Abstract
We have identified genes encoding candidate proteins involved in iron storage (pcl1+), the tricarboxylic acid cycle (sdh4+), and iron-sulfur cluster assembly (isa1+) that are negatively regulated in response to iron deprivation. Promoter deletion and site-directed mutagenesis permitted identification of a new cis-regulatory element in the promoter region of the pcl1+ gene. This cis-acting regulatory sequence containing the pentanucleotide sequence CCAAT is responsible for transcriptional repression of pcl1+ under low iron supply conditions. In Schizosaccharomyces pombe, the CCAAT-binding factor is a heteromeric DNA-binding complex that contains three subunits, designated Php2, Php3, and Php5. Inactivation of the php2+ locus negatively affects the transcriptional competency of pcl1+. A fourth subunit, designated Php4, is not essential for the transcriptional activation of target genes under basal and iron-replete conditions. We demonstrate that, in response to iron-limiting conditions, Php4 is required for down-regulation of pcl1+, sdh4+, and isa1+ mRNA levels. In vivo RNase protection studies reveal that the expression of php4+ is negatively regulated by iron and that this regulated expression requires a functional fep1+ gene. The results of these studies reveal that Fep1 represses php4+ expression in response to iron. In contrast, when iron is scarce, Fep1 becomes inactive and php4+ is expressed to act as a regulatory subunit of the CCAAT-binding factor that is required to block pcl1+, sdh4+, and isa1+ gene transcription.
Iron is a biological constituent required by most organisms (22). As a cofactor in a wide range of biochemical reactions, iron is important (46). For example, iron is present in the active site of enzymes involved in metabolic processes such as the tricarboxylic acid (TCA) cycle, the cellular respiratory chain, oxygen transport, and photosynthesis. Iron's value resides in the reactivity of the Fe2+/Fe3+ redox couple, which enables it to donate or accept an electron to catalyze enzymatic reactions. Paradoxically, the same redox-active nature of iron renders it biotoxic. This is due to its ability to react with oxygen species to generate the hydroxyl radical (19). Production of this free reactive oxygen species can damage cellular components such as DNA, proteins, and membrane lipids (20). Therefore, organisms need efficient mechanisms to acquire iron but also to keep its reactivity in check.
Iron presents an additional problem in that it is found under aerobic conditions as insoluble ferric hydroxides, making it biologically unavailable (10, 14). Consequently, organisms have evolved different iron-scavenging systems for solubilizing iron and transporting it into cells. Many bacteria and fungi synthesize and excrete low-molecular-weight iron chelators known as siderophores (15, 18, 24, 58). Subsequent to excretion of the siderophores, iron-siderophore complexes are efficiently recaptured by microorganisms via specific cell surface receptors (28, 46). Other iron assimilation mechanisms include reduction via a cell surface reductase and subsequent transport of the iron across the membrane by iron uptake proteins (2, 11, 12, 17, 29, 32, 54), proteolytic degradation of the host iron-binding proteins (49, 53), and surface receptors for mammalian iron carriers such as transferrin, lactoferrin, and heme (20).
In the model organism Schizosaccharomyces pombe, two types of iron uptake systems have been found (3, 48, 52). The first system consists of two genes, sib1+ (SPAC23G3.02c) and sib2+ (SPAC23G3.03), which are involved in the acquisition of iron from the hydroxamate-type siderophore ferrichrome (52). The ability of fission yeast cells to produce and excrete ferrichrome is consistent with the existence of S. pombe Str1, which confers ferrichrome uptake when ectopically expressed in a Saccharomyces cerevisiae fet3Δ arn1-4Δ mutant strain (44). Two other proteins found in the S. pombe proteome exhibit sequence similarity to Str1. These two homologs were designated Str2 and Str3 (44). Although Str2 and Str3 may participate in the mobilization of iron bound to siderophores, their substrate specificity has not been determined. Under low environmental iron conditions, a second system is used by S. pombe to accumulate iron. It is divided into multiple steps. A first step is the reduction of ferric iron by a cell surface electron transporter encoded by the frp1+ gene (48). Then, subsequent to reduction, ferrous iron is captured by an oxidase-permease complex formed by Fio1 and Fip1 (3). Fio1 converts ferrous iron to ferric iron, which is then transported across the membrane by the iron permease Fip1 (3).
Under iron-deficient conditions, the extent of transcription of frp1+, fio1+, fip1+, str1+, str2+, and str3+ increases several times (∼8- to 18-fold), whereas when environmental iron is abundant, expression of these genes is extinguished (3, 43, 44, 48). Conserved cis-acting elements in the frp1+, fio1+, fip1+, str1+, str2+, and str3+ promoters with the sequence 5′-(A/T)GATAA-3′ are required for iron-mediated repression of gene expression (43, 44). Fep1 is a GATA transcription factor that has been shown to negatively regulate iron uptake by repressing frp1+, fio1+, fip1+, str1+, str2+, and str3+ gene expression under iron-replete conditions (43, 44). Deletion of fep1+ (fep1Δ) results in constitutive expression of these iron transport genes. Based on the ability of the iron chelator BPS to preclude its binding, Fep1 has been shown to associate with DNA in an iron-dependent manner (43). The N-terminal 241 amino acids of Fep1 contain its DNA-binding domain. At its C terminus, Fep1 contains a leucine zipper domain that mediates Fep1 dimerization and increases its potency as a transcriptional repressor (45).
In the fission yeast S. pombe, following glucose depletion, a protein complex comprising the Php2, Php3, and Php5 proteins positively regulates many of the genes involved in oxidative phosphorylation, including cyc1+, which encodes the cytochrome c oxidase protein (38, 42, 60). The S. pombe Php2, Php3, and Php5 proteins are orthologous to Saccharomyces cerevisiae Hap2, Hap3, and Hap5, respectively (37, 39). Consistently, expression of S. pombe php2+, php3+, and php5+ functionally complements S. cerevisiae hap2Δ, hap3Δ, and hap5Δ mutants, respectively, for growth on a nonfermentable carbon source (38, 41, 60). The S. pombe Php2, Php3, and Php5 proteins contain short regions of homology with their S. cerevisiae counterparts that correspond to evolutionarily conserved core domains (38). In S. cerevisiae, the DNA-binding capability of the CCAAT-binding complex is conferred by the Hap2/Hap3/Hap5 subunits (39). In addition, a fourth subunit, denoted Hap4, enhances the Hap2/Hap3/Hap5 complex activity via its trans-activation domain (16). The S. cerevisiae heteromeric CCAAT-binding complex is modulated via the expression of Hap4, which is repressed in the presence of glucose and induced in media containing nonfermentable carbon sources such as lactate, ethanol, and glycerol. This suggests that Hap4 is a key regulator of CCAAT complex activity in response to carbon source in S. cerevisiae. Until recently, Hap4 homologs had not been identified in other fungi. A recent in silico study has revealed that such homologs exist in the genomes of Saccharomyces species closely related to S. cerevisiae, as well as two more distantly related Saccharomyces species (55). Furthermore, functional complementation studies revealed that Hap4 homologs exist in Kluyveromyces lactis (8) and Hansenula polymorpha (56).
The strict requirement for appropriate intracellular iron concentrations suggests that there may be regulatory mechanisms that prevent futile expression of the genes encoding iron-requiring proteins when iron is limiting. In Escherichia coli, a small noncoding regulatory RNA, termed RyhB, is induced under conditions of iron starvation (35). It hybridizes with its target mRNAs and causes their degradation (34). RyhB targets encode iron storage and iron-containing proteins (36). Transcription of the ryhB gene is repressed by the iron-Fur complex (33). A similar process has been described for the action of two small RNAs, called prrF1 and prrF2, in the control of iron-regulatable genes in Pseudomonas aeruginosa (57). In S. cerevisiae, the RNA-binding protein Cth2 has been shown to mediate the degradation of transcripts encoding iron-containing proteins in response to iron deprivation (47). Under iron-adequate conditions, CTH2 expression is down-regulated. In contrast, increased CTH2 gene expression occurs during iron scarcity and depends on the iron-responsive transcription factors Aft1 and Aft2 (47).
In this study, we have identified a set of genes (pcl1+, sdh4+, and isa1+) whose expression is down-regulated under iron starvation conditions. Analysis of regions in the pcl1+ promoter demonstrated that a CCAAT-type regulatory sequence is necessary for its transcriptional regulation. Consistently, inactivation of the php2+ locus that encodes a subunit of the heteromeric CCAAT-binding factor negatively affects the transcriptional competency of pcl1+. To examine the function of the CCAAT-binding factor in S. pombe, we cloned php4+ and generated a php4Δ mutant strain. We found that in contrast to its activator function in S. cerevisiae, the S. pombe Php4 factor appeared to function as a repressor of genes encoding iron-using and iron storage proteins. We determined that php4+ is regulated at the level of gene transcription: it is induced under conditions of iron deprivation and turned off under conditions of iron repletion. Furthermore, its iron-dependent regulated expression requires a functional fep1+ gene. Taken together, these results reveal the existence of a transcription factor cascade composed of Fep1 and the heteromeric CCAAT-binding factor to regulate gene expression in response to iron deficiency in fission yeast.
MATERIALS AND METHODS
Strains and growth conditions.
S. pombe strains used in this work were the wild-type strain FY435 (h+ his7-366 leu1-32 ura4-Δ18 ade6-M210) (6) and the pcl1Δ (isogenic to FY435 plus pcl1Δ::Kanr), php2Δ (isogenic to FY435 plus php2Δ::Kanr), php3Δ (isogenic to FY435 plus php3Δ::Kanr), php4Δ (isogenic to FY435 plus php4Δ::Kanr), php5Δ (isogenic to FY435 plus php5Δ::Kanr), and zfs1Δ (isogenic to FY435 plus zfs1Δ::Kanr) disruption strains. The gene disruptions were created by replacing the coding region of pcl1+, php2+, php3+, php4+, php5+, or zfs1+ with a loxP-KANMX2-loxP cassette through homologous recombination (21). An isogenic fep1Δ disruption strain (h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 fep1Δ::ura4+) was also used as described previously (43). Yeast strains were cultivated at 30°C in yeast extract plus supplements or in selective Edinburgh minimal medium lacking specific nutrients required for plasmid selection (1). For iron starvation or iron repletion experiments, S. pombe strains were grown to mid-logarithmic phase (A600 of ∼1.0) and then incubated for 90 min in the presence of 250 μM 2,2′-dipyridyl (Dip) or 100 μM FeCl3, respectively. After treatments, 15-ml samples were withdrawn from the cultures for subsequent steady-state mRNA analysis.
Analysis of gene expression.
Total RNA was extracted by the hot phenol method as described previously (4). RNAs were quantified spectrophotometrically, and 15 μg of RNA per sample was used in the RNase protection protocol, which was carried out as described previously (5). 32P-labeled antisense pcl1+, sdh4+, isa1+, php2+, php3+, php5+, fio1+, and zfs1+ RNAs were produced from the BamHI-linearized plasmids pSKpcl1+, pSKsdh4+, pSKisa1+, pSKphp2+, pSKphp3+, pSKphp5+, pSKfio1+, and pSKzfs1+, respectively. 32P-labeled antisense lacZ RNA was generated from the HindIII-linearized plasmid pKSlacZ (26). The riboprobe derived from the plasmid pSKact1+ (25) was used to probe act1+ mRNA as an internal control for normalization during quantification of the RNase protection products.
Plasmids.
Plasmid pSKpcl1+ was created by inserting a 170-bp BamHI-EcoRI fragment from the pcl1+ gene into the same sites of pBluescript SK (Stratagene, La Jolla, CA). The antisense RNA hybridizes to the region between +18 and +188 downstream from the initiator codon of pcl1+. Plasmid pSKsdh4+ was constructed by ligating a 178-bp BamHI-EcoRI fragment from the sdh4+ gene into the BamHI-EcoRI sites of pBluescript SK. This fragment corresponds to the region between +3 and +181 down to the first base of the translational start codon of sdh4+. To generate pSKisa1+, a 188-bp fragment from the isa1+ gene (corresponding to the coding region between +3 and +191) was amplified and cloned into the BamHI-EcoRI sites of pBluescript SK. pSKphp2+ was made by ligating a PCR product containing a 174-bp fragment from the php2+ gene (corresponding to the coding region between +51 and +225) into the BamHI-EcoRI sites of pBluescript SK. Plasmid pSKphp3+ was created by inserting a 183-bp BamHI-EcoRI fragment from the php3+ gene (matching to the coding region between +54 and +237) into the corresponding sites of pBluescript SK. Plasmid pSKphp4+ was constructed by inserting a 182-bp BamHI-EcoRI fragment of the php4+ gene into the same sites of pBluescript SK. The antisense RNA pairs to the region between +336 and +518 downstream from the A of the start codon of php4+. pSKphp5+ was generated by ligating a 196-bp fragment from the php5+ gene into the BamHI-EcoRI sites of pBluescript SK. The latter plasmid was used to produce an antisense RNA probe that hybridizes specifically to php5+ mRNA (positions +307 to +503). Plasmid pSKzfs1+ was generated by inserting a 184-bp fragment from the zfs1+ gene (corresponding to the coding region between +11 and +194) into pBluescript SK using the BamHI and EcoRI sites. Plasmids pKSlacZ, pSKfio1+, and pSKact1+, for determining lacZ, fio1+, and act1+ mRNA levels, respectively, were described elsewhere (25, 43).
To generate the pSP1pcl1+ plasmid, a 2,472-bp XhoI-BamHI PCR-amplified DNA segment containing the S. pombe pcl1+ locus starting at −1060 from the translational start codon up to +683 after the stop codon was inserted into the XhoI and BamHI sites of pSP1 (9). The plasmid pSP1pcl1+-1398lacZ was constructed by introducing a BamHI-Asp718 PCR-amplified fragment from the pcl1+ promoter containing 1,398 bp of the 5′-noncoding region and the first 10 codons of the pcl1+ gene into the BamHI-Asp718-cut Yep357R vector (40). Once generated, the pcl1+ promoter region was isolated from Yep357Rpcl1+-1398lacZ with BamHI and Bsu36I and swapped for the equivalent DNA restriction fragment in pSP1fio1+-1155lacZ (43) to generate pSP1pcl1+-1398lacZ. Six plasmids (pSP1pcl1+-680lacZ, pSP1pcl1+-478lacZ, pSP1pcl1+-364lacZ, pSP1pcl1+-247lacZ, pSP1pcl1+-211lacZ, and pSP1pcl1+-173lacZ) harboring sequential deletions from the 5′ end of the pcl1+ promoter were created by PCR from plasmid pSP1pcl1+-1398lacZ. Each PCR product obtained was purified, digested with BamHI and Bsu36I, and used to replace the equivalent DNA restriction fragment in pSP1pcl1+-1398lacZ. Plasmids pSP1pcl1+-247lacZ and pSP1pcl1+-211lacZ were used to introduce mutations in the CCAAT box (positions −205 to −201 with respect to the A of the ATG codon of pcl1+). Precisely, the oligonucleotide 5′-TCCCGGGCCCCAGTACTTAAACAAGGTTCTTAAGCCGCGTTAATCCCGTTGAAACCGCAAAAGGC-3′ or 5′-CGCGGATCCCGTTGAAACCGCAAAAGGCTTCTATATCAAATATTTATTTTGACACC-3′ was utilized in combination with another oligonucleotide (5′-CGTTGCACCACAGATGAAACGC-3′) to generate by PCR two pcl1+ promoter DNA fragments containing a mutant CCAAT box. Letters that are underlined in the oligonucleotides represent multiple point mutations in the CCAAT element. Subsequently, the BamHI-Bsu36I mutant DNA restriction fragment was exchanged with an identical DNA region into the pSP1pcl1+-247lacZ or pSP1pcl1+-211lacZ plasmid. To create both the wild-type and mutant pCF83pcl1-247lacZ fusion plasmids, the pcl1 promoter region (positions −247 to −52) was PCR amplified from the wild-type and mutant pSP1pcl1-247lacZ constructs. Both PCR products were purified and inserted in their natural orientations into the XmaI and XhoI sites of the CYC1-lacZ fusion plasmid pCF83 (25). The php2+ locus starting at −1092 from the initiator codon up to the stop codon was isolated by PCR from genomic DNA of the wild-type strain FY435. The purified DNA fragment was cloned via XhoI and BamHI sites into the corresponding sites of pSP1. After verification by dideoxy sequencing of the integrity of the php2+ DNA sequence, the php2+ locus was subcloned into pGEM-7Zf (Promega, Madison, WI) with the XhoI and BamHI sites. The resulting plasmid, denoted pGEMphp2+, was subsequently digested with XbaI and BamHI, and then the DNA fragment containing the php2+ gene and its regulatory region was inserted into the corresponding sites of pJK148 (23). Therefore, we ensured that the php2+ gene was under the control of its own promoter, once integrated in the genome. To generate the pJK148php4+ plasmid, a 1,915-bp BamHI-SalI PCR-amplified DNA segment containing the S. pombe php4+ locus starting at −1027 from the translational start codon up to the stop codon was inserted into the BamHI and SalI sites of pJK148.
Expression and purification of recombinant proteins.
The DNA containing the php2+ codons 2 to 70 was amplified by PCR, purified, and inserted in-frame into the pMAL-c2X vector (New England BioLabs, Beverly, MA) with the BamHI and PstI restriction enzymes. Plasmid pMAL-2php2+70 was transformed into E. coli BL21. Fresh transformants of BL21 cells containing the plasmid pMAL-c2X or pMAL-2php2+70 were grown to an A600 of 0.5. At this early growth phase, the cells were induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside for 18 h at 25°C. Harvested cells were washed once in ice-cold water and resuspended in A buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 0.5 mg/ml lysozyme, and a protease inhibitor cocktail [Sigma; P-8340]). The mixture was incubated for 20 min at 4°C. Cell lysis was achieved by sonication, and insoluble material was removed by centrifugation at 15,000 rpm for 20 min. The supernatant was applied to a 1-ml column of amylose resin (New England BioLabs, Beverly, MA) that had been equilibrated with CBG200 buffer containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 200 mM NaCl, and 10% glycerol. The column was washed with 10 ml of CBG200 buffer and then eluted stepwise with CBG200 buffer containing 10 and 20 mM maltose. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis analysis showed that the affinity-purified MBP-2Php270 protein was recovered predominantly in the 10 mM maltose eluate fractions. The above-mentioned procedure was also used to express and purify the recombinant MBP-2Php3117 and MBP-2Php5191 proteins. Expression and purification of the glutathione S-transferase (GST) and GST-2Php4295 proteins in E. coli were carried out as described previously (63). Protein concentrations were determined by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard. For in vitro cross-linking experiments, purified recombinant proteins were incubated with increasing concentrations of EGS (BioLynx, Brockville, Ontario, Canada) as described previously (45). The cross-linked complexes were resolved by SDS-polyacrylamide gel electrophoresis under denaturing conditions and analyzed by immunoblotting with the monoclonal anti-MBP antibody (New England BioLabs, Beverly, MA) and polyclonal anti-GST antibody Z-5 (Santa Cruz Biotechnology, Santa Cruz, CA).
Preparation of S. pombe extracts and EMSAs.
S. pombe cells were grown to an A600 of ∼1.0 at 30°C. Cells were broken with glass beads in extraction buffer [200 mM Tris-HCl (pH 8.0), 400 mM (NH4)2SO4, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 7 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride] in the presence of a protease inhibitor cocktail (P-8340; Sigma) followed by centrifugation at 10,000 rpm at 4°C for 5 min. Typically, binding reactions contained 35 to 40 μg of whole-cell extract in 1× binding buffer [50 mM Tris-HCl (pH 7.9), 100 mM (NH4)2SO4, 2.5 mM MgCl2, 1.2 mM EDTA, 50 mM NaCl], 1 μg of poly(dI-dC)2, and ∼1.0 ng of radiolabeled probe in a final reaction volume of 20 μl. Reactions were incubated at room temperature for 30 min, and the protein-DNA complexes were resolved by gel electrophoresis (3 h at 40 V) on 4% polyacrylamide gels (acrylamide/bisacrylamide ratio, 37.5:1) in 0.25× Tris-borate at 4°C. After electrophoresis, the gels were fixed, dried, and subjected to PhosphorImager analysis. For reaction mixtures containing a purified recombinant Php2, the conditions were identical, except that the protein was present at ∼200 ng. For reaction mixtures containing affinity-purified MBP-Fep1 (∼200 ng), expression of the MBP-Fep1 fusion protein in E. coli, preparation of extracts, and electrophoretic mobility shift assays (EMSAs) were carried out as described previously (43). Furthermore, purified proteins were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting as described previously (43). Oligonucleotides 5′-AGCTTAATCCCGTTGACCAATCAAAAGGCTTCT-3′ and 5′-CTAGAGAAGCCTTTTGATTGGTCAACGGGATTA-3′ were complementary strands derived from the CCAAT box region of the pcl1+ promoter that had 5′-AGCT and 5′-CTAG overhangs after annealing. Likewise, 5′CTAGACTTAGATCAGATATAATTTAATCGTATCTCTTTATCAGATTAAAAC-3′ and 5′TCGAGTTTTAATCTGATAAAGAGATACGATTAAATTATATCTGATCTAAGT-3′ were complementary oligonucleotides, except that they were derived from the GATA box region of the php4+ promoter and they had 5′-CTAG and 5′-TCGA overhangs after annealing. Once annealed, DNA probes were end labeled with [α-32P]dCTP (6,000 Ci/mmol) (Perkin Elmer, Boston, MA) with the Klenow fragment. When indicated, cold competitors to the concentrations specified in Fig. 6 and 9 were added together with the probe.
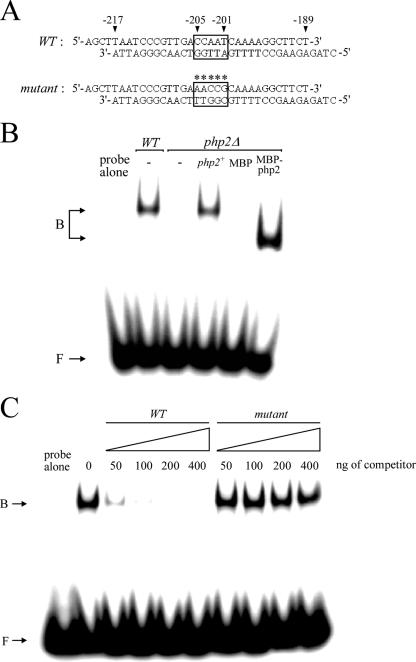
FIG. 6.
The php2+ gene product is required for the DNA-binding activity of the CCAAT-binding factor. (A) Sequences of the synthetic oligomers used. The box indicates the wild-type element CCAAT of the pcl1+ promoter, whereas the asterisks indicate that the element contains five substitutions. The nucleotide numbers refer to the position relative to the A of the initiator codon of the pcl1+ open reading frame. (B) DNA mobility shift assays were carried out with DNA-binding reaction mixtures containing the radiolabeled wild-type CCAAT box-containing probe (as shown in panel A) incubated with S. pombe extracts prepared from the wild-type (WT) strain or a php2Δ mutant strain. An empty vector (−) or a wild-type copy of the php2+ gene was reintegrated (php2+) and tested for the ability to restore DNA-binding activity to php2Δ extracts. Purified recombinant MBP or MBP-2Php270 was included in the DNA-binding reaction mixtures prepared with php2Δ extracts. The positions of the CCAAT-binding complexes are indicated on the left (B). (C) Electrophoretic mobility-shifted gel of a representative competition experiment using whole-cell extracts prepared from strain FY435 (php2+). Competition was performed with double-stranded DNA unlabeled oligomers corresponding to wild-type (WT) and mutant elements. The amount of competitor used in each reaction is shown over the lanes, and the probe concentration was 1 ng/reaction. B, bound probe DNA; F, free probe DNA.
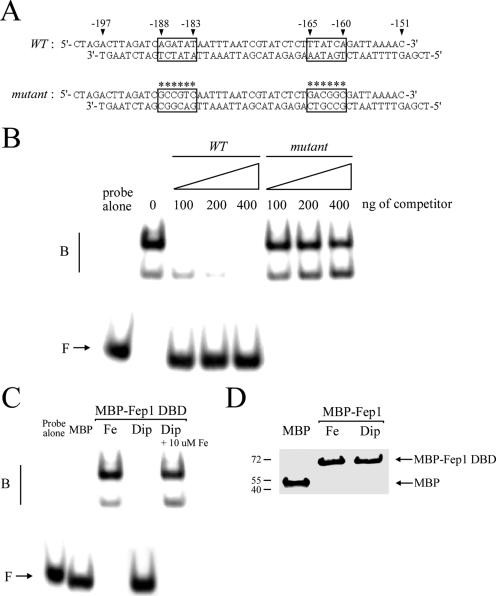
FIG. 9.
Fep1 interacts with the php4+ GATA sequences in an iron-dependent manner. (A) Sequences of the synthetic oligomers used that are derived from the php4+ promoter. The wild-type (WT) GATA elements are boxed, whereas boxes marked with asterisks indicate that each of the two GATA elements contains six substitutions (mutant). The nucleotide numbers refer to the position relative to the A of the initiator codon of the php4+ open reading frame. (B) Representative EMSA analysis using affinity-purified MBP-2Fep1241. MBP-2Fep1241 was produced and isolated from iron-treated (100 μM FeCl3) E. coli cells. Binding reactions containing the indicated amount of competitor were carried out with wild-type (WT) or mutant unlabeled oligomers. B, bound probe DNA; F, free probe DNA. (C) MBP-2Fep1241 fusion protein purified from E. coli cultures grown in the presence of 100 μM FeCl3 (Fe) or 250 μM Dip (Dip) was analyzed by gel retardation using the wild-type GATA probe. For the chelated MBP-2Fep1241 fusion peptide, its DNA-binding activity was restored by exogenous FeCl3 (10 μM Fe) (last lane). As a control, EMSA was conducted using chromatographic fractions prepared from E. coli cells expressing MBP alone. (D) Affinity-purified MBP and MBP-2Fep1241 proteins used above for panel C were analyzed by immunoblotting with anti-MBP antibody. MBP-2Fep1241 was detected with an apparent molecular mass of ∼69.5 kDa, whereas MBP fused to α peptide displays a faster electrophoretic mobility, with a molecular mass of ∼50.0 kDa.
RESULTS
pcl1+, sdh4+, and isa1+ mRNA levels are repressed under conditions of iron deprivation.
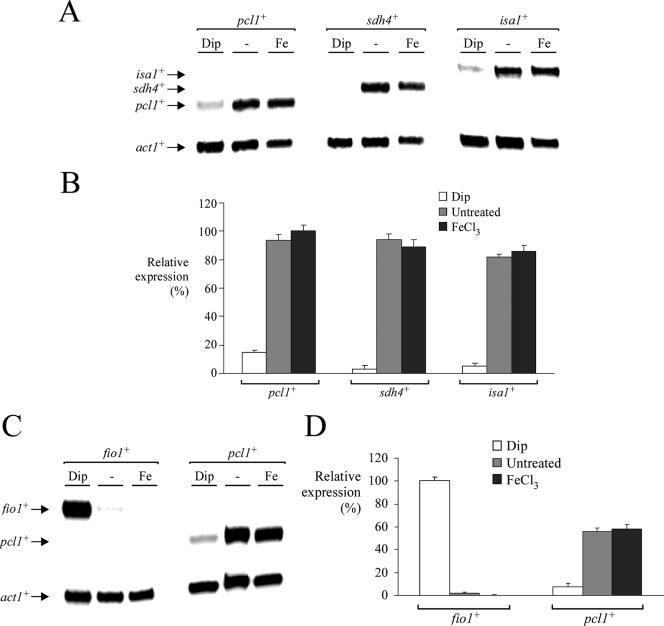
In S. pombe, previous studies have revealed that genes encoding components of the reductive (e.g., fip1+, fio1+, and frp1+) and nonreductive (e.g., str1+, str2+, and str3+) iron transport systems are induced by iron starvation and repressed by iron repletion (43, 44). However, little is known of the expression profiles of genes involved in storage and utilization of iron. To investigate the latter aspect, we characterized the regulation of genes encoding proteins that may play a role in iron detoxification (pcl1+), the TCA cycle (sdh4+), and Fe-S cluster assembly (isa1+) as a function of iron availability. Pcl1 (for “pombe Ccc1-like” protein) exhibits homology to S. cerevisiae Ccc1 from residues 25 to 69 (62% similarity) and from residues 149 to 231 (50% similarity). These two regions encompass five putative transmembrane domains (called the DUF125 module). Pcl1 and Ccc1 are the only known proteins of S. pombe and S. cerevisiae, respectively, that belong to the DUF125 transmembrane protein family. Importantly, as observed for the intracellular iron transporter encoded by the CCC1 gene in S. cerevisiae, deletion of the pcl1+ gene in S. pombe renders cells sensitive to iron compared with the wild-type strain (A. Mercier, B. Pelletier, and S. Labbé, unpublished data). Clearly, this result reveals that Pcl1 plays a role in protecting cells against iron toxicity. Examination of the S. pombe genome database suggests that the open reading frame SPBP23A10.16 encodes a putative ortholog of S. cerevisiae Sdh4. Both proteins are predicted to be structurally related, with 35% sequence homology with each other. Based on that observation, it was inferred from homology that the S. pombe Sdh4 protein represents the mitochondrial membrane anchor subunit of the succinate dehydrogenase, which is a key iron-sulfur protein of the TCA cycle. Excluding the mitochondrial targeting sequence, the S. pombe Isa1 protein shows 71% and 64% sequence similarity to S. cerevisiae Isa1 and E. coli IscA, respectively. Furthermore, it has been demonstrated that S. pombe Isa1 is implicated in iron-sulfur cluster assembly paths (59). We found that in the presence of the iron chelator Dip, pcl1+, sdh4+, and isa1+ mRNA levels were strongly repressed, by ∼7-, ∼33-, and ∼17-fold, respectively, compared with their basal levels of expression observed in untreated cells as assayed by RNase protection (Fig. 1A and B). Conversely, under iron-replete conditions, transcription from pcl1+, sdh4+, and isa1+ remained approximately equal to those observed in untreated cells (Fig. 1A and B). As expected, fio1+ mRNA levels (assayed as a control) were up- and down-regulated after treatment with Dip and iron, respectively (Fig. 1C and D). These results reveal that the pcl1+, sdh4+, and isa1+ genes, encoding proteins that may function in sequestration and utilization of iron, are regulated by iron-limiting conditions in a direction opposite that of the iron transport genes.
FIG. 1.
pcl1+, sdh4+, and isa1+ gene expression is down-regulated under conditions of iron depletion. (A) Wild-type strain FY435 was grown to mid-logarithmic phase in yeast extract plus supplements. Total RNA from Dip (250 μM), control (−), or FeCl3 (Fe) (100 μM) cultures was isolated. Shown is a representative RNase protection assay of pcl1+, sdh4+, isa1+, and act1+ (as control) mRNA steady-state levels. Results shown are representative of three independent experiments. (B) Quantification of lacZ levels after treatments shown in panel A. Values are the averages of triplicate determinations ± standard deviations. (C) Representative RNase protection assay of fio1+ (used as a control gene known to be induced under conditions of iron starvation) and pcl1+ mRNA steady-state levels. (Bottom) act1+ mRNA as an internal control. Total RNA was extracted from aliquots of cultures incubated in the absence (−) or presence of 250 μM Dip or 100 μM FeCl3 (Fe) for 90 min at 30°C. (D) Graphic representation of quantification of three independent RNase protection assays, including the experiment shown in panel C. The values are the means of three replicates ± standard deviations.
Identification of the cis-acting element necessary for activation of pcl1+ gene expression under basal and iron-replete conditions.
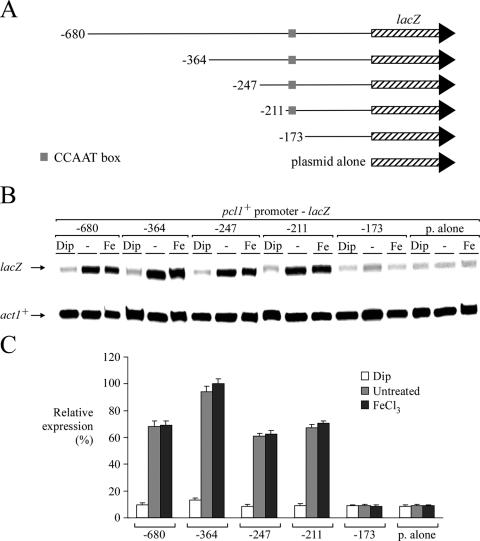
In order to determine the regulatory sequence responsible for the iron-replete-mediated expression of pcl1+, sdh4+, and isa1+, we examined several regions of the pcl1+ promoter as well as the 3′ untranslated region of the pcl1+ gene. To ascertain whether the pcl1+ promoter was involved in pcl1+ regulation in response to changes in iron levels, a series of pcl1+ promoter deletion mutants were fused upstream of and in-frame to the lacZ gene in pSP1-lacZ (43). Gene expression from these plasmids was analyzed by RNase protection experiments. As shown in Fig. 2, removal of DNA sequences between −680 and −211 of the pcl1+ promoter had no effect on the iron-regulatable expression of pcl1+-lacZ fusions. In the presence of Dip, plasmids pSP1pcl1+-680lacZ, pSP1pcl1+-364lacZ, pSP1pcl1+-247lacZ, and pSP1pcl1+-211lacZ were still repressed by approximately sevenfold compared with the basal level of expression observed during testing under both untreated and iron-replete conditions (Fig. 2). Furthermore, for all of the above-mentioned pcl1+-lacZ promoter derivatives, basal and elevated iron concentrations resulted in high constitutive levels of pcl1+-lacZ mRNA expression (Fig. 2). When the pcl1+ promoter was deleted to position −173, iron-regulatable expression was completely abolished. Furthermore, truncation to position −173 also abolished the highly expressed steady-state level of pcl1+-lacZ mRNA in the presence of iron, lowering its expression to a minimal threshold (Fig. 2).
FIG. 2.
Analysis of pcl1+ promoter sequences required to activate gene expression under basal and iron-replete conditions. (A) Schematic representation of nested 5′ deletions of pcl1+ promoter sequences. Nucleotide numbers refer to positions relative to the initiator codon of the pcl1+ gene. The gray box represents the wild-type CCAAT sequence, and the hatched box indicates the lacZ gene. (B) Total RNA was isolated from transformants of strain FY435 harboring the indicated pcl1+-lacZ promoter derivatives, and steady-state mRNA levels of lacZ and act1+ (indicated with arrows) were analyzed by RNase protection experiments. Where indicated, cells were untreated (−) or treated with Dip (250 μM) or FeCl3 (100 μM). Data illustrated are representative of three independent experiments. (C) The graph indicates the normalized expression levels of pcl1+-lacZ mRNA. The values represent averages of three separate determinations ± standard deviations.
On the other hand, we also analyzed wild-type pcl1+ containing its own 3′ untranslated region and pcl1+ coding sequence with its 3′ untranslated region replaced by the CYC1 3′ region. Both constructs were expressed under the control of the wild-type pcl1+ promoter and both were down-regulated (approximately sevenfold) under low-iron conditions (Mercier et al., unpublished). Although we have identified a putative S. pombe ortholog to Cth2, designated Zfs1 (encoded by the locus SPBC1718.07c), we determined that zfs1+ mRNA is not regulated by iron addition or depletion (Mercier et al., unpublished). Furthermore, we ascertained that iron deprivation-repressed pcl1+ expression is independent of zfs1+ (Mercier et al., unpublished). Total RNA isolated from an isogenic pair of strains, one carrying an inactivated copy of zfs1+ and the other harboring a wild-type copy of the gene, showed no apparent effect on pcl1+ transcript levels or its regulation by iron availability. Consistently, inspection of the pcl1+ 3′ untranslated region did not allow the identification of putative AU-rich elements (47).
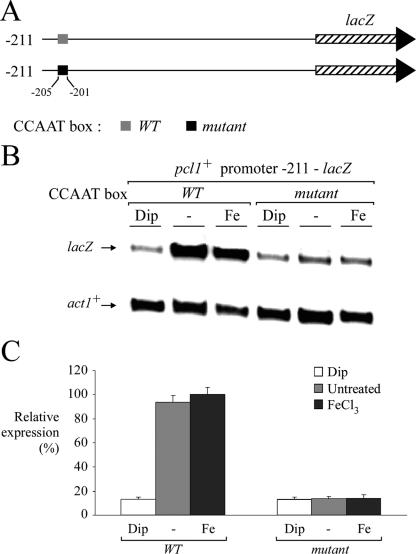
Because of the observation that the presence of the promoter region between −211 and −173 was required for governing iron-replete-mediated expression of the pcl1+-lacZ fusion gene, we examined whether a pcl1+ promoter segment including this region could contain a cis-acting element responsible for such iron-regulatable gene expression. Interestingly, the pcl1+ promoter region between −205 and −201 harbors a copy of the sequence CCAAT that is identical to the binding site for the CCAAT-binding transcription factor (30). To examine whether this CCAAT sequence could mediate gene expression as a function of iron availability, we inserted multiple point mutations that mimic changes known to abolish binding of the CCAAT-binding factor to CCAAT boxes (31). Mutation of the base pairs within the−205CCAAT−201 element (AACCG instead of CCAAT) dramatically reduced the steady-state level of pcl1+-211lacZ mRNA under both basal and iron-replete conditions (Fig. 3). Furthermore, there was a complete lack of iron responsiveness of the reporter gene (Fig. 3). In contrast, the wild-type pcl1+-211lacZ fusion promoter segment was readily expressed from control (untreated) or iron-treated cells, with lacZ mRNA down-regulation (approximately sevenfold) seen only in iron-limited cells (Fig. 3). Furthermore, the iron limitation-dependent down-regulation expression observed with the plasmid pSP1pcl1+-211lacZ was virtually identical to that observed for the endogenous pcl1+ gene (Mercier et al., unpublished).
FIG. 3.
Mutagenesis of the pcl1+ promoter CCAAT sequence abrogates repression under conditions of iron deprivation. (A) Diagrammatic representation of the pcl1+ promoter, indicating the position of the CCAAT sequence relative to the initiator codon of pcl1+. The gray box depicts the wild-type CCAAT sequence, whereas the filled box represents its mutant version, AACCG. (B) Cultures of the wild-type strain FY435 transformed with pSP1pcl1+-211lacZ or pSP1pcl1+-211mutantlacZ were incubated in the absence (−) or presence of Dip (250 μM) or FeCl3 (100 μM) for 90 min. After total RNA extraction, the lacZ and act1+ steady-state mRNA levels were analyzed by RNase protection assays. (C) The histogram shows the normalized expression levels of pcl1+-211lacZ and pcl1+-211mutantlacZ mRNAs. Values are the averages of triplicate measurements ± standard deviations.
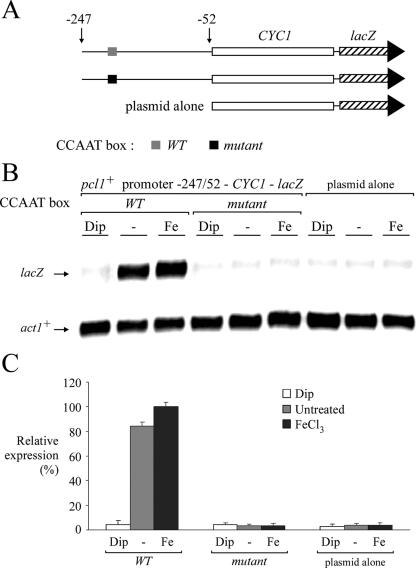
To ascertain whether the CCAAT sequence could regulate a heterologous reporter gene in an iron-dependent manner, a short 195-bp DNA segment derived from the pcl1+ promoter (positions −247 to −52) was inserted in its natural orientation upstream of the minimal promoter of the CYC1 gene fused to lacZ in pCF83 (25). This promoter fusion was able to activate lacZ mRNA expression under both standard (untreated) and iron-replete conditions. Conversely, under conditions of iron starvation, lacZ mRNA expression was strongly repressed (∼20- to 23-fold) compared with the level of transcript detected from either control (untreated) or iron-treated cells (Fig. 4). For the −247pcl1+−52-CYC1-lacZ fusion reporter derivative, the integrity of the CCAAT sequence was essential because a CCAAT box mutant abrogates any expression and regulation in response to changes in iron levels (Fig. 4). Consistently, CCAAT boxes were also identified in the sdh4+ and isa1+ promoters. The sdh4+ promoter region contains three putative CCAAT sequences (positions −1037 to −1033, −820 to −816, and −451 to −447), whereas the 5′ flanking region of isa1+ harbors four putative CCAAT sequences (positions −1098 to −1094, −1007 to −1003, −762 to −758, and −207 to −203). To ascertain whether some of these elements play a role in sdh4+ and isa1+ regulation as a function of iron availability, we constructed CYC1 minimal promoter-lacZ fusion genes harboring either a 229-bp fragment from the sdh4+ promoter that contains one CCAAT element (positions −451 to −447) or the same fragment in which the CCAAT element has been mutagenized. Likewise, we constructed CYC1 minimal promoter-lacZ fusion genes harboring either a 187-bp fragment from the isa1+ promoter that contains one CCAAT element (positions −207 to −203) or the same fragment in which the CCAAT box has been mutated. As shown in Fig. S1 in the supplemental material, CYC1-lacZ expression from the wild-type reporter plasmids was expressed in the presence of iron and down-regulated in the presence of Dip. When we inserted multiple point mutations that mimic changes known to abolish binding of CCAAT-binding factor to CCAAT sequences in both elements within the sdh4+ and isa1+ promoters, a low and constitutive basal level of expression was observed. In fact, there was a complete lack of either down- or up-regulation of the sdh4+-CYC1-lacZ and isa1+-CYC1-lacZ fusions. Collectively, these results indicate that CCAAT sequences in pcl1+, sdh4+, and isa1+ promoters are required to confer iron-regulated pcl1+, sdh4+, and isa1+ gene expression.
FIG. 4.
The pcl1+ promoter CCAAT element regulates the heterologous minimal promoter CYC1-lacZ as a function of iron availability. (A) Schematic representation of the plasmid derivatives assayed in the RNase protection assay. The gray box indicates the wild-type CCAAT element, whereas the filled box represents its mutant version (AACCG). (B) The steady-state levels of CYC1-lacZ mRNA from the wild-type and mutant CCAAT fusions were analyzed in the absence (−) or presence of Dip (250 μM) or FeCl3 (100 μM). CYC1-lacZ and act1+ (as control) mRNA levels are indicated with arrows. (C) Reporter gene activity values are the averages of triplicate determinations ± standard deviations.
pcl1+, sdh4+, and isa1+ genes require the Php2 subunit of the heteromeric CCAAT-binding factor for expression under conditions of iron availability.
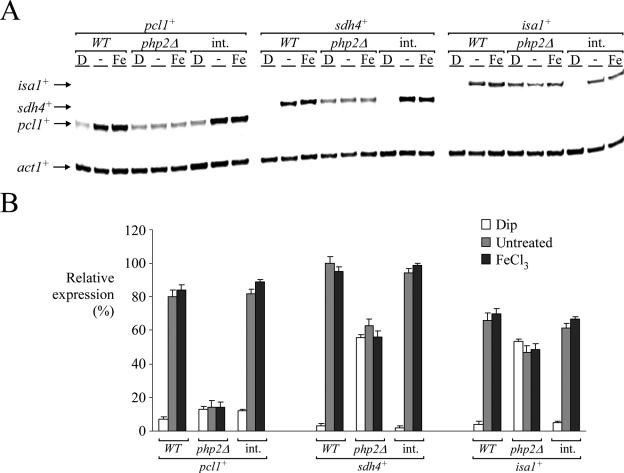
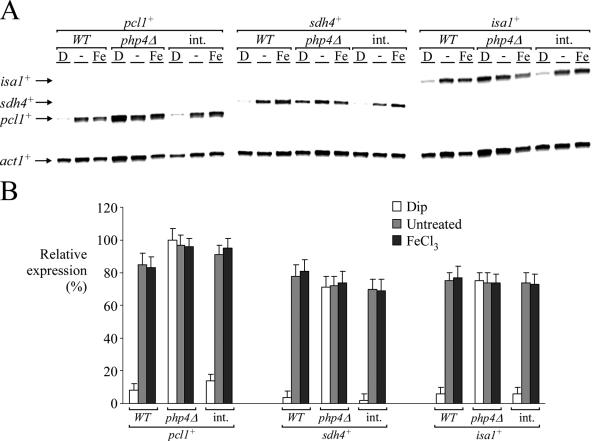
Based on the gene expression data we obtained, we predicted that the CCAAT-binding complex of S. pombe, which contains products of the php2+, php3+, and php5+ genes, may be required to regulate the expression of pcl1+. To test this hypothesis, and because Php2, Php3, and Php5 are essential for the formation of the CCAAT-binding complex in S. pombe (38), we first deleted the php2+ gene (php2Δ). pcl1+ mRNA was barely detected in php2Δ cells and not regulated by iron deprivation (Fig. 5). Importantly, iron limitation-dependent down-regulation of pcl1+ gene expression was corrected by integrating a php2+ allele expressed from its own promoter (Fig. 5). As a control, under conditions of iron deficiency, pcl1+ expression was found to be reduced (approximately sevenfold) compared with the basal level of pcl1+ transcript detected in untreated wild-type cells (php2+) (Fig. 5). Cells harboring an inactivated php2+ gene (php2Δ) also failed to mediate iron starvation-dependent repression of sdh4+ and isa1+. Interestingly, in php2Δ cells the magnitude of the steady-state basal level of sdh4+ or isa1+ mRNA was greater than that observed for pcl1+. This suggests the presence of additional transcription factors for basal gene expression of sdh4+ and isa1+. As shown in Fig. 5, the analysis of sdh4+ and isa1+ mRNA levels in a strain expressing php2+ showed complete repression of both sdh4+ and isa1+ expression in the presence of the iron chelator Dip. In php2Δ cells, sdh4+ or isa1+ gene expression was not completely repressed after treatment with 250 μM Dip. This observation clearly suggests a requirement for the Php2/Php3/Php5 complex to repress gene expression during iron deficiency. Consistently, mutations in the php3+- and php5+-encoded CCAAT-binding proteins were phenocopies of php2Δ (Mercier et al., unpublished). Taken together, these data establish a requirement for the presence of the CCAAT-binding complex to control the level of pcl1+, sdh4+, and isa1+ expression as a function of iron availability.
FIG. 5.
Disruption of the php2+ gene decreases gene expression levels and renders cells unable to down-regulate pcl1+, sdh4+, and isa1+ mRNA levels in response to iron starvation. (A) pcl1+, sdh4+, isa1+, and act1+ mRNA steady-state levels (indicated with arrows) were determined in a wild-type strain of S. pombe (WT) and an S. pombe php2Δ disruption strain in which an empty vector or a wild-type copy of the php2+ gene was reintegrated (int.). Results shown are representative of three independent experiments. (B) Quantification of pcl1+, sdh4+, and isa1+ mRNA levels after the treatments shown in panel A. The values are the averages of triplicate determinations ± standard deviations.
The pcl1+ promoter CCAAT element is recognized by the CCAAT-binding complex.
To further investigate the function of the CCAAT-binding factor in this regulatory pathway, an oligomer spanning the CCAAT box derived from the pcl1+ promoter (Fig. 6A) was end labeled and used in an EMSA with extracts derived from php2+ wild-type and php2Δ cells and from php2Δ cells expressing a php2+ that was reintegrated within the genome. As shown in Fig. 6B, the pcl1+ promoter CCAAT element formed a DNA-protein complex when extracts were prepared from php2+ wild-type cells, whereas the extracts prepared from the php2Δ deletion strain showed loss of the complex. When extracts were prepared from php2Δ cells in which a wild-type copy of php2+ was returned by integration, a DNA-protein complex was formed and had an electrophoretic mobility similar to that observed with extracts derived from wild-type cells. Together, the results suggest that Php2 is necessary for the assembly and DNA-binding activity of the DNA-protein complex. To determine whether CCAAT-binding activity could be reconstituted by the addition of recombinant Php2 to php2Δ extracts, we expressed a conserved core domain of Php2 (residues 2 to 70) fused to MBP in E. coli cells. The polypeptide was purified to near homogeneity by two rounds of one-step affinity chromatography based on MBP affinity for maltose. The fusion protein was used in a mobility shift assay with extracts prepared from the php2Δ deletion strain. As shown in Fig. 6B, the addition of MBP-2Php270 fusion protein, but not MBP alone, reconstituted DNA-binding activity. To determine the specificity of the DNA-protein complex formation, we carried out competition experiments with unlabeled oligomers using either wild-type CCAAT or CCAAT with multiple point mutations within the 33-bp DNA fragment specified in Fig. 6A. Formation of the DNA-protein complex was inhibited by incubation with excess wild-type oligomer but not by the mutant competitor, indicating that the complex was formed by sequence-specific interactions (Fig. 6C). Taken together, these data indicate that the Php2 N-terminal 70 amino acid residues contain CCAAT recognition and subunit association domains and, therefore, are likely required for CCAAT-dependent transcription of pcl1+.
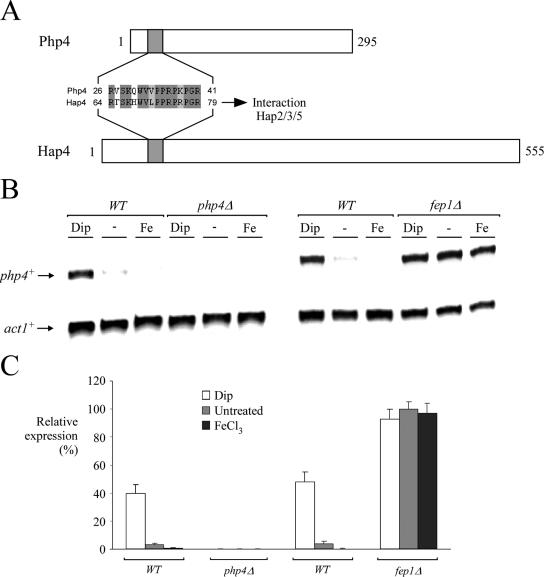
Php4 is required for down-regulation of pcl1+, sdh4+, and isa1+ mRNA levels under low-iron conditions.
In S. cerevisiae, a feature of the CCAAT-binding factor is the presence of a fourth subunit in addition to subunits 2, 3, and 5. This fourth subunit of the complex, denoted Hap4, is down-regulated in the presence of glucose and induced in its absence, while the expression of HAP2 (subunit 2), HAP3 (subunit 3), and HAP5 (subunit 5) is constitutive (13). Although it has been observed that Hap4-like proteins exist in other fungi, the sequence homology between these proteins is very limited (37, 56). Analysis of genomic DNA sequence from the S. pombe Genome Project revealed one locus (SPBC16E9.01c) that encodes a putative Hap4 homolog, which we have termed Php4. Although Php4 exhibits only 8.5% identity on the amino acid level with the Hap4 sequence from S. cerevisiae, one conserved motif found in Php4 (residues 26 to 41), which corresponds to residues 64 to 79 of Hap4, is strongly conserved (see Fig. 8A). This 16-amino-acid domain is known in S. cerevisiae to be essential for the interaction of Hap4 with the CCAAT-binding complex that is composed of Hap2, Hap3, and Hap5 (37). Interestingly, using the cross-linking agent EGS, we found that the Php4 protein associates with the Php2/Php3/Php5 complex (Mercier et al., unpublished). To investigate the role of Php4 in fission yeast, we deleted the php4+ gene (php4Δ). Inactivation of php4+ gave rise to a sustained level of pcl1+, sdh4+, and isa1+ gene expression without any change in response to iron starvation (Fig. 7). On the contrary, php4Δ cells in which a wild-type php4+ allele was reintegrated regained the capacity to down-regulate pcl1+, sdh4+, and isa1+ gene expression when cells were grown during iron starvation (Fig. 7). These data strongly suggest that repression of S. pombe iron metabolic genes during iron deficiency depends on the presence of Php4.
FIG. 8.
php4+ gene expression is regulated by cellular iron status, and a functional fep1+ gene is required for its iron-mediated repression. (A) Common primary structural features of the Php4 and Hap4 proteins. The top of the panel shows a schematic representation of the Php4 protein. The gray box indicates the location of a putative domain for interaction with the Php2/Php3/Php5 complex. The bottom part of the panel is a schematic representation of the primary structure of the Hap4 protein. Hap4 has a domain (gray box) known to be important for association with the Hap2/Hap3/Hap5 heterotrimer. (B) php4+ transcripts in wild-type strain FY435 (WT) are down-regulated in the presence of 100 μM FeCl3 and up-regulated under conditions of iron deficiency (250 μM Dip). In an isogenic fep1Δ strain, the constitutive steady-state levels of php4+ mRNA are unaffected by exogenous Dip (250 μM) or FeCl3 (100 μM). No php4+ transcript was observed in the disruption strain (php4Δ). The php4+ and act1+ mRNA steady-state levels are indicated with arrows. The results shown are representative of three independent experiments. (C) Quantification of php4+ levels after the treatments shown in panel B. The values are the averages of triplicate determinations ± standard deviations.
FIG. 7.
The php4+ gene is required for iron limitation-dependent down-regulation of pcl1+, sdh4+, and isa1+ transcripts. (A) Mid-logarithmic-phase cultures of isogenic strains FY435 (php4+) and AMY15 (php4Δ) were grown in the presence of FeCl3 (0 and 100 μM) or under conditions of iron deprivation (250 μM Dip) at 30°C. Fifteen-milliliter samples were taken after 90 min of treatment. RNA was extracted from each sample and analyzed by RNase protection assays. mRNA steady-state levels of pcl1+, sdh4+, isa1+, and act1+ (indicated with arrows) were analyzed with respect to the php4+ allele status. As a positive control, php4Δ cells were also transformed with an integrating plasmid (int.) expressing S. pombe php4+ under the control of its own promoter and assayed for iron limitation-dependent repression of specific mRNAs (pcl1+, sdh4+, isa1+, and act1+) under the same conditions. (B) Graphic representation of quantification of three independent RNase protection assays, including the experiment shown in panel A.
Given these results, we analyzed steady-state mRNA levels of php2+, php3+, php5+, and php4+ as a function of iron availability in the presence of glucose. php2+, php3+, and php5+ steady-state mRNA levels were found to be constitutive and unresponsive to cellular iron status (Mercier et al., unpublished). To determine whether the php4+ gene has a similar pattern of expression, we carried out RNase protection experiments to detect php4+ mRNA from untreated cells or from S. pombe cells incubated in the presence of 250 μM Dip or 100 μM FeCl3. As shown in Fig. 8B, php4+ transcript levels were barely detectable from iron-replete cells. In contrast, in cells treated with Dip, php4+ steady-state mRNA levels were increased approximately fivefold over basal levels detected in untreated cells. Consistently, php4+ mRNA was absent in php4Δ cells. Because php4+ was repressed in the presence of exogenous iron, we carried out RNase protection assays with wild-type and fep1Δ cells to ascertain whether Fep1 plays a role in php4+ gene regulation. In wild-type cells, php4+ mRNA levels were clearly derepressed in the presence of 250 μM Dip. However, in the presence of 100 μM FeCl3, the steady-state levels of php4+ were down-regulated (Fig. 8B and C). Interestingly, in a fep1Δ strain, php4+ mRNA was highly expressed and unresponsive to iron for repression (Fig. 8B and C). Taken together, these data reveal that Fep1 is required for iron-mediated repression of php4+ gene expression, which itself is involved in repressing pcl1+, sdh4+, and isa1+ gene expression under conditions of iron deprivation.
Iron-induced Fep1 binding to the php4+ GATA elements.
Based on the iron-dependent regulation of php4+ gene expression we obtained (Fig. 8 and Fig. S2 in the supplemental material), we predicted that Fep1 interacts with the sequences −188AGATAT−183 and −165TGATAA−160 found in the php4+ promoter. To determine whether such interaction occurs, we produced the N-terminal 241 amino acids of Fep1 fused to MBP in E. coli cells grown in the presence of either 100 μM FeCl3 or 250 μM Dip. Subsequent to its purification, the recombinant Fep1 fusion protein was used for binding analyses. As shown in Fig. 9 by a representative EMSA, the wild-type 32P-end-labeled 51-bp php4+ promoter fragment, which contains the above-mentioned GATA sequences, forms a DNA-protein complex in the presence of metallated Fep1. To ascertain the specificity of this complex formation, we carried out competition experiments with unlabeled oligomers using either wild-type GATA or GATA with multiple point mutations within the 51-bp DNA fragment (Fig. 9A). Formation of the DNA-protein complexes was inhibited by incubation with excess wild-type oligomer but not by the mutant competitor, indicating that the complexes were formed by sequence-specific interactions (Fig. 9B). To test whether Fep1 binds to GATA elements derived from the php4+ promoter in an iron-dependent manner, EMSAs were conducted using iron- and Dip-treated purified protein preparations. As shown in Fig. 9C, the 32P-end-labeled double-stranded php4+ oligomer formed a complex with MBP-Fep1 when the fusion protein was purified from iron-treated E. coli extracts. In contrast, no complex was formed when the purified fusion protein was isolated from Dip-treated E. coli extracts (Fig. 9C). Furthermore, a reconstitution experiment was carried out to determine the ability of iron to restore MBP-Fep1 GATA-binding activity. Dip-treated MBP-Fep1 peptide was incubated with 10 μM FeCl3 for 30 min. A 20-μl portion of the treated sample was assayed by gel shift retardation assay. As shown in Fig. 9C (last lane), Fep1 GATA-binding activity was restored by iron. The fusion proteins from iron- and Dip-treated cells were probed with monoclonal antibodies against MBP to confirm their presence in the chromatographic fractions (Fig. 9D). Taken together, these results indicate that the N-terminal 241 amino acids of Fep1 associate with GATA promoter elements from php4+ in an iron-mediated manner.
DISCUSSION
In this paper, we present a novel homeostatic mechanism whereby the fission yeast S. pombe regulates gene expression in response to iron deficiency. We identified three genes encoding putative iron-containing proteins (pcl1+, sdh4+, and isa1+) that are down-regulated at the mRNA level in response to iron depletion. Our results showed that the pcl1+, sdh4+, and isa1+ promoters require a CCAAT-type regulatory sequence for expression and regulation by iron starvation. Given the fact that multiple point mutations within the pcl1+, sdh4+, and isa1+ promoter CCAAT-type sequences abolished iron starvation-dependent repression of pcl1+, sdh4+, and isa1+, we tested the possibility that the CCAAT-binding complex of S. pombe plays a role in the regulation of pcl1+, sdh4+, and isa1+ gene expression. Indeed, we demonstrated that cells bearing a disruption of php2+, which encodes one subunit of the CCAAT-binding factor, are defective in the regulation of pcl1+, sdh4+, and isa1+ mRNA levels. To further understand how the S. pombe CCAAT-binding factor functions to regulate target gene expression in response to iron deprivation, we looked for clues about a potential mechanism. First, we found similar levels of php2+, php3+, and php5+ transcripts in low- and high-iron-grown cells of S. pombe, indicating that transcription of php2+, php3+, and php5+ is independent of cellular iron status. Second, we observed no variation of Php2, Php3, and Php5 protein steady-state levels in cells grown in the absence or presence of iron (Mercier et al., unpublished). Third, because EMSAs using extracts from Dip-treated, control, or iron-treated cells revealed the presence of a specific CCAAT-protein complex (Mercier et al., unpublished), we concluded that the DNA-binding activity of the S. pombe CCAAT-binding factor was insensitive to iron depletion. Thus, the question of how lack of iron within cells affects the CCAAT-binding complex was still an enigma. From there, we hypothesized that this might occur through an upstream component that could produce an alteration in activity of the Php2/Php3/Php5 heterotrimer. Based on a report that a putative homologue of S. cerevisiae Hap4 had been identified in the genome of S. pombe (56), we tested the possibility that php4+ plays a role in the regulation of pcl1+, sdh4+, and isa1+ gene expression during iron deficiency. php4+ encodes a protein of 295 amino acids harboring a conserved 16-amino-acid region, 26RVSKQWVVPPRPKPGR41. The conserved motif that is present in Hap4 is known to be required for its association with Hap2/Hap3/Hap5. Although Php4 bears this N-terminal 16-amino-acid motif found within S. cerevisiae Hap4 as well as other Hap4 homologues (55, 56), the rest of Php4 exhibits a limited overall sequence homology with Hap4 (8.5% identity, 12.8% similarity). Furthermore, four notable differences exist between the S. pombe Php4 and S. cerevisiae Hap4 products. First, under glucose conditions, php4+ mRNA is readily detectable in fission yeast. Although php4+ mRNA was unregulated by cellular iron status, under glucose conditions and in the absence of Fep1, its expression was clearly observed. This contrasts with the situation in S. cerevisiae, where the HAP4 gene has been shown to be repressed in the presence of glucose and derepressed when cells are grown on nonfermentable carbon sources (16). Second, in S. pombe, inactivation of php4+ (php4Δ) does not affect the ability of cells to grow on nonfermentable carbon sources (Mercier et al., unpublished), while S. cerevisiae hap4Δ deletion cells are defective in growth on nonfermentable carbon sources (16). Third, in S. pombe, we observed that induction of cyc1+ expression by a shift to a nonfermentable carbon source is independent of Php4 (Mercier et al., unpublished). In S. cerevisiae cells containing a hap4 null allele, genes involved in mitochondrial electron transport such as CYC1 are not induced when cells are shifted from glucose to a nonfermentable carbon source (16). Fourth, the S. pombe Php4 protein is predicted at neutral pH to have a net charge of +9 (pI = 9.9) without any indication of an acidic cluster region. As opposed to this situation, the S. cerevisiae Hap4 protein is quite hydrophilic, with a predicted isoelectric point of 5.2. Furthermore, two regions capable of stimulating transcription have been mapped within Hap4, one between residues 359 and 476 and the other between residues 124 and 329 (55). Taken together, these differences led us to hypothesize that the Php4 protein in S. pombe may play a regulatory function different from that of the S. cerevisiae Hap4 protein.
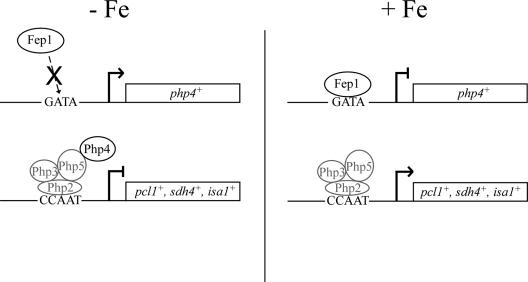
Consistent with a role for Php4 as a regulatory protein in iron-regulated gene expression, we determined that its expression is under the control of the iron-regulatory transcription factor Fep1. We identified two GATA-like elements (positions −188 to −183 and −165 to −160) in the php4+ promoter that can be specifically bound by Fep1. Furthermore, as previously observed for the interaction between Fep1 and fio1+ promoter GATA sequences (43), Fep1 interacts with the php4+ promoter GATA elements in an iron-dependent manner. Based on our previous (43) and current observations, we propose a model for the regulation of genes encoding iron-containing proteins during iron deficiency (Fig. 10). In the absence of iron, an inactivated Fep1 fails to bind GATA elements in the php4+ promoter. The Php4 protein is synthesized and associates with the Php2/Php3/Php5 heterotrimer already present on the promoters of genes required for iron utilization. As a result, the Php2/Php3/Php5/Php4 complex blocks target gene expression, presumably to avoid a futile expenditure of energy in producing iron-using proteins that lack the necessary cofactor (iron) to function. On the other hand, under conditions of iron excess, Fep1 interacts with GATA elements in the php4+ promoter, repressing php4+ gene expression. Lack of Php4 allows the CCAAT-binding Php2/Php3/Php5 heterotrimer to activate gene expression. Recently, using DNA microarrays, Lan et al. (27) have demonstrated that the Candida albicans HAP43 gene (orf19.8298/orf9.681), which encodes a putative transcriptional regulator orthologous to S. pombe Php4, is regulated in response to iron status in the same manner as php4+. Furthermore, it has been shown that the iron-responsive transcriptional control of HAP43 is mediated by the C. albicans Fep1 ortholog, Sfu1 (27). These findings with C. albicans support our results that Php4 is an iron-regulated protein. Furthermore, this also suggests that C. albicans may have a closer relationship to S. pombe than to S. cerevisiae with respect to the ability to establish and maintain normal iron homeostasis.
FIG. 10.
Proposed transcriptional mechanism for regulation of genes encoding iron-using proteins as a function of iron availability in fission yeast. In the absence of iron, Fep1 is inactive and fails to repress php4+ gene expression. The Php4 that is synthesized forms a complex with Php2, Php3, and Php5, which represses expression of genes encoding iron-using proteins. In contrast, in the presence of iron, Fep1 interacts with GATA elements in the php4+ promoter to inactivate transcription. This inactivation of php4+ enables iron-using proteins to be expressed via the Php2/Php3/Php5 heterotrimeric CCAAT-binding factor.
All organisms share a requirement for the regulation of iron metabolism during iron deficiency. In bacteria, a posttranscriptional down-regulation of iron-using pathways, which depends on small noncoding regulatory RNAs, has been uncovered (35, 57). In S. cerevisiae, two transcription factors, Aft1 and Aft2, are iron sensors that activate genes encoding components of iron transport systems in response to iron deprivation (7, 50, 51, 61, 62). Cth2, an RNA-binding protein, is also synthesized when the availability of iron is limited. Induction of Cth2 is mediated by increased transcript levels, and the Aft1 and Aft2 transcription factors play an essential role in this process (47). In response to low environmental iron levels, Puig et al. (47) have shown that Cth2 binds to specific mRNAs and triggers them for degradation. The AU-rich elements through which Cth2 exerts its function are found in the 3′ untranslated regions of targeted mRNAs, which encode several iron-containing components, including enzymes involved in the TCA cycle, Fe-S cluster assembly, and heme biosynthesis (47). Although we have found a potential S. pombe ortholog to Cth2, named Zfs1, its deletion had no apparent effect on pcl1+, sdh4+, and isa1+ transcript levels or their regulation by iron availability. Thus, this suggested that S. pombe has a distinct regulator for iron limitation-dependent down-regulation of gene expression.
Optimizing the utilization of limited available iron represents a critical challenge for all organisms, and it is therefore not surprising that various solutions have evolved. The results presented here describe a novel regulatory mechanism that governs cellular metabolism during iron deficiency in S. pombe. The question of whether a similar basic mechanism exists in other organisms represents a pertinent area for future investigation.
Supplementary Material
Acknowledgments
We are grateful to Kevin A. Morano for critically reading the manuscript and for valuable suggestions. We thank Éric Massé for advice regarding the manuscript.
A.M. and B.P. are recipients of studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds de la Recherche en Santé du Québec (FRSQ), respectively. This work was supported by NSERC grant 238238-01 to S.L. S.L. is a Scholar from the FRSQ.
Footnotes
Published ahead of print on 8 September 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast, a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Askwith, C., D. Eide, A. Van Ho, P. S. Bernard, L. Li, S. Davis-Kaplan, D. M. Sipe, and J. Kaplan. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Askwith, C., and J. Kaplan. 1997. An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J. Biol. Chem. 272:401-405. [DOI] [PubMed] [Google Scholar]
- 4.Beaudoin, J., and S. Labbé. 2001. The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J. Biol. Chem. 276:15472-15480. [DOI] [PubMed] [Google Scholar]
- 5.Beaudoin, J., A. Mercier, R. Langlois, and S. Labbé. 2003. The Schizosaccharomyces pombe Cuf1 is composed of functional modules from two distinct classes of copper metalloregulatory transcription factors. J. Biol. Chem. 278:14565-14577. [DOI] [PubMed] [Google Scholar]
- 6.Bezanilla, M., S. L. Forsburg, and T. D. Pollard. 1997. Identification of a second myosin-II in Schizosaccharomyces pombe: Myp2p is conditionally required for cytokinesis. Mol. Biol. Cell 8:2693-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaiseau, P. L., E. Lesuisse, and J. M. Camadro. 2001. Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J. Biol. Chem. 276:34221-34226. [DOI] [PubMed] [Google Scholar]
- 8.Bourgarel, D., C. C. Nguyen, and M. Bolotin-Fukuhara. 1999. HAP4, the glucose-repressed regulated subunit of the HAP transcriptional complex involved in the fermentation-respiration shift, has a functional homologue in the respiratory yeast Kluyveromyces lactis. Mol. Microbiol. 31:1205-1215. [DOI] [PubMed] [Google Scholar]
- 9.Cottarel, G., D. Beach, and U. Deuschle. 1993. Two new multi-purpose multicopy Schizosaccharomyces pombe shuttle vectors, pSP1 and pSP2. Curr. Genet. 23:547-548. [DOI] [PubMed] [Google Scholar]
- 10.Dancis, A. 1998. Genetic analysis of iron uptake in the yeast Saccharomyces cerevisiae. J. Pediatr. 132:S24-S29. [DOI] [PubMed] [Google Scholar]
- 11.Dancis, A., R. D. Klausner, A. G. Hinnebusch, and J. G. Barriocanal. 1990. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dancis, A., D. G. Roman, G. J. Anderson, A. G. Hinnebusch, and R. D. Klausner. 1992. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc. Natl. Acad. Sci. USA 89:3869-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 14.Eide, D. J. 1998. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu. Rev. Nutr. 18:441-469. [DOI] [PubMed] [Google Scholar]
- 15.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 16.Forsburg, S. L., and L. Guarente. 1989. Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev. 3:1166-1178. [DOI] [PubMed] [Google Scholar]
- 17.Georgatsou, E., and D. Alexandraki. 1994. Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas, H. 2003. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 62:316-330. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell, B., and J. M. Gutteridge. 1992. Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lett. 307:108-112. [DOI] [PubMed] [Google Scholar]
- 20.Hentze, M. W., M. U. Muckenthaler, and N. C. Andrews. 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285-297. [DOI] [PubMed] [Google Scholar]
- 21.Iwaki, T., and K. Takegawa. 2004. A set of loxP marker cassettes for Cre-mediated multiple gene disruption in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 68:545-550. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, J., D. McVey Ward, R. J. Crisp, and C. C. Philpott. 2006. Iron-dependent metabolic remodeling in S. cerevisiae. Biochim. Biophys. Acta 1763:646-651. [DOI] [PubMed] [Google Scholar]
- 23.Keeney, J. B., and J. D. Boeke. 1994. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 136:849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosman, D. J. 2003. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 47:1185-1197. [DOI] [PubMed] [Google Scholar]
- 25.Labbé, S., M. M. O. Peña, A. R. Fernandes, and D. J. Thiele. 1999. A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. J. Biol. Chem. 274:36252-36260. [DOI] [PubMed] [Google Scholar]
- 26.Labbé, S., Z. Zhu, and D. J. Thiele. 1997. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J. Biol. Chem. 272:15951-15958. [DOI] [PubMed] [Google Scholar]
- 27.Lan, C. Y., G. Rodarte, L. A. Murillo, T. Jones, R. W. Davis, J. Dungan, G. Newport, and N. Agabian. 2004. Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53:1451-1469. [DOI] [PubMed] [Google Scholar]
- 28.Lesuisse, E., S. A. Knight, M. Courel, R. Santos, J. M. Camadro, and A. Dancis. 2005. Genome-wide screen for genes with effects on distinct iron uptake activities in Saccharomyces cerevisiae. Genetics 169:107-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesuisse, E., and P. Labbé. 1989. Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 135:257-263. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani, R. 1998. A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res. 26:1135-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani, R. 1999. The molecular biology of the CCAAT-binding factor NF-Y. Gene 239:15-27. [DOI] [PubMed] [Google Scholar]
- 32.Martins, L. J., L. T. Jensen, J. R. Simon, G. L. Keller, and D. R. Winge. 1998. Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J. Biol. Chem. 273:23716-23721. [DOI] [PubMed] [Google Scholar]
- 33.Massé, E., and M. Arguin. 2005. Ironing out the problem: new mechanisms of iron homeostasis. Trends Biochem. Sci. 30:462-468. [DOI] [PubMed] [Google Scholar]
- 34.Massé, E., F. E. Escorcia, and S. Gottesman. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 17:2374-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massé, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massé, E., C. K. Vanderpool, and S. Gottesman. 2005. Effect of RyhB small RNA on global iron use in Escherichia coli. J. Bacteriol. 187:6962-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNabb, D. S., and I. Pinto. 2005. Assembly of the Hap2p/Hap3p/Hap4p/Hap5p-DNA complex in Saccharomyces cerevisiae. Eukaryot. Cell 4:1829-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNabb, D. S., K. A. Tseng, and L. Guarente. 1997. The Saccharomyces cerevisiae Hap5p homolog from fission yeast reveals two conserved domains that are essential for assembly of heterotetrameric CCAAT-binding factor. Mol. Cell. Biol. 17:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNabb, D. S., Y. Xing, and L. Guarente. 1995. Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev. 9:47-58. [DOI] [PubMed] [Google Scholar]
- 40.Myers, A. M., A. Tzagoloff, D. M. Kinney, and C. J. Lusty. 1986. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene 45:299-310. [DOI] [PubMed] [Google Scholar]
- 41.Olesen, J. T., J. D. Fikes, and L. Guarente. 1991. The Schizosaccharomyces pombe homolog of Saccharomyces cerevisiae HAP2 reveals selective and stringent conservation of the small essential core protein domain. Mol. Cell. Biol. 11:611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olesen, J. T., and L. Guarente. 1990. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 4:1714-1729. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier, B., J. Beaudoin, Y. Mukai, and S. Labbé. 2002. Fep1, an iron sensor regulating iron transporter gene expression in Schizosaccharomyces pombe. J. Biol. Chem. 277:22950-22958. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier, B., J. Beaudoin, C. C. Philpott, and S. Labbé. 2003. Fep1 represses expression of the fission yeast Schizosaccharomyces pombe siderophore-iron transport system. Nucleic Acids Res. 31:4332-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelletier, B., A. Trott, K. A. Morano, and S. Labbé. 2005. Functional characterization of the iron-regulatory transcription factor Fep1 from Schizosaccharomyces pombe. J. Biol. Chem. 280:25146-25161. [DOI] [PubMed] [Google Scholar]
- 46.Philpott, C. C. 2006. Iron uptake in fungi: a system for every source. Biochim. Biophys. Acta 1763:636-645. [DOI] [PubMed] [Google Scholar]
- 47.Puig, S., E. Askeland, and D. J. Thiele. 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120:99-110. [DOI] [PubMed] [Google Scholar]
- 48.Roman, D. G., A. Dancis, G. J. Anderson, and R. D. Klausner. 1993. The fission yeast ferric reductase gene frp1+ is required for ferric iron uptake and encodes a protein that is homologous to the gp91-phox subunit of the human NADPH phagocyte oxidoreductase. Mol. Cell. Biol. 13:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouault, T. A. 2004. Pathogenic bacteria prefer heme. Science 305:1577-1578. [DOI] [PubMed] [Google Scholar]
- 50.Rutherford, J. C., S. Jaron, E. Ray, P. O. Brown, and D. R. Winge. 2001. A second iron-regulatory system in yeast independent of Aft1p. Proc. Natl. Acad. Sci. USA 98:14322-14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutherford, J. C., S. Jaron, and D. R. Winge. 2003. Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J. Biol. Chem. 278:27636-27643. [DOI] [PubMed] [Google Scholar]
- 52.Schrettl, M., G. Winkelmann, and H. Haas. 2004. Ferrichrome in Schizosaccharomyces pombe—an iron transport and iron storage compound. Biometals 17:647-654. [DOI] [PubMed] [Google Scholar]
- 53.Skaar, E. P., M. Humayun, T. Bae, K. L. DeBord, and O. Schneewind. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626-1628. [DOI] [PubMed] [Google Scholar]
- 54.Stearman, R., D. S. Yuan, Y. Yamaguchi-Iwai, R. D. Klausner, and A. Dancis. 1996. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271:1552-1557. [DOI] [PubMed] [Google Scholar]
- 55.Stebbins, J. L., and S. J. Triezenberg. 2004. Identification, mutational analysis, and coactivator requirements of two distinct transcriptional activation domains of the Saccharomyces cerevisiae Hap4 protein. Eukaryot. Cell 3:339-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sybirna, K., B. Guiard, Y. F. Li, W. G. Bao, M. Bolotin-Fukuhara, and A. Delahodde. 2005. A new Hansenula polymorpha HAP4 homologue which contains only the N-terminal conserved domain of the protein is fully functional in Saccharomyces cerevisiae. Curr. Genet. 47:172-181. [DOI] [PubMed] [Google Scholar]
- 57.Wilderman, P. J., N. A. Sowa, D. J. FitzGerald, P. C. FitzGerald, S. Gottesman, U. A. Ochsner, and M. L. Vasil. 2004. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA 101:9792-9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkelmann, G. 2002. Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30:691-696. [DOI] [PubMed] [Google Scholar]
- 59.Wu, G., S. S. Mansy, C. Hemann, R. Hille, K. K. Surerus, and J. A. Cowan. 2002. Iron-sulfur cluster biosynthesis: characterization of Schizosaccharomyces pombe Isa1. J. Biol. Inorg. Chem. 7:526-532. [DOI] [PubMed] [Google Scholar]
- 60.Xing, Y., J. D. Fikes, and L. Guarente. 1993. Mutations in yeast HAP2/HAP3 define a hybrid CCAAT box binding domain. EMBO J. 12:4647-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi-Iwai, Y., A. Dancis, and R. D. Klausner. 1995. AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J. 14:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi-Iwai, Y., R. Stearman, A. Dancis, and R. D. Klausner. 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 15:3377-3384. [PMC free article] [PubMed] [Google Scholar]
- 63.Znaidi, S., B. Pelletier, Y. Mukai, and S. Labbé. 2004. The Schizosaccharomyces pombe corepressor Tup11 interacts with the iron-responsive transcription factor Fep1. J. Biol. Chem. 279:9462-9474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.