Abstract
An Aspergillus nidulans mutation, designated nmdA1, has been selected as a partial suppressor of a frameshift mutation and shown to truncate the homologue of the Saccharomyces cerevisiae nonsense-mediated mRNA decay (NMD) surveillance component Nmd2p/Upf2p. nmdA1 elevates steady-state levels of premature termination codon-containing transcripts, as demonstrated using mutations in genes encoding xanthine dehydrogenase (hxA), urate oxidase (uaZ), the transcription factor mediating regulation of gene expression by ambient pH (pacC), and a protease involved in pH signal transduction (palB). nmdA1 can also stabilize pre-mRNA (unspliced) and wild-type transcripts of certain genes. Certain premature termination codon-containing transcripts which escape NMD are relatively stable, a feature more in common with certain nonsense codon-containing mammalian transcripts than with those in S. cerevisiae. As in S. cerevisiae, 5′ nonsense codons are more effective at triggering NMD than 3′ nonsense codons. Unlike the mammalian situation but in common with S. cerevisiae and other lower eukaryotes, A. nidulans is apparently impervious to the position of premature termination codons with respect to the 3′ exon-exon junction.
The process of nonsense-mediated decay (NMD), whereby an mRNA containing a premature translation termination codon resulting from a frameshift or “nonsense” mutation is preferentially degraded, has been investigated in a variety of eukaryotic organisms (reviewed in references 1, 2, 17, 21, 26-28, 40, and 47). The role of this process lies not only in its ability to preclude the synthesis of incomplete proteins but also in its involvement in regulating the levels of many wild-type transcripts (20, 24, 43, 48). Although the proteins involved in NMD vary from organism to organism, a complex consisting of Upf1, Nmd2/Upf2, and Upf3 (where Upf abbreviates up-frameshift) plays a central role in every case (see the reviews mentioned above). Nmd2 acts as a bridge between Upf1 and Upf3, and regions important for the interactions have been determined in all three proteins of Saccharomyces cerevisiae (19). The interaction between the human Upf2 and Upf3 proteins has been characterized structurally (23). Here we describe a loss-of-function mutation in the gene encoding the orthologue of the Nmd2/Upf2 protein in the ascomycete Aspergillus nidulans and establish isofunctionality by showing that it can affect both the steady-state level and the half-life of mRNA containing premature translation termination codons. We also show that this mutation can increase the stability of pre-mRNA (unspliced) and also that of certain wild-type mRNAs. This extends the range of organisms in which NMD has been identified to filamentous fungi. Of particular interest is the finding that, in at least one respect, NMD in A. nidulans appears to be more similar than NMD in S. cerevisiae to NMD in mammalian cells.
MATERIALS AND METHODS
A. nidulans strains and genetic techniques.
Aspergillus nidulans strains carried markers in standard use (9, 30). Standard genetic techniques (8, 39) were used. Growth media were as described by Cove (11).
Selection of nmdA1.
The nmdA1 mutation was selected, after UV mutagenesis in a strain of genotype inoB2 pacC+/−230 fwA1, as largely alleviating the molybdate hypersensitivity due to pacC+/−230 (36) on appropriately supplemented glucose-minimal medium containing 25 mM sodium molybdate and 5 mM ammonium (+)-tartrate as nitrogen source.
Cloning of nmdA.
As the A. nidulans genome sequence was not yet available, nmdA was cloned using standard transformation procedures (44) and linkage group I-containing cosmids from a chromosome-allocated cosmid library (4) in combination with the argB+-containing plasmid pILJ16 (22). Arginine-independent transformants were selected in a strain of genotype nmdA1 argB2 pantoB100 on regeneration medium containing 10 mM NaNO3 as a nitrogen source, and nmdA+ transformants were recognized directly as having a wild-type growth rate and morphology.
Transcript analysis.
Growth of mycelia, inhibition of transcription, RNA preparation, and quantitative Northern analysis were as described previously (32, 38). For analysis of palB transcript levels, mycelia were harvested after 12 h growth at 37°C with 10 mM NH4+ [as the (+)-tartrate] as a nitrogen source. For analysis of pacC, hxA, and uaZ transcript levels, mycelia were grown for 14 h at either 37°C or 27°C (as indicated) with 10 mM NH4+ [as the (+)-tartrate] as nitrogen source. The mycelia were harvested, washed with medium at the appropriate temperature, and transferred to fresh flasks containing as the sole N source either NH4+ (10 mM) for pacC analysis or uric acid (0.1 mg/ml) for hxA and uaZ analysis. They were then incubated for an additional 2 h prior to transcriptional inhibition and sampling. With the exception of the short time course analyses for uaZ and hxA mRNA, transcription was inhibited with proflavine for 10 min prior to commencement of the time course. For the short time course experiments, proflavine was added 30 seconds prior to taking the first samples. Proflavine has been shown to inhibit transcription of a variety of A. nidulans genes, and its effectiveness has been established by demonstrating that in the presence of cycloheximide, which dramatically reduces transcript degradation, no increase in transcript levels is observed (12, 27, 31, 33, I. Y. Morozov and M. X. Caddick, unpublished data). For Northern blots of pacC, the probe was a fragment of plasmid p4 (44). Probes used for Northern analysis of hxA, uaZ, and palB were produced by PCR, using oligonucleotides CTTTGCGGGTTTCACTCTCGTCAT and CCATTTCGCCGTCTTCTGCTTTAG for hxA, CGCGCATACCAACATCATCACG and GAAGCCCCAGAATTGCGAACC for uaZ, and CCCGGCACGTTCTGGATGG and GGCTGCGGACCCTGGCTATG for palB. As a reference for Northern analysis, the levels of 18S rRNA were determined using a probe constructed by PCR with oligonucleotides GGGGCTCTTTTGGGTCTC and CCATACTCCCCCCAGAAC. For quantitative real-time PCR, DNase I-treated total RNA was reverse transcribed from random hexamer primers by using SuperScript II reverse transcriptase (Invitrogen, Paisley, Scotland, United Kingdom) according to the manufacturer's instructions. PCR was performed using a Rotor-Gene 3000 (Corbett Research Ltd., Cambridge, United Kingdom). Each 10-μl reaction mixture contained 6 μl of the SYBR Green Jump Start (Sigma) PCR mix, cDNA, and two specific oligonucleotides. Unspliced uaZ mRNA was monitored using the forward oligonucleotide CCGCTATGGTAAGGACAATG with TCGGCTTTGGTGTAGCTGTG, which spans the 3′ splice site of the second intron. Unspliced hxA mRNA was monitored using the forward oligonucleotide GTCGTTTCGCAAATCAATCCG with AGAGCGCTAGAGACTCTT, which spans the 3′ splice site of the second intron. These primer pairs gave a positive signal with cDNA and genomic DNA but not the DNase-treated total RNA used for cDNA synthesis. Spliced uaZ mRNA was monitored using the forward oligonucleotide in combination with TCGGCTTTGGTGTAGGAAGT, which spans the processed splice sites of the second intron. Spliced hxA mRNA was monitored using the forward oligonucleotide in combination with AAGAGCGCTAGAGACTCATC, which spans the processed splice sites of the second intron. These primer pairs failed to give any PCR product with genomic DNA or DNase-treated total RNA but gave a robust signal with cDNA. The efficiency of amplification for each set of primers was determined beforehand by measuring the abundance of transcripts from a cDNA dilution series. For loading controls, levels of actin-encoding mRNA were monitored by reverse transcription-PCR (RT-PCR) using oligonucleotides ACCGTATGCAGAAGGAAATC and AAGGACCGCTCTCATCGTA. Efficiencies were computed for each primer set by using REST (37) (http://www.wzw.tum.de/gene-quantification/). Each RNA sample was assayed in triplicate, and RNAs were assayed from three biological repeats. The transcript abundance levels were normalized to actin by using Q-gene software (http://bioinformatics.gene-quantification.info/) (33).
The genotypes of strains used for transcript analysis were yA2 pantoB100 (wild type), uaZ14 pantoB100 (uaZ14), yA2 uaZ14 nmdA1 pantoB100 (uaZ14 nmdA1), yA2 nmdA1 pantoB100 (nmdA1), yA2 hxA1 pantoB100 (hxA1), yA2 nmdA1 hxA1 pantoB100 (hxA1 nmdA1), yA2 hxA5 pantoB100 (hxA5), yA2 nmdA1 hxA5 pantoB100 (hxA5 nmdA1), yA2 hxA18 pantoB100 (hxA18), yA2 nmdA1 hxA18 pantoB100 (hxA18 nmdA1), pantoB100 pacC+/−230 (pacC230), pabaA1 nmdA1 pacC+/−230 (pacC230 nmdA1), inoB2 glrA1 chaA1 palB7 (palB7), nmdA1 inoB2 glrA1 chaA1 palB7 (palB7 nmdA1), pantoB100 palB37 (palB37), yA2 nmdA1 pantoB100 palB37 (palB37 nmdA1), pantoB100 palB38 (palB38), and nmdA1 pantoBl00 palB38 (palB38 nmdA1).
RESULTS AND DISCUSSION
Selection and characterization of the nmdA1 mutation.
The nmdA1 mutation was selected as partially alleviating the hypersensitivity to molybdate toxicity resulting from pacC+/−230. pacC+/−230 is an acidity-mimicking mutation in the transcription factor gene pacC which mediates regulation of gene expression by ambient pH (44). pacC+/−230 strains exhibit poor growth at high pH, reduced alkaline phosphatase activity, and elevated acid phosphatase activity in addition to molybdate hypersensitivity (30, 36). pacC+/−230 is a −1 frameshift mutation resulting in a protein containing the N-terminal 238 residues of the PacC protein followed by 55 out-of-frame residues (30). In addition to its effect on molybdate tolerance in pacC+/−230 strains, nmdA1 partially suppresses the lack of alkaline phosphatase and elevation of acid phosphatase in plate tests. In contrast, it reduces growth of pacC+/−230 strains at pH 8. In a pacC+ background, nmdA1 leads to slow growth, which is considerably exacerbated under alkaline growth conditions such as pH 8 or medium containing nitrate as a nitrogen source.
Parasexual analysis located nmdA1 to chromosome I. From a cross of relevant genotype yA2 niiC628 × biA1 nmdA1, 148 random progeny plus 10 progeny selected as niiC+ nmdA+ by being able to utilize nitrate as a nitrogen source (and thus niiC+) and to grow at a normal rate despite the alkalinization resulting from nitrate reduction (and thus nmdA+) were analyzed. This showed nmdA1 to map at 4.1 cM centromere proximal to niiC628 on the right arm, with the gene order being niiC-nmdA-biA-yA.
Identification of the nmdA gene and the nmdA1 mutation.
Starting with pools of chromosome I-containing cosmids, cosmid W11E02 was identified as containing nmdA1-rescuing activity, which was localized to a 7.5-kb BglII-XhoI fragment with overlapping subclones enabling further localization. Sequencing of the region rescuing nmdA1 revealed a gene homologous to S. cerevisiae NMD2/UPF2. This gene, designated nmdA, corresponds to autocalled gene AN6695.2 in the A. nidulans genome sequence (http://www.broad.mit.edu/annotation/fungi/aspergillus). This homology immediately suggests a possible explanation for the slow-growth phenotype of nmdA1 strains (in addition to problems associated with lack of mRNA surveillance of aberrant and wild-type transcripts), as de Pinto et al. (13) have shown that S. cerevisiae nmd2 null mutations (as well as those in UPF1 and UPF3) impair respiration and A. nidulans is an obligate aerobe.
Figure 1A shows an alignment between NmdA, S. cerevisiae Nmd2p/Upf2p, and their human homologue. By tblastn, NmdA shares 27% identity over 875 residues with S. cerevisiae Nmd2p and 33% identity over 913 residues with human Upf2. The nmdA1 mutation is a C-to-T transition in nucleotide 3065, resulting in a Gln-to-amber stop in codon 589. It is likely to be a null mutation. First, it removes nearly half of the 1,171 residues from the NmdA protein. Second, it removes a majority of one MIF4G domain (residues 506 to 696) and all of a second MIF4G domain (residues 712 to 918) (PFAM domain PF02854; http://www.sanger.ac.uk/Software/Pfam/index.shtml). In this alignment, the first of these two MIF4G domains overlaps a putative transmembrane domain in S. cerevisiae Nmd2p (18, 29), which would be missing in the NmdA1 protein. The second of these MIF4G domains aligns with the third MIF4G domain of human Upf2, which has been shown to bind both human Upf3 and RNA (23). Arg-734 and Lys-735 of NmdA, which are missing in NmdA1, correspond in alignment to Arg-796 and Lys-797 of human Upf2 (Fig. 1A), where their double substitution by glutamate abolishes RNA binding (23). Third, the NmdA1 protein lacks regions corresponding to those in Nmd2p that are implicated in interaction with Upf1p (Nmd2p residues 933 to 1089) and Upf3p (Nmd2p residues 564 to 771 and 879 to 923) (19). Included among the residues missing in NmdA1 is Glu-796, which corresponds in alignment with Glu-858 of human Upf2, whose E858R substitution abolishes its interaction with Upf3 (23). Finally, even deletion of the C-terminal 56 residues of Nmd2p is sufficient to prevent NMD (18), and NmdA1 lacks the equivalent of the C-terminal 631 Nmd2p residues. The slow-growth phenotype of nmdA1 is recessive in diploids, suggesting that the truncated NmdA1 protein itself does not adversely affect NMD or other cellular functions, consistent with the likelihood that it is unable to bind mRNA or the A. nidulans Upf1 and Upf3 orthologues.
FIG.1.
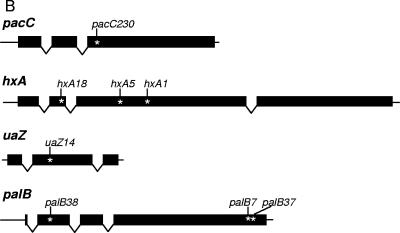
(A) Alignment of NmdA with yeast Nmd2p/Upf2p and human hUpf2 in ClustalW format, obtained by the method of T-Coffee (34), version 2.11 (http://www.ch.embnet.org/software/TCoffee.html). The NmdA sequence shown here is the conceptual translation of AN6695.2 obtained from http://www.broad.mit.edu/annotation/fungi/aspergillus/geneindex.html. The two MIF4G domains (PFAM domain PF02854; http://www.sanger.ac.uk/Software/Pfam/index.shtml) in the A. nidulans sequence, residues 506 to 696 and 712 to 918 are in white and shaded in black. S. cerevisiae residues 564 to 771 and 879 to 923, implicated in interaction with Upf3p, and residues 933 to 1089, implicated in interaction with Upf1p, are shaded in light gray. The nmdA1 Q589* substitution, resulting in loss of function; the hUpf2 E858R substitution, which abolishes hUpf3 binding; and the hUpf2 R796E K797E double substitution, which abolishes RNA binding, are shown. (B) Schematic representation of the pacC, hxA, uaZ, and palB transcription units. Intron positions and sites of translation termination codons for mutations used in this work are indicated.
Effect of nmdA1 on pacC+/−230 mRNA.
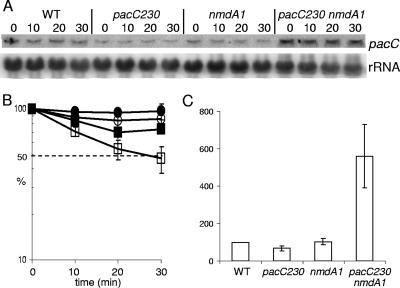
In view of the in vivo suppression of pacC+/−230 by nmdA1, the pacC+/−230 mRNA was an obvious first candidate for detecting an effect of nmdA1 on mRNA stability. A schematic representation of pacC+/−230 and other mutations used to establish the role of nmdA in NMD is shown in Fig. 1B. Figure 2 shows that there is partial stabilization of both pacC+ and pacC+/−230 transcripts by nmdA1. Surprisingly, the pacC+/−230 transcript appeared to be more stable than that of pacC+ in the nmdA+ background. However, the most striking effect of nmdA1 is on the pacC+/−230 mRNA level, which is elevated more than eightfold. This degree of elevation almost certainly explains the selection of nmdA1 and probably reflects a combination of the ability of the PacC230 protein to mimic PacC27 (the activated, processed form of PacC) (14, 30) and the autogenous transcriptional regulation of pacC (44) rather than reflecting uniquely mRNA stabilization. Autogenous activation of pacC expression by processed PacC230 (30) is probably also a factor in determining pacC transcript levels in pacC+/−230 nmdA+ strains. These factors render pacC+/−230 (and many other pacC mutant alleles) poorly suited for studying NMD.
FIG. 2.
Quantitative Northern analysis of pacC transcript levels at 37°C. RNA was extracted from wild-type (WT), pacC+/−230, nmdA1, and pacC+/−230 nmdA1 strains. Transcription was inhibited prior to commencement of the time course, and samples were taken over a 30-min period, as indicated. (A) pacC mRNA levels were monitored by Northern analysis. The phosphorimager data from triplicate experiments were compiled for further analysis. (B) pacC transcript degradation rates for the four strains were plotted. Over the 30-min time course, the WT (pacC+ nmdA+) (□) pacC transcript appeared to be more labile than those of the pacC+ nmdA1 (○), pacC+/−230 nmdA+ (▪), and pacC+/−230 nmdA1 (•) strains. The standard error is given for each value. (C) The relative levels of pacC in the four strains at time zero were determined from these data. Surprisingly, the pacC+/−230 strain had an expression level similar to that of the wild type; however, in the double mutant significantly higher expression levels were observed.
Effects of nmdA1 on premature nonsense codon-containing mRNAs transcribed from mutant alleles of hxA, the structural gene for xanthine dehydrogenase (purine hydroxylase I).
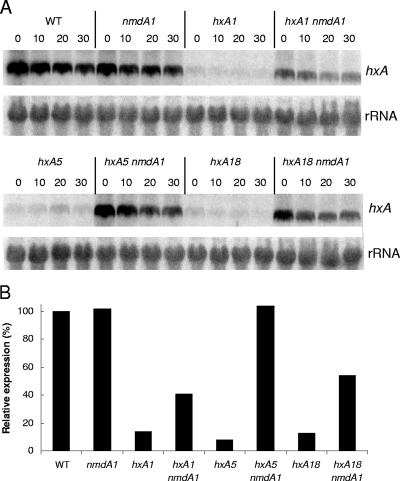
The effects of nmdA1 on messengers from the following hxA alleles were examined: hxA18, which deletes G1061, resulting in a protein with 142 in-frame and 7 out-of-frame residues; hxA5, which is a C1790T transition, truncating the protein after residue 369; and hxA1, which deletes A1953, resulting in a protein of 423 in-frame and 47 out-of-frame residues compared to the wild-type HxA protein of 1,364 residues (6, 16). Translation termination in the case of hxA18 would occur in exon 2, and that in the cases of hxA5 and hxA1 would occur in exon 3. nmdA1 clearly increases mRNA levels for all three hxA mutant alleles, most strikingly in the case of hxA5 (Fig. 3). Although the hxA mutant alleles greatly reduce transcript levels, Northern analysis did not provide sufficient sensitivity to demonstrate the kinetics of transcript degradation in the nmdA+ background, even though steady-state mRNA levels are considerably elevated by the absence of NMD. However, at least in the case of hxA5, kinetic evidence given below indicates that nmdA1 does stabilize the mRNA.
FIG. 3.
Quantitative Northern analysis of hxA mRNA. (A) Strains bearing three hxA mutant alleles (hxA1, hxA5, and hxA18) in both the nmdA+ and nmdA1 backgrounds were compared to the wild type (WT) and the nmdA1 single mutant for hxA transcript levels over a 30-min time course at 37°C after a 2-h induction period using uric acid as the sole nitrogen source. Although dramatic differences in the levels of hxA transcript are apparent, no obvious differences in the rates of transcript degradation were observed. (B) The relative levels of hxA transcript at time zero are presented for each strain. nmdA1 partially or completely restores hxA expression levels for all three alleles.
Effect of nmdA1 on mRNA transcribed from a premature chain termination allele of uaZ, the structural gene for urate oxidase: stability of a transcript escaping NMD, a mammalian similarity.
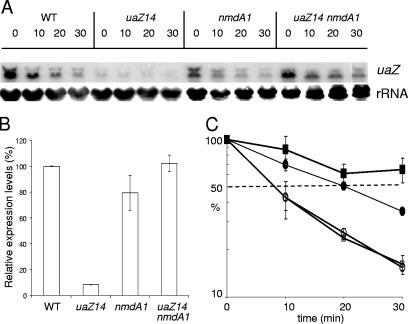
Oestreicher and Scazzocchio (35) showed that uaZ14, an ochre mutation terminating the 301-residue UaZ protein after residue 131 in exon 2, leads to extremely low mRNA levels. Figure 4 shows that nmdA1 has no apparent effect on the stability of uaZ+ mRNA but, nevertheless, increases the steady-state level of uaZ14 mRNA more than 12-fold. In view of the disparities between nmdA1 elevation of mRNA levels and its apparent lack of mRNA stabilization, we considered the possibility that NMD might have occurred in the interval between the inhibition of transcription and sampling. If so, the implication would be that at least some premature nonsense codon-containing mRNAs escape NMD and are relatively stable. Although stability of mRNAs escaping NMD has not, to our knowledge, been reported for yeast (25), it has a precedent from certain transcripts in mammalian cells, where it was originally attributed to an absence of cytoplasmic NMD such that, once the mRNAs have left the nucleus and escaped nuclear NMD, they are relatively stable (7, 41). Alternatively, this mRNA stability might be a consequence of dissociation of the exon junction complex which is required for NMD (2, 40, 42, 45). It is tempting to speculate that this apparently greater similarity of NMD in A. nidulans to that in mammalian cells than to that in yeast is related to a higher frequency of introns and a greater use of alternative splicing and its possible link to gene regulation (24). Differential splicing is a feature, for example, of the A. nidulans areB gene (10). Alternative splicing in fungi has been most recently discussed by Galagan et al. (15).
FIG. 4.
Analysis of uaZ transcript levels at 37°C. RNA was extracted from wild-type (WT), uaZ14, nmdA1, and uaZ14 nmdA1 strains after induction using uric acid as the sole nitrogen source for 2 h at 37°C. Transcription was inhibited for 10 min prior to commencement of the time course, and samples were taken over a 30-min period, as indicated. (A) uaZ levels were monitored by Northern analysis. (B) The relative levels of uaZ transcript in the four strains at time zero were determined using RT-PCR, which was used to monitor specifically the level of spliced mRNA. (C) RT-PCR was also used to monitor degradation rates. uaZ mRNA appears to be significantly more labile in the WT (uaZ+ nmdA+) (□) and uaZ+ nmdA1 (○) strains than in the uaZ14 nmdA+ (▪) and uaZ14 nmdA1 (•) strains. Quantitative analysis of the Northern data gave similar results (data not shown). Standard errors are indicated.
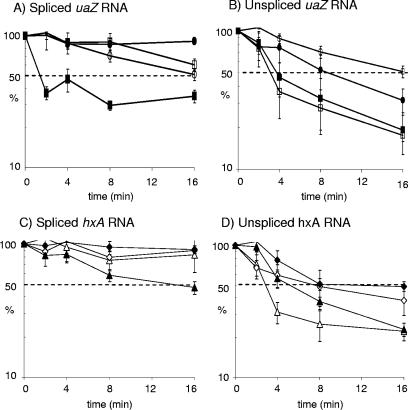
To examine the possibility that NMD had escaped detection by preceding our first sampling, further experiments over a compressed time scale and at a lower growth temperature were performed using uaZ14 and hxA5, and both mRNA and pre-mRNA (unspliced) were monitored using real time RT-PCR (see Materials and Methods). Figure 5A shows that decay of uaZ14 mRNA occurs rapidly. More than half the uaZ14 mRNA is degraded within the first 2 minutes at 27°C, but the remaining transcript is then more stable than the wild-type transcript and is equivalent in stability to the uaZ14 transcript in a strain also carrying nmdA1. Thus, nmdA1 does stabilize the uaZ14 transcript, consistent with the considerable elevation in steady-state uaZ14 mRNA levels seen in the presence of nmdA1. The hxA5 mRNA decays with a half-life of ∼14 min but is stabilized by the presence of nmdA1 (Fig. 5C). In contrast, nmdA1 does not stabilize uaZ+ or hxA+ mRNA levels; however, it does markedly stabilize uaZ+, uaZ14, hxA+, and hxA5 pre-mRNA levels (Fig. 5B and D). Probably relevant to this pre-mRNA stabilization is the presence of three in-frame stop codons in intron l of uaZ (35) and two in-frame stop codons in intron 1 of hxA (16). Pre-mRNA containing an in-frame intron stop codon in S. cerevisiae is subjected to NMD (43; reviewed in reference 26). It is intriguing that both pacC+/−230 and uaZ14 mutant transcripts apparently become more stable than those of the corresponding wild-type transcripts, irrespective of the nmdA genotype. Yeast transcripts with premature stop codons accumulate in polysomes in the absence of NMD (25), which is likely to impede translation generally. Polysome association might account for mutant transcript stabilization (although stabilization of pre-mRNA remains puzzling). NMD might therefore minimize disruption of translation resulting from the accumulation of aberrant transcripts as well as minimizing synthesis of aberrant polypeptides.
FIG. 5.
Short time course analysis of uaZ and hxA transcript levels at 27°C. RNA was extracted from wild-type (WT), uaZ14, nmdA1, uaZ14 nmdA1, hxA5, and hxA5 nmdA1 strains after induction using uric acid as the sole nitrogen source for 2 h at 27°C. Transcription was inhibited for 30 seconds prior to commencement of the time course, and samples were taken over a 16-min period, as indicated. Using RT-PCR, the levels of spliced uaZ (A), unspliced uaZ (B), spliced hxA (C), and unspliced hxA (D) were monitored, relative to actin as a standard. The data were compiled from three separate experiments, and standard errors are indicated. With respect to the spliced transcripts, in the nmdA+ background the uaZ14 mRNA (▪) is very unstable in the first 2 minutes but subsequently stabilizes. In the nmdA1 background the uaZ14 transcript (•) is very stable throughout. The uaZ+ mRNA shows a gradual rate of degradation over the 16-min time course in both the nmdA+ (□) and nmdA1 (○) backgrounds. With respect to the spliced hxA transcripts, in the nmdA+ background the hxA5 mRNA (▴) is significantly less stable than the wild type, with a half-life of ∼14 min, compared to an estimated half-life for the wild type of ∼40 min. In the nmdA1 background the hxA5 transcript (⧫) is very stable throughout. The hxA+ mRNA shows only limited degradation over the 16-min time course in both the nmdA+ (▵) and nmdA1 (⋄) backgrounds. For both unspliced uaZ and hxA transcripts, the nmdA1 background leads to a general increase in stability. Standard errors are indicated.
nmdA1 does not affect l-glutamine-signaled degradation of the mRNA for the nitrogen metabolism regulatory gene areA.
The mRNA for areA, which encodes a transcription factor mediating nitrogen metabolite repression of the expression of many genes involved in nitrogen utilization (reviewed in reference 46), undergoes regulated degradation when l-glutamine is added to cultures (31, 32, 38). A region(s) within the 3′ untranslated region (UTR) of areA mRNA is responsible for this glutamine-signaled degradation (31, 32, 38). Three different nmdA1 strains were compared to an nmdA+ wild-type strain for the effects of l-glutamine on areA mRNA stability at 37°C. However, nmdA1 had no significant effect on the degradation process (data not shown). This shows that NmdA is not involved in all mRNA decay processes in A. nidulans.
NMD in A. nidulans, resembling that in S. cerevisiae, is not influenced by exon-exon junctions.
A characteristic difference between NMD in S. cerevisiae, Schizosaccharomyces pombe, Caenorhabditis elegans, and Drosophila melanogaster and that in mammals is that a premature termination codon fewer than 50 to 55 nucleotides upstream of the 3′ exon-exon junction usually does not trigger NMD in mammalian cells, whereas it does in the lower eukaryotes (reviewed in references 26 and 28). The termination codons resulting from hxA1, hxA5, hxA18, and uaZ14 all occur more than 55 nucleotides upstream of the respective hxA and uaZ 3′ exon-exon junctions. However, the termination codon resulting from pacC+/−230 occurs in the 3′ exon, and there is therefore no exon-exon junction downstream from it (Fig. 1B). Nevertheless, the pacC+/−230 mRNA is clearly stabilized by nmdA1 (Fig. 2). Thus, at least in this case, NMD in A. nidulans resembles that in S. cerevisiae (as well as that in S. pombe, C. elegans, and D. melanogaster) rather than that in mammals.
As it is in yeast, NMD in A. nidulans is polar.
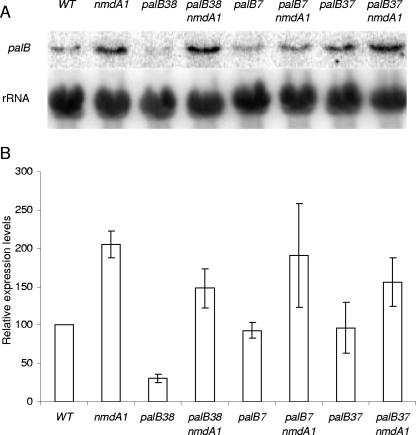
Cao and Parker (5) have shown that 5′ nonsense mutations trigger higher rates of mRNA decapping than 3′ nonsense mutations in S. cerevisiae, thus providing a mechanism to explain why 3′ nonsense mutations can fail to provoke NMD, since 5′-to-3′ exonucleolytic decay is the usual course of NMD. In the faux UTR model for NMD (reviewed in reference 1), the proximity of the premature termination codon to the normal termination codon [and hence to proteins binding the 3′ UTR, such as the poly(A)-binding protein] determines the decapping rate, thus explaining in an alternative way the greater susceptibility of 5′ nonsense codon-containing mRNAs to NMD. Polarity of NMD is also observed in A. nidulans. In the palB gene, encoding the putative signaling protease of the ambient pH signal transduction pathway (14, 36), palB38 in the first 10% of the coding region strongly provokes NMD, whereas palB7 and palB37 in the last 10% do not (Fig. 1B and 6). In contrast to wild-type hxA and uaZ transcripts but like that of pacC, the palB wild-type transcript is stabilized by nmdA1, and this premature termination codon-independent stabilization has to be taken into consideration in assessing the palB mutant transcript data (Fig. 6). The palB7 mutation terminates the 847-residue PalB protein after residue 791 (M. A. Peñalva, as cited in reference 3). Sequence changes resulting from palB37 and palB38 (E. Reoyo, M. A. Peñalva, and H. N. Arst, Jr., unpublished data) will be reported elsewhere. In the cases of two other pH signal transduction genes, palF and palH, Northern blots gave no indication that premature chain termination mutations throughout the coding regions of these genes provoke NMD (data not shown), suggesting that the mRNAs of these genes are not subject to NMD surveillance.
FIG. 6.
Quantitative Northern analysis of palB mRNA. (A) Steady-state palB transcript levels were compared for the wild type (WT) and three loss-of-function alleles, palB7, palB37, and palB38, in both nmdA+ and nmdA1 backgrounds. (B) The relative levels of palB transcript are shown for each strain. The wild-type transcript is stabilized twofold in the presence of nmdA1, and this degree of stabilization (or somewhat less) is also seen for the palB7 and palB37 transcripts. A clearly greater degree of stabilization by nmdA1 is seen in the case of the palB38 transcript, indicating that in this case the premature termination codon provokes NMD. Standard errors are indicated.
Uses of nmdA1.
We believe that nmdA1 constitutes a useful addition to the tools available for working with A. nidulans. It will facilitate further studies of NMD and investigation of the role of the NMD surveillance complex in regulating mRNA levels in this organism. It could potentially enable elevated levels of prematurely terminated homologous or heterologous mutant proteins to be obtained. It can be used to determine whether mRNA instability rather than protein truncation or instability is responsible for a premature termination mutant phenotype and thus to enable more precise interpretation of specific mutant allele phenotypes. We anticipate that tools improving the manipulability of A. nidulans will add to its attractiveness as a model organism.
Acknowledgments
This work was funded by the BBSRC through grant support for I.Y.M. (grant P14059) and a grant to H.N.A. (grant 60/P11494) and by the Wellcome Trust through a grant to H.N.A. and J.T. (grant 067878).
We are very grateful to Claudio Scazzocchio for suggesting the use of and supplying hxA and uaZ mutant alleles for investigating NMD. Helpful suggestions were provided by Miguel Peñalva and three anonymous referees. Valuable technical assistance was provided by Adebola Akintade, Lily Stanton, and Tatiana Munera Huertas.
Footnotes
Published ahead of print on 8 September 2006.
REFERENCES
- 1.Amrani, A., M. S. Sachs, and A. Jacobson. 2006. Early nonsense: mRNA decay solves a translational problem. Nat. Rev. Mol. Cell. Biol. 7:415-425. [DOI] [PubMed] [Google Scholar]
- 2.Behm-Ansmant, I., and E. Izaurralde. 2006. Quality control of gene expression: a stepwise assembly pathway for the surveillance complex that triggers nonsense-mediated mRNA decay. Genes Dev. 20:391-398. [DOI] [PubMed] [Google Scholar]
- 3.Bignell, E., S. Negrete-Urtasun, A. M. Calcagno, K. Haynes, H. N. Arst, Jr., and T. Rogers. 2005. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol. Microbiol. 55:1072-1084. [DOI] [PubMed] [Google Scholar]
- 4.Brody, H. J., J. Griffith, A. J. Cuticchia, M. Arnold, and W. E. Timberlake. 1991. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. Nucleic Acids Res. 19:3105-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, D., and R. Parker. 2003. Computational modeling and experimental analysis of nonsense-mediated decay in yeast. Cell 113:533-545. [DOI] [PubMed] [Google Scholar]
- 6.Charbonnier-Glatigny, A. 1993. Thèse de Doctorat. Université de Paris-Sud (XI), Orsay, France.
- 7.Cheng, J., and L. E. Maquat. 1993. Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol. Cell. Biol. 13:1892-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clutterbuck, A. J. 1974. Aspergillus nidulans, p. 447-510. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 9.Clutterbuck, A. J. 1993. Aspergillus nidulans, p. 3.71-3.84. In S. J. O'Brien (ed.), Genetic maps. Locus maps of complex genomes, 6th ed., Mvol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 10.Conlon, H., I. Zadra, H. Haas, H. N. Arst, Jr., M. G. Jones, and M. X. Caddick. 2001. The Aspergillus nidulans GATA transcription factor gene areB encodes at least three proteins and features three classes of mutations. Mol. Microbiol. 40:361-375. [DOI] [PubMed] [Google Scholar]
- 11.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. [DOI] [PubMed] [Google Scholar]
- 12.Cybis, J., and P. Weglenski. 1972. Arginase induction in Aspergillus nidulans. Eur. J. Biochem. 30:262-268. [DOI] [PubMed] [Google Scholar]
- 13.de Pinto, B., R. Lippolis, R. Castaldo, and N. Altamira. 2004. Overexpression of Upf1p compensates for mitochondrial splicing deficiency independently of its role in mRNA surveillance. Mol. Microbiol. 51:1129-1142. [DOI] [PubMed] [Google Scholar]
- 14.Díez, E., J. Álvaro, E. A. Espeso, L. Rainbow, T. Suárez, J. Tilburn, H. N. Arst, Jr., and M. A. Peñalva. 2002. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 21:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galagan, J. E., M. R. Henn, L.-J. Ma, C. A. Cuomo, and B. Birren. 2005. Genomics of the fungal kingdom: insights into eukaryotic biology. Genome Res. 15:1620-1631. [DOI] [PubMed] [Google Scholar]
- 16.Glatigny, A., and C. Scazzocchio. 1995. Cloning and molecular characterization of hxA, the gene coding for the xanthine dehydrogenase (purine hydroxylase I) of Aspergillus nidulans. J. Biol. Chem. 270:3534-3550. [DOI] [PubMed] [Google Scholar]
- 17.González, C. I., A. Bhattacharya, W. Wang, and S. W. Peltz. 2001. Nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Gene 274:15-25. [DOI] [PubMed] [Google Scholar]
- 18.He, F., A. H. Brown, and A. Jacobson. 1996. Interaction between Nmd2p and Upf1p is required for activity but not for dominant-negative inhibition of the nonsense-mediated decay pathway in yeast. RNA 2:153-170. [PMC free article] [PubMed] [Google Scholar]
- 19.He, F., A. H. Brown, and A. Jacobson. 1997. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol. Cell. Biol. 17:1580-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, F., X. Li, P. Spatrick, R. Casillo, S. Dong, and A. Jacobson. 2003. Genome-wide analysis of mRNAs regulated by the 5′ to 3′ mRNA decay pathways in yeast. Mol. Cell 12:1439-1452. [DOI] [PubMed] [Google Scholar]
- 21.Holbrook, J. A., G. Neu-Yilik, M. W. Hentze, and A. Kulozik. 2004. Nonsense-mediated decay approaches the clinic. Nat. Genet. 36:801-808. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone, I. L., S. G. Hughes, and A. J. Clutterbuck. 1985. Cloning an Aspergillus nidulans developmental gene by transformation. EMBO J. 4:1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadlec, J., E. Izaurralde, and S. Cusack. 2004. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nat. Struct. Mol. Biol. 11:330-337. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, B. P., R. E. Green, and S. E. Brenner. 2003. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA 100:189-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maderazo, A. B., J. P. Belk, F. He, and A. Jacobson. 2003. Nonsense-containing mRNAs that accumulate in the absence of a functional nonsense-mediated mRNA decay pathway are destabilized rapidly upon its restitution. Mol. Cell. Biol. 23:842-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maquat, L. E. 2004. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell. Biol. 5:89-99. [DOI] [PubMed] [Google Scholar]
- 27.Maquat, L. E. 2004. Nonsense-mediated decay: a comparative analysis of different species. Curr. Genomics 5:175-190. [Google Scholar]
- 28.Maquat, L. E. 2005. Nonsense-mediated mRNA decay in mammals. J. Cell Sci. 118:1773-1776. [DOI] [PubMed] [Google Scholar]
- 29.Mendell, J. T., S. M. Medghalchi, R. G. Lake, E. N. Noensie, and H. C. Dietz. 2000. Novel Upf2p orthologues suggest a functional link between translation initiation and nonsense surveillance complexes. Mol. Cell. Biol. 20:8944-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mingot, J.-M., J. Tilburn, E. Diez, E. Bignell, M. Orejas, D. A. Widdick, S. Sarkar, C. V. Brown, M. X. Caddick, E. A. Espeso, H. N. Arst, Jr., and M. A. Peñalva. 1999. Specificity determinants of proteolytic processing of Aspergillus PacC transcription factor are remote from the processing site, and processing occurs in yeast if pH signalling is bypassed. Mol. Cell. Biol. 19:1390-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morozov, I. Y., M. Galbis-Martinez, M. G. Jones, and M. X. Caddick. 2000. A defined sequence within the 3′UTR of the areA transcript is sufficient to mediate nitrogen metabolite repression signalling via accelerated deadenylation. Mol. Microbiol. 37:1248-1257. [DOI] [PubMed] [Google Scholar]
- 32.Morozov, I. Y., M. Galbis-Martinez, M. G. Jones, and M. X. Caddick. 2001. Characterization of nitrogen metabolite signalling in Aspergillus via the regulated degradation of areA mRNA. Mol. Microbiol. 42:269-277. [DOI] [PubMed] [Google Scholar]
- 33.Muller, P. Y., H. Janovjak, A. R. Miserez, and S. Dobbie. 2002. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques 32:1372-1379. [PubMed] [Google Scholar]
- 34.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignments. J. Mol. Biol. 302:205-217. [DOI] [PubMed] [Google Scholar]
- 35.Oestreicher, N., and C. Scazzocchio. 1993. Sequence, regulation and mutational analysis of the gene encoding urate oxidase in Aspergillus nidulans. J. Biol. Chem. 268:23382-23389. [PubMed] [Google Scholar]
- 36.Peñalva, M. A, and H. N. Arst, Jr. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66:426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl, M. W., G. W. Horgan, and L. Demple. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt, A., T. Langdon, H. N. Arst, Jr., D. Kirk, D. Tollervey, J. M. Mates Sanchez, and M. X. Caddick. 1996. Nitrogen metabolite signalling involves the C-terminus and the GATA domain of the Aspergillus transcription factor AREA and the 3′ untranslated region of its mRNA. EMBO J. 15:2791-2801. [PMC free article] [PubMed] [Google Scholar]
- 39.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. Macdonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 40.Singh, G., and J. Lykke-Andersen. 2003. New insights into the formation of active nonsense-mediated decay complexes. Trends Biochem. Sci. 28:464-466. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson, L. S., and L. E. Maquat. 1996. Cytoplasmic mRNA for human triosephosphate isomerase is immune to nonsense-mediated decay despite forming polysomes. Biochimie 78:1043-1047. [DOI] [PubMed] [Google Scholar]
- 42.Tange, T. Ø., A. Nott, and M. J. Moore. 2004. The ever increasing complexities of the exon junction complex. Curr. Opin. Cell Biol. 16:279-284. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, R., B. W. Kabaara, T. Nazarenus, A. Jones, R. Yamanaka, R. Uhrenholdt, J. P. Wendler, and A. L. Atkin. 2005. Gene set coregulated by the Saccharomyces cerevisiae nonsense-mediated mRNA decay pathway. Eukaryot. Cell 4:2066-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Peñalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner, E., and J. Lykke-Andersen. 2002. mRNA surveillance: the perfect persist. J. Cell Sci. 115:3033-3038. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, R. A, and H. N. Arst, Jr. 1998. Mutational analysis of AREA, a transcriptional activator mediating nitrogen metabolite repression in Aspergillus nidulans and a member of the “streetwise” GATA family of transcription factors. Microbiol. Mol. Biol. Rev. 62:586-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilusz, C. J., W. Wang, and S. W. Peltz. 2001. Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev. 15:2781-2785. [DOI] [PubMed] [Google Scholar]
- 48.Wittmann, J., E. M. Hol, and H.-M. Jäck. 2006. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol. Cell. Biol. 26:1272-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]