Abstract
The plasmodial surface anion channel (PSAC), induced on human erythrocytes by the malaria parasite Plasmodium falciparum, is an important target for antimalarial drug development because it may contribute to parasite nutrient acquisition. However, known antagonists of this channel are quite nonspecific, inhibiting many other channels and carriers. This lack of specificity not only complicates drug development but also raises doubts about the exact role of PSAC in the well-known parasite-induced permeability changes. We recently identified a family of new PSAC antagonists structurally related to dantrolene, an antagonist of muscle Ca++ release channels. Here, we explored the mechanism of dantrolene's actions on parasite-induced permeability changes. We found that dantrolene inhibits the increased permeabilities of sorbitol, two amino acids, an organic cation, and hypoxanthine, suggesting a common pathway shared by these diverse solutes. It also produced parallel reductions in PSAC single-channel and whole-cell Cl− currents. In contrast to its effect on parasite-induced permeabilities, dantrolene had no measurable effect on five other classes of anion channels, allaying concerns of poor specificity inherent to other known antagonists. Our studies indicate that dantrolene binds PSAC at an extracellular site distinct from the pore, where it inhibits the conformational changes required for channel gating. Its affinity for this site depends on ionic strength, implicating electrostatic interactions in dantrolene binding. In addition to the potential therapeutic applications of its derivatives, dantrolene's specificity and its defined mechanism of action on PSAC make it a useful tool for transport studies of infected erythrocytes.
Infection of human red blood cells (RBCs) with the malaria parasite Plasmodium falciparum, leads to markedly increased uptake of anions (28), amino acids (6), sugars (21), purines (57), vitamins (50), and precursors for phospholipid biosynthesis (2). Although these permeability changes have been known for more that 50 years (43) and are proposed to function in nutrient acquisition from serum (12), the precise molecular mechanism for uptake remains under debate. One model is that a single unusual ion channel, the plasmodial surface anion channel (PSAC), serves as a common route for the uptake of all these solutes. Because PSAC is likely encoded by the intracellular parasite and has biophysical properties highly conserved in phylogenetically divergent parasites (1, 35), this model proposes that PSAC serves an essential function for the parasite and is an ideal target for antimalarial development.
There are a number of other models for how the permeability changes may be manifest, primarily via the activation of quiescent human channels. One of these alternative models is that there are at least three different human channels activated through nonspecific parasite oxidative stress (16). Other models propose activation through the action of parasite protein kinases (18) or via a link to the human cystic fibrosis transport regulator (CFTR) chloride channel (58).
With most of the models, channels identified with electrophysiological methods have been linked to organic solute uptake via parallel effects of a number of small molecule antagonists including furosemide, 5-nitro-2-3-phenylpropylaminobenzoic acid (NPPB), glybenclamide, and phloridzin. For example, a precise concordance between the dose responses for furosemide inhibition of single-PSAC recording, whole-cell voltage clamp, sorbitol-induced osmotic lysis, and uptake of [14C]lactate supports the model of a single shared channel (1). These same inhibitors have also been used to implicate other putative ion channels seen in electrophysiological recordings in different laboratories.
An important concern is that these four commonly available inhibitors are highly nonspecific. For example, furosemide inhibits multiple co- and counter-transporters and a diverse collection of ion channels (44, 41, 30, 39). NPPB, although primarily used for its effects on anion channels, is also known to inhibit Ca++ channels, K+ channels, gap junctions, and cyclooxygenase (15, 20, 48, 5). Glybenclamide and phloridzin may be even less specific, with known effects on soluble metabolic factors, exocytosis, and even protein-free planar lipid bilayers (7, 56, 49). In light of these highly promiscuous effects, it has been argued that parallel effects on patch-clamp currents and organic solute permeability may be coincidences rather than evidence for a mechanistic link (23). Moreover, in vitro parasite growth inhibition with each of these agents, though providing circumstantial evidence in support of an essential role for parasite-induced channel(s), could simply be due to effects on other parasite activities (11).
Identification of more specific antagonists therefore represents a major hurdle in this field. Although there have been several attempts to find such agents (27, 54), those identified so far are not broadly available, as required for basic research studies. In search of such an antagonist, we screened a library of known channel blockers for their ability to inhibit parasite-induced permeability changes. Dantrolene, a clinically used muscle relaxant that inhibits the sarcoplasmic reticulum Ca++ release channel (ryanodine receptor [RyR]), was the most potent compound identified. This finding was unexpected because RyR is not thought to share functional properties or inhibitory profiles with either PSAC or any of the proposed modified host channels. This observation has already been extended by identifying two dantrolene derivatives with substantial activity against in vitro parasite growth (26). Interestingly, both of these derivatives inhibited PSAC in single-channel and whole-cell patch-clamp recordings.
Although dantrolene may be a starting point for future antimalarial drugs, important unknowns include the range of its effects on the various parasite-induced permeability changes, its precise mechanism of inhibition, and whether it is as promiscuous as other available antagonists. Here, we found that dantrolene inhibits the full spectrum of permeability changes previously attributed to either PSAC or other putative ion channels. Electrophysiological studies revealed direct action on PSAC at an extracellular site distinct from the channel pore. Ionic strength effects on dantrolene's affinity for this site implicate charge-charge interactions in binding and channel inhibition. Using the Xenopus oocyte expression system, we also found that dantrolene has no measurable effect on any of five divergent anion channels from other organisms, achieving a higher level of specificity for PSAC than previously demonstrated. Finally, we used this new and more specific antagonist as a tool to further substantiate a central role of PSAC in the parasite-induced uptake of sugars, amino acids, organic cations, and purines. Such mechanistic studies will be important if therapeutic agents targeting this unusual ion channel are to be successfully developed.
MATERIALS AND METHODS
Osmotic lysis assays.
The kinetics of infected RBC osmotic lysis in solutions of permeant solutes was followed as previously described (59). Trophozoite-infected RBCs were enriched by percoll-sorbitol separation, washed, and suspended at 0.5% hematocrit in test permeant solute with or without candidate antagonist. Each solution had a nominal osmolarity of ∼310 mosM and contained permeant solute supplemented with only 20 mM Na-HEPES and 0.1 mg/ml bovine serum albumin, pH 7.4 (buffer A). The percent transmittance (T) of 700 nm light through this cell suspension was then continuously followed as a marker of osmotic swelling and lysis.
Radioisotope uptake.
Infected RBCs were enriched, washed, and used in uptake of [3H]hypoxanthine (4 μCi/ml) at 5% hematocrit in 150 mM NaCl, 20 mM Na-phosphate, and 150 μM unlabeled hypoxanthine with indicated dantrolene concentrations. Uptake was performed at room temperature and terminated by transfer of 40 μl of cell suspension to 1 ml of tracer-free uptake buffer with 5 mM furosemide and centrifuged at 14,000 × g through dibutyl phthalate. Cell pellets were digested and counted as described previously (13). Measurements were taken in duplicate, and the counts were averaged. Parallel experiments with uninfected RBCs were identically executed.
Electrophysiology.
Patch-clamp of infected RBCs was performed as described previously (1, 12) using bath and pipette solutions as indicated in each figure legend. In addition to physiological or supraphysiological concentrations of charge-carrying ions, all solutions contained 10 mM MgCl2, 5 mM CaCl2, and 20 mM Na-HEPES, pH 7.4 (buffer B). Where used, hypertonic salt solutions were designed to permit detection of currents through single PSAC molecules; hypertonic solutions achieve improved signal-to-noise ratios by permitting higher Cl− permeation rates through this small conductance channel and by lowering access resistance in the small-geometry patch pipettes required for durable seals on human RBCs. Previous studies indicate that these solutions do not measurably alter PSAC gating and power spectra, selectivity, voltage dependence, or inhibition by furosemide and phloridzin (1, 11). Pipettes were pulled from quartz glass to tip diameters of <0.5 μm and resistances of 1 to 4 MΩ. Seal resistances were >100 GΩ. Voltage clamp recordings were obtained with an Axopatch 200B amplifier (Axon Instruments), filtered at 5 kHz with an eight-pole Bessel filter, digitized online at 100 kHz (Digidata 1322A), and recorded with Clampex 9.0 software (Axon Instruments). Whole-cell recordings were similarly acquired after application of brief, high-voltage pulses to achieve rupture of the membrane patch under the pipette tip (12).
In some experiments, we used a specially designed chamber to permit very slow perfusion of the bath around the patch-clamped cell because the fragile seal on human erythrocytes does not tolerate the turbulence invariably associated with rapid bulk perfusion of the bath (1). The design of this chamber permitted complete solution changes in separate troughs without aggressive bulk perfusion. Under these conditions, we could sustain high resistance seals for up to 1 h on single infected erythrocytes.
Analysis.
To determine mean single-channel open probabilities (Po), we pooled between 24 and 65 s of recordings at a membrane potential (Vm) of −100 mV from up to four single-channel patches at each dantrolene concentration in two separate patch-clamp solutions. The analysis used locally developed code that identifies transitions between channel open and closed states by the 50% threshold-crossing technique. The results were then compared to the measured Po in the absence of dantrolene, 43 ± 2% (1).
Because PSAC openings at positive Vm are poorly resolved, we used integration of currents relative to the closed channel baseline to examine the voltage dependence of dantrolene's effects in single-channel recordings. Here, the code determines the mean current at the closed level, subtracts this value from the trace, and then averages the residual current samples at each voltage. This average open-channel current corresponds to the product of the single channel amplitude and the Po. The main advantage of this integration method over the above mid-threshold algorithm is that it does not require definition of open-channel current levels.
Dwell time distributions were obtained with code that uses consecutive midthreshold crossings to determine event durations. These durations were corrected for sampling error through linear interpolation of sampled currents immediately before and after each crossing as described previously (10). We also quantified the nonzero response time of our recording equipment by measuring an overall rise time, Tr, of 66.4 μs, consistent with Gaussian filtering at 5 kHz. Equipment rise times in this range are problematic for very short channel openings or closings (<80 μs), where the numbers of events are underestimated. To correct for this error, we transformed measured event durations with the equation
 |
(1) |
where wo and wt are the true and measured event durations, respectively; a1, a2, and a3 are empirical constants that depend on Tr as 0.5382 × Tr, 0.837 × Tr−2, and 1.120 × Tr−3, respectively (9). Measurements of short events generated with simple resistor capacitor circuits confirmed the validity of this approach with our equipment and filtering conditions (10). Histogram ordinate values were normalized to the percentage of the total number of events detected under each condition and are displayed on a square root-logarithmic plot (53). On these plots, an exponentially decaying probability density function, expected for any kinetic process with a single reversible transition, would be given by the equation
 |
(2) |
where b and c are constants and t is the duration of channel events. Importantly, the peak of f(t) occurs at ln(t) = b, corresponding to the time constant of the kinetic process, τ, through
 |
(3) |
Heterologous expression of chloride channels.
Functional studies of five divergent anion channels expressed in Xenopus oocytes were performed with the two-electrode voltage clamp method (55). CLC-1 and CLC-5 were cloned into the pTLN vector (36) and transcribed from the SP6 RNA promoter. CFTR and MOD-1 (modulation of locomotion chloride channel in Caenorhabditis elegans) were cloned into the pGEM 3Z vector (33) and transcribed from the T7 RNA promoter. For each heterologous channel, oocytes were microinjected with 4 ng of in vitro transcribed cRNA flanked by the 3′ and 5′ untranslated regions of a Xenopus β-globin gene to improve expression (33). After incubation at 18°C for 3 days, electrophysiological recordings were obtained in 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM Na-HEPES, 0.1 mg/ml gentamicin, and 0.55 mg/ml pyruvate, pH 7.4, at 22°C. For endogenous Ca++-activated Cl− channel (eCaCC), uninjected oocytes were preincubated in 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM Na-HEPES, and 0.5 mM EGTA, pH 7.4, with 5 μM A23187 before recording in 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM Na-HEPES, pH 7.4, with either 0.5 mM EGTA or 4 mM CaCl2. For CFTR, currents were activated with 10 μM forskolin and 200 μM IBMX (3-isobutyl-1-methylxanthine).
Oocyte recordings were obtained with a GeneClamp 500 amplifier (Axon Instruments), filtered at 5 kHz, and digitized at 100 kHz. Pulses (50 ms) from a holding potential of −60 mV to voltages between −100 and +100 mV were applied. Effects of 50 μM dantrolene were then evaluated by applying the same voltage protocol after several minutes of perfusion to allow dantrolene access. Because the rapid inactivation of MOD-1 prevents accurate determination of current voltage relationships, we recorded the kinetics of response to 1 μM serotonin at a holding potential of −60 mV in the presence and absence of dantrolene; both the kinetics and the peak currents in response to serotonin were compared to evaluate possible effects of dantrolene. eCaCC undergoes partial inactivation after induction of currents by extracellular addition of Ca++; both peak currents at −60 mV and the subsequent steady-state current-voltage relationships were used to examine possible effects of dantrolene on this channel.
RESULTS
Dantrolene inhibits both sorbitol-induced lysis and PSAC-mediated Cl− currents.
Trophozoite-stage infected RBCs undergo osmotic lysis in isotonic sorbitol with a halftime of 7 to 10 min (Fig. 1 A, upper trace), depending on parasite maturity. This halftime is a quantitative marker of this solute's markedly increased permeability after infection. We used a simple light-scattering assay designed to track lysis of RBCs (59) and found that dantrolene produces a concentration-dependent slowing of sorbitol-induced lysis, with a concentration of 10 μM almost completely abolishing these transmittance changes (bottom trace). Over a range of concentrations, inhibition was adequately fitted by the equation
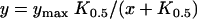 |
(4) |
as expected for a 1:1 interaction between dantrolene and a single passive mechanism of sorbitol uptake. The fitted K0.5 was 1.2 μM (Fig. 1 B).
FIG. 1.
Dantrolene inhibits PSAC-mediated osmotic lysis. (A) Kinetics of osmotic lysis of infected RBCs in 280 mM sorbitol plus buffer A and 0, 1, 3, and 10 μM dantrolene (top to bottom traces, respectively) at 37°C. Ordinate corresponds to percent transmittance of 700 nm light (%T) through each suspension of cells, prepared from identical aliquots of a single parasite culture. (B) Dose response for lysis inhibition, calculated as previously described (59). Normalized sorbitol permeabilities, Ps, are shown as mean ± standard error of the mean (up to 5 measurements at each concentration). Solid line represents the best fit to y = K0.5/(K0.5+ x) with a K0.5 of 1.2 μM. (C) Osmotic lysis time courses in 280 mM sorbitol plus buffer A with 0, 0.5, 1, 2, 5, or 10 μM azumolene (top to bottom traces, respectively) at 37°C. The chemical structure shows the similar structures of dantrolene (R is NO2) and azumolene (R is Br). The dashed rectangle highlights the scaffold previously implicated in PSAC inhibition (26). (D) Effects of RyR modulators on sorbitol-induced osmotic lysis with normalization to 100% for no addition. Results for 100 nM and 10 μM ryanodine (agonist and antagonist concentrations for RyR, respectively), 1 mM caffeine, 1 mM hydantoin, 0.5 mM EGTA, and 10 μM ruthenium red are shown (mean ± standard error of the mean; for each agent, n = 2 to 4). Hydantoin is not known to affect RyR but was tested because it is structurally related to dantrolene.
We then wondered if there are other pharmacological similarities between the parasite-induced permeabilities and RyR. Although azumolene, a bromo-phenyl analog of dantrolene, also inhibits both of these functionally divergent transport mechanisms, we found no other pharmacological similarities (Fig. 1C and D). Ryanodine, an agonist for skeletal muscle Ca++ release at low concentrations and an antagonist at high concentrations, had no effect on sorbitol-induced osmotic lysis. The RyR agonist caffeine, the RyR antagonist ruthenium red, and other modulators of Ca++ release channels also had no significant effects.
While polymorphisms in PSAC gating suggest it is parasite encoded (1), other channels proposed to mediate organic solute uptake after infection are believed to result from modifications of host channel proteins (18, 17, 58). Our searches of the completed P. falciparum genome database did not find any clear homologues of a short 20-residue region in RyR where dantrolene binds (45), leaving open the question of how the parasite induces a dantrolene-sensitive sorbitol permeability.
To explore possible mechanisms of dantrolene's effects, we used cell-attached patch-clamp on trophozoite-stage-infected RBCs. Because PSAC has a small single channel conductance and fast-flickering transitions with mean open durations of only 200 μs, detection of single-channel molecule events requires hypertonic recording solutions containing chloride salts, seal resistances of ≥100 GΩ, and optimized low-noise circuitry (1). Similar experiments using isotonic salt solutions reveal characteristic inward-rectifying channel noise in multichannel patches but do not permit single-channel detection (12, 1). Here, we began with low noise recordings using a total nominal chloride concentration of 1,145 mM and detected a 20-pS inward rectifying channel with fast-flickering gating (Fig. 2 A, top two traces). This channel behavior was detected as one or more functional copies in about half of all successful patches. Other channel types, such as activity consistent with proposed outward rectifying channels (17), were never detected under these experimental conditions (n = 604 patches).
FIG. 2.
(A) Single PSAC recordings in 1,000 mM choline-Cl, 115 mM NaCl plus buffer B, and indicated dantrolene concentrations in bath and pipette. Vm was +100 mV for the top trace and −100 mV for all others. Closed channel levels are marked with dashes on both sides of each trace. At a Vm of +100 mV, PSAC openings are infrequent and poorly resolved because of the channel's intrinsic inward-rectifying voltage dependence. Notice that increasing dantrolene concentrations decrease the frequency of openings, visible as downward deflections from the closed level for Vm of −100 mV. Horizontal and vertical scale bars represent 150 ms and 2.0 pA, respectively. (B) Averaged single-channel current (i) versus imposed Vm for recordings without dantrolene (filled circles) or with 5 μM (filled triangles) or 20 μM dantrolene (open circles). Each symbol represents the mean single-channel current ± standard error of the mean determined by integration of open-channel currents as described in Materials and Methods. At each voltage, between 2.9 and 12 s of recording was averaged. (C) Pooled single-channel Po in the same solution with 0, 5, 10, and 20 μM dantrolene in both bath and pipette. Symbols represent mean ± standard error of the mean and were calculated from up to 61 s of recording at each concentration. Vm was −100 mV in these recordings. Solid line represents the best fit of single-channel Po values to equation 4. The dashed line shows the profile expected based on the inhibition dose response for sorbitol-induced lysis (Fig. 1B).
Identical experiments performed with up to 20 μM dantrolene in both pipette and bath solutions produced PSAC activity with visibly different gating behavior (Fig. 2 A, bottom three traces). Dantrolene added a population of relatively long closings without affecting open-channel amplitude. This change was most apparent at negative Vm values, where nearly all of PSAC's intrinsic closings are less than 5 ms long (10). Because PSAC openings are less frequent at positive Vm, we integrated open-channel currents to determine that increasing concentrations produce progressively greater inhibition at all examined voltages (Fig. 2B). As this approach is limited to analyses of relatively short recordings from single-channel molecules, we did not attempt to quantify inhibition here. Nevertheless, these recordings provide pharmacological evidence for a mechanistic link between sorbitol-induced osmotic lysis and PSAC channel activity.
To more quantitatively determine dantrolene's affinity for inhibition in single-channel recordings, we measured PSAC open probability (Po) in up to 61 s of recording at each dantrolene concentration as described in Materials and Methods. Without the addition of antagonist, PSAC exhibits a mean Po of 43 ± 2% at a Vm of −100 mV under these recording conditions (1). Consistent with the above averaging of open-channel currents, the mean Po decreased as the dantrolene concentration was raised. By pooling measurements from a total of 11 single channel patches, the Po versus dantrolene dose response was adequately fitted by equation 4 with a K0.5 of 4.0 μM (Fig. 2 C). Although this value is similar to the K0.5 of 1.2 μM for inhibition of sorbitol-induced lysis (Fig. 1 B), the levels of inhibition in our single-channel recordings at each dantrolene concentration were somewhat less than expected from the sorbitol-induced lysis experiments. We were further puzzled because similar measurements with furosemide (1) and phloridzin (11) have produced more precise correlations between inhibitory effects on osmotic lysis and single channel recordings. Indeed, furosemide interaction and inhibition are also precisely reproduced in independent whole-cell patch-clamp and tracer uptake experiments (1).
Why might dantrolene not have produced similarly reproducible levels of inhibition? One possibility is that uptake of sorbitol occurs via a pathway other than PSAC and that both are inhibited by dantrolene, albeit with somewhat differing K0.5 values. Another possibility is that dantrolene's ability to inhibit PSAC may be influenced by the differing experimental conditions used in the lysis and single-channel transport assays. These differences include the compositions of solutions, the temperatures used, and the membrane potentials channels encounter.
We examined the effect of solution composition on dantrolene inhibition. While the lysis experiments are performed in very low ionic strength solutions with almost no charged ions, single-channel detection of PSAC requires a supraphysiological ionic-strength solution. If dantrolene binds PSAC through interaction with charged residues on the channel protein, then increasing the solution ionic strength would be expected to decrease the affinity of block. We tested this possibility by performing single PSAC recordings in a solution with an intermediate ionic strength (Fig. 3 A, right column of traces). This solution has a nominal Cl− concentration of 530 mM and produces reduced PSAC single-channel amplitudes still clearly resolved above the baseline noise. As previously reported (1), PSAC gating and voltage dependence were not affected by lowering ionic strength. We then examined the effect of 20 μM dantrolene under these conditions and found it produced a statistically significant greater inhibition (Fig. 3 B) (P value of 0.7%, two-tailed Student's t test).
FIG. 3.
Solution effects on dantrolene inhibition of single PSAC recordings. (A) Single-channel recordings in buffer B with either 1,000 mM choline-Cl, 115 mM NaCl (left column), or 500 mM NaCl (right column). In each column, the top trace was recorded without dantrolene while the lower two traces were with 20 μM dantrolene added to bath and pipette. Opening events (downward transitions) have smaller amplitudes in 500 mM NaCl because of a lower Cl− concentration. Notice that openings occur with similar frequencies in these two solutions when dantrolene is absent; inhibition by dantrolene, however, is improved by lowering the ionic strength. Closed-channel levels are indicated with dashes at the ends of each trace. Imposed Vm is −100 mV. Scale bar, 100 ms/2 pA. (B) Single-channel Po with 20 μM dantrolene in these solutions. Bars represent mean Po ± standard error of the mean, each determined from ∼ 60-s recordings from three or four single-channel patches in each solution.
Because of the suboptimal signal-to-noise ratio in this lower ionic strength solution and because of the inability to detect single PSAC transitions in more physiological solutions, we performed additional experiments using the whole-cell patch-clamp configuration. The whole-cell configuration not only permits detection of PSAC in isotonic salt solutions, but it avoids concerns about conclusions based on analyses of a small number of ion channel molecules. Here, we obtained high resistance seals on infected erythrocytes in one of four different ionic strength solutions, achieved the whole-cell configuration, and used slow perfusion of the bath with the same solution supplemented with dantrolene concentrations between 2 μM and 40 μM. Current-voltage profiles, recorded after stabilization at each dantrolene concentration, revealed an unambiguous ionic strength effect on dantrolene inhibition (Fig. 4 A, B, and C). We tallied the dose responses in each recording solution, fitted each with equation 4, and found that the dantrolene K0.5 increased monotonically as a nonlinear function of the Cl− concentration (Fig. 4E). As the Cl− concentration was lowered to physiological values, dantrolene inhibition approached the level seen in our osmotic lysis experiments (2.3 ± 0.2 μM in physiological saline versus 1.2 μM in the salt-free sorbitol solution). The parallel effects observed in our single-channel and whole-cell recordings indicate that ionic strength modulates dantrolene's affinity for inhibition of PSAC. Preliminary whole-cell recordings after addition of the impermeant anion gluconate to physiological saline yielded similar increases in K0.5 (data not shown), further suggesting electrostatic interactions between dantrolene and charged residues on the channel's binding site.
FIG. 4.
Ionic strength effects in whole-cell recordings. Whole-cell currents in buffer B plus 115 mM NaCl supplemented with 1,000 mM or 370 mM choline-Cl (A and B, respectively) or without choline-Cl (C). In each panel, the four groups of traces represent currents after slow perfusion of no inhibitor, 4 μM dantrolene, 10 μM dantrolene, and 2 mM furosemide (left to right groups of traces, respectively). Each group of traces depicts the current responses to application of Vm between −100 and +100 mV in 10-mV increments. The x-y plots in each panel show the corresponding mean whole-cell current versus Vm profiles without dantrolene and for 4 and 10 μM dantrolene (filled circles, triangles, and open circles, respectively). With each cell, multiple solution changes between differing dantrolene concentrations were used to confirm reproducibility. Furosemide was used at saturating concentrations to determine seal quality. Scale bars represent 50 ms (horizontal) and 2742 pA, 1688 pA, and 500 pA (panels A, B, and C, respectively). In these slow perfusion experiments, we found that there was an initial run-down of whole-cell currents. The experiments shown and all analyses were carried out after stabilization of whole-cell currents. (D) Whole-cell chord conductances versus nominal Cl− concentrations without dantrolene for the experiments in panels A to C and in identical experiments using buffer B plus 115 mM NaCl and 70 mM choline-Cl. Chord conductances were calculated between Vm values of −100 mV and 0 mV. Solid line represents the best fit to y = a × x/(x + b) and confirms the weakly saturating behavior of Cl− permeation through PSAC (1). (E) Mean ± standard error of the mean K0.5 values for dantrolene inhibition in each solution (n = 2, 4, 5, and 7 cells at [Cl−] of 145, 215, 515, and 1145 mM, respectively). Notice that dantrolene affinity decreases as the ionic strength increases.
PSAC's unique pharmacological profile.
Dantrolene block of PSAC might reflect either this channel's unusual pharmacological profile or a previously unknown effect on a larger subset of anion channels. We distinguished these possibilities by expressing a diverse collection of anion channels or transporters on Xenopus oocytes. We tested CFTR, ClC-1, ClC-5, the oocyte's eCaCC, and MOD-1, a serotonin-gated Cl− channel (24, 52, 61, 47). Transfected oocytes exhibited currents with mean reversal potentials between −25 and −30 mV, consistent with selective Cl− conduction (not shown). Although these channels exhibited divergent voltage and ligand dependencies as well as differing levels of expression on oocytes, none were measurably inhibited by 50 μM dantrolene (Fig. 5), the limit of its solubility under these conditions and some 45 times its K0.5 for inhibition of PSAC-mediated sorbitol lysis. In contrast, previously known PSAC antagonists are less specific. Glybenclamide (50 μM) produced 60% inhibition of CFTR (mean of n = 3 cells; data not shown), consistent with previous studies (60). NPPB is also known to have broad activity against these and other anion channels (24).
FIG. 5.
Dantrolene does not inhibit other anion channels or transporters. (A) Current-voltage profile for expressed CFTR before stimulation with forskolin and IBMX (open circles) and with and without 50 μM dantrolene after stimulation (triangles and filled circles, respectively). Current-voltage profiles for ClC-1 (B) and ClC-5 (C) with and without 50 μM dantrolene (triangles and filled circles, respectively). (D) Time courses of eCaCC-mediated current response for untransfected oocytes pretreated with A23187 upon changing the bathing solution from Ca++-free (with 0.5 mM EGTA) to 4 mM Ca++. (E) Time courses of MOD-1 current response to application of 1 μM serotonin. In panels D and E, oocytes were clamped at a Vm of −60 mV. Traces, shown beginning with the onset of solution change, are with and without 50 μM dantrolene (right and left traces in each panel, respectively). Horizontal/vertical scale bars represent 5 s/1.5 μA in panels D and E. Statistical analyses of chord conductances or peak currents revealed no significant effect of dantrolene on any of these five divergent Cl− conductances (n = 9 to 23 each; not shown).
Site and mechanism of dantrolene action on PSAC.
We next used whole-cell patch-clamp measurements on infected RBCs to examine dantrolene's site of action on the PSAC molecule. In these experiments, rapid equilibration between the relatively small RBC cytosolic volume (∼100 femtoliters) and the larger volume of the pipette solution (∼10 μl in our experiments) permits the selective application of dantrolene to one membrane face of PSAC at a time. To avoid errors due to leakage of inhibitor between bath and pipette compartments, these experiments also required high seal resistances, as previously described (1). In the absence of dantrolene, whole-cell currents were greater at negative membrane potentials (Fig. 6, left group of traces), consistent with PSAC's voltage-dependent gating (12); we calculated a whole-cell chord conductance of 54 ± 5 nS between −100 and 0 mV under these conditions (n = 18 cells). Dantrolene (20 μM), when added to both intracellular and extracellular solutions, reduced this conductance by 80% (11 ± 0.9 nS, n = 8 cells). Extracellular addition alone matched this level of inhibition (11 ± 1 nS, n = 4 cells), while intracellular addition alone produced significantly less inhibition (48 ± 6 nS, n = 6 cells; P < 10−5, two-tailed Student's t test), suggesting that dantrolene's binding site is on PSAC's extracellular face.
FIG. 6.
(A) Whole-cell currents for infected RBCs without (left group of traces) or with 20 μM dantrolene added to both bath and pipette, to bath only, or to pipette only (second, third, and fourth groups, respectively). Groups of traces represent the current in response to Vm pulses from −100 to +100 mV (10-mV increments) and reveal PSAC's inward rectifying behavior (12). Bath and pipette contained 500 mM NaCl plus buffer B. Notice that addition of dantrolene to the bath alone produces marked inhibition in whole-cell currents, while addition to the pipette has a negligible effect. Scale bars, 25 ms/2,000 pA for all groups of traces. (B) Corresponding current-voltage profiles for no dantrolene (open circles) or 20 μM dantrolene in both bath and pipette (filled circles), in bath only (open triangles), or in pipette only (closed triangles). (C) Mean ± standard error of the mean of whole-cell chord conductances calculated between a membrane potential of −100 and 0 mV with indicated dantrolene concentrations (in μM). Asterisks denote statistical significance relative to measurements with no dantrolene (n = 4 to 18 cells under each condition).
Dantrolene inhibition of PSAC is unusual, distinguishing this channel from known human anion channels. How is this inhibition achieved? Analysis of open and closed event durations in single-channel recordings with multiple concentrations of an inhibitor can be used to categorize the agent as a simple pore blocker, a simple nonluminal inhibitor, or a compound with a more complicated mechanism (3). Pore blockers produce inhibition by directly occluding an open-channel pore. Because the resulting steric hindrance to ion flow bypasses the channel's intrinsic gating, measured durations of open-channel events decrease as the concentration of a pore blocker is increased. In contrast, a simple nonluminal inhibitor reduces currents by binding to a site on the channel separate from the pore used by permeating solutes. Its binding there prohibits transitions to the open state by stabilizing one or more closed conformations. As a result, nonluminal inhibitors increase the durations of closed events without affecting open-channel durations. If this distant effect involves a 1:1 interaction with first-order rate constants, the nonluminal inhibitor will add a single exponentially decaying population of closings whose mean duration does not vary with inhibitor concentration. Any other combination of effects on open and closed channel durations would suggest a more complicated mechanism of inhibitor action, as commonly reported (46).
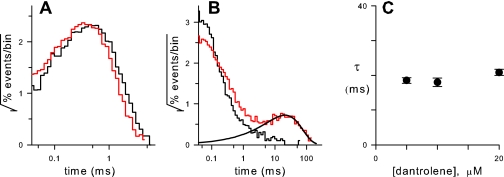
We used analysis of single PSAC recordings to probe dantrolene's mechanism of inhibition. In the absence of inhibitors, PSAC exhibits one open and multiple closed states (10). The distribution of open-channel durations was not affected by the presence of up to 20 μM dantrolene (Fig. 7 A), excluding a simple pore-blocking mechanism. The distribution of closed durations, on the other hand, was markedly affected with the addition of a large fraction of closed events greater than 10 ms (Fig. 7 B, red distribution). This fraction was adequately fitted by the theoretical distribution for a single dantrolene-bound state with an exponentially decaying rate constant of 21 ± 1 ms for unbinding (Fig. 7 B, smooth curve). Because this rate constant did not vary with inhibitor concentration (Fig. 7 C), dantrolene's effects on the channel can be conservatively explained by a simple nonluminal interaction with a single site on PSAC's extracellular face that prevents the conformational changes needed for channel opening. (Although the duration of individual inhibitory event does not depend on dantrolene concentration, the frequency of these events increases with concentration. This higher frequency of blocked events produces the greater inhibition observed in macroscopic measurements.)
FIG. 7.
Open (A) and closed (B) dwell time distributions for single PSAC recordings without (black histogram lines) or with 20 μM dantrolene (red histogram lines). Dwell time analyses used single-channel recordings obtained in the cell-attached configuration with 1000 mM choline-Cl, 115 mM NaCl plus buffer B in the bath and pipette. The smooth black curve in panel B shows the best fit of events longer than 4.4 ms to the distribution predicted for a single dantrolene-bound state (equation 2) and corresponds to a mean duration of PSAC block (τ) by dantrolene of 21 ms (equation 3). (C) Mean τ ± standard error of the mean versus dantrolene concentration (n between 7,000 and 11,000 closing events each). The lack of effect on open durations combined with a single population of blocked events with concentration-independent τ values suggests simple nonluminal inhibition of the channel.
Although osmotic lysis, tracer flux, and electrophysiological measurements with furosemide, a known PSAC antagonist, suggest that PSAC mediates the uptake of solutes as diverse as anions, sugars, amino acids, and bulky organic cations (1), some workers remain skeptical because furosemide is nonspecific; a highly nonspecific antagonist might inhibit multiple different pathways induced by the parasite and falsely implicate a common route (23). Moreover, electrophysiological studies in other laboratories suggest that channels with distinct biophysical properties may also be induced by the parasite (17, 18, 58). Because dantrolene is more specific than previously characterized inhibitors, we tested whether it can also inhibit the increased permeabilities of solutes other than sorbitol and Cl−. Using the osmotic lysis assay, we found that it inhibited the parasite-induced permeability of two amino acids and of the organic cation phenyl-trimethyl-ammonium at concentrations that inhibit sorbitol uptake (Fig. 8 A). It also inhibited the parasite-induced component of [3H]hypoxanthine tracer uptake (Fig. 8 B). Because this purine is required for in vitro parasite growth (14), dantrolene inhibition is consistent with a role for PSAC in nutrient acquisition.
FIG. 8.
(A) Osmotic lysis time courses in the buffer A with 280 mM sorbitol, 145 mM phenyl-trimethyl-ammonium chloride (PhTMA+ Cl−), 280 mM isoleucine, or 280 mM alanine as indicated. In each panel, upper and lower traces reflect the lysis kinetics at 20°C without and with 15 μM dantrolene, respectively. Each ordinate was normalized to percent lysis to permit comparison of solute transport rates. Dantrolene effectively inhibits the uptake of each solute. (B) [3H]hypoxanthine accumulation in 90 s in infected cells without or with 15 μM dantrolene at room temperature. Accumulation in uninfected RBCs under identical conditions is shown to illustrate the endogenous permeability prior to infection. Results shown are typical of three independent experiments. (C) Osmotic lysis time course in 280 mM sorbitol plus buffer A without (top trace) or with 20 μM dantrolene (lower two traces) at 37°C. The middle trace was recorded with 10% serum added to the lysis solution to evaluate dantrolene adsorption. This serum concentration did not affect lysis kinetics in the absence of dantrolene (not shown).
The broad inhibitory effects of dantrolene on parasite-induced permeability changes, when combined with its known inhibitory effects on parasite growth in culture (32, 26), suggest it may be a starting point for development of future antimalarial drugs that kill parasites by inhibition of PSAC. As with a number of previous antagonists of this channel, we found that dantrolene inhibition is adversely affected by the addition of serum (Fig. 8 C), presumably because of adsorption to lipids and hydrophobic proteins. Drug development programs will need to identify derivatives with sustained availability in serum to achieve in vivo PSAC inhibition and parasite killing.
DISCUSSION
Because PSAC does not share other properties with the skeletal RyR Ca++ release channels, dantrolene inhibition of PSAC is quite unexpected. Indeed, inhibitory effects of dantrolene on parasite growth, known for a number of years (32), were attributed to yet undiscovered Ca++ channels without consideration of possible effects on known parasite-induced channels. In light of our findings, dantrolene's ability to inhibit in vitro parasite growth can be more conservatively explained by its effects on PSAC.
Since PSAC's identification (12), other electrophysiological and biophysical studies (17, 18, 58, 22) have generated much controversy over whether a single ion channel could be responsible for the increased permeabilities of diverse solutes of both positive and negative charge. One of the main arguments for a single shared pathway has been nearly universal inhibition by a collection of quite nonspecific inhibitors. Furosemide (which inhibits both anion and cation channels as well as carriers), NPPB (which inhibits many different anion channels and other enzymes [5]), and phloridzin (which inhibits many diverse transporters and even affects the permeability of pure lipid bilayers [38]) were among the most used agents to argue for one pathway. Workers were rightly skeptical of these agents. Our studies indicate that dantrolene is significantly more specific (Fig. 5). Its ability to inhibit the uptake of multiple solutes in the various assays used here allays the concerns arising from poor specificity. Moreover, its parallel effects on PSAC activity detected in both single channel and whole-cell electrophysiological experiments suggest a solid pharmacological link between PSAC and the generally accepted organic solute permeability changes.
A previous screen of 165 dantrolene derivatives implicated the nitro-phenyl furan group of dantrolene in PSAC inhibition (Fig. 1 C, dashed rectangle). Here, we found that azumolene, which has a bromo-phenyl group in the place of the critical nitro-phenyl, also inhibits PSAC with comparable affinity. This observation suggests that the strong electron donating abilities of both these groups are critical for PSAC inhibition. This suggestion is also consistent with our identification of a modest, but statistically significant ionic strength effect on dantrolene's affinity for the channel.
Ionic strength effects on inhibitor binding have also been seen with other enzymes, including a number of ion channels (4, 19, 29, 37, 42). They represent a classical test of electrostatic interactions between fixed charges on the enzyme's binding site and polar or charged groups on the inhibitor. In one elegant study (19), engineered mutations of a negatively charged residue on a K+ channel altered the effect of ionic strength on inhibitor affinity in a predictable fashion, definitively confirming electrostatic interactions with that residue. Those workers also determined that the ionic strength effect was primarily via effects on the inhibitor association rate constant, a finding which suggested a detailed mechanism of inhibitor action on the channel. While similar studies with PSAC must wait for both the cloning of its gene(s) and the development of a suitable heterologous expression system, the ionic strength effect on dantrolene affinity is most conservatively explained by interaction with charged residues at the channel extracellular face, some of which have been identified using covalent modification with N-hydroxysulfosuccinimide esters (8). Alternative explanations are unlikely because neither ionic strength nor Cl− concentration affects the affinity of furosemide (1) or phloridzin (11). They also do not alter PSAC gating, voltage dependence, or selectivity, suggesting that there are not global changes in channel structure under high-salt conditions (12, 1).
Because dantrolene and its derivatives kill in vitro parasite cultures, they may be lead compounds for antimalarial development (26). Several observations suggest this approach should be explored actively. First, while it remains debated whether the permeability changes after infection result from a parasite-encoded protein or a modified host protein, functional and biophysical conservation on erythrocytes infected with the phylogenetically distant Plasmodium knowlesi is consistent with an important biological role after infection (35). Second, the plasma-exposed location of PSAC reduces concerns about acquired resistance through extrusion of unmetabolized drug from the infected erythrocyte complex, a mechanism with substantial supportive evidence for chloroquine (31, 51, 40) and possibly for other antimalarial drugs (25). Other mechanisms of resistance, such as selection of mutations in the antagonist binding pocket, remain possible. Third, PSAC's location on the surface of the infected RBC complex reduces drug design constraints such as those proposed by Lipinski et al. (34). These constraints propose that an ideal drug should not have a molecular weight or cLogP (a marker of aqueous and membrane solubility) outside of empirically determined ranges to facilitate access to intracellular targets. A drug binding site directly exposed to bloodstream concentrations, such as now demonstrated for the dantrolene site on PSAC, would presumably not need to meet these recommendations.
Acknowledgments
We thank J. Gonzalez and K. Oades for help with screening ion channel inhibitors and T. Jentsch, D. Gadsby, and H. Horvitz for providing the Xenopus expression constructs. We thank S. Baylor and K. Swartz for helpful suggestions and their comments on the manuscript.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Alkhalil, A., J. V. Cohn, M. A. Wagner, J. S. Cabrera, T. Rajapandi, and S. A. Desai. 2004. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 104:4279-4286. [DOI] [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Parant, M. J. Thuet, J. R. Philippot, and H. J. Vial. 1991. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 273:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias, H. R. 1996. Luminal and non-luminal non-competitive inhibitor binding sites on the nicotinic acetylcholine receptor. Mol. Membr. Biol. 13:1-17. [DOI] [PubMed] [Google Scholar]
- 4.Arias, H. R. 1996. Temperature and ionic strength dependence of quinacrine binding and quinacrine displacement elicited by high concentrations of agonists on the nicotinic acetylcholine receptor. Arch. Biochem. Biophys. 333:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Breuer, W., and K. L. Skorecki. 1989. Inhibition of prostaglandin E2 synthesis by a blocker of epithelial chloride channels. Biochem. Biophys. Res. Commun. 163:398-405. [DOI] [PubMed] [Google Scholar]
- 6.Cabantchik, Z. I. 1990. Properties of permeation pathways induced in the human red cell membrane by malaria parasites. Blood Cells 16:421-432. [PubMed] [Google Scholar]
- 7.Caro, J. F. 1990. Effects of glyburide on carbohydrate metabolism and insulin action in the liver. Am. J. Med. 89:17S-25S. [DOI] [PubMed] [Google Scholar]
- 8.Cohn, J. V., A. Alkhalil, M. A. Wagner, T. Rajapandi, and S. A. Desai. 2003. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol. Biochem. Parasitol. 132:27-34. [DOI] [PubMed] [Google Scholar]
- 9.Colquhoun, D., and F. J. Sigworth. 1983. Fitting and statistical analysis of single-channel records, p. 191-263. In B. Sakmann and E. Neher (ed.), Single-channel recording. Plenum Press, New York, N.Y.
- 10.Desai, S. A. 2005. Open and closed states of the plasmodial surface anion channel. Nanomed. Nanotechnol. Biol. Med. 1:58-66. [DOI] [PubMed] [Google Scholar]
- 11.Desai, S. A., A. Alkhalil, M. Kang, U. Ashfaq, and M. L. Nguyen. 2005. PSAC-independent phloridzin resistance in Plasmodium falciparum. J. Biol. Chem. 280:16861-16867. [DOI] [PubMed] [Google Scholar]
- 12.Desai, S. A., S. M. Bezrukov, and J. Zimmerberg. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005. [DOI] [PubMed] [Google Scholar]
- 13.Desai, S. A., P. H. Schlesinger, and D. J. Krogstad. 1991. Physiologic rate of carrier-mediated Ca2+ entry matches active extrusion in human erythrocytes. J. Gen. Physiol. 98:349-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divo, A. A., T. G. Geary, N. L. Davis, and J. B. Jensen. 1985. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J. Protozool. 32:59-64. [DOI] [PubMed] [Google Scholar]
- 15.Doughty, J. M., A. L. Miller, and P. D. Langton. 1998. Non-specificity of chloride channel blockers in rat cerebral arteries: block of the L-type calcium channel. J. Physiol. 507:433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duranton, C., S. Huber, V. Tanneur, K. Lang, V. Brand, C. Sandu, and F. Lang. 2003. Electrophysiological properties of the Plasmodium falciparum-induced cation conductance of human erythrocytes. Cell Physiol. Biochem. 13:189-198. [DOI] [PubMed] [Google Scholar]
- 17.Duranton, C., S. M. Huber, V. Tanneur, V. B. Brand, C. Akkaya, E. V. Shumilina, C. D. Sandu, and F. Lang. 2004. Organic osmolyte permeabilities of the malaria-induced anion conductances in human erythrocytes. J. Gen. Physiol. 123:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egee, S., F. Lapaix, G. Decherf, H. M. Staines, J. C. Ellory, C. Doerig, and S. L. Thomas. 2002. A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum. J. Physiol. 542:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobar, L., M. J. Root, and R. MacKinnon. 1993. Influence of protein surface charge on the bimolecular kinetics of a potassium channel peptide inhibitor. Biochemistry 32:6982-6987. [DOI] [PubMed] [Google Scholar]
- 20.Fioretti, B., E. Castigli, I. Calzuola, A. A. Harper, F. Franciolini, and L. Catacuzzeno. 2004. NPPB block of the intermediate-conductance Ca2+-activated K+ channel. Eur. J. Pharmacol. 497:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Ginsburg, H., S. Kutner, M. Krugliak, and Z. I. Cabantchik. 1985. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 14:313-322. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg, H., and W. D. Stein. 2004. The new permeability pathways induced by the malaria parasite in the membrane of the infected erythrocyte: comparison of results using different experimental techniques. J. Membr. Biol. 197:113-134. [DOI] [PubMed] [Google Scholar]
- 23.Ginsburg, H., and W. D. Stein. 2005. How many functional transport pathways does Plasmodium falciparum induce in the membrane of its host erythrocyte? Trends Parasitol. 21:118-121. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch, T. J., V. Stein, F. Weinreich, and A. A. Zdebik. 2002. Molecular structure and physiological function of chloride channels. Physiol. Rev. 82:503-568. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, D. J., D. A. Fidock, M. Mungthin, V. Lakshmanan, A. B. Sidhu, P. G. Bray, and S. A. Ward. 2004. Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol. Cell 15:867-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang, M., G. Lisk, S. Hollingworth, S. M. Baylor, and S. A. Desai. 2005. Malaria parasites are rapidly killed by dantrolene derivatives specific for the plasmodial surface anion channel. Mol. Pharmacol. 68:34-40. [DOI] [PubMed] [Google Scholar]
- 27.Kirk, K., and H. A. Horner. 1995. In search of a selective inhibitor of the induced transport of small solutes in Plasmodium falciparum-infected erythrocytes: effects of arylaminobenzoates. Biochem. J. 311:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk, K., H. A. Horner, B. C. Elford, J. C. Ellory, and C. I. Newbold. 1994. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 269:3339-3347. [PubMed] [Google Scholar]
- 29.Knaus, H. G., R. O. Koch, A. Eberhart, G. J. Kaczorowski, M. L. Garcia, and R. S. Slaughter. 1995. [125I]margatoxin, an extraordinarily high affinity ligand for voltage-gated potassium channels in mammalian brain. Biochemistry 34:13627-13634. [DOI] [PubMed] [Google Scholar]
- 30.Korpi, E. R., T. Kuner, P. H. Seeburg, and H. Luddens. 1995. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol. Pharmacol. 47:283-289. [PubMed] [Google Scholar]
- 31.Krogstad, D. J., I. Y. Gluzman, D. E. Kyle, A. M. Oduola, S. K. Martin, W. K. Milhous, and P. H. Schlesinger. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283-1285. [DOI] [PubMed] [Google Scholar]
- 32.Krogstad, D. J., S. P. Sutera, J. S. Marvel, I. Y. Gluzman, C. W. Boylan, J. R. Colca, J. R. Williamson, and P. H. Schlesinger. 1991. Calcium and the malaria parasite: parasite maturation and the loss of red cell deformability. Blood Cells 17:229-241. [PubMed] [Google Scholar]
- 33.Liman, E. R., J. Tytgat, and P. Hess. 1992. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9:861-871. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3-26. [DOI] [PubMed] [Google Scholar]
- 35.Lisk, G., and S. A. Desai. 2005. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot. Cell 4:2153-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz, C., M. Pusch, and T. J. Jentsch. 1996. Heteromultimeric CLC chloride channels with novel properties. Proc. Natl. Acad. Sci. USA 93:13362-13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKinnon, R., R. Latorre, and C. Miller. 1989. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry 28:8092-8099. [DOI] [PubMed] [Google Scholar]
- 38.Melnik, E., R. Latorre, J. E. Hall, and D. C. Tosteson. 1977. Phloretin-induced changes in ion transport across lipid bilayer membranes. J. Gen. Physiol. 69:243-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer, G., S. Doppierio, P. Vallin, and L. Daffonchio. 1996. Effect of frusemide on Cl− channel in rat peritoneal mast cells. Eur. Respir. J. 9:2461-2467. [DOI] [PubMed] [Google Scholar]
- 40.Naude, B., J. A. Brzostowski, A. R. Kimmel, and T. E. Wellems. 2005. Dictyostelium discoideum expresses a malaria chloroquine resistance mechanism upon transfection with mutant, but not wild-type, Plasmodium falciparum transporter PfCRT. J. Biol. Chem. 280:25596-25603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nazaret, C., J. Diez, P. A. Hannaert, M. O. Christen, N. Wierzbicki, and R. P. Garay. 1987. Inhibition of the Cl−/NaCO3− anion exchanger by xipamide in human red blood cells. Eur. J. Pharmacol. 144:353-362. [DOI] [PubMed] [Google Scholar]
- 42.Neumeyer, T., F. Tonello, M. F. Dal, B. Schiffler, F. Orlik, and R. Benz. 2006. Anthrax lethal factor (LF) mediated block of the anthrax protective antigen (PA) ion channel: effect of ionic strength and voltage. Biochemistry 45:3060-3068. [DOI] [PubMed] [Google Scholar]
- 43.Overman, R. R. 1948. Reversible cellular permeability alterations in disease. In vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am J. Physiol. 152:113-121. [DOI] [PubMed] [Google Scholar]
- 44.Palfrey, H. C., and M. E. O'Donnel. 1992. Characteristics and regulation of the Na/K/2Cl cotransporter. Cell Physiol. Biochem. 2:293-307. [Google Scholar]
- 45.Paul-Pletzer, K., T. Yamamoto, M. B. Bhat, J. Ma, N. Ikemoto, L. S. Jimenez, H. Morimoto, P. G. Williams, and J. Parness. 2002. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J. Biol. Chem. 277:34918-34923. [DOI] [PubMed] [Google Scholar]
- 46.Prince, R. J., R. A. Pennington, and S. M. Sine. 2002. Mechanism of tacrine block at adult human muscle nicotinic acetylcholine receptors. J. Gen. Physiol. 120:369-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranganathan, R., S. C. Cannon, and H. R. Horvitz. 2000. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408:470-475. [DOI] [PubMed] [Google Scholar]
- 48.Ripps, H., H. Qian, and J. Zakevicius. 2004. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol. Neurobiol. 24:647-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rokitskaya, T. I., E. A. Kotova, and Y. N. Antonenko. 2002. Membrane dipole potential modulates proton conductance through gramicidin channel: movement of negative ionic defects inside the channel. Biophys. J. 82:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saliba, K. J., H. A. Horner, and K. Kirk. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273:10190-10195. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez, C. P., W. Stein, and M. Lanzer. 2003. Trans stimulation provides evidence for a drug efflux carrier as the mechanism of chloroquine resistance in Plasmodium falciparum. Biochemistry 42:9383-9394. [DOI] [PubMed] [Google Scholar]
- 52.Sheppard, D. N., and M. J. Welsh. 1999. Structure and function of the CFTR chloride channel. Physiol. Rev. 79:S23-S45. [DOI] [PubMed] [Google Scholar]
- 53.Sigworth, F. J., and S. M. Sine. 1987. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 52:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staines, H. M., B. C. Dee, M. O'Brien, H. J. Lang, H. Englert, H. A. Horner, J. C. Ellory, and K. Kirk. 2004. Furosemide analogues as potent inhibitors of the new permeability pathways of Plasmodium falciparum-infected human erythrocytes. Mol. Biochem. Parasitol. 133:315-318. [DOI] [PubMed] [Google Scholar]
- 55.Stuhmer, W. 1992. Electrophysiological recording from Xenopus oocytes. Methods Enzymol. 207:319-339. [DOI] [PubMed] [Google Scholar]
- 56.Tian, Y. A., G. Johnson, and S. J. Ashcroft. 1998. Sulfonylureas enhance exocytosis from pancreatic beta-cells by a mechanism that does not involve direct activation of protein kinase C. Diabetes 47:1722-1726. [DOI] [PubMed] [Google Scholar]
- 57.Upston, J. M., and A. M. Gero. 1995. Parasite-induced permeation of nucleosides in Plasmodium falciparum malaria. Biochim. Biophys. Acta 1236:249-258. [DOI] [PubMed] [Google Scholar]
- 58.Verloo, P., C. H. Kocken, W. A. van der, B. C. Tilly, B. M. Hogema, M. Sinaasappel, A. W. Thomas, and H. R. De Jonge. 2004. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J. Biol. Chem. 279:10316-10322. [DOI] [PubMed] [Google Scholar]
- 59.Wagner, M. A., B. Andemariam, and S. A. Desai. 2003. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys. J. 84:116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamazaki, J., and J. R. Hume. 1997. Inhibitory effects of glibenclamide on cystic fibrosis transmembrane regulator, swelling-activated, and Ca2+-activated Cl− channels in mammalian cardiac myocytes. Circ. Res. 81:101-109. [DOI] [PubMed] [Google Scholar]
- 61.Young, G. P., J. D. Young, A. K. Deshpande, M. Goldstein, S. S. Koide, and Z. A. Cohn. 1984. A Ca2+-activated channel from Xenopus laevis oocyte membranes reconstituted into planar bilayers. Proc. Natl. Acad. Sci. USA 81:5155-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]