Abstract
Dendritic cells (DCs) play a pivotal role in generating protective host immunity to Mycobacterium tuberculosis. Few studies have addressed DC function in the context of active tuberculosis (TB), largely due to technical constraints in obtaining adequate numbers of DC from sick patients. We quantitated peripheral blood myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) in the whole blood of patients with active TB and show that blood from patients with pleural TB was characterized by high circulating levels of mDCs. We also developed and optimized a novel whole-blood assay to study mDC production of the Th1-promoting cytokine interleukin 12 (IL-12) and upregulation of the maturation marker CCR7 after incubation with mycobacteria. We found that pleural TB was associated with increased IL-12 production and CCR7 expression compared to lung parenchymal disease. Our findings suggest important differences in innate immunity between patients with different forms of active TB, and this may contribute to the differences in natural history observed between the two groups.
Tuberculosis (TB) remains the major bacterial cause of mortality worldwide, accounting for approximately 2 million deaths annually (3). Although predominantly a disease of the lung parenchyma (i.e., pulmonary TB [PTB]), TB can involve a number of extrapulmonary sites such as lymph nodes, brain, and bone, as well as structures adjacent to the lung such as the pleural space (pleural TB). Tuberculous pleural effusions classically present 3 to 7 months after infection (postprimary) but may present at any time during the course of infection (17). Recent reports suggest an increasing incidence of postprimary pleural TB in human immunodeficiency virus (HIV)-seronegative individuals from areas of high TB endemicity (11). Studies from the preantibiotic era have highlighted differences in severity and prognosis in patients with pleural TB compared to those with PTB (16). In particular, patients with pleural TB showed a higher rate of spontaneous resolution of disease than did patients with PTB. These findings point to important differences in host protective immunity in patients with the two forms of active disease.
Most immunological studies of pleural TB have concentrated on the adaptive T-cell response, demonstrating compartmentalization of T-cell subsets (2, 24) and increased levels of gamma interferon (IFN-γ) secretion into pleural fluid (2, 5, 18, 24). However, few studies have addressed the role of innate cellular immunity that drives this adaptive T-cell response. Dendritic cells (DCs) comprise a key population of innate immune cells that bridge innate and adaptive immunity to pathogens through the presentation of microbial antigens to naive and memory T cells (20). Myeloid DCs (mDCs) comprise the major DC population and are found in blood, tissues, and lymph nodes. A number of pathogens are capable of eliciting or subverting DC responses (see reviewed by Palucka and Banchereau [14]). DCs sense microbial pathogens such as Mycobacterium tuberculosis by recognition of pathogen-associated molecular patterns via Toll-like receptors. Binding of pathogens to DC Toll-like receptors induces DC maturation, a series of coordinated events leading to upregulation of the cell surface major histocompatibility complex and costimulatory molecule expression required for antigen presentation. In addition, the upregulation of chemokine receptors such as CCR7, which direct the antigen-presenting cells to regional lymph nodes where interaction with T cells can occur, is observed. The production of cytokines such as interleukin-12 (IL-12), which are central in mediating a potentially protective Th1 adaptive immune response, is also a result (20). Any impairment in the ability of DCs to undergo maturation and to produce IL-12 may result in impaired stimulation of Th1 immunity.
The study of mDC function in vivo is hampered by the rarity of these cells and the difficulty involved in obtaining tissue specimens containing them. Ex vivo studies generally rely on culturing monocyte-derived DCs from peripheral blood monocytes, an in vitro correlate of “immature” DCs. This procedure requires obtaining large volumes of blood from a given individual, making such experiments difficult to undertake in the setting of sick patients with active TB. In addition, such assays involve much ex vivo manipulation of cells, which increases the likelihood of experimental artifacts (12). To address these issues, we have quantified DCs in peripheral blood and have studied the function of mDCs directly by means of a novel whole-blood assay (WBA). Two DC populations circulate in blood: mDCs are identified by cell surface expression of HLA-DR+ CD123− CD11c+ cells, whereas plasmacytoid DCs (pDCs) are characterized as HLA-DR+ CD123+ CD11c− cells. pDCs are the major type 1 IFN-producing cells in the body and play a major role in the immune response to viral infection (19).
We describe here quantitative and functional differences in blood mDC populations from patients with pleural TB and PTB. We show that pleural TB is associated with preservation of mDC number and function in contrast to mDCs from patients with PTB, which are functionally impaired in their ability to upregulate IL-12 production and CCR7 expression when stimulated ex vivo. These results identify important differences in the innate immune response in patients with pleural and lung parenchymal TB. These differences may contribute to the observed variation in natural history between the two groups.
MATERIALS AND METHODS
Human participants and blood collection.
Written informed consent was obtained from patients and healthy blood donors for these studies. The protocol was approved by the institutional review boards of The Rockefeller University of New York (a previous affiliation of M. Mendelson, W. A. Hanekom, and G. Kaplan), The Public Health Research Institute of New Jersey, the University of Cape Town, and the U.S. Department of Health and Human Sciences. Adult patients were recruited from TB clinics of the Southwest Peninsula Board of Healthcare, Western Cape, South Africa. Participants were enrolled prior to starting standard quadruple anti-TB therapy. All patients underwent a chest radiograph, and two sputum samples were analyzed for the presence of M. tuberculosis by acid-fast staining and microscopy and by M. tuberculosis culture. Where appropriate, pleural fluid or biopsy material was also cultured for the growth of M. tuberculosis. All patients underwent voluntary counseling and testing for HIV infection by trained HIV counselors in the clinic. The exclusion criteria were: HIV infection, pregnancy, steroids within the preceding month, alcohol abuse, concurrent diabetes mellitus or other chronic health problem, and age <16 or >65 years. Healthy HIV-seronegative tuberculin skin test-positive adults were included as control participants; their blood was also used for developing and optimizing the WBA. Seven milliliters of blood was collected directly into a sodium-heparinized Vacutainer tube (BD Biosciences) for the WBA, and 4 ml of blood was collected into an EDTA-containing tube for a complete blood count.
Quantitation of blood DCs.
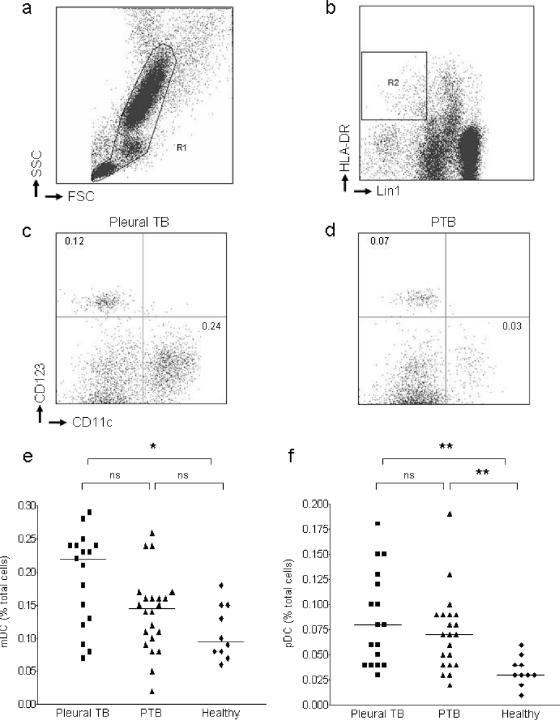
Red cells were lysed, and white cells were fixed by incubating 1 ml of sodium-heparinized blood in 9 ml of fluorescence-activated cell sorting (FACS) lysing solution (BD Biosciences) for 10 min at room temperature. Cells were pelleted and washed in “FACS-wash,” which is comprised of 3% fetal calf serum (Gemcell) and 0.09% sodium azide (Sigma) in phosphate-buffered saline (PBS) (BioWhittaker). The cell suspension was transferred to a 4-ml FACS tube (Falcon). Cells were pelleted, the supernatant was discarded, and the tubes were inverted onto a paper towel to remove excess supernatant. Cells were resuspended in the remaining 50 μl of FACS-wash and stained for 30 min at 4°C with the following directly conjugated monoclonal antibodies: Lin1-fluorescein isothiocyanate (FITC) (CD3, CD14, CD16, CD19, CD20, and CD56), CD123-phycoerythrin (PE), HLA-DR-PerCP, and CD11c-APC (all from BD Biosciences). After incubation, cells were washed once in FACS-wash, and the pellet was resuspended in 1% paraformaldehyde in PBS while being vortex mixed. Four-color flow cytometric analysis was undertaken on a FACSCalibur flow cytometer (BD Biosciences). Compensation for the overlap of fluorescence detection was performed with cells processed similarly to the test samples, using a single fluorescence-conjugated antibody for each color. Acquisition and analysis was completed by using CellQuest software (BD Biosciences). Dead cells and debris were excluded on the basis of forward and side scatter characteristics, and 1.5 × 106 events in gate R1 were analyzed (Fig. 1a). Blood DCs were identified as cells within R1 that were Lin1− HLA-DR+ (R2, Fig. 1b). Basophils, which are also included in this gate, were excluded from further analysis on the basis of their subsequent double negative staining for CD11c and CD123. mDCs were identified as Lin1− HLA-DR+ CD11c+ CD123− and pDCs as Lin1− HLA-DR+ CD11c− CD123+.
FIG. 1.
Quantification of dendritic cells in whole blood. (a) Viable cells were selected on the basis of their forward and side scatter characteristics (R1). (b) Blood DCs were identified within R1 as negative for lineage markers CD3, CD14, CD16, CD19, CD20, and CD56 (Lin1) and positive for HLA-DR (gate R2). Lin1− HLA-DR+ blood DCs were phenotyped as mDC (CD11c+ CD123−) or plasmacytoid (CD11c− CD123+). (c and d) Representative plots indicating levels of circulating blood DCs from a single patient with pleural TB (c) and PTB (d). Values are expressed as the percentage of total cells in lysed blood. (e and f) Phenotypic analysis of mDC (e) and pDC (f) subsets in blood of patients with pleural TB, patients with PTB, or healthy controls. Each point represents a single donor, and horizontal bars represent the median. Statistical differences between groups are indicated as follows: *, P < 0.05; and **, P < 0.01 (using Dunn's comparison of multiple groups as post-test analysis of the Kruskal-Wallis test [see Materials and Methods]).
Optimal stimulation and flow cytometry analysis of mDC function in whole blood.
Two milliliter screw-top polypropylene tubes (Sarstedt) were prepared to contain 7.5 × 106 CFU of M. tuberculosis strain CDC1551 which had been rapidly thawed from an aliquot cryopreserved from log-phase culture and sonicated prior to use. CDC1551 was used as the stimulant in this assay since previous experiments from our laboratory have shown it to be a potent stimulator of DC maturation (M. Mendelson, unpublished results). Brefeldin A (Sigma) at 10 μg/ml was added to each tube to capture IL-12 formed during the assay within the Golgi apparatus of the cell. A negative control tube was set up containing brefeldin A with an equal volume of medium instead of CDC1551. One milliliter of heparinized blood was added to each tube. Tubes were vortexed for 10 s and then transferred to a 37°C water bath that was programmed to switch off and cool down to room temperature to stop the stimulation of the cells after 12 h, a time point which often occurred in the middle of the night. Blood was harvested 10 h after the water bath was switched off. A portion (100 μl) of 20 mM EDTA (Sigma) in PBS was added to the tube to detach adherent cells, followed by high-speed vortexing and 15 min of incubation at room temperature. Each sample was transferred to a 15-ml centrifuge tube (Falcon) to which 9 ml of FACS lysing solution was added to lyse red blood cells and fix white cells. After 10 min of incubation at room temperature, the cells were pelleted and resuspended in 2 ml of 0.1% bovine serum albumin (Sigma) plus 0.1% saponin (Sigma) in PBS (permeabilization solution). The sample was transferred to a 4-ml FACS tube (Falcon) and incubated for 10 min at room temperature to permeabilize the white cells. Thereafter, cells were again pelleted, and the supernatant was resuspended in 50 μl of permeabilization solution for staining at 4°C with DC marker blood dendritic cell antigen 1 (BDCA-1)-FITC (Miltenyi Biotec), CD19-APC, HLA-DR-PerCP, and IL-12-PE (all from BD Biosciences). After 30 min of incubation at 4°C, the cells were washed in permeabilization solution, pelleted, and resuspended in 250 μl of 1% paraformaldehyde prior to flow cytometry. For each subject, another set of tubes was set up without the addition of brefeldin A to study cell surface expression of the chemokine receptor CCR7. Cells were not permeabilized but washed in FACS-wash and stained with BDCA-1-FITC, CD19-APC, HLA-DR-PerCP, and CCR7-PE (BD Biosciences).
Flow cytometric analysis of mDCs producing IL-12 and expressing CCR7 was completed by acquiring 1.5 × 106 fixed white cells on a FACSCalibur flow cytometer and completing analysis using CellQuest software. The same method of compensation was used as for the DC quantitation outlined above. Dead cells and debris were excluded by virtue of forward and side scatter characteristics, and the remaining cells were analyzed for expression of the B-cell marker CD19. CD19− cells, defined on a CD19/forward scatter plot, were then analyzed on a BDCA-1/HLA-DR plot to define BDCA-1bright HLA-DR+ mDCs. Finally, this population of mDCs was analyzed for IL-12 expression on an IL-12/forward scatter plot or for CCR7 expression on a CCR7/forward scatter plot.
In some experiments, variables that could affect the outcome of the assay were optimized. Therefore, different concentrations of CDC1551 (0 to 100 × 106 organisms/ml), and different durations of stimulation of whole blood (6 to 18 h) were tested. We also studied the effect of delay in incubating the whole blood with antigen (for 0 to 180 min) on assay outcome, and the effect of the temperature at which the collected blood was kept prior to stimulation (room temperature versus 37°C).
Statistical analysis.
Statistical analysis was undertaken using GraphPad Prism version 3.02 for Windows. The Kruskal-Wallis test for nonparametric analysis of ≥2 unpaired samples was used and Dunn's multiple comparison test was used for post-test analysis of all paired groups.
RESULTS
Participant characteristics.
We included 21 patients with PTB (all confirmed by sputum culture) and 17 patients with pleural TB (confirmed by culture of pleural fluid or pleural biopsy) in our analysis. Patients presenting with pleural TB were all negative on sputum smears and culture. In addition, posteroanterior and lateral chest radiographs taken at presentation and after 1 month of treatment showed no evidence of underlying lung parenchymal involvement in patients with pleural TB. The demographic characteristics of the two patient populations were similar (Table 1), with no statistically significant differences apparent between either group or the healthy controls (Kruskal-Wallis test).
TABLE 1.
Patient characteristics
| Characteristica | PTB patients | Pleural TB patients | Healthy controls |
|---|---|---|---|
| No. | 21 | 17 | 10 |
| Median age in yr (range) | 35 (20-55) | 28 (20-62) | 32 (24-52) |
| Sex (male/female) | 14/7 | 12/5 | 5/5 |
| Ethnicity (no. [%]) | |||
| Black | 3 (14) | 6 (35) | 2 (20) |
| Colored | 18 (86) | 9 (53) | 3 (30) |
| White | 0 | 2 (12) | 5 (50) |
| Median symptom duration in days (range) | 56 (14-336) | 28 (14-96) | |
| Median hematology (interquartile range) | |||
| Hemoglobin (g/dl) | 13.2 (12.6-14.1) | 11.8 (11.0-14.1) | 12.3 (11.4-15.2) |
| WBC (109/liter) | 8.1 (6.5-9.6) | 6.2 (5.3-8.5) | 7.3 (4.9-9.2) |
| Monocytes (109/liter) | 0.73 (0.46-0.93) | 0.64 (0.46-0.89) | 0.57 (0.39-0.99) |
| Lymphocytes (109/liter) | 2.03 (1.33-2.34) | 1.41 (1.04-1.78) | 1.94 (1.16-2.28) |
| Platelets (109/liter) | 345 (289-413) | 340 (314-504) | 361 (277-495) |
WBC, white blood cell count.
Quantitation of blood DCs in patients with pleural TB and PTB.
We quantified peripheral blood DCs in whole blood by flow cytometry (Fig. 1). Analysis of variance between the three groups of subjects using the Kruskal-Wallis test identified a significant difference in circulating mDC frequencies (P = 0.01). Post-test analysis confirmed an increased frequency of circulating mDCs in patients with pleural TB compared to healthy controls but no difference between patients with PTB and healthy controls (Fig. 1e). Despite not reaching statistical significance, there was a trend toward higher mDC frequencies in pleural TB patients (median, 0.22 [interquartile range of 0.12 to 0.24]) compared to those with PTB (median, 0.14 [interquartile range of 0.09 to 0.16]). Similar analysis of frequencies of circulating pDCs demonstrated significantly greater levels in patients with either form of TB compared to healthy controls but no difference between the two TB groups (Fig. 1f).
To determine whether differences in the populations observed were part of a generalized alteration in blood cell lineages, we analyzed complete blood counts from patients with pleural TB, patients with PTB, and controls (Table 1). No differences (using the Kruskal-Wallis test) were observed in any of the cell lineages tested. We conclude that patients with pleural TB maintain higher circulating frequencies of mDCs than healthy individuals, with a trend toward a greater frequency of mDCs in pleural TB versus PTB patients. In addition, both forms of TB are associated with raised circulating frequencies of pDCs compared to healthy controls.
Optimization of flow cytometry assay to study functional characteristics of mDC in a WBA.
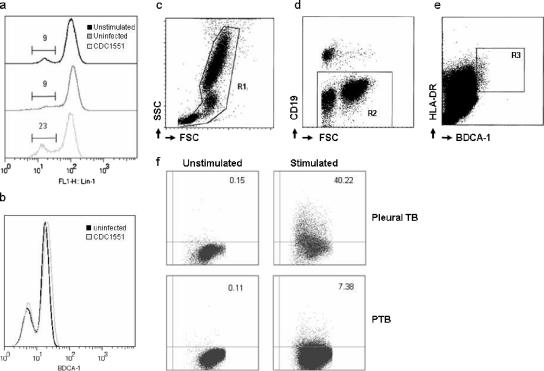
To identify whether quantitative differences in mDCs observed in pleural TB patients are associated with functional impairment, we adapted an existing T-cell WBA (8) to study mDCs in peripheral blood. Initial experiments focused on choosing the right surface markers for detecting peripheral blood mDCs after the incubation of whole blood with virulent mycobacteria. One option was to use the anti-Lin1 cocktail of antibodies, which we used in quantitating mDCs and which identifies non-mDC such as monocytes, T cells, and B cells, in the gating strategy. We showed that incubation of whole blood with mycobacteria resulted in downregulation of Lin1 expression (Fig. 2a), predominantly due to the downregulation of CD14 on the surface of peripheral blood monocytes (data not shown). Therefore, using this strategy might have incorporated monocytes, which share some of the functional responses of mDCs to stimulation with virulent mycobacteria, into the Lin1-negative gate. Since we were unable to use anti-Lin1 antibodies, we determined whether antibodies against an alternative mDC marker, BDCA-1 (CD1c), expressed on the majority of circulating mDCs and a subpopulation of CD19+ B lymphocytes (4), could be used in this setting. Incubation of whole blood with mycobacteria was not associated with significantly altered expression of BDCA-1 (Fig. 2b). Our gating strategy therefore included exclusion of dead cells and debris (Fig. 2c), followed by exclusion of CD19+ B lymphocytes (Fig. 2d). CD19 is coexpressed on only a very small subpopulation of BDCA-1+ mDCs (4). Consequently, this subpopulation was excluded from our mDC analysis. Myeloid DCs were then identified as BDCA-1bright HLA-DR+ cells lying within the CD19− population (Fig. 2e). To determine IL-12 expression in mDCs from whole blood incubated with mycobacteria, the expression of the mDC population in unstimulated whole blood was used from each participant to determine where the cutoff between IL-12+ and IL-12− cells should be (Fig. 2f).
FIG. 2.
Analysis strategy for determining mDC functional response by flow cytometry after ex vivo stimulation with virulent mycobacteria. (a) Whole blood was analyzed for expression of anti-Lin1 antibody prior to incubation (unstimulated), after 12 h of incubation without addition of CDC1551 (uninfected), and after stimulation with CDC1551. Values refer to the percentage of cells within the gate drawn. (b) Analysis of BDCA-1 expression after the incubation of uninfected or CDC1551-stimulated blood was assessed for changes in the expression profile. (c) For the functional mDC WBA, dead cells and debris were excluded, and the remaining cells (R1) were analyzed by virtue of forward and side scatter characteristics. (d) CD19+ B lymphocytes, a subset of cells that express cell surface BDCA-1, were excluded from further analysis by negative gating (R2). (e) BDCA-1bright HLA-DR+ mDCs (R3) were analyzed for intracellular IL-12 production. (f) Representative sample from a patient with pleural TB and lung parenchymal TB. Figures indicate the percentage of mDC within R3 expressing IL-12 in control samples (unstimulated) and in whole blood stimulated with CDC1551 (stimulated).
Optimization of whole-blood incubation with mycobacteria to determine functional characteristics of mDCs.
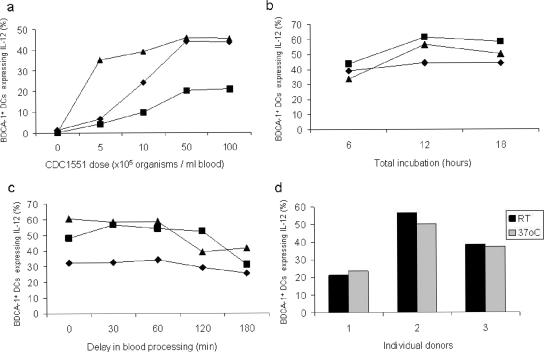
A number of key variables affecting the performance of the WBA were optimized. The optimal dose of CDC1551 (7.5 × 106 CFU) was titrated using blood from three healthy donors (Fig. 3a). The optimal duration of incubation of blood with the bacilli for a maximal response was 12 h (Fig. 3b). White cell viability assessed by trypan blue exclusion at this time point using the optimal dose of CDC1551 was >98%. Since previous studies had shown that a delay in incubation of collected whole blood with specific antigens resulted in significantly reduced IFN-γ expression in T cells (8), we assessed the effect of a time delay to incubation of whole blood in the mDC assay on the functional response. A delay of more than 60 min prior to incubation with mycobacteria resulted in reduced IL-12 production by mDCs (Fig. 3c). In addition, there was no difference in the results of the assay when blood was kept in the Vacutainer tube at ambient temperature or at 37°C during the first 60 min after venesection (Fig. 3d). Hence, blood samples were transferred to the laboratory at ambient temperature and processed within 1 h of obtaining the sample in the clinic.
FIG. 3.
Optimization of WBA parameters. (a) Dose response to CDC1551 in whole blood from three healthy adult volunteers. After 12 h of stimulation, the frequency of IL-12-expressing mDCs was determined. (b) The optimal duration of whole-blood stimulation for a maximum mDC IL-12 response was assessed. (c) Effect of delay in blood processing after collection. (d) Ambient temperature at which blood (in panel c) is kept within the first hour after collection. The results are shown for three separate healthy volunteers in each experiment.
Myeloid DC IL-12 production and CCR7 expression, as a functional correlate of type of clinical TB.
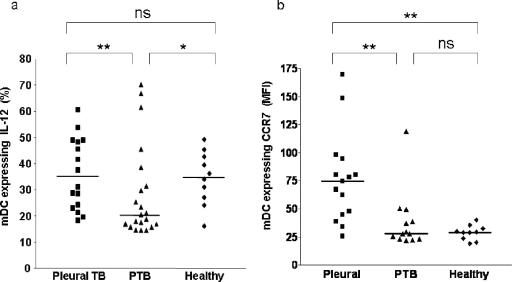
The process of maturation is the key functional response of the DC after pathogen recognition and ultimately leads to activation of adaptive immunity. We assayed DC IL-12 and CCR7 upregulation, as markers of the maturation response, in patients with different forms of TB. Patients with pleural TB demonstrated an increased upregulation of IL-12 production by mDCs after ex vivo stimulation (Fig. 4a). In contrast, the ability of mDCs from patients with PTB to upregulate IL-12 was impaired. The upregulation of cell surface CCR7 followed the same pattern as IL-12 upregulation; mDCs from patients with pleural TB showed a greater level of upregulation after stimulation than mDCs from patients with PTB (Fig. 4b).
FIG. 4.
Expression of markers of DC function in patients with TB. (a and b) Analysis of intracellular IL-12 production (a) and CCR7 surface expression (b) by mDCs in the whole blood of patients with TB and in healthy controls after ex vivo stimulation. Each point represents a single donor, and horizontal bars represent the median. Statistical differences between groups are indicated as follows: *, P < 0.05; and **, P < 0.01 (Dunn's comparison of multiple groups [see Materials and Methods]).
We conclude that patients with PTB exhibit an impaired peripheral blood mDC functional response after ex vivo stimulation with virulent M. tuberculosis compared to patients presenting with pleural disease, whose sustained circulating frequency of mDCs is accompanied by a greater functional response.
DISCUSSION
We have shown that patients with pleural TB have improved function of mDCs in peripheral blood compared to patients with PTB. To achieve this, we optimized a WBA to study DC function directly ex vivo, thereby reducing the likelihood of possible artifacts introduced as a consequence of PBMC isolation and DC purification. The assay is based on the work of a number of different groups who have previously described WBAs on individual blood cell populations (7, 8, 18, 21, 23). The assay is robust, allows for limited time delay in transferring samples from the field to the laboratory, and is able to quantitate different aspects of the DC maturation response. In addition, the assay has the potential to use very small blood volumes (down to 250 μl [data not shown]), which would make it applicable to studies in pediatric, as well as adult, populations and in ill patients. The assay uses a novel gating strategy for flow cytometric analysis of mDC function: we have avoided markers that may be downregulated during the incubation process, allowing monocytes to be misidentified as mDC. Ultimately, markers of DC maturation may be studied directly ex vivo, providing correlates of innate immune function in individuals. Although we did not test whether mDCs in peripheral blood were actually infected with M. tuberculosis prior to or after ex vivo stimulation, it has been demonstrated that M. tuberculosis can infect DCs (6), making it possible that stimulation of mDCs in our assay may have been via direct infection. However, an important caveat is that the stimulation of bystander cells such as monocytes or natural killer cells that are also present in whole blood may be responsible in part or in whole for the induction of IL-12 upregulation and CCR7 expression by mDCs observed in our assay.
The criteria we used for determining whether pleural TB patients had underlying parenchymal lung disease was the inability to culture M. tuberculosis from two sputum samples and resolution of the pleural effusion at 1 month of antituberculous treatment with no radiological evidence of parenchymal involvement in the underlying lung. It remains a possibility that occult active parenchymal TB was present despite our best efforts to exclude this. However, in participants who we classified as having pleural TB, the clinical findings were more consistent with primary disease not affecting lung parenchyma rather than reactivation of parenchymal TB with an associated secondary pleural effusion. Studies of the natural history of TB from the preantibiotic era found that the rate of spontaneous resolution of TB in patients presenting with pleural disease was greater than in patients presenting with PTB (16). This implies that differences in host protective immunity may exist between patients manifesting TB at different sites of the body. Little attention has been given to the innate immune response in this regard. Uehira et al. showed that patients with predominantly pulmonary TB had reduced numbers of peripheral blood mDCs compared to age-matched controls (22). These authors noted an increase in DCs within the regional lymph nodes of patients. In contrast, our findings suggest that patients with pleural TB have increased numbers of mDCs compared to healthy controls. Furthermore, we observed a trend toward greater numbers of mDCs in patients presenting with pleural TB than in those with PTB. It is unclear whether the higher circulating level of mDCs in pleural TB patients than those with PTB reflects the premorbid state or whether PTB patients have increased recruitment of mDCs to the site of disease. A longitudinal prospective study would be required to answer this question. However, it is interesting that our findings are in keeping with the historical differences in the prognosis of active disease in patients with the two forms of TB prior to the advent of antibiotics. Furthermore, the differential response of mDCs from patients with pleural TB correlates with a number of studies showing increased antigen-specific T-cell responses in patients with pleural TB (9, 24).
The discrepancy between our results and those of Uehira et al. (22) may relate to the methods used. We looked directly at the frequency of DCs in whole blood ex vivo, whereas Uehira et al. separated PBMC from whole blood prior to analysis. In addition, patients analyzed by Uehira et al. included an unspecified number of patients presenting with TB at extrathoracic sites, which have not formed part of our analysis. Our studies were novel in that we were also able to describe functional aspects of mDCs in the peripheral blood of patients with different forms of TB disease. We showed that mDCs from patients with PTB are less able to upregulate IL-12 production or CCR7 expression after ex vivo stimulation than mDCs from patients with pleural TB. We did not find any differences in the baseline phenotype of blood DCs to explain our findings (data not shown). Our finding of upregulation of CCR7 on mDC in response to exposure to M. tuberculosis is in keeping with previous ex vivo studies of CCR7 expression in response to PAMPs (10). We hypothesize that the maturation of mDCs in blood either in direct response to bacteria during bacteremia or in response to a proinflammatory milieu will direct maturing mDCs to lymph nodes, thereby positively affecting the immune response (13). It is unclear at present why mDCs from healthy controls upregulated IL-12 expression in common with pleural TB patients and yet did not upregulate CCR7 in a similar manner. It is interesting to speculate that a stimulus present in the blood of pleural TB patients that is required to induce CCR7 upregulation is missing from the blood of PTB patients or healthy individuals. Overall, preservation of the circulating mDC number and function in pleural TB may be an important factor contributing to the favorable outcome of this disease form versus PTB.
Another potential contributing factor that could influence the site of disease and outcome of infection with M. tuberculosis is the type of infecting strain. It has become increasingly recognized that the ability of different M. tuberculosis strains to cause disease varies. Hypervirulence of W-Beijing strains in mice, for example, is related to the presence of a phenolic glycolipid, whose disruption is associated with a loss of virulence (15). Very few published studies have related strain variation to specific sites of presentation of TB. One study used restriction fragment length polymorphism analysis to show that the type 1 restriction pattern was more common in central nervous system TB (1). We were unable to evaluate clustering of strains in either of our TB cohorts, nor could we evaluate a potential predominance of one type of strain family by restriction fragment length polymorphism and spoligotyping (data not shown). However, larger prospective studies are now ongoing within our laboratory to address this question.
There are a number of limitations to our study. First, we were unable to study cells from the site of infection in the pleura and lung parenchyma, which may have yielded interesting comparisons with the alterations observed in blood DCs. Second, by using whole M. tuberculosis, we did not define which ligands of M. tuberculosis induced the maturation of mDCs in patient groups or which maturation signals may have been defective. Lastly, our studies were restricted to patients with TB, and therefore we are unable to comment on whether alterations in blood DC numbers are restricted to this group or also occur in patients with other lung diseases. Such information would be important if such assays are to be developed for use in the field.
In summary, our findings highlight quantitative and functional differences in mDC populations between patients with two forms of TB and with healthy controls that we suggest contribute to determining the outcome of the disease. We propose that the mDC WBA that we have optimized may be used to define a correlate of innate immunity in other infectious diseases, and further studies are warranted to evaluate the use of the assay in longitudinal studies to assess the efficacy of antimycobacterial therapy.
Acknowledgments
This study was supported by NIAID grants AI 22616 and AI 54361 to G.K. W.A.H. was supported by the NIH (AI 65653), as well as EDCTP and the Dana Foundation.
We thank Rose Hendry and all of the staff of TB clinics in the Southwest Peninsula Board of Healthcare for their help in recruiting patients into the study.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Arvanitakis, Z., R. L. Long, E. S. Hershfield, J. Manfreda, A. Kabani, D. Kunimoto, and C. Power. 1998. Mycobacterium tuberculosis molecular variation in CNS infection: evidence for strain-dependent neurovirulence. Neurology 6:1827-1832. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. F., S. D. Mistry, C. L. Cooper, C. Pirmez, T. H. Rea, and R. L. Modlin. 1989. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J. Immunol. 142:1114-1119. [PubMed] [Google Scholar]
- 3.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 4.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037-6046. [DOI] [PubMed] [Google Scholar]
- 5.Ellner, J. 1978. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann. Intern. Med. 89:932-933. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek, T. B. H., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, M. J. E. Vandenbrouke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godoy-Ramirez, K., K. Franck, S. Mahdavifar, L. Andersson, and H. Gaines. 2004. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J. Immunol. Methods 292:1-15. [DOI] [PubMed] [Google Scholar]
- 8.Hanekom, W. A., J. Hughes, M. Mavinkurve, M. Mendillo, M. Watkins, H. Gameldien, S. J. Gelderbloem, M. Sidbana, N. Mansoor, V. Davids, R. A. Murray, A. Hawkridge, P. A. Haslett, S. Ress, G. D. Hussey, and G. Kaplan. 2004. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods 291:185-195. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, C. S., Z. Toossi, J. L. Johnson, H. Luzze, L. Ntambi, P. Peters, M. McHugh, A. Okwera, M. Joloba, P. Mugyenyi, R. D. Mugerwa, P. Terebuh, and J. J. Ellner. 2001. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J. Infect. Dis. 183:788-799. [DOI] [PubMed] [Google Scholar]
- 10.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallustro, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 11.Liam, C. K., K. H. Lim, and C. M. Wong. 1999. Tuberculous pleurisy as a manifestation of primary and reactivation disease in a region with a high prevalence of tuberculosis. J. Tuberc. Lung Dis. 3:816-822. [PubMed] [Google Scholar]
- 12.Liu, Y. J. 2004. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275-306. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Fontecha, A., S. Sebastian, U. E. Hopken, M. Uguccioni, M. Lipp, A. Lanzavecchia, and F. Sallustro. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paluka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 15.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-88. [DOI] [PubMed] [Google Scholar]
- 16.Roper, W. H., and J. J. Waring. 1955. Primary serofibrinous pleural effusion in military personnel. Am. Rev. Tuberc. 71:616-634. [DOI] [PubMed] [Google Scholar]
- 17.Sahn, S. A. 1998. State of the art-the pleura. Am. Rev. Respir. Dis. 138:184-234. [DOI] [PubMed] [Google Scholar]
- 18.Shimokata, K., H. Kishimoto, E. Takagi, and H. Tsunekawa. 1986. Determination of the T-cell subset producing gamma-interferon in tuberculous pleural effusions. Microbiol. Immunol. 30:353-361. [DOI] [PubMed] [Google Scholar]
- 19.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Atonenko, and Y. J. Liu. 1999. The nature of the peripheral type I interferon-producing cells in human blood. Science 284:1835-1839. [DOI] [PubMed] [Google Scholar]
- 20.Steinman, R. M. 2001. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai. J. Med. 68:106-166. [PubMed] [Google Scholar]
- 21.Suni, M. A., L. J. Picker, and V. C. Maino. 1998. Detection of antigen-specific T-cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 212:89-96. [DOI] [PubMed] [Google Scholar]
- 22.Uehira, K., R. Amakawa, T. Ito, K. Tajima, S. Naitoh, Y. Ozaki, T. Shimizu, K. Yamaguchi, Y. Uemura, H. Kitajima, S. Yonezu, and S. Fukuhara. 2002. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin. Immunol. 105:296-303. [DOI] [PubMed] [Google Scholar]
- 23.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T-cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson, K. A., R. J. Wilkinson, A. Pathan, K. Ewer, M. Prakash, P. Klenerman, N. Maskell, R. Davies, G. Pasvol, and A. Lalvani. 2005. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin. Infect. Dis. 40:184-187. [DOI] [PubMed] [Google Scholar]