in countries where the disease is endemic, is associated with more than 50% of the cases of sporadic acute hepatitis. The disease is self limiting but sometimes has severe complications and a high case fatality rate, particularly among pregnant women (about 20%) (11, 18, 24). Traditionally, North America and Europe have been considered regions of nonendemicity where most HEV infections were thought to be imported, although the seroprevalences in these areas range from 1 to 5% (11, 18).
The availability of diagnostic serologic tests has permitted the epidemiology of the infection to be better known. It is estimated that the prevalence of infection in regions of endemicity ranges between 3 and 26%, and the estimates suppose that this infection accounts for more than 50% of sporadic cases of acute hepatitis. In regions of nonendemicity, prevalences of infection range between 1 and 3% (11, 14, 15, 16, 18, 19, 24, 25, 30).
Infections are more frequent in countries with deficient hygienic conditions and may present as waterborne or food-borne outbreaks (1, 7, 26, 29) or as sporadic cases. The highest disease incidence rates are observed for young and middle-aged adults, and anicteric and subclinical forms are more frequent in children and adolescents (7). In developed countries, most cases are detected in travelers coming from regions where the disease is endemic.
At present, no risk factors associated with sporadic cases have been identified, although person-to-person transmission seems to be infrequent (29).
Although initial evidence suggested that HEV was an enterically transmitted virus with transmission mechanisms similar to those of the hepatitis A virus (HAV), the differences in the prevalences of infection of the two viruses and their differential distributions in specific population groups have led to a search for risk factors associated with HEV infection (1, 10). The fact that other enterically transmitted viruses, such as the hepatitis A virus, can occasionally be transmitted parenterally has led to the suggestion that HEV could also be transmitted by this route. Some studies have shown a prevalence among hemodialyzed patients higher than that among blood donors or the general population (1, 6). However, there are no conclusive studies for this transmission mechanism and it seems that the risk, if it exists, would be low (1, 8, 28).
The objective of this study was to study the prevalence of HEV infection in a representative sample of the adult population of Catalonia and to determine the associated demographic and behavioral factors.
MATERIALS AND METHODS
Sample.
The study was carried out in 2002 in Catalonia, a region in the northeast of Spain with a population of more than six million. A representative sample of the adult population of Catalonia that was ≥15 years of age was obtained by a two-stage procedure. In the first stage, 97 municipalities were randomly selected. In the second stage, participants were selected randomly from municipal censuses. The number of participants selected in each municipality was proportional to its classification as urban (>10,000 inhabitants) or rural (<10,000 inhabitants).
The sample size calculated for an expected prevalence of 50%, an alpha error of 5%, and a precision of ±0.025 was 1,536 people. Informed consent was obtained from all participants.
Serological tests.
Blood samples were obtained from all participants by venipuncture, and the sera obtained were frozen at −20°C until serologic testing. Anti-HEV antibodies were determined by an immunoenzymatic method (Bioelisa HEV IgG; BIOKIT, Barcelona, Spain) in which immunoglobulin G (IgG) antibodies to HEV are captured by three recombinant antigens, derivatives of a Burma strain and a Mexico strain, that correspond to the structural region of HEV. A goat anti-human IgG combined with a peroxidase that unites with the antigen-antibody complex was used as the secondary antibody. Anti-HEV antibodies were detected by incubating the complex with an enzymatic substrate and a chromogen. The intensity of the resulting color is proportional to the amount of anti-HEV IgG content. A sample was considered positive if the absorbance was superior to a cutoff value calculated by adding 0.500 to the average absorbance of the negative control. A repeatedly positive result indicated the presence of antibodies in the sample.
Sociodemographic, behavioral, and clinical variables.
Data for sociodemographic and clinical variables were obtained from all participants by use of a questionnaire. The sociodemographic variables studied were age, sex, place of birth, place of residence, and occupation. Places of residence were classified as rural (<10,000 inhabitants) or urban (>10,000 inhabitants). Socioeconomic levels were determined by occupation and classified into six sociodemographic groups (I to VI) (23). The questionnaire was also used to obtain information on risk factors for parenteral and sexual infection.
Statistical analysis.
The prevalence of anti-HEV antibodies and their 95% confidence intervals (95% CIs) for different age and sex groups, the sociodemographic variables, and the prevalence of risk factors were calculated. The chi-square test was used to analyze the differences. A P of <0.05 was considered statistically significant.
The odds ratios (ORs) of the statistically significant variables in the univariate analysis and their 95% CIs were calculated. Multiple logistic regression analysis was carried out to adjust the odds ratios and to determine which variables were independently associated with the prevalence of HEV infection.
The statistical analyses were carried out using the SPSS statistical program (SPSS Inc., Chicago, Ill.).
RESULTS
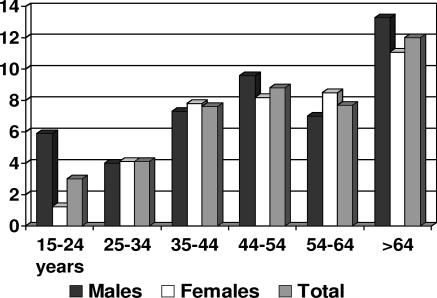
The number of people included in the sample was 1,280, which represented a participation of 83%. IgG antibodies to HEV were detected in 96 (7.3%) of the patient samples analyzed. The presence of antibodies in males (7.8%) was greater than that in women (7%). Figure 1 shows the distributions according to age and sex. Antibody positivities increased with age, both for women and men, ranging between 1.2 and 5.9%, respectively, in the 15- to 24-year age group, and 11.1 and 13.2%, respectively, in the ≥65-year age group.
FIG. 1.
Prevalence of antibodies to the hepatitis E virus in the adult population of Catalonia in 2002.
With respect to the sociodemographic behavioral and clinical variables studied, the bivariate analysis showed an association between the prevalence of anti-HEV antibodies and a history of minor surgery, abdominal surgery, and, for women, being uniparous or multiparous (Table 1). No differences in the prevalences of anti-HEV antibodies were observed with respect to place of birth, urban or rural habitat, or risk factors, such as injecting-drug use or blood transfusions. The only variable statistically associated with the presence of anti-HEV antibodies in the multivariate analysis was minor surgery (OR, 1.68; 95% CI, 1.03 to 2.70).
TABLE 1.
Variables associated with the prevalence of antibodies to the hepatitis E virus in the adult population of Catalonia
| Risk factor parameter | Prevalence (%) (95% CI) | No. of people | OR (95% CI), OR aj.a (95% CI) | Pb |
|---|---|---|---|---|
| Minor surgery | ||||
| Yes | 11.6 (7.7-15.5) | 259 | 1.96 (1.24-3.11), 1.68 (1.03-2.70) | 0.003 |
| No | 6.3 (4.8-7.8) | 1,008 | 0.035 | |
| Abdominal surgery | ||||
| Yes | 10.4 (7.0-13.8) | 317 | 1.74 (1.12-2.73), 1.38 (0.86-2.20) | 0.013 |
| No | 6.2 (4.7-7.7) | 947 | 0.183 | |
| Uniparous or multiparous woman | ||||
| Yes | 8.3 (5.9-10.7) | 517 | 2.84 (1.19-6.79), 1.81 (0.76-4.33) | 0.014 |
| No | 3.1 (0.7-5.5) | 194 | 0.177 | |
| Sex | ||||
| Male | 7.8 (5.6-10.0) | 567 | ||
| Female | 7.0 (5.1-8.9) | 713 | NS | |
| Habitat | ||||
| Urban | 7.5 (5.9-9.1) | 1,060 | ||
| Rural | 6.4 (3.2-9.6) | 220 | NS | |
| Social classc | ||||
| I to III | 6.5 (4.3-8.7) | 489 | ||
| IV to V | 7.0 (5.0-9.0) | 610 | NS | |
| Place of birth | ||||
| Spain | 7.1 (5.7-8.5) | 1,220 | ||
| Other | 8.3 (0-17.3) | 36 | NS | |
| Injecting-drug user | ||||
| Yes | 0.0 | 5 | ||
| No | 7.4 (5.9-8.9) | 1,233 | NS | |
| Tattoos | ||||
| Yes | 6.0 (0-12.6) | 50 | ||
| No | 7.4 (5.9-8.9) | 1,222 | NS | |
| Transfusions | ||||
| Yes | 7.8 (2.6-13.0) | 102 | ||
| No | 7.2 (5.7-8.7) | 1,167 | NS | |
| Injections | ||||
| Yes | 8.2 (5.8-10.6) | 524 | ||
| No | 7.0 (5.1-8.9) | 697 | NS | |
| Hospitalizations | ||||
| Yes | 8.6 (5.9-11.2) | 429 | ||
| No | 6.8 (5.1-8.5) | 838 | NS | |
| Accidents causing bleeding wounds | ||||
| Yes | 6.0 (4.0-8.0) | 550 | ||
| No | 8.2 (6.2-10.2) | 720 | NS | |
| Health professional | ||||
| Yes | 4.3 (0-10.1) | 47 | ||
| No | 7.4 (5.9-8.9) | 1,222 | NS | |
| Endoscopy(ies) | ||||
| Yes | 7.8 (4.1-11.5) | 205 | ||
| No | 7.2 (5.6-8.7) | 1,068 | NS | |
| Hemodialysis | ||||
| Yes | 0.0 | 6 | ||
| No | 7.3 (5.9-8.7) | 1,266 | NS | |
| Administration of gamma globulins | ||||
| Yes | 4.6 (1.0-8.2) | 130 | ||
| No | 7.8 (6.2-9.4) | 1,083 | NS | |
| Blood diseases | ||||
| Yes | 2.6 (0-5.1) | 39 | ||
| No | 7.8 (5.8-9.8) | 691 | NS | |
| Surgical interventions | ||||
| Yes | 7.9 (6.0-9.8) | 783 | ||
| No | 6.4 (4.2-8.5) | 497 | NS | |
| Dental extractions | ||||
| Yes | 7.9 (6.2-9.5) | 1,032 | ||
| No | 4.6 (1.9-7.2) | 240 | NS | |
| Abortions | ||||
| Yes | 9.2 (0-18.9) | 153 | ||
| No | 6.3 (4.3-8.3) | 556 | NS | |
| Acupuncture | ||||
| Yes | 7.6 (3.6-11.6) | 170 | ||
| No | 7.3 (5.8-8.8) | 1,103 | NS | |
| Piercing | ||||
| Yes | 4.8 (0-11.3) | 42 | ||
| No | 7.5 (6.0-9.0) | 1,228 | NS | |
| Multiple sexual partners | ||||
| Yes | 11.8 (1.0-22.6) | 34 | ||
| No | 7.3 (5.8-8.8) | 1,154 | NS | |
| Sex-worker client | ||||
| Yes | 10.0 (0-28.6) | 10 | ||
| No | 7.3 (5.8-8.8) | 1,161 | NS | |
| Condom use | ||||
| Yes | 5.5 (3.2-7.8) | 382 | ||
| No | 8.4 (6.5-10.2) | 862 | NS | |
| Hepatitis in spouse/partner | ||||
| Yes | 7.6 (1.2-14.0) | 66 | ||
| No | 7.4 (5.9-8.9) | 1,150 | NS | |
| HIV infection | ||||
| Yes | 6.5 (1.8-11.2) | 107 | ||
| No | 4.4 (0.2-8.6) | 90 | NS |
OR aj., odds ratio adjusted by multiple logistic regression analysis including significant variables in the univariate analysis.
NS, not significant.
Social classes include professional (I), managerial and technical (II), skilled (III), partly skilled (IV), and unskilled (V) occupations.
DISCUSSION
This is the first large Spanish study of the prevalence of IgG antibodies to HEV, which reveal a past infection or exposure to HEV. The prevalence of anti-HEV antibodies was lower than 10%, but this is substantially higher than the 1 to 3% prevalence found in a Spanish study analyzing blood donors, although that study had a smaller sample size (22).
In patients with acute hepatitis of unknown etiology, the prevalence of IgM anti-HEV antibodies or HEV RNA, markers associated with acute hepatitis E, is also very low. In a study carried out by our group, anti-HEV antibodies were detected in only 5.6% of cases of non-A, non-B, and non-C acute hepatitis and in 4% of cases of acute hepatitis A (5, 16, 30). These data contrast with the 43% prevalence of HEV detected in sewage in different years, a prevalence considered high for a region where HEV infection is nonendemic (30). The discrepancies between the low seroprevalence of HEV in the general population and cases of acute hepatitis and the high level of HEV in sewage water may be due to various reasons.
Most of the existing assays for the detection of antibodies to HEV are enzyme immunoassays which use recombinantly expressed proteins or synthetic peptides representing antigenic domains from Orf2 and Orf3, commonly from strains of at least two geographically distinct HEV strains (4, 12, 17, 20, 21, 27). The HEV strains used in these tests are representative of those from countries of endemicity and show some differences with recently identified strains in the amino acid sequence of some of the major epitopes, such as the region near the carboxyl ends of Orf2 and Orf3. This diversity could be producing a lower level of sensitivity in the serological assays for these infections. A study by the Hepatitis E Virus Antibody Serum Panel Evaluation Group (21) concluded that differing results with blood donor sera indicate that anti-HEV seroprevalence data in countries of nonendemicity may be unreliable and should be interpreted with caution.
Another explanation could be the reduction of IgG HEV antibodies to undetectable levels (17). The humoral response generated against HEV infection has a variable duration. During the acute HEV infection phase, IgM anti-HEV antibodies appear in the early phase of clinical illness, preceding IgG anti-HEV by a few days, and disappear over a 4- to 5-month period (13). In one study, 100%, 50%, and 40% of sera collected from patients during hepatitis outbreaks 1 to 40 days, 3 to 4 months, and 6 to 12 months after the onset of jaundice, respectively, tested positive for IgM anti-HEV (4). The IgG response appears shortly after the IgM response, and its titer increases throughout the acute phase and into the convalescence phase and remains high from 1 to 4.5 years after the acute phase (9, 13). In one study, anti-HEV was detected in 47% of individuals 14 years after acute HEV infection (17), but the exact duration of persistence of anti-HEV is not known. In a previous study, our group demonstrated that IgG antibodies are reduced in 64% of cases a few years after infection (5). In some cases, this occurs after only 3 to 4 months, which makes the diagnosis of hepatitis E virus infection extremely difficult, particularly if HEV RNA or IgM anti-HEV antibodies are not used (5, 17).
In addition, reports suggest that some people may not produce a detectable antibody response and that the prevalence of anti-HEV in areas where the disease is endemic is much lower than expected, at a rate of 2.8 to 20.2%, with a very high proportion of HAV-seropositive individuals (3, 8). In industrialized countries, anti-HEV antibodies have regularly been found at a rate below 5%, and in Spain, a level of 1 to 3% is reported in blood donors (22). The low prevalence of IgG antibodies to HEV in patients with acute hepatitis during the study period probably indicates that some cases of acute hepatitis E are asymptomatic and that the serological tests currently applied may not have adequate sensitivity for the strains circulating in industrialized countries.
HEV is transmitted by fecal-oral routes in a way similar to that of hepatitis A virus. However, the seroprevalence of anti-HAV antibodies in Spain is much higher (67.8%) (4a) than that detected for HEV, and the presence of HAV in sewage samples collected from 1994 to 2001 was 57.4%, suggesting that a similar relationship with HEV would occur.
Finally, our cross-sectional study demonstrated that the only variable associated with a risk of HEV was minor surgery. It is difficult to explain the role of this factor and the lack of a relationship with rural habitat or low levels of education, variables usually found in developing countries associated with HEV infection. Hygienic and health conditions are probably important factors in the reduction of the prevalence of HEV infection, but a prospective study of the risk of infection in people undergoing this type of operation would be necessary to determine their true relevance.
In conclusion, this study found a prevalence of anti-HEV antibodies of 7.3% in the adult Catalan population, which increased with age to almost 12% in people ≥65 years of age. In addition, the prevalence of anti-HEV antibodies was higher among subjects with a history of minor surgery.
Acknowledgments
The study was funded by the Directorate of Public Health, Generalitat of Catalonia.
We thank the staff of the Sub-Directorate of Health Promotion, the Sub-Directorate of Health Planning of the Department of Health, and the Health Regions of the Catalan Health Service for their help in carrying out the health examination in Catalonia and obtaining the blood samples.
We have no commercial or other associations that might pose a conflict of interest.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Arankalle, V. A., M. S. Chadha, S. D. Chitambar, A. M. Walimbe, L. P. Chobe, and S. S. Gandhe. 2001. Changing epidemiology of hepatitis A and hepatitis E in urban and rural India (1982-98). J. Viral Hepat. 8:293-303. [DOI] [PubMed] [Google Scholar]
- 2.Arankalle, V. A., S. A. Tsarev, M. S. Chadha, D. W. Alling, S. U. Emerson, K. Banerjee, and R. H. Purcell. 1995. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J. Infect. Dis. 171:447-450. [DOI] [PubMed] [Google Scholar]
- 3.Balayan, M. S. 1997. Epidemiology of hepatitis E virus infection. J. Viral Hepat. 4:155-165. [DOI] [PubMed] [Google Scholar]
- 4.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Bruguera, M., L. Salleras, P. Plans, J. Vidal, E. Navas, A. Domínguez, J. Batalla, J. L. Taberner, and J. Espuñes. 1999. Changes in seroepidemiology of hepatitis A virus infection in Catalonia in the period 1989-1996. Implications for new vaccination strategy. Med. Clin. (Barcelona) 112:406-408. (In Spanish.) [PubMed] [Google Scholar]
- 5.Buti, M., P. Clemente, R. Jardi, M. Formiga-Cruz, M. Schaper, A. Valdes, F. Rodriguez-Frias, R. Esteban, and R. Girones. 2004. Sporadic cases of acute autochthonous hepatitis E in Spain. J. Hepatol. 41:126-131. [DOI] [PubMed] [Google Scholar]
- 6.Cengiz, K., E. Ozyilkan, A. M. Cosar, and M. Gunaydin. 1996. Seroprevalence of hepatitis E in hemodialysis patients in Turkey. Nephron 74:731-732. [DOI] [PubMed] [Google Scholar]
- 7.Corwin, A. L., H. B. Khiem, E. T. Clayson, K. S. Pham, T. T. Vo, T. Y. Vu, T. T. Cao, D. Vaughn, J. Merven, T. L. Richie, M. P. Putri, J. He, R. Graham, F. S. Wignall, and K. C. Hyams. 1996. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am. J. Trop. Med. Hyg. 54:559-562. [DOI] [PubMed] [Google Scholar]
- 8.Dalekos, G. N., E. Zervou, M. Elisaf, N. Germanos, E. Galanakis, K. Bourantas, K. C. Siamopoulos, and E. V. Tsianos. 1998. Antibodies to hepatitis E virus among several populations in Greece: increased prevalence in an hemodialysis unit. Transfusion 38:589-595. [DOI] [PubMed] [Google Scholar]
- 9.Dawson, G. J., K. H. Chau, C. M. Cabal, P. O. Yarbough, G. R. Reyes, and I. K. Mushahwar. 1992. Solid-phase enzyme linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J. Virol. Methods 38:175-186. [DOI] [PubMed] [Google Scholar]
- 10.Ding, X., T. C. Li, S. Hayashi, N. Masaki, T. H. Tran, M. Hirano, M. Yamaguchi, M. Usui, N. Takeda, and K. Abe. 2003. Present state of hepatitis E virus epidemiology in Tokyo, Japan. Hepatol. Res. 27:169-173. [DOI] [PubMed] [Google Scholar]
- 11.Emerson, S., and R. Purcell. 2004. Running like water—the omnipresence of hepatitis E. N. Engl. J. Med. 351:2367-2368. [DOI] [PubMed] [Google Scholar]
- 12.Erker, J. C., S. M. Desai, and I. K. Mushahwar. 1999. Rapid detection of hepatitis E virus RNA by reverse transcription-polymerase chain reaction using universal oligonucleotide primers. J. Virol. Methods 81:109-113. [DOI] [PubMed] [Google Scholar]
- 13.Favorov, M. O., Y. E. Khudyakov, E. E. Mast, T. L. Yashina, C. N. Shapiro, N. S. Khudyakova, D. L. Jue, G. G. Onischenko, H. S. Margolis, and H. A. Fields. 1996. IgM and IgG antibodies to hepatitis E virus (HEV) detected by an enzyme immunoassay based on an HEV-specific artificial recombinant mosaic protein. J. Med. Virol. 50:50-58. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, T. J., G. M. Dusheiko, and A. J. Zuckerman. 2004. Hepatitis viruses, p. 199-247. In A. J. Zuckerman, J. E. Banetvale, J. R. Pattison, P. D. Griffiths, and B. D. Scoub (ed.), Principles and practice of clinical virology, 5th ed. John Wiley & Sons, Chichester, United Kingdom.
- 15.Heymann, D. L. 2004. Hepatitis, viral, p. 247-268. In D. L. Heymann (ed.), Control of communicable diseases manual, 18th ed. American Public Health Association, Washington, D.C.
- 16.Jardi, R., M. Buti, F. Rodriguez-Frias, and R. Esteban. 1993. Hepatitis E infection in acute sporadic hepatitis in Spain. Lancet 341:1355-1356. [DOI] [PubMed] [Google Scholar]
- 17.Khuroo, M. S., S. Kamili, M. Y. Dar, R. Moecklii, and S. Jameel. 1993. Hepatitis E and long-term antibody status. Lancet 341:1355. [PubMed] [Google Scholar]
- 18.Krawczynski, K. 1993. Hepatitis E. Hepatology 17:932-941. [PubMed] [Google Scholar]
- 19.Kwo, P. Y., G. G. Schlauder, H. A. Carpenter, P. J. Murphy, J. E. Rosenblatt, G. J. Dawson, et al. 1997. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clin. Proc. 72:1133-1136. [DOI] [PubMed] [Google Scholar]
- 20.Lin, C. H., J. C. H. Wu, T. T. Chang, W. Y. Chang, M. L. Yu, A. W. Tam, S. C. H. Wang, Y. H. Huang, F. Y. Chang, and S. D. Lee. 2000. Diagnostic value of immunoglobulin G (IgG) and IgM anti-hepatitis E virus (HEV) tests based on HEV RNA in an area where hepatitis E is not endemic. J. Clin. Microbiol. 38:3915-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mast, E. E., M. J. Alter, P. V. Holland, and R. H. Purcell. 1998. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatology 27:857-861. [DOI] [PubMed] [Google Scholar]
- 22.Mateos, M. L., C. Camarero, E. Lasa, J. L. Teruel, N. Mir, and F. Baquero. 1999. Hepatitis E virus: relevance in blood donors and risk groups. Vox Sang. 76:78-124. [DOI] [PubMed] [Google Scholar]
- 23.Office of Population Censuses and Surveys. 1980. Classification of occupations. HMSO, London, England.
- 24.Paul, D. A., M. F. Knigge, A. Ritter, R. Gutierrez, T. Pilot-Matías, K. H. Chau, et al. 1994. Determination of hepatitis E virus seroprevalence by using recombinant fusion proteins and synthetic peptides. J. Infect. Dis. 169:801-806. [DOI] [PubMed] [Google Scholar]
- 25.Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. [DOI] [PubMed] [Google Scholar]
- 26.Purcell, R. H., and S. U. Emerson. 2005. Hepatitis E virus, p. 2204-2217. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Elsevier, Philadelphia, Pa.
- 27.Schlauder, G. G., and I. K. Mushahwar. 2001. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 65:282-292. [DOI] [PubMed] [Google Scholar]
- 28.Sylvan, S., S. Jacobson, and B. Christenson. 1998. Prevalence of antibodies to hepatitis E virus among hemodialysis patients in Sweden. J. Med. Virol. 54:38-43. [DOI] [PubMed] [Google Scholar]
- 29.Wong, K. H., Y. M. Liu, P. S. P. Ng, B. W. Y. Young, and S. S. Lee. 2004. Epidemiology of hepatitis A and hepatitis E infection and their determinants in adult Chinese community in Hong Kong. J. Med. Virol. 72:538-544. [DOI] [PubMed] [Google Scholar]
- 30.Zanetti, A. R., G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. [DOI] [PubMed] [Google Scholar]