Abstract
ERK5 is a mitogen-activated protein (MAP) kinase regulated in human cells by diverse mitogens and stresses but also suspected of mediating the effects of a number of oncogenes. Its expression in the slt2Δ Saccharomyces cerevisiae mutant rescued several of the phenotypes caused by the lack of Slt2p (Mpk1p) cell integrity MAP kinase. ERK5 is able to provide this cell integrity MAP kinase function in yeast, as it is activated by the cell integrity signaling cascade that normally activates Slt2p and, in its active form, able to stimulate at least one key Slt2p target (Rlm1p, the major transcriptional regulator of cell wall genes). In vitro ERK5 kinase activity was abolished by Hsp90 inhibition. ERK5 activity in vivo was also lost in a strain that expresses a mutant Hsp90 chaperone. Therefore, human ERK5 expressed in yeast is an Hsp90 client, despite the widely held belief that the protein kinases of the MAP kinase class are non-Hsp90-dependent activities. Two-hybrid and protein binding studies revealed that strong association of Hsp90 with ERK5 requires the dual phosphorylation of the TEY motif in the MAP kinase activation loop. These phosphorylations, at positions adjacent to the Hsp90-binding surface recently identified for a number of protein kinases, may cause a localized rearrangement of this MAP kinase region that leads to creation of the Hsp90-binding surface. Complementation of the slt2Δ yeast defect by ERK5 expression establishes a new tool with which to screen for novel agonists and antagonists of ERK5 signaling as well as for isolating mutant forms of ERK5.
Mitogen-activated protein kinase (MAPK) modules consist of 3 protein kinases that stimulate each other in series (MAP3K → MAP2K → MAPK), resulting in the activation of the terminal and often multifunctional MAPK. These signaling cascades are key components of the highly interactive protein kinase networks in eukaryotic cells. As their name implies, some MAPKs initiate a proliferative response. Others control pathways of embryogenesis, differentiation, stress responses, and cell death (42). Association of MAPK modules with scaffold proteins appears to be one way of ensuring that each MAPK only becomes activated in response to the correct extracellular stimuli or stress signals (52). Once activated, this MAPK can then proceed to phosphorylate its substrates, the latter being often involved in both short-term and longer-term (e.g., transcription-mediated) cellular changes. Many MAPKs have substrates in both the cytoplasm and the nucleus, such that nuclear import/export mechanisms frequently govern their accessibility to their substrates. The final outcome of any MAPK activation event is presumably dictated by this substrate availability in any given cell type, by an intrinsic substrate specificity directed by the docking interaction of the MAPK with its substrates, and by signal attenuation. The latter involves the intervention of the protein phosphatases that, by dephosphorylating and thereby deactivating the MAPK, modulate both the intensity and the duration of the activation signal.
Mammalian systems possess at least four MAPK subfamilies: extracellular-regulated protein kinases 1 and 2 (ERK1/2), ERK5, c-Jun NH2-terminal protein kinases (JNKs), and p38s. All are activated in response to the dual Thr/Tyr phosphorylation of a Thr-X-Tyr (TXY) motif (X corresponding to a Glu in ERKs, a Pro in JNK, and a Gly in p38). This TXY phosphorylation is catalyzed by the appropriate MAP2K, an enzyme that is often in physical interaction with its MAPK substrate, at least until it can activate the latter. Gene knockout experiments with mice have revealed that these different MAPK subfamilies mediate different biological responses. The ERK subfamilies are mostly associated with cell proliferation and survival, whereas JNKs and p38s are activated mainly in response to cytokines and stress, being important for pathogenesis and apoptosis (42). The least studied of these MAPK subfamilies was, until recently, ERK5. This is a MAPK activated in response to a variety of signals, including chemical activators (e.g., serum, epidermal, and nerve growth factors, lysophosphatidic acid, and phorbol esters), oxidative stress (e.g., hydrogen peroxide and UV radiation), and physical stress (e.g., sorbitol, vascular shear stress, and ischemia) (1, 60). Recently ERK5 has become the subject of intense interest following the discovery that it is essential for cardiovascular development and an important contributor to cell survival mechanisms. ERK5-null mice display defective endothelial cell morphology, blood vessel formation, and cardiac development, leading to embryonic lethality (18). ERK5 activation has been found to prevent endothelial cell apoptosis by stimulating phosphorylation of Bad in the cytoplasm and thereby inhibiting caspase-3 activity (43). Moreover, analysis of human tumors has revealed a link between abnormal levels of ERK5 expression and cancer, leading to the suspicion that ERK5 signaling may be important for mediating the effects of several oncogenes (see references 19 and 56 for reviews).
Appreciation of the full importance of ERK5 has been relatively slow in coming. This is due, at least in part, to the lack of biological tools, such as antibodies, specific inhibitors of ERK5 signaling, and systems to unravel this MAPK genetically. To this end, we have investigated the functionality of ERK5 in the Saccharomyces cerevisiae system. So extensive is the sequence conservation among MAPKs of different species that the expression of a mammalian MAPK in yeast will often rescue a defect in one the yeast MAPKs (all of which are nonessential for growth). For example, Hog1p, the MAPK of the yeast high osmolarity glycerol pathway can be functionally replaced by either JNKs (13) or p38 (17). Also, an activated human ERK1 expressed in yeast partially mimics the active form of the native Kss1p, though it is unresponsive to the endogenous activators of this Kss1p (3). In this study, we demonstrate that human ERK5 expression in yeast complements the lack of Slt2p (Mpk1p), the stress-activated MAPK of the cell integrity signaling pathway. ERK5 is able to confer this Slt2p function as it becomes activated in yeast by the MAP2K activator of Slt2p and, once activated, is able to stimulate at least one major Slt2p target (the transcriptional regulator of yeast cell wall genes, Rlm1p) (14, 22, 23).
We show, furthermore, that heat shock protein 90 (Hsp90) is needed for ERK5 to be an active protein kinase and provide cell integrity MAPK function in yeast. Hsp90, an essential molecular chaperone, catalyzes the final activation step of many key regulatory proteins in eukaryotic cells (the Hsp90 “clients”) (reviewed at http:///www.picard.ch) (41, 48). Genomic studies with yeast have recently addressed the breadth of the Hsp90 clientele, revealing that up to 10% of the proteome may be subject to Hsp90 regulation (34, 63). Despite Hsp90 being essential for many important protein kinase activities, MAPKs are still generally considered nonclients of Hsp90 (6, 45). We recently found, though, that the cell integrity MAPK of yeast, Slt2p, is an Hsp90 client (34). In revealing that ERK5 is also Hsp90 dependent in yeast, this investigation provides the first evidence for a mammalian MAPK being Hsp90 dependent, raising the issue of whether ERK5 is also an Hsp90 client in mammals. Assuming this is the case, yeast expressing ERK5 in place of the native Slt2p may provide a model system in which to study how Hsp90 assists the activation of a mammalian MAPK.
MATERIALS AND METHODS
Yeast strains and yeast growth.
The yeast strains used in this study are listed in Table 1. PP30 slt2Δ and PP30 mkk1Δmkk2Δslt2Δ were constructed by hphMX4 cassette (15) deletion of the SLT2 gene in wild-type and mkk1Δmkk2Δ versions of PP30 (44). Liquid cultures used either YP (2% [wt/vol] Bacto peptone, 1% yeast extract, 20 mg/liter adenine) containing 2% glucose (YPD) or 3% glycerol (YPGlycerol) or dropout glucose medium (DO) (2). Plate growth at the indicated temperatures used the same media with 1.5% agar, with or without the indicated inhibitor additions. α-Factor treatment involved treating liquid YPD cultures containing 40 mM sodium citrate, pH 4.5 (6 h 30°C), with 0.5 μM α-factor (Sigma).
TABLE 1.
The yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| Strains with a single gene for wild-type or T22I mutant yeast Hsp90 | ||
| P82a | W303-1a hsc82::LEU2 hsp82::LEU2 HIS3-HSP82a | 38 |
| T22I | W303-1a hsc82::LEU2 hsp82::LEU2 HIS3-hsp82(T22I)a | 38 |
| P82aslt2Δ | P82a slt2ΔkanMX4 | 34 |
| T22Islt2Δ | T22I slt2ΔkanMX4 | 34 |
| PP30slt2Δ | MATatrp1-289 leu2-3,112 his3-200 ura3-52 ade2-101oc lys2-801am hsc82::kanMX4 hsp82::kanMX4 slt2Δ::hphMX4 [pHSC82]b | This study |
| PP30 mkk1Δ mkk2Δ slt2Δ | MATatrp1-289 leu2-3,112 his3-200 ura3-52 ade2-101oc lys2-801am hsc82::kanMX4 hsp82::kanMX4 mkk1::kanMX4 mkk2::HIS3 slt2Δ::hphMX4 [pHSC82]b | This study |
| Two-hybrid strains | ||
| PJ694a | MATatrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ | 21 |
| PJ694α | MATα trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ | 21 |
Expression vectors for ERK2 and ERK5 expression in yeast.
Expression vectors for ERK2 and ERK5 expression in yeast were constructed by PCR amplifying the coding sequences of ERK2 (from plasmid pGEX4T-ERK2) (33) and ERK5a (from image clone 4300124; GenBank accession no. BC009963) (primer sequences available on request). TRP1 plasmid vectors (pG1-ERK2 and pG1-ERK5, respectively), for expression of these MAPKs in yeast under the control of the constitutive TDH1 promoter, were constructed by digesting the products of these PCRs with BamHI plus SalI prior to their insertion into BamHI- plus XhoI-cleaved pG1 (49). Vectors for expression of a C-terminally truncated ERK5 (the 1 to 407 region) and also the T218A, Y220F double mutant of this C-terminally truncated ERK5 [ERK5(1-407)(AEF)] were constructed by PCR amplifying regions of cDNA clones previously described by Barros and Marshall (4). These were cloned into BamHI- plus SalI-cut pUT36 (34) to generate vectors for MET25 promoter-regulated expression of these sequences with an N-terminal 12× His tag [pHis-ERK5(1-407) and pHis-ERK5(1-407(AEF)), respectively]. The same ERK5 sequences were also cloned into PEW415 (this is pRS415 [50] with a TPI promoter multiple-cloning site [3× hemagglutinin {HA}] insert) (E. Hettema, unpublished) to generate plasmids for expression of the same sequences with a C-terminal 3× HA tag [pERK5(1-407)-HA and pERK5(1-407(AEF))-HA, respectively]. The vector for MET25 promoter-regulated MKK1-P386 expression was as previously described (34).
Protein analysis.
Preparation of yeast soluble protein extracts was as described previously (40). His-ERK5(1-407) and His-ERK5(1-407(AEF)) in these extracts was isolated by nickel-nitrilotriacetate chromatography (His-select columns; Sigma); the ERK5(1-407)-HA was isolated by immunoprecipitation using anti-HA immunoglobulin G-coated agarose beads (Sigma). Western blot analysis used the following as primary antibodies: for MAPK detection, anti-ERK2 and anti-ERK5 antisera (sc-5626 and sc-154, respectively; Santa Cruz); for His12-tagged proteins, mouse His4 antibody (QIAGEN); for yeast Hsp90 and Sba1p, rabbit polyclonal antisera raised against the latter proteins (34). The secondary antibody was horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G (Amersham) diluted 2,000-fold. Assays of MAPK activity on ERK5(1-407)-HA immunoprecipitated from yeast cell extracts were as previously described for an HA-tagged Slt2p (24), using 50 μg of ERK5(1-407)-HA protein per assay. Where present, 1 μM radicicol was added at the start of incubation.
Two-hybrid studies.
Construction of PJ694α (Table 1) expressing wild-type and mutant forms of the Hsp82-BD fusion was described earlier (34-36). PJ694a cells expressing either a wild-type or a nonphosphorylatable AD-ERK5 fusion [AD-ERK5; AD-ERK5(AEF), respectively] were generated by homologous recombination within yeast, essentially as described previously (36, 54). These PJ694a transformants were mated to the PJ694α strains expressing the Hsp82-BD fusions, with the resultant PJ69-4 diploids (now expressing both the AD and BD fusions) being selected on DO lacking histidine and tryptophan. Automated measurement of the β-galactosidase activity due to the interaction-responsive, GAL7 promoter-regulated lacZ gene of PJ69-4 was as previously described (34, 35). Data in Fig. 5B and C show the mean and standard deviations (SD) of results from eight individual assays on separate aliquots of each culture. The control diploid PJ69-4 cells contained pBDC (36) lacking a gene insert and the plasmid for the AD fusion expression (since the low basal lacZ expression levels in this system are generally due to the AD protein fusion, with the even lower lacZ expression level in cells containing an Hsp82-BD “bait” and empty AD vector [pOAD] being essentially unaffected by stress [35]).
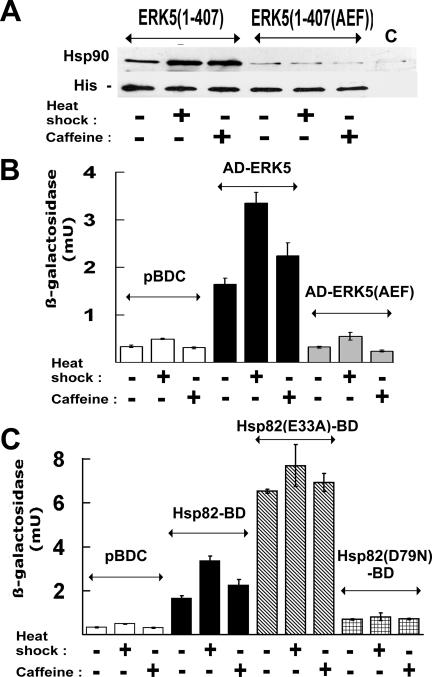
FIG. 5.
Strong Hsp90 binding is selective for the phosphorylated state of ERK5. (A) Levels of Hsp90 associated with nickel resin-retained, wild-type or nonphosphorylatable AEF mutant His-ERK5(1-407). Extracts were from cultures of P82aslt2Δ mutant cells expressing these His-ERK5(1-407) forms either in growth at 25°C, heat shocked from 25 to 39°C for 1 h, or treated with 8 mM caffeine for 1 h at 25°C, The control lane (C) is the extract from unstressed, non-ERK5-expressing P82aslt2Δ. (B) Two-hybrid interaction of a functional Hsp82-BD bait with the control (pBDC), wild type (AD-ERK5), or nonphosphorylatable [AD-ERK5(AEF)] prey fusions in cultures either unstressed, heat shocked, or caffeine treated as for panel A, showing that Hsp90-ERK5 interaction is moderately strengthened by stress and selective for the phosphorylatable form of the MAPK. (C) Effects of the E33A and D79N mutations in the Hsp82-BD bait fusion on two-hybrid interaction with AD-ERK5. The control cells (pBDC) contained wild-type AD-ERK5 together with the empty pBDC vector, since the basal lacZ expression in this system is mainly due to the AD fusion (35).
RESULTS
Diverse slt2Δ mutant phenotypes are suppressed by ERK5 expression.
Human ERK5 and yeast Slt2p exhibit striking sequence identity within their N-terminal MAPK domains (49.7% between amino acids 32 and 357 [ERK5] and 1 and 326 [Slt2p]). Nevertheless, MAPKs are in general so extremely conserved that this sequence identity is no more than that seen with human ERK1/2 or with the other MAPKs of yeast. It is within the protein kinase activation segment (defined as the region between, and including, two conserved tripeptide motifs [DFG…APE]) (39) that the primary sequence comparisons indicate ERK5 as a possible mammalian ortholog of the yeast Slt2p (Table 2). ERK5 and Slt2p are also set aside from the other MAPKs of humans and yeast, respectively, by virtue of unusually large sequence extensions C-terminal to the MAPK domain. These C-terminal regions are important for certain of the events of transcription factor activation by ERK5 in humans and by Slt2p in yeast (see Discussion).
TABLE 2.
Alignment of the activation loop regions of the five yeast MAPKs, human ERK5, and human ERK2
| MAPK | Sequencea |
|---|---|
| Kss1p | DFGLARCLASSSDSRETLVG---FMTEYVATRWYRAPE |
| Fus3p | DFGLARIIDESAADNSEPTGQQSGMTEYVATRWYRAPE |
| Smk1p | DFGLARGIHAGFFKCHSTVQP--HITNYVATRWYRAPE |
| Hog1p | DFGLARIQDPQ-------------MTGYVSTRYYRAPE |
| Slt2p | DFGLARGYSENPVENSQ------FLTEYVATRWYRAPE |
| ERK5 | DFGMARGLCTSPAEHQY------FMTEYVATRWYRAPE |
| ERK2 | DFGLARVADPDHDHTG-------FLTEYVATRWYRAPE |
| Identityb | ***:** :* **:**:***** |
A G. …P…E motif common to Slt2p and ERK5 is in boldface type; the TXY motif that is dually Thr/Tyr phosphorylated by the activating MAP2K is underlined.
A highly flexible loop anchored between conserved DFG and APE tripeptide sequences in protein kinases (39).
Initially, we sought to test whether ERK5 expression would suppress the effects of Slt2p loss in yeast (phenotypes of the slt2Δ mutant). The mouse erk5 gene spans ∼5.6 kb and comprises 6 exons and 5 introns. Alternative splicing of its transcripts generates three ERK5 isoforms, a, b, and c, but of these, only ERK5a is an active kinase (60). It was therefore the human equivalent of this ERK5a that we expressed (together with ERK2) in yeast. The expression vector pG1-ERK2 or pG1-ERK5 (see Materials and Methods) was found to lead to the synthesis of proteins recognized by anti-ERK2 or anti-ERK5 antisera, respectively (Fig. 1A and B).
FIG. 1.
ERK5 expression complements loss of Slt2p function in yeast. (A and B) Protein extracts from strain P82a transformed with pG1, pG1-ERK2, or pG1-ERK5, analyzed by Western blotting using anti-ERK2 (A) or anti-ERK5 (B) antisera. Sba1p was used as a loading control. (C) Comparison of 3-day YPD growth either at 37°C or at 30°C with or without caffeine for wild type (WT; strain P82a) cells transformed with pG1 as well as P82aslt2Δ transformed with pG1, pG1-ERK2, or pG1-ERK5. (D) Growth (3 days, 30°C) of P82aslt2Δ transformed with pG1 or pG1-ERK5 on glycerol, a respiratory carbon source, or on YPD without or containing 50 μg ml−1 calcofluor white (CFW), 50 μg ml−1 hygromycin B (HygB), 160 mM or 200 mM hydroxyurea (HU), or 5 ng ml−1 rapamycin. (E) Analysis of the formation of mating pheromone projections in P82aslt2Δ transformed with either pG1 or pG1-ERK5.
Early studies revealed a number of phenotypes generated by Slt2p loss in yeast, including temperature and caffeine sensitivity (24, 27, 32), as well as an inability to form mating projections in response to the pheromone α-factor (62). To see whether ERK2 or ERK5 expression would rescue these defects, pG1-ERK2, pG1-ERK5, and the empty vector lacking any gene insert (pG1) were transformed into P82a and P82aslt2Δ (Table 1), strains isogenic but for the presence or absence of the SLT2 gene. pG1-ERK5, but not pG1-ERK2, restored caffeine resistance and a capacity for high-temperature growth in the P82aslt2Δ mutant (Fig. 1C). Other slt2Δ mutant phenotypes were also suppressed by this ERK5 expression (but not the ERK2 expression), including the lack of growth on respiratory carbon sources (57); sensitivities to compounds that affect the cell wall, such as calcofluor white (9, 28) and hygromycin B (8); and sensitivities to rapamycin and hydroxyurea (29, 47) (Fig. 1D). ERK5 expression also restored to slt2Δ cells the capacity to form mating projections in response to mating pheromone α-factor (Fig. 1E). ERK5 expression rescuing such a diverse spectrum of slt2Δ mutant phenotypes revealed that this expression is providing many, if not all, of the functions of the native Slt2p cell integrity MAPK in yeast.
Expressed in yeast in place of the native Slt2p, ERK5 stimulates a native Slt2p target, the Rlm1p transcriptional regulator of cell wall genes.
In its active, dually Thr/Tyr-phosphorylated state, the yeast cell integrity MAPK binds to its recognition (docking) domains on both substrates (targets of Slt2p-mediated phosphorylation) and the protein phosphatases that will eventually restore this Slt2p to the state of an inactivated MAPK (7, 12, 16). In the cytosol, this active Slt2p is recruited to the cell cortex (55), while in the nucleus, it activates two transcription factor regulators of cell integrity genes, Rlm1p and Swi4p (30). Rlm1p appears to be the major transactivator of cell wall genes in yeast (14, 22, 23).
slt2Δ mutant cells exhibit a pronounced Rlm1p activity defect (58). To determine if the expression of ERK5 was rescuing this defect, a reporter gene of Rlm1p activity (YIL117c-lacZ) (23) was monitored in SLT2+ (strain P82a) and slt2Δ (strain P82aslt2Δ) cells containing either pG1 or pG1-ERK5. Rlm1p activity was substantially restored to the slt2Δ mutant by the presence of the pG1-ERK5 expression vector, with the levels of this activity being increased with two stresses that stimulate cell integrity signaling (heat shock and caffeine) (Fig. 2A).
FIG. 2.
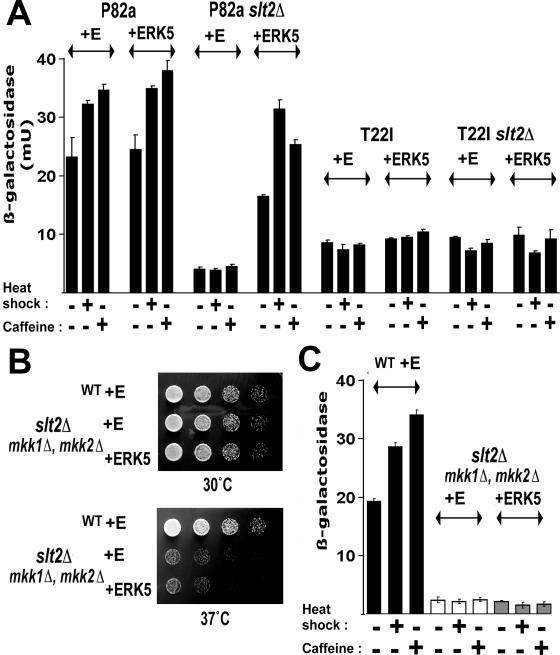
ERK5 restores Rlm1p activity in the slt2Δ mutant, with activity rescue abolished by the expression of a T22I mutant Hsp90 or the loss of Mkk1/2p. (A) Expression of a YIL117c-lacZ reporter of Rlm1p activity in strains P82a, P82aslt2Δ, T22I, and T22Islt2Δ, either containing empty pG1 vector (E) or the pG1-ERK5 expression vector (ERK5). (B and C) Analysis of 37°C growth (B) and Rlm1p activity (C) in a slt2Δ mkk1Δ mkk2Δ triple mutant transformed with either empty pG1 (E) or pG1-ERK5 (ERK5) compared to the PP30 parent strain (WT). YIL117c-lacZ expression measurements were on cells either in growth at 25°C (unstressed), heat shocked from 25°C to 37°C for 1 h, or exposed to 8 mM caffeine for 1 h at 25°C.
Activity of ERK5 in yeast requires the MAP2K of the cell integrity pathway.
Slt2p, the MAPK functionally complemented by this ERK5 expression in yeast, is part a signaling cascade composed of a MAP3K (Bck1p), a pair of redundant MAP2Ks (Mkk1/2p), and Slt2p (reviewed in reference 30). To confirm that ERK5 was becoming activated in yeast by this cell integrity cascade (i.e., by the MAP2K Mkk1/2p), both a full-length ERK5 and a C-terminally truncated ERK5 [ERK5(1-407)] (the latter a form partially functional in vivo; see below) were expressed in a slt2Δ mkk1Δ mkk2Δ triple-mutant strain (Table 1). As with the slt2Δ single mutant, this triple mutant displays the characteristic phenotypes of loss of Slt2p-mediated signaling. Whereas ERK5 expression could suppress these phenotypes when the signaling components of the cell integrity pathway upstream of Slt2p were still intact (slt2Δ cells) (Fig. 1C to E; Fig. 3A), it was unable to rescue these phenotypes in this slt2Δ mkk1Δ mkk2Δ triple-mutant background (the lack of any rescue of high-temperature growth or Rlm1p activity is shown in Fig. 2B and C). Therefore, ERK5 becomes activated in yeast by the MAP2K of the cell integrity cascade.
FIG. 3.
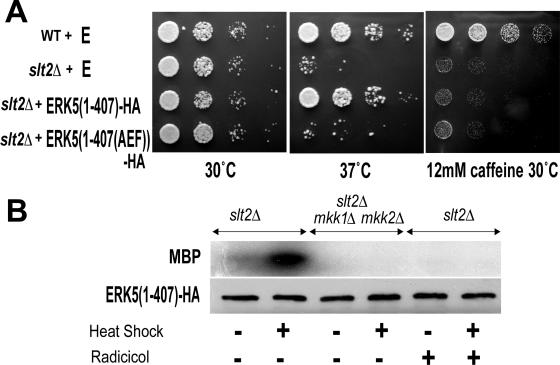
Analysis of a C-terminally truncated ERK5 expressed in yeast. (A) Expression of a MET25 promoter-regulated ERK5(1-407)-HA restores high temperature growth, but not caffeine resistance, in the slt2Δ mutant. Wild-type (WT, strain p82a) and P82aslt2Δ (slt2Δ) cells containing either empty PEW415 vector (E), pERK5(1-407)-HA, or pERK5(1-407(AEF))-HA were grown for 3 days at the indicated temperature on DO medium lacking uracil and methionine. (B) Phosphorylation of myelin basic protein (MBP) by the ERK5(1-407)-HA immunoprecipitated from extracts of unstressed or heat-shocked (1 h at 37°C) slt2Δ single-mutant and slt2Δ mkk1Δ mkk2Δ triple-mutant cells. Immunoprecipitated fractions were also Western blotted and probed with anti-HA antiserum.
Activity of ERK5 in yeast cell extracts is abolished with the loss of Mkk1/2p and with Hsp90 inhibitor treatment.
Our initial attempts to demonstrate ERK5 activity in yeast by standard MAPK assay were thwarted by an inability to obtain the full-length ERK5 in soluble form in cell extracts. Such problems may have been caused by the known oligomerization properties of ERK5 (56). We had previously found, though, that in the case of the native Slt2p, the in vitro MAPK activity is preserved in a C-terminally truncated, dually TEY-phosphorylated protein that comprises just the N-terminal MAPK domain (34; also unpublished data). By expressing in the yeast a C-terminally truncated, HA-tagged ERK5 [ERK5(1-407)-HA] (see Materials and Methods), the solubility problems experienced with the full-length ERK5 were overcome. As with the corresponding C-terminally truncated form of Slt2p (28, 51), ERK5(1-407)-HA is partially functional in vivo, rescuing Swi4p-dependent, but not Rlm1p-dependent, transcription of slt2Δ cells. Thus, when expressed in slt2Δ cells, this ERK5(1-407)-HA is able to rescue the growth at high temperatures and on respiratory medium but not the caffeine sensitivity or the lack of mating projection formation (Fig. 3A; also data not shown).
ERK5(1-407)-HA was immunoprecipitated from extracts of slt2Δ single-mutant and slt2Δ mkk1Δ mkk2Δ triple-mutant cells and assayed for MAPK activity (Fig. 3B). The activity of the ERK5(1-407)-HA expressed in slt2Δ cells was increased by heat shock of the cells prior to extract preparation (a stress that activates cell integrity signaling) (Fig. 3B). Importantly, this activity from slt2Δ cells was inhibited by the presence of the highly selective Hsp90 inhibitor radicicol (Fig. 3B), revealing ERK5(1-407)-HA to be an Hsp90-dependent protein kinase (the native yeast Slt2p cell integrity MAPK is also an Hsp90 client) (34). There was no ERK5(1-407)-HA activity in the extracts from the slt2Δ mkk1Δ mkk2Δ triple mutant (Fig. 3B), consistent with this MAPK being activated by the MAP2K of the cell integrity pathway.
Expression of T22I mutant Hsp90 abolishes ERK5 activity in yeast.
T22I (Table 1) is a yeast mutant that expresses a T22I point mutant form of Hsp90 as its sole form of Hsp90 chaperone (38). This strain is defective in the maintenance of cell integrity, displaying a phenotype similar to that of mutants defective in cell integrity pathway signaling (34). T22I exhibits Mkk1/2-directed phosphorylation of Slt2p in response to the activation of cell integrity signaling, but its defect in Hsp90 prevents this phosphorylated Slt2p from activating Rlm1p (34) (Fig. 2A).
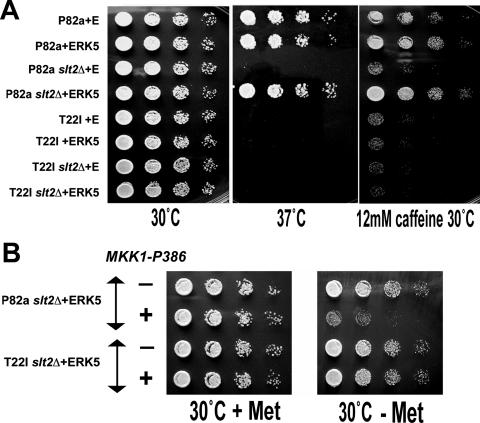
Strains lacking the endogenous Slt2p MAPK and expressing either a wild-type or a T22I mutant Hsp90 (P82aslt2Δ and T22Islt2Δ, respectively) (Table 1) were transformed with pG1-ERK5 and the control empty vector (pG1). ERK5 expression restored both high-temperature growth and caffeine resistance to the slt2Δ cells containing the wild-type Hsp90 but provided no such rescue in the cells isogenic but for their possession of T22I mutant Hsp90 (compare P82aslt2Δ and T22Islt2Δ) (Fig. 4A and B). ERK5 rescue of the Rlm1p activity in the slt2Δ mutant was similarly abolished by this T22I mutation in Hsp90 (compare the Rlm1p activity data of P82aslt2Δ and T22Islt2Δ) (Fig. 2A).
FIG. 4.
An Hsp90 mutation compromises ERK5 activity in yeast. (A) Growth at 37°C and caffeine resistance of P82a and T22I, strains expressing wild-type or T22I mutant Hsp90, and Slt2p-deficient forms of these strains (P82aslt2Δ and T22Islt2Δ) transformed with either the pG1-ERK5 expression vector (ERK5) or the control pG1 (E). (B) MET25 promoter-regulated MKK1-P386 expression exerts a dominant-negative effect in ERK5-expressing cells with the wild-type Hsp90 (P82aslt2Δ) but not those with T22I mutant Hsp90 (T22Islt2Δ). Growth (3 days, 30°C) was on DO medium either lacking only uracil or lacking uracil and methionine.
Excessively high MAPK activity levels are generally extremely detrimental for yeast (16, 57, 58). Such an effect is seen with the expression of MKK1-P386, the gene for an overactive point mutant form of Mkk1p cell integrity MAP2K (57). The toxicity of this MKK1-P386 is, in turn, suppressed by defects in either Slt2p or Rlm1p (57). As shown in Fig. 4B, MKK1-P386 expression exerted a strong dominant-negative effect on the growth of ERK5-expressing, Slt2p-deficient cells containing the wild-type Hsp90 (P82aslt2Δ). This effect was, though, absent in cells isogenic but for their expression of the T22I mutant form of Hsp90 (T22Islt2Δ) (Fig. 4B). Replacement of wild-type Hsp90 by this T22I mutant Hsp90 also abolishes the toxicity of MKK1-P386 in cells with the native Slt2p (34). Therefore, the high cell integrity MAPK activity (ERK5 or Slt2p) that is normally generated by MKK1-P386P in vivo is abolished by the T22I mutation in Hsp90. T22I mutant cells display essentially normal Slt2p MAPK phosphorylation in response to the activation of cell integrity signaling (34). Their partially compromised Hsp90 activity, though, acts to prevent the in vivo activation of Rlm1p (34) by this phosphorylated Slt2p (34) or the heterologously expressed ERK5 (Fig. 2A).
Strong Hsp90 association is selective for the dually TEY-phosphorylated state of ERK5.
Through protein binding studies, using wild-type and mutant forms of both Hsp90 and Slt2p, and also a study of the two-hybrid interaction of the corresponding wild-type and mutant forms of Hsp90-Gal4p DNA binding domain (Hsp82-BD) and Gal4p activator domain (AD) Slt2p fusions, we recently demonstrated that Hsp90 binding is selective for the dually Tyr,Thr-phosphorylated state of Slt2p (34). This was the first study to show that an Hsp90 client can acquire its capacity to bind Hsp90 through regulatory phosphorylation. It indicates that phosphorylation of Slt2p by the upstream Mkk1/2p MAP2K leads to the creation of an Hsp90-binding surface on this MAPK. In vivo Hsp90-Slt2p interaction is therefore strengthened with stimulation of cell integrity signaling and, therefore, increases in Mkk1/2p activity. Upon finding that ERK5 expression would rescue many of the effects of loss of this Slt2p in vivo (Fig. 1), we investigated whether ERK5 would also bind Hsp90 and whether such binding is affected by the loss of activating Tyr,Thr phosphorylation of ERK5.
A His-tagged, C-terminally truncated ERK5 was expressed in slt2Δ yeast both in native [His-ERK5(1-407)] and nonphosphorylatable T218A,Y220F double-mutant [His-ERK5(1-407(AEF)] form (see Materials and Methods). The His-ERK5(1-407) present in extracts of these cells was found to be associated with appreciable amounts of Hsp90, with this Hsp90 binding being increased under conditions of heat or caffeine stress (Fig. 5A). In contrast, the nonphosphorylatable His-ERK5(1-407(AEF)) exhibited only weak Hsp90 binding, irrespective of whether the cells had been stressed prior to extract preparation (Fig. 5A). These findings indicated that Mkk1/2p-directed phosphorylation of ERK5 is a requirement for strong Hsp90 binding by this MAPK in cell extracts.
To confirm that the dual phosphorylation of ERK5 is also a prerequisite for Hsp90 binding to this MAPK in vivo, yeasts expressing the Hsp82-BD “bait” fusion and either a wild-type (AD-ERK5) or a nonphosphorylatable [AD-ERK5(AEF)] “prey” fusion were generated for two-hybrid purposes (see Materials and Methods). The former fusion is substantially a functional form of Hsp90 in vivo (36). These yeast strains were then used to determine the relative strengths of basal and stress-induced Hsp82-BD-AD-ERK5 interaction. Figures 5B and C show these interaction measurements, determined from levels of expression of the interaction-responsive, GAL7 promoter-regulated lacZ gene of the two-hybrid strain (34, 35). As with the corresponding two-hybrid interaction of AD-Slt2p with this same Hsp90 bait (Hsp82-BD-AD-Slt2p) (34), Hsp82-BD exhibited a stress-reinforced in vivo interaction with the native AD-ERK5 “prey” fusion, while mutations that prevent phosphorylation of this fusion [the double-mutant form, AD-ERK5(AEF)] substantially abolished this Hsp82-BD-AD-ERK5 interaction (Fig. 5B).
The Hsp82-BD-AD-ERK5 two-hybrid interaction was considerably reinforced by the presence of the E33A mutation in the Hsp82-BD fusion [Hsp82(E33A)-BD] (Fig. 5C). E33A is an Hsp90 mutation that arrests the Hsp90 chaperone cycle, allowing this cycle to progress to a late-stage complex where the client protein is stabilized in tight association with the ATP-bound state of Hsp90 but where the final ATPase-dependent release of the activated client from the chaperone complex is blocked (40). In contrast, two-hybrid Hsp82-BD-AD-ERK5 interaction was abolished by the D79N mutation in the Hsp82-BD fusion [Hsp82(D79N)-BD] (Fig. 5C), a mutation that prevents ADP/ATP binding to Hsp90 (40).
Taken together, the data in Fig. 5 reveal that, as for the native Slt2p MAPK (34), it is the dually Tyr/Thr-phosphorylated form of ERK5 that engages in a strong association with the Hsp90 chaperone. Furthermore, when the chaperone cycle is inhibited by the E33A mutation in Hsp90, a late-stage chaperone cycle complex accumulates in vivo that comprises this phosphorylated ERK5 in association with the mutant Hsp90 (Fig. 5C). Such physical interaction of Hsp90 with the phosphorylated ERK5 (Fig. 5) is almost certainly a key step in Hsp90 activation of this MAPK (Fig. 3B and 4).
DISCUSSION
This study reveals that an expression of human ERK5 rescues many of the phenotypes resulting from the loss of the native Slt2p MAPK in slt2Δ yeast (Fig. 1). ERK5 can confer cell integrity MAPK function in yeast, as it is capable both of being activated by the cell integrity signaling pathway and, in its activated state, stimulating transcription factor targets of Slt2p (Rlm1p [Fig. 2 and 3] and Swi4p [data not shown]). We show, furthermore, that ERK5 expressed in yeast is a client of the Hsp90 chaperone system, the first evidence of Hsp90 dependence for a true mammalian MAPK. Hsp90 binding has been shown to stabilize Mok1p, a mammalian protein kinase moderately related structurally to the conventional MAPKs (37). A number of the kinases of various MAPK signaling cascades are also known to be Hsp90 dependent (6, 31, 45, 53). In these cascades, it appears that several MAP3Ks are Hsp90 clients (see reference 6 for literature). In contrast, the MAP2Ks and MAPKs are generally nonclients (6), though there appear to be some exceptions to this rule (e.g., Wis1p, an MAP2K of Schizosaccharomyces pombe [53] and the Slt2p/ERK5 MAPKs that we have been studying). To date, assignations of Hsp90 client status to mammalian protein kinases have generally been based on identifying those kinases that become destabilized when cells are treated with Hsp90 inhibitor drugs (6, 46). In such a screen, ERK5 is not destabilized and is therefore not designated an Hsp90 client (6). This lack of destabilization with Hsp90 inhibitor drug treatment might, though, merely reflect much of the ERK5 in cells not being in its MAP2K-phosphorylated state. Our findings (Fig. 5) indicate that only the Mkk1/2p-phosphorylated form of ERK5 is in strong association with Hsp90, at least in the yeast system.
This study and our earlier investigation of the Slt2p-Hsp90 interaction (34) are the first to show that Hsp90 clients can be rendered Hsp90 binding as the result of regulatory phosphorylation. Slt2p and ERK5 both become strong Hsp90-binding agents upon Mkk1/2p-catalyzed dual Thr/Tyr phosphorylation of the TEY motif in their activation loop (Table 2; Fig. 5). These phosphorylations, at sites in close proximity to the common Hsp90-binding surface recently identified on a number of protein kinases (6), may cause localized structural rearrangement of this region, leading to the creation of the Hsp90-binding surface on Slt2p and ERK5. Alternatively, displacement of the activating MAP2K may uncover such a surface.
ERK5 expression complementing the mutational defects of slt2Δ yeast provides a model experimental system for investigating ERK5 activity. It can be used to analyze the Hsp90 dependence of a mammalian MAPK. Also, the strong suppression of several slt2Δ mutant phenotypes through ERK5 expression (Fig. 1) might be used to screen for ERK5 inhibitors. In addition, it should allow screens for constitutively active, gain-of-function (GOF) and dominant-negative mutant forms of ERK5. Once isolated, these mutant forms can then be transferred to mammalian cells for an analysis of the effects of altered ERK5 activity in a variety of cell culture systems. The utility of yeast for direct selection of such MAPK mutations is already very well established, e.g., Fus3p (see reference 11 for literature) and Hog1p (59). In contrast, it has proven much more difficult to isolate mutant MAPKs using mammalian culture systems, even though GOF mutations in the mammalian MAPKs can be associated with malignancy (20). Indeed, the only successful engineering of mammalian MAPKs for GOF to date appears to have been by the introduction of mutations originally identified for yeast Fus3p into rat ERK2 (all at residues precisely conserved between Fus3p and ERK2) (11). Such studies reveal just how valuable yeast screens for altered MAPK activity can be when attempting to engineer the mammalian MAPKs.
A shared property of the two MAPKs so far shown to be Hsp90 dependent (ERK5 [this study] and yeast Slt2p [34]) is that they stimulate MADS (MCM1, agamous deficiens serum response factor) box transcription factors, with these being among their best-characterized substrates. In mammals, ERK5 regulates the myocyte enhancer factor (MEF) family of transcriptional regulators (MEF2A, C, and D), with direct ERK5 phosphorylation of MEF2C acting to increase the transcriptional activity of the latter and, therefore, c-jun expression (26). In yeast, the MADS box transcription factor Rlm1p is similarly activated through direct, in this case Slt2p-mediated, phosphorylation (23). Remarkably, yeast Rlm1p shares with the mammalian MEF2s the same in vitro DNA binding specificity [CTA(T/A)4TAG] (10) and MAPK docking domain sequence (residues 324 to 329 in Rlm1p [23] and 273 to 278 in MEF2A [61]). The N-terminal kinase domains of ERK5 and Slt2p are, though, not the only regions of these MAPKs that interact with MADS box transcription factors. Loss of sequences C-terminal to the MAPK domain on these MAPKs, though it does not prevent in vitro MAPK activity (Fig. 3B), prevents in vivo activation of MEF2 (60) or Rlm1p (our unpublished data) by ERK5 or Slt2p, respectively. Within this C-terminal tail region on ERK5 is a MEF2-interacting region (residues 440 to 501) and transcriptional activation domain (residues 664 to 789) (25, 61). The C-terminal tail of Slt2p also has transcriptional activation potential (28, 51). Therefore, the C-terminal sequence extensions that set ERK5 and Slt2p apart from the other MAPKs of mammalian cells and yeast appear to be of rather similar function. This, in turn, probably contributes to the ability of ERK5 to provide cell integrity MAPK function when expressed in yeast in place of the native Slt2p (Fig. 1). Despite this, these C-terminal regions display no obvious sequence similarities. That of Slt2p is much smaller (Slt2p is only 484 amino acids long, whereas ERK5 is 789 amino acids). Also, it has no MAPK phosphorylation consensus, whereas the much larger C-terminal region of ERK5 contains no less than 10 potential MAPK phosphorylation sites (ERK5 undergoes autophosphorylation) (5) and 2 proline-rich regions. The second of these ERK5 proline-rich regions (124 amino acids with 44 prolines) includes several proline-alanine repeats, a motif also present in myosin light chain kinase, where it interacts directly with actin. Thus, like the nuclear localization signal of the ERK5 C-terminal tail (5, 60), this region may have a role in the targeting of ERK5 to specific locations in the cell (60).
Hsp90 dependence may be limited to just a few MAPKs. Whether the Hsp90 requirement of ERK5 and Slt2p is associated with the role of the large C-terminal sequence regions on these MAPKs is at present unclear, although loss of the latter region on ERK5 does not abolish either Hsp90 binding (34) (Fig. 5A) or the susceptibility of the kinase activity to Hsp90 inhibitors (Fig. 3B).
Acknowledgments
We are indebted to S. Fields, J. Francois, J. Hegemann, E. Hettema, D. Levin, S. Lindquist, C. Marshall, M. Molina, K. Matsumoto, and B. Panaretou for gifts of strains, plasmids, and antisera.
This work was supported by grants from the Wellcome Trust (074575/Z/04/Z) and BBSRC (C506721/1).
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Abe, J., M. Kusuhara, R. J. Ulevitch, B. C. Berk, and J. D. Lee. 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 271:16586-16590. [DOI] [PubMed] [Google Scholar]
- 2.Adams, A., D. E. Gottschling, C. A. Kaiser, and T. Stearns. 1997. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Atienza, J. M., M. Suh, I. Xenarios, R. Landgraf, and J. Colicelli. 2000. Human ERK1 induces filamentous growth and cell wall remodeling pathways in Saccharomyces cerevisiae. J. Biol. Chem. 275:20638-20646. [DOI] [PubMed] [Google Scholar]
- 4.Barros, J. C., and C. J. Marshall. 2005. Activation of either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of the actin cytoskeleton. J. Cell Sci. 118:1663-1671. [DOI] [PubMed] [Google Scholar]
- 5.Buschbeck, M., and A. Ullrich. 2005. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J. Biol. Chem. 280:2659-2667. [DOI] [PubMed] [Google Scholar]
- 6.Citri, A., D. Harari, G. Shochat, P. Ramakrishnan, J. Gan, M. Eisenstein, A. Kimchi, D. Wallach, S. Pietrokovski, and Y. Yarden. 2006. Hsp90 recognizes a common surface on client kinases. J. Biol. Chem. 281:14361-14369. [DOI] [PubMed] [Google Scholar]
- 7.Collister, M., M. P. Didmon, F. MacIsaac, M. J. Stark, N. Q. MacDonald, and S. M. Keyse. 2002. YIL113w encodes a functional dual-specificity protein phosphatase which specifically interacts with and inactivates the Slt2/Mpk1p MAP kinase in S. cerevisiae. FEBS Lett. 527:186-192. [DOI] [PubMed] [Google Scholar]
- 8.Dean, N. 1995. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc. Natl. Acad. Sci. USA 92:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 10.Dodou, E., and R. Treisman. 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emrick, M. A., A. N. Hoofnagle, A. S. Miller, L. F. Ten Eyck, and N. G. Ahn. 2001. Constitutive activation of extracellular signal-regulated kinase 2 by synergistic point mutations. J. Biol. Chem. 276:46469-46479. [DOI] [PubMed] [Google Scholar]
- 12.Flandez, M., I. C. Cosano, C. Nombela, H. Martin, and M. Molina. 2004. Reciprocal regulation between Slt2 MAPK and isoforms of Msg5 dual-specificity protein phosphatase modulates the yeast cell integrity pathway. J. Biol. Chem. 279:11027-11034. [DOI] [PubMed] [Google Scholar]
- 13.Galcheva-Gargova, Z., B. Derijard, I. H. Wu, and R. J. Davis. 1994. An osmosensing signal transduction pathway in mammalian cells. Science 265:806-808. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, R., C. Bermejo, C. Grau, R. Perez, J. M. Rodriguez-Pena, J. Francois, C. Nombela, and J. Arroyo. 2004. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 279:15183-15195. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, J. S., and D. J. Thiele. 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 277:21278-21284. [DOI] [PubMed] [Google Scholar]
- 17.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, M., S. W. Kim, K. Imanaka-Yoshida, T. Yoshida, E. D. Abel, B. Eliceiri, Y. Yang, R. J. Ulevitch, and J. D. Lee. 2004. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J. Clin. Investig. 113:1138-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi, M., and J. D. Lee. 2004. Role of the BMK1/ERK5 signaling pathway: lessons from knockout mice. J. Mol. Med. 82:800-808. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda, H., N. Ohta, K. Furukawa, H. Miyazaki, L. Wang, K. Kuribayashi, L. J. Old, and H. Shiku. 1997. Mutated mitogen-activated protein kinase: a tumor rejection antigen of mouse sarcoma. Proc. Natl. Acad. Sci. USA 94:6375-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 23.Jung, U. S., A. K. Sobering, M. J. Romeo, and D. E. Levin. 2002. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46:781-789. [DOI] [PubMed] [Google Scholar]
- 24.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 25.Kasler, H. G., J. Victoria, O. Duramad, and A. Winoto. 2000. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell. Biol. 20:8382-8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J. D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16:7054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirchrath, L., A. Lorberg, H. P. Schmitz, U. Gengenbacher, and J. J. Heinisch. 2000. Comparative genetic and physiological studies of the MAP kinase Mpk1p from Kluyveromyces lactis and Saccharomyces cerevisiae. J. Mol. Biol. 300:743-758. [DOI] [PubMed] [Google Scholar]
- 29.Krause, S. A., and J. V. Gray. 2002. The protein kinase C pathway is required for viability in quiescence in Saccharomyces cerevisiae. Curr. Biol. 12:588-593. [DOI] [PubMed] [Google Scholar]
- 30.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louvion, J. F., T. Abbas-Terki, and D. Picard. 1998. Hsp90 is required for pheromone signaling in yeast. Mol. Biol. Cell 9:3071-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 33.McDonald, O. B., W. J. Chen, B. Ellis, C. Hoffman, L. Overton, M. Rink, A. Smith, C. J. Marshall, and E. R. Wood. 1999. A scintillation proximity assay for the Raf/MEK/ERK kinase cascade: high-throughput screening and identification of selective enzyme inhibitors. Anal. Biochem. 268:318-329. [DOI] [PubMed] [Google Scholar]
- 34.Millson, S. H., A. Truman, V. King, C. Prodromou, L. Pearl, and P. W. Piper. 2005. A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in activity of a stress-activated MAP kinase, Slt2p(Mpk1p). Eukaryot. Cell 4:849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millson, S. H., A. Truman, F. Wolfram, V. King, B. Panaretou, C. Prodromou, L. H. Pearl, and P. W. Piper. 2004. Investigating the protein-protein interactions of the yeast Hsp90 chaperone system by two hybrid analysis: potential uses and limitations of this approach. Cell Stress Chaperones 9:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millson, S. H., A. Truman, and P. W. Piper. 2003. Vectors for N- or C-terminal positioning of the yeast Gal4p DNA binding or activator domains. BioTechniques 35:60-64. [DOI] [PubMed] [Google Scholar]
- 37.Miyata, Y., Y. Ikawa, M. Shibuya, and E. Nishida. 2001. Specific association of a set of molecular chaperones including HSP90 and Cdc37 with MOK, a member of the MAP kinase superfamily. J. Biol. Chem. 16:16. [DOI] [PubMed] [Google Scholar]
- 38.Nathan, D. F., and S. Lindquist. 1995. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 15:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolen, B., S. Taylor, and G. Ghosh. 2004. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15:661-675. [DOI] [PubMed] [Google Scholar]
- 40.Panaretou, B., C. Prodromou, S. M. Roe, R. O'Brien, J. E. Ladbury, P. W. Piper, and L. H. Pearl. 1998. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17:4829-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearl, L. H., and C. Prodromou. 2006. Structure and mechanism of the hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75:271-294. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 43.Pi, X., G. Garin, L. Xie, Q. Zheng, H. Wei, J. Abe, C. Yan, and B. C. Berk. 2005. BMK1/ERK5 is a novel regulator of angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ. Res. 96:1145-1151. [DOI] [PubMed] [Google Scholar]
- 44.Piper, P. W., S. H. Millson, M. Mollapour, B. Panaretou, G. Siligardi, L. H. Pearl, and C. Prodromou. 2003. Sensitivity to Hsp90-targeting drugs can arise with mutation to the Hsp90 chaperone, cochaperones and plasma membrane ATP binding cassette transporters of yeast. Eur. J. Biochem. 270:4689-4695. [DOI] [PubMed] [Google Scholar]
- 45.Prince, T., and R. L. Matts. 2004. Definition of protein kinase sequence motifs that trigger high affinity binding of Hsp90 and Cdc37. J. Biol. Chem. 279:39975-39981. [DOI] [PubMed] [Google Scholar]
- 46.Prince, T., and R. L. Matts. 2005. Exposure of protein kinase motifs that trigger binding of Hsp90 and Cdc37. Biochem. Biophys. Res. Commun. 338:1447-1454. [DOI] [PubMed] [Google Scholar]
- 47.Queralt, E., and J. C. Igual. 2005. Functional connection between the Clb5 cyclin, the protein kinase C pathway and the Swi4 transcription factor in Saccharomyces cerevisiae. Genetics 171:1485-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riggs, D. L., M. B. Cox, J. Cheung-Flynn, V. Prapapanich, P. E. Carrigan, and D. F. Smith. 2004. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit. Rev. Biochem. Mol. Biol. 39:279-295. [DOI] [PubMed] [Google Scholar]
- 49.Schena, M., D. Picard, and K. R. Yamamoto. 1991. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 194:389-398. [DOI] [PubMed] [Google Scholar]
- 50.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soler, M., A. Plovins, H. Martin, M. Molina, and C. Nombela. 1995. Characterization of domains in the yeast MAP kinase Slt2 (Mpk1) required for functional activity and in vivo interaction with protein kinases Mkk1 and Mkk2. Mol. Microbiol. 17:833-842. [DOI] [PubMed] [Google Scholar]
- 52.Tanoue, T., and E. Nishida. 2003. Molecular recognitions in the MAP kinase cascades. Cell. Signal. 15:455-462. [DOI] [PubMed] [Google Scholar]
- 53.Tatebe, H., and K. Shiozaki. 2003. Identification of Cdc37 as a novel regulator of the stress-responsive mitogen-activated protein kinase. Mol. Cell. Biol. 23:5132-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uetz, P., G. Cagney, D. Lockshon, A. Qureshi-Emili, D. Conover, M. Johnston, and S. Fields. 2000. A protein array for genomewide screens of protein-protein interactions. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 55.van Drogen, F., and M. Peter. 2002. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 12:1698-1703. [DOI] [PubMed] [Google Scholar]
- 56.Wang, X., A. J. Merritt, J. Seyfried, C. Guo, E. S. Papadakis, K. G. Finegan, M. Kayahara, J. Dixon, R. P. Boot-Handford, E. J. Cartwright, U. Mayer, and C. Tournier. 2005. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol. Cell. Biol. 25:336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe, Y., G. Takaesu, M. Hagiwara, K. Irie, and K. Matsumoto. 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaakov, G., M. Bell, S. Hohmann, and D. Engelberg. 2003. Combination of two activating mutations in one HOG1 gene forms hyperactive enzymes that induce growth arrest. Mol. Cell. Biol. 23:4826-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan, C., H. Luo, J. D. Lee, J. Abe, and B. C. Berk. 2001. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 276:10870-10878. [DOI] [PubMed] [Google Scholar]
- 61.Yang, C. C., O. I. Ornatsky, J. C. McDermott, T. F. Cruz, and C. A. Prody. 1998. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 26:4771-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao, R., M. Davey, Y. C. Hsu, P. Kaplanek, A. Tong, A. B. Parsons, N. Krogan, G. Cagney, D. Mai, J. Greenblatt, C. Boone, A. Emili, and W. A. Houry. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120:715-727. [DOI] [PubMed] [Google Scholar]