Abstract
Mycobacterium bovis is the causative agent of bovine tuberculosis, a disease that is increasing in incidence in United Kingdom cattle herds. In addition to increasing economic losses, the rise in bovine tuberculosis poses a human health risk. There is an urgent requirement for effective strategies for disease eradication; this will likely involve vaccination in conjunction with current test and slaughter policies. A policy involving vaccination would require an accurate diagnosis of M. bovis-infected animals and the potential to distinguish these animals from vaccinates. Currently used diagnostic tests, the skin test and gamma interferon (IFN-γ) blood test, have a sensitivity of up to 95%. A further complication is that M. bovis BCG-vaccinated animals are also scored positive by these tests. We tested the hypothesis that the quantification of IFN-γ-producing lymphocytes by flow cytometric analysis of intracellular IFN-γ expression would provide a more accurate discrimination of M. bovis-infected animals from BCG vaccinates. Significant numbers of IFN-γ-expressing CD4+ T cells were detected following culture of heparinized blood from M. bovis-infected animals, but not from BCG vaccinates, with purified protein derived from M. bovis (PPD-B) or live mycobacteria. Only 1 of 17 BCG-vaccinated animals had a significant number of CD4+ T lymphocytes expressing IFN-γ, compared with 21/22 M. bovis-infected animals. This assay could allow an accurate diagnosis of M. bovis and allow the discrimination of BCG-vaccinated cattle from infected cattle.
The increase in incidence in bovine tuberculosis, caused by infection with Mycobacterium bovis, in developed countries such as the United Kingdom, New Zealand, and the United States poses significant economic problems and increasing risks to human health. Despite the continued use of the skin test and slaughter policies in the United Kingdom, the incidence of bovine tuberculosis (TB) in United Kingdom cattle herds is now increasing rapidly. There is now clearly a requirement for improved diagnostic tests and/or vaccination of cattle to halt the spread of this disease and thus reduce economic losses and risks to human health. Currently, the two most widely used diagnostic tests for tuberculosis in cattle are based on the detection of established cell-mediated immune responses: (i) the delayed-type hypersensitivity (DTH) response, detected by the comparative tuberculin skin test (25), and (ii) the in vitro synthesis of gamma interferon (IFN-γ) by whole blood cultured with mycobacterial antigens (purified protein derivative [PPD]), which can be subsequently detected by the Bovigam enzyme-linked immunosorbent assay (ELISA) (41). However, it is well documented that both diagnostic techniques are only partially successful in distinguishing infected cattle from healthy, uninfected animals, with reported sensitivities ranging from 68 to 95% for the skin test (25). Several factors also affect the sensitivity of the IFN-γ test; these factors may include the proximity of blood sampling to the time of skin test and the time between blood sampling and assay setup (11, 30, 31). However, major factors underlying the lack of sensitivity of both diagnostic tests is prior exposure of cattle to other mycobacteria present within the environment that share common antigens with M. bovis and nonspecific responses possibly due to NK cells in young animals (13). Incorrect diagnosis may cause significant problems; for example, where false positives exist, the movement restrictions that are placed on farms with herd breakdowns have significant negative economic impacts on those affected farms. Infected cattle that are “missed” by the skin test (false negatives) are a possible source of cattle-to-cattle transmission of M. bovis.
To date, there are no commercial vaccines available for tuberculosis in cattle. The human vaccine strain M. bovis BCG produces significant protection (2, 3) but has been shown to have variable efficacy in cattle, as in humans. This variation, like the failure of diagnostic tests, has been attributed to the effects of environmental sensitization to other mycobacterial species. More recently, alternative vaccination strategies such as neonatal vaccination (15, 22, 36) and heterologous prime-boost vaccination (7, 23, 24, 38) have significantly improved the efficacy of BCG such that it is now being considered for additional trials in humans and cattle. However, BCG-vaccinated individuals test positive by both the skin test and the standard IFN-γ test, and therefore, there is an important requirement for the development of quick, sensitive diagnostic tests that can distinguish vaccinated subjects from those that are infected with virulent mycobacteria. Discrimination of BCG-vaccinated cattle from M. bovis-infected cattle has been achieved through the use of antigens such as ESAT-6 and CFP-10 (35), which are present in virulent M. bovis strains but are absent from BCG. Reactivity to ESAT-6 and/or CFP-10 was shown to correlate with disease severity and could be used to distinguish protected BCG vaccinates from M. bovis-infected animals (4, 37). However, reactivity to these antigens is not observed in all M. bovis-infected animals (29) and is significantly affected by prior sensitization of cattle to environmental mycobacteria (14). Thus, more specific antigens or more sensitive assay systems are required for the accurate diagnosis of bovine TB and the distinction of vaccinated individuals.
It is well documented that cytokine secretion, and IFN-γ production in particular, is associated with protective immune responses to Mycobacterium tuberculosis and M. bovis infections (18, 27). The requirement for IFN-γ was elegantly demonstrated in IFN-γ knockout mice, which are extremely susceptible to mycobacterial infections and may even succumb to fatal BCG infections (5, 8). Classically, IFN-γ has been detected in infected animals, or humans, by ELISA measurement of IFN-γ secreted in vitro following stimulation of blood or peripheral blood mononuclear cells (PBMC) with mycobacterial antigens. The enzyme-linked immunospot (ELISPOT) technique, which detects numbers of IFN-γ-secreting cells, allows the estimation of the precursor frequency of antigen-specific IFN-γ-secreting cells and has been suggested to accurately differentiate M. tuberculosis- or M. bovis-infected individuals from BCG-vaccinated humans (24, 37). More recently, the development of cytokine flow cytometry (CFC) has proven to be an extremely sensitive methodology for assaying numbers of antigen-specific cytokine-expressing cells in PBMC and whole blood (32, 39). These assays, where cells can be induced to produce IFN-γ in response to antigen in vitro, are potentially useful indicators of immune status. CFC has been compared with alternative measures of T-cell immunity such as cell proliferation assays, cytotoxic-T-lymphocyte assays, major histocompatibility complex oligomer assays, and ELISPOT (6, 17, 19, 28), and there is relatively good correlation with each technique.
In this study, we describe the development of bovine CFC for the detection of mycobacterial antigen-specific IFN-γ-secreting cells. We hypothesized that M. bovis-infected animals would have higher numbers of persisting circulating antigen-specific cells than BCG-vaccinated or control animals. The expression of IFN-γ by CD4, CD8, and WC1+ γδ T lymphocytes was measured in cattle experimentally infected with M. bovis or vaccinated with BCG. We demonstrated that cells from infected cattle consistently showed higher numbers of CD4+ T cells expressing IFN-γ than BCG-vaccinated cattle, which were detectable by CFC following in vitro stimulation of whole blood for 24 h with PPD or BCG. These data suggest that this method could accurately diagnose M. bovis-infected cattle in the field.
MATERIALS AND METHODS
Inoculation of animals with BCG or M. bovis.
Cattle were British Holstein-Friesian calves (Bos taurus) bred at the Institute for Animal Health. The Institute for Animal Health herd has been confirmed to be free of bovine TB for more than 10 years.
Calf groups were (i) noninoculated control animals, (ii) calves inoculated subcutaneously with 1 × 106 CFU BCG Pasteur as previously described (15), (iii) calves inoculated intranasally with 1 × 104 CFU virulent M. bovis AF2122/97 (15) (experiment 1), and (iv) calves inoculated intratracheally with 5 × 103 CFU M. bovis (37) (experiment 2).
Animals were vaccinated with BCG at approximately 1 month of age. For challenge experiments, animals were between 4 and 9 months old at the time of inoculation. Comparisons were made using age-matched animals either vaccinated with BCG or infected with M. bovis. The control and immunized animals were housed separately in standard animal accommodations, and the infected animals were kept in high-security (Advisory Committee on Dangerous Pathogens category 3) units. Animals were sampled at various time points postinfection, as indicated in Results. The experiments were approved by the local ethics committee according to national United Kingdom guidelines.
Antigens and mycobacteria.
Purified protein derivative from M. bovis (PPD-B) was obtained from the Tuberculin Production Unit at the Veterinary Laboratories Agency, Weybridge, United Kingdom. BCG Pasteur was diluted from a previously titrated frozen (−70°C) stock grown in Middlebrook 7H9 broth containing 10% albumin-dextrose-catalase supplement (12). Numbers of CFU were determined on 7H10 agar plates. M. bovis strain AF2122/97 (9), grown in 7H9 broth for 7 days, was frozen at −70°C. A 1-ml aliquot was thawed and cultured in 10 ml of 7H9 broth for 7 days at 37°C to produce log-phase cells. The optical density at 600 nm was measured, and the culture was diluted in 7H9 broth to give an estimated 5 × 103 CFU per ml based on a comparison with a previously established standard curve. The number of CFU was determined on 7H11 agar containing 10% oleic acid-albumin-dextrose-catalase supplement and 4.16 g pyruvate per liter.
Collection of blood.
Blood samples were taken by venepuncture at the indicated times postvaccination or postchallenge. Sodium heparin (10 units per ml; Leo Laboratories, Princes Risborough, United Kingdom) was used as an anticoagulant. Cultures were set up within 2 h of collection.
CFC.
The CFC protocol employed was a modification of that described previously by Suni et al. (32). Whole-blood samples were incubated for 20 to 24 h at 37°C with 2.5 × 105 CFU M. bovis or 2.5 × 106 CFU BCG per ml of blood, 20 μg/ml PPD-B, or an appropriate volume of phosphate-buffered saline (PBS) as a control. The numbers of CFU of BCG and M. bovis added per ml blood were calculated to give CFU/cell ratios of approximately 10:1 and 1:1, respectively. These CFU/cell ratios were previously determined to be optimal in this laboratory. For the final 4 h of the incubation period, 10 μg/ml brefeldin A was added. Following this, red blood cells were lysed for 30 min with FACSlyse solution (1 volume 10× FACSlyse mixed with 8 volumes of water and added to 1 volume of blood; Becton Dickinson, San Jose, CA). Following two washes in PBS, the cells were permeabilized for 20 min in FACS permeabilizing solution (1 ml per 1 ml blood; Becton Dickinson). After a further two washes in PBS, cells were resuspended in PBS-1% bovine serum albumin-0.1% sodium azide. Aliquots of the cell suspensions were transferred to microtiter plates and stained using a standard indirect immunofluorescence protocol. The following mouse anti-bovine monoclonal antibodies (mAbs) were used: CD4, CC30 (immunoglobulin G1 [IgG1]) or CC8 (IgG2a), CD8, CC63 (IgG2a), WC1 (26), CC39 (IgG1) or CC15 (IgG2a), IFN-γ, CC302 (IgG1) (Serotec, Kidlington, United Kingdom), CC302-fluorescein isothiocyanate (Serotec), or 6H5 (IgG2a) (40). In some instances, mAbs were directly coupled to allophycocyanin using a Phycolink APC conjugation kit (ProZyme, San Leandro, CA). Isotype- and concentration-matched controls were mouse anti-avian mAb as previously described (13). Where nonconjugated mAbs were used, specific binding was detected with goat anti-mouse IgG1 phycoerythrin and goat anti-mouse IgG2a fluorescein isothiocyanate (Southern Biotech). The cells were assayed using a flow cytometer (FACSCalibur; Becton Dickinson), and the data were analyzed using FCS Express (De Novo Software, Ontario, Canada). The mononuclear cells were identified by light-scattering properties, and the percentages of CD4, CD8, or WC1 cells that produced IFN-γ were calculated.
RESULTS
Detection of intracytoplasmic IFN-γ in response to M. bovis, BCG, and PPD-B.
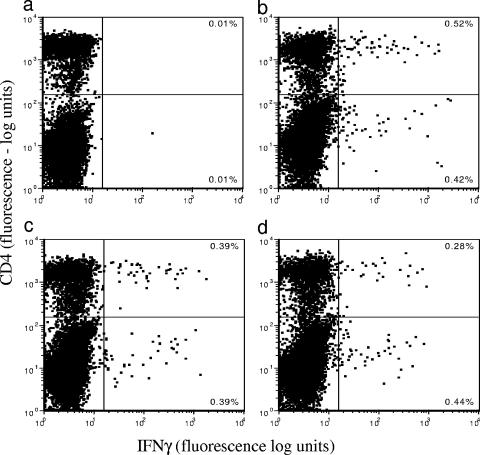
Blood from M. bovis-infected animals was cultured for 24 h with M. bovis, BCG, or PPD-B, and the expression of IFN-γ within CD4+ T cells was examined (Fig. 1). Each of these antigens stimulated significant increases in IFN-γ-expressing cells compared to the control (Fig. 1a). The in vitro responses to PPD (Fig. 1b) (P < 0.05), BCG (Fig. 1c) (P < 0.05), and M. bovis (Fig. 1d) (P < 0.05) varied slightly in terms of the number of responsive cells and the intensity of staining. For further studies, PPD-B and BCG were utilized in preference to M. bovis to minimize the handling of virulent mycobacteria. We also reasoned that a routine diagnostic test requiring the use of M. bovis would be impracticable.
FIG. 1.
Detection of intracytoplasmic IFN-γ in whole-blood cultures. Blood taken from M. bovis-infected animals 12 weeks postinfection was cultured in vitro for 24 h with PBS (a), PPD-B (b), BCG (c), or M. bovis (d). Expression of intracellular IFN-γ within CD4+ T cells was examined by flow cytometry. Percentages of cells in each quadrant are illustrated. One representative animal of three is shown.
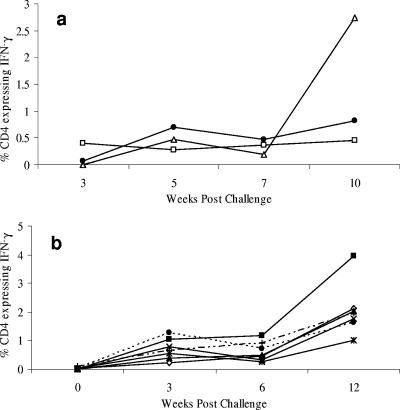
Kinetics of the IFN-γ response to M. bovis.
The optimal time after mycobacterial exposure for the measurement of in vitro responses to antigen was assessed in two groups of animals. In the first experiment (Fig. 2a), blood was taken from three animals infected with M. bovis by the intranasal route at 3, 5, 7, and 10 weeks postchallenge. The responses were determined by measuring the percentage of CD4 T cells producing IFN-γ in response to in vitro stimulation with PPD-B (Fig. 2a). The percentage of IFN-γ-expressing CD4+ T lymphocytes was undetectable or low at 3 weeks postinfection (mean, 0.16%), but this was increased at week 5 (mean, 0.48%). Although there was a significant degree of variability between animals, the trend was for increasing numbers of CD4+ cells expressing IFN-γ in relation to time (Fig. 2a). A second group of seven animals (Fig. 2b) challenged with M. bovis by the intratracheal route showed similar kinetics, with maximum numbers of IFN-γ-secreting cells detected 12 weeks postinfection. Significant increases in the percentages of CD4 T cells producing IFN-γ in response to in vitro stimulation with PPD-B were detected from week 3 postinfection (P < 0.01 at all time points). A degree of animal-to-animal variation was observed; this may reflect the extent of disease within these animals. In BCG-vaccinated animals, responses were very low or undetectable, and the kinetics of the response could not be accurately determined. In subsequent experiments, animals were sampled at 12 weeks postinfection or postvaccination.
FIG. 2.
Kinetics of the IFN-γ response to M. bovis. Blood was taken from M. bovis-infected animals at the indicated time points postinfection and was cultured in vitro for 24 h with PPD-B. Expression of intracellular IFN-γ within CD4+ T cells was examined by flow cytometry. Animals were inoculated with M. bovis by the intranasal route (a) or intratracheally (b). Data for individual animals are shown.
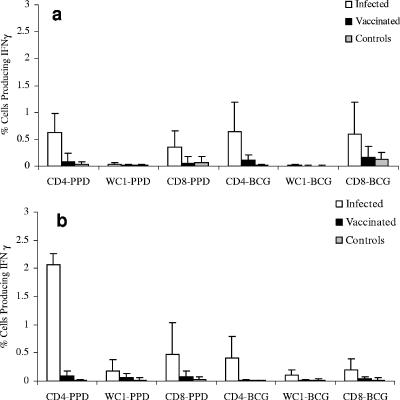
Detection of IFN-γ by CFC allows differentiation of cattle infected with M. bovis from BCG vaccinates.
Blood samples from M. bovis-infected cattle, BCG-vaccinated animals, and healthy controls were compared by CFC for numbers of CD4, CD8, and WC1 T lymphocytes that expressed IFN-γ following culture with either live BCG or PPD-B. Animals from two experiments were studied. The initial data compared animals infected intranasally with M. bovis (Fig. 3a); subsequently, data were generated from animals infected intratracheally (Fig. 3b). In each case, IFN-γ production was found in a higher proportion of cells in M. bovis-infected animals (Fig. 3, white bars) than in vaccinated animals (Fig. 3, black bars) in response to both PPD and BCG. No significant responses were observed in age-matched control calves. The differences between infected and vaccinated groups were highly significant for CD4 cells following culture with either PPD-B (P = <0.001) or BCG (P = <0.01). Differences in IFN-γ expression by CD8+ T cells from infected and vaccinated animals were also evident, although relatively high intra-animal variations in CD8 responses were observed, and overall, these differences were not significant. The expression of IFN-γ by WC1 T cells from most animals was low or undetectable.
FIG. 3.
IFN-γ responses of T-lymphocyte subsets following culture with PPD-B and BCG. Whole blood from animals infected with M. bovis intranasally (a, n = 15) or intratracheally (b, n = 7), from BCG-vaccinated animals (a, n = 10; b, n = 7), or from control animals (a, n = 5; b, n = 6) was stimulated in vitro with PPD-B or BCG. Intracellular IFN-γ expression was assessed in CD4+ (white bars), CD8+ (black bars), and WC1+ (gray bars) γδ T cells. Results are expressed as the mean percentages of IFN-γ-expressing T cells (CD4, CD8, and WC1) ± standard deviations.
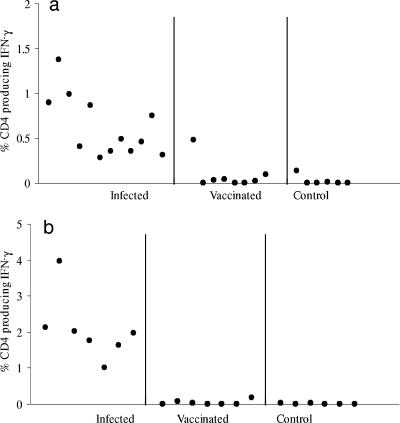
Individual animals (Fig. 4) showed variable percentages of CD4+ cells expressing IFN-γ in response to PPD-B stimulation. This was observed in animals inoculated with M. bovis both intranasally (Fig. 4a) and intratracheally (Fig. 4b). Nevertheless, the responses of M. bovis-infected animals were largely nonoverlapping with those of BCG-vaccinated or control animals. Only one vaccinated animal showed a significant response (Fig. 4a). The differential between vaccinated and infected animals was most evident in the second experiment (Fig. 4b), where all of the animals were closely age matched. The mean (plus 2 standard deviations) was calculated for vaccinated animals in each experiment and determined to be 0.3 and 0.19, respectively. Using these values as a “cutoff,” 14/15 M. bovis-infected animals were determined to be infected in the first experiment, with 1 BCG-vaccinated animal also showing a significant response. In the second experiment, 7/7 infected animals and 0/7 vaccinated animals were diagnosed as being infected.
FIG. 4.
Expression of intracytoplasmic IFN-γ by CD4+ T cells in response to PPD-B discriminates M. bovis-infected animals from BCG-vaccinated animals. Whole blood was taken and stimulated as described in the legend of Fig. 3. Each point represents the percentage of CD4 cells expressing IFN-γ 24 h following culture with PPD-B. Samples were assessed 12 weeks postinfection with M. bovis via either the intranasal (a) or intratracheal (b) route or at 12 weeks after vaccination with BCG. In each case, infected and control animals were tested in parallel. Control animals were age-matched, nontreated animals.
DISCUSSION
The incidence of M. bovis infections in United Kingdom cattle herds is increasing rapidly. The failure to control the spread of TB in cattle has many contributory factors including the presence of a wildlife reservoir of disease as well as limitations on the sensitivities of diagnostic tests. In addition, potential strategies for the use of BCG in cattle, such as neonatal vaccination (15, 22, 36) and heterologous prime-boost vaccination (7, 23, 24, 38), are hampered by the fact that BCG-vaccinated animals test positive by both the skin test and the IFN-γ test. Differentiation of BCG-vaccinated cattle from M. bovis-infected cattle may be achieved in assays using ESAT-6 and CFP-10 (35). However, reactivity to these antigens is not observed in all M. bovis-infected animals (29) and is significantly affected by prior sensitization of cattle to environmental mycobacteria (14). There is an important requirement for the development of sensitive diagnostic tests that can accurately distinguish vaccinated subjects from those that are infected with virulent mycobacteria.
The measurement of antigen-specific IFN-γ by CFC has been suggested as an extremely sensitive methodology for assaying antigen-specific cells in PBMC and whole blood (10, 16, 17, 19-21, 28, 32-34, 39, 40). A number of studies have compared CFC with other methods to detect T-cell immunity including proliferation assays, cytotoxic-T-lymphocyte assays, major histocompatibility complex oligomer assays, and ELISPOT (6, 17, 19, 28), and there is relatively good correlation between these techniques. The ELISPOT technique, which detects numbers of IFN-γ-secreting cells, allows the estimation of the precursor frequency of antigen-specific IFN-γ-secreting cells and has been suggested to accurately differentiate M. tuberculosis- or M. bovis-infected individuals from BCG vaccinates (24, 37). However, this is a time-consuming, and expensive, technique. In addition, the detection of cytokine secretion by ELISPOT reflects the balance between cytokine secretion, utilization, and degradation and may be significantly affected by changes in cell subset distribution.
One major advantage of CFC is that it not only allows the enumeration of responding cells but also provides information on the phenotypes of these cells. CFC also offers the possibility of measuring more than one cytokine simultaneously, which may increase the diagnostic potential. While the majority of studies assessing the use of CFC as a diagnostic test utilized separated PBMC, recently, Suni et al. (32) described a modification using whole blood. The results obtained with whole blood were comparable to those observed using isolated PBMC preparations, and it was suggested that increased frequencies of responding T cells were observed in whole-blood cultures (32). The use of whole blood reduces costs and cell preparation time and eliminates the possibility of cell activation through separation techniques.
In this study, we described the development of bovine CFC for the detection of mycobacterial antigen-specific IFN-γ-secreting cells in whole blood. We demonstrated that M. bovis-infected animals had significantly higher numbers of circulating antigen-specific cells than BCG-vaccinated or control animals, detectable by CFC following in vitro stimulation of whole blood with PPD or BCG. These results are in contrast to those assessing the potential of CFC for the diagnosis of M. tuberculosis infection. In a number of such studies where cells were stimulated with PPD, it was not possible to distinguish BCG-vaccinated patients from TB patients (1, 16, 33, 34). Accurate diagnosis was observed only where ESAT-6 was used as the stimulating antigen, and an overall sensitivity of between 90 and 95% was achieved. The number of responding CD4+ T cells ranged from 0.1 to approximately 1%, although overall responses to ESAT-6 were very low (1, 16, 33, 34). Although ESAT-6 has been suggested as a potential diagnostic tool for distinguishing M. bovis-infected cattle from BCG-vaccinated animals, it is clear that only a proportion of infected animals respond to this antigen (15). In our studies, CFC of whole blood stimulated by PPD-B gave a sensitive discrimination between vaccinated and M. bovis-infected animals, with upwards of 95% animals being diagnosed positive. Only one BCG-vaccinated animal showed a significant response using this assay. By comparison, each of the BCG-vaccinated animals showed strong IFN-γ secretion by ELISA (data not shown), and we have previously shown that although BCG vaccination induced lower skin test reactivity than M. bovis infection, all BCG vaccinates scored positive by this test (15). This suggests that CFC could be a sensitive method for differential diagnosis.
Another important consideration is the ability to accurately distinguish those vaccinated animals that are protected from M. bovis infection from vaccinated animals that will go on to develop disease. It is clear from a number of studies that although on a group/herd basis, BCG has the potential to induce a significant level of protection, some animals still show evidence of disease, and these animals could be a potential source of cattle-to-cattle transmission of M. bovis. Accurate identification of such animals would be essential for the overall success of BCG vaccination strategies in the field. Early indications from this laboratory suggest that CFC using PPD-B can accurately detect BCG-vaccinated animals that are infected with M. bovis, although at present, only low numbers of such animals have been assessed, and studies are ongoing. These data suggest that this method could accurately diagnose M. bovis-infected cattle in the field.
Acknowledgments
This work was funded by the Department for the Environment, Food, and Rural Affairs (DEFRA), United Kingdom.
We gratefully acknowledge the staff in the animal care facilities at the Institute for Animal Health.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Antas, P. R., J. S. Sales, K. C. Pereira, E. B. Oliveira, K. S. Cunha, E. N. Sarno, and E. P. Sampaio. 2004. Patterns of intracellular cytokines in CD4 and CD8 T cells from patients with mycobacterial infections. Braz. J. Med. Biol. Res. 37:1119-1129. [DOI] [PubMed] [Google Scholar]
- 2.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desombere, I., P. Meuleman, H. Rigole, A. Willems, J. Irsch, and G. Leroux-Roels. 2004. The interferon gamma secretion assay: a reliable tool to study interferon gamma production at the single cell level. J. Immunol. Methods 286:167-185. [DOI] [PubMed] [Google Scholar]
- 7.Ferraz, J. C., E. Stavropoulos, M. Yang, S. Coade, C. Espitia, D. B. Lowrie, M. J. Colston, and R. E. Tascon. 2004. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect. Immun. 72:6945-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godoy-Ramirez, K., K. Franck, S. Mahdavifar, L. Andersson, and H. Gaines. 2004. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J. Immunol. Methods 292:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Gormley, E., M. B. Doyle, K. McGill, E. Costello, M. Good, and J. D. Collins. 2004. The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet. Immunol. Immunopathol. 102:413-420. [DOI] [PubMed] [Google Scholar]
- 12.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285-291. [DOI] [PubMed] [Google Scholar]
- 13.Hope, J. C., P. Sopp, and C. J. Howard. 2002. NK-like CD8(+) cells in immunologically naive neonatal calves that respond to dendritic cells infected with Mycobacterium bovis BCG. J. Leukoc. Biol. 71:184-194. [PubMed] [Google Scholar]
- 14.Hope, J. C., M. L. Thom, B. Villarreal-Ramos, H. M. Vordermeier, R. G. Hewinson, and C. J. Howard. 2005. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin. Exp. Immunol. 141:432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope, J. C., M. L. Thom, B. Villarreal-Ramos, H. M. Vordermeier, R. G. Hewinson, and C. J. Howard. 2005. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 139:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes, A. J., P. Hutchinson, T. Gooding, N. J. Freezer, S. R. Holdsworth, and P. D. Johnson. 2005. Diagnosis of Mycobacterium tuberculosis infection using ESAT-6 and intracellular cytokine cytometry. Clin. Exp. Immunol. 142:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson, A. C., J. N. Martin, S. R. Younger, B. M. Bredt, L. Epling, R. Ronquillo, A. Varma, S. G. Deeks, J. M. McCune, D. F. Nixon, and E. Sinclair. 2003. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J. Immunol. Methods 283:141-153. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura, I., H. Tsukada, H. Yoshikawa, M. Fujita, K. Nomoto, and M. Mitsuyama. 1992. IFN-gamma-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J. Immunol. 148:2887-2893. [PubMed] [Google Scholar]
- 19.Maino, V. C., and H. T. Maecker. 2004. Cytokine flow cytometry: a multiparametric approach for assessing cellular immune responses to viral antigens. Clin. Immunol. 110:222-231. [DOI] [PubMed] [Google Scholar]
- 20.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207-215. [DOI] [PubMed] [Google Scholar]
- 21.Maino, V. C., M. A. Suni, and J. J. Ruitenberg. 1995. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry 20:127-133. [DOI] [PubMed] [Google Scholar]
- 22.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 23.McShane, H., and A. Hill. 2005. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 7:962-967. [DOI] [PubMed] [Google Scholar]
- 24.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinburgh) 85:47-52. [DOI] [PubMed] [Google Scholar]
- 25.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 26.Morrison, W. I., and W. C. Davis. 1991. Individual antigens of cattle. Differentiation antigens expressed predominantly on CD4− CD8− T lymphocytes (WC1, WC2). Vet. Immunol. Immunopathol. 27:71-76. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 28.Pahar, B., J. Li, T. Rourke, C. J. Miller, and M. B. McChesney. 2003. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J. Immunol. Methods 282:103-115. [DOI] [PubMed] [Google Scholar]
- 29.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 30.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1992. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust. Vet. J. 69:1-4. [DOI] [PubMed] [Google Scholar]
- 31.Ryan, T. J., B. M. Buddle, and G. W. De Lisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 69:57-61. [DOI] [PubMed] [Google Scholar]
- 32.Suni, M. A., L. J. Picker, and V. C. Maino. 1998. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 212:89-98. [DOI] [PubMed] [Google Scholar]
- 33.Tesfa, L., F. W. Koch, W. Pankow, H. D. Volk, and F. Kern. 2004. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytom. B Clin. Cytom. 60:47-53. [DOI] [PubMed] [Google Scholar]
- 34.Tilley, P. A., and J. N. Menon. 2000. Detection of Mycobacterium-specific interferon-gamma-producing human T lymphocytes by flow cytometry. APMIS 108:57-66. [DOI] [PubMed] [Google Scholar]
- 35.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 37.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vordermeier, H. M., S. G. Rhodes, G. Dean, N. Goonetilleke, K. Huygen, A. V. Hill, R. G. Hewinson, and S. C. Gilbert. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weynants, V., K. Walravens, C. Didembourg, P. Flanagan, J. Godfroid, and J. J. Letesson. 1998. Quantitative assessment by flow cytometry of T-lymphocytes producing antigen-specific gamma-interferon in Brucella immune cattle. Vet. Immunol. Immunopathol. 66:309-320. [DOI] [PubMed] [Google Scholar]
- 41.Wood, P. R., and S. L. Jones. 2001. BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinburgh) 81:147-155. [DOI] [PubMed] [Google Scholar]