Abstract
Leishmania is a protozoan parasite that causes serious morbidity and mortality in humans worldwide. The ability of these parasites to survive within the phagolysosomes of mammalian macrophages is dependent on the developmental regulation of a variety of genes. Identifying genomic sequences that are preferentially expressed during the parasite's intracellular growth would provide new insights about the mechanisms controlling stage-specific gene regulation for intracellular development of the parasite. Using a genomic library that differentially hybridized to probes made from total RNA from Leishmania infantum amastigote or promastigote life cycle stages, we identified a new class of noncoding RNAs (ncRNAs) ranging from ∼300 to 600 nucleotides in size that are expressed specifically in the intracellular amastigote stage. These ncRNAs are transcribed by RNA polymerase II from genomic clusters of tandem head-to-tail repeats, which are mainly located within subtelomeric regions. Remarkably, both the sense and antisense orientations of these ncRNAs are transcribed and are processed by trans splicing and polyadenylation. The levels of antisense transcripts are at least 10-fold lower than those of the sense transcripts and are tightly regulated. The sense and antisense ncRNAs are cytosolic as shown by fluorescence in situ hybridization studies and cosediment with a small ribonucleoprotein complex. Amastigote-specific regulation of these ncRNAs possibly occurs at the level of RNA stability. Interestingly, overexpression of these ncRNAs in promastigotes, as part of an episomal expression vector, failed to produce any transcript, which further highlights the instability of these RNAs in the promastigote stage. This is the first report describing developmentally regulated ncRNAs in protozoan parasites.
Leishmania protozoan parasites are the etiological agents of human leishmaniasis (22). A measure of the seriousness of this parasitic disease is the two million new cases of human leishmaniasis that appear annually (22). Infection with these parasites causes a wide spectrum of pathologies that range from self-healing cutaneous lesions to severe lethal visceral infections. Treatment of leishmaniasis cases typically involves the use of chemotherapeutics based on pentavalent antimony formulations. However, these classes of drugs are now being found to no longer be effective in some parts of the world (65). This problem is exacerbated, since there are no effective practical vaccines against the Leishmania parasites.
Leishmania parasites are transmitted to humans through the bite of an infected sand fly vector where they exist as flagellated promastigotes. After entry into the mammalian host, the promastigotes differentiate into the nonflagellated amastigotes within the phagolysosomal compartment of macrophages. Differentiation of promastigotes into amastigotes is a critical step for the establishment of infection in macrophages. Hence, characterization of genomic sequences that are preferentially expressed during the parasite's intracellular growth may facilitate determination of the mechanisms controlling stage-specific gene regulation and the intracellular life of the parasite. Several genes, such as the A2 gene and members of the amastin gene family, have already been reported to be specifically expressed in amastigotes of Leishmania (9, 55, 75). However, to date, there have been no reports of noncoding genomic sequences that are expressed in a stage-specific manner during the development of Leishmania. In other eukaryotes, noncoding RNAs (ncRNAs) have been found to be differentially expressed in tissues involved in development and associated with disease states such as cancer (reviewed in references 13, 21, 38, and 47). The sequencing of the Leishmania major Friedlin genome has been recently completed, and 911 RNA genes have been identified. This total comprises 83 tRNAs, 63 rRNAs, 63 spliced leader (SL) RNAs, 6 snRNAs, 695 snoRNAs, and a single signal recognition particle RNA (26). The snoRNAs are a family of constitutively expressed small nucleolar RNAs that act as guides for other rRNAs destined for 2′-O methylation and pseudouridilation (68). The SL RNA genes encode a 39-nucleotide (nt) miniexon that is fused to the 5′ ends of all expressed RNAs by the process of trans splicing (1, 56, 69). Successful completion of the trans splicing process requires the participation of the snRNAs (73). In addition to the above ncRNAs, there is the class of the guide RNAs that are encoded by the minicircles of the mitochondrial DNA in trypanosomatids and are used as templates in the uridine insertion/deletion RNA editing of maxicircle transcripts (61, 64).
In this work we identified a novel class of developmentally regulated noncoding RNAs that are transcribed from Leishmania infantum genomic clusters of tandem head-to-tail repeats, mainly located within subtelomeric regions, and have no homology to other known eukaryotic noncoding RNAs. To date, stage-specific noncoding RNAs (ss ncRNAs) have not been reported in trypanosomatid protozoa. We have further characterized these novel ncRNAs with respect to their transcription, 5′- and 3′-end processing, localization within the amastigote cell, and putative ability to bind proteins.
MATERIALS AND METHODS
Cell lines and culture of Leishmania.
The Leishmania infantum MHOM/MA/67/ITMAP-263 strain used in this study was described previously (59). L. infantum promastigotes were cultured at 25°C in RPMI 1640 (Invitrogen) medium (pH 7.4) supplemented with 10% fetal calf serum (Multicell; Wisent Inc.) and 5 μg/ml hemin. In order to obtain axenically cultured amastigotes of this species, stationary-phase parasites were transferred into MAA/20 cell-free medium (60), buffered to maintain the pH at 5.5, and grown for up to 7 or 8 days at 37°C in 5% CO2 as described previously (60). Axenic amastigotes were maintained in culture for several subpassages. After three or four passages on average, axenic amastigotes were recycled back to promastigotes to maintain infectivity. Promastigotes of Leishmania major LV39, L. major MHOM/IL/80/FRIEDLIN, Leishmania tropica, Leishmania tarentolae TARII, Leishmania donovani Sudanese 1S2D, and L. donovani LV39 strains were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated calf serum and 5 μg/ml hemin (29). Promastigotes were grown at pH 7.0 and 25°C. Intracellular amastigotes were obtained from the spleens of golden Syrian hamsters ∼1 month following injection of 5 × 107 L. donovani LV39 late-stationary-phase promastigotes into the intraperitoneal cavity.
Nucleic acid manipulations.
Intact Leishmania chromosomes were prepared from cells at mid to late log phase, washed, and lysed in situ in 1% low-melting-point agarose plugs as previously described (49). Intact Leishmania chromosomes migrated through 1% ultrapure agarose (Bio-Rad) gels in Tris-borate-EDTA using a Bio-Rad CHEF-DR III apparatus at 5.9 V/cm, 120° separation angle, and switch times ranging from 35 to 120 s for 25 to 30 h. Genomic DNA was obtained by using DNAzol reagent and following the instructions of the company (Invitrogen). Total RNA from parasites was obtained by using TRIzol reagent (Invitrogen) and following the instructions of the manufacturer. Purified samples were incubated twice with DNase I (New England BioLabs) for 20 min at 37°C. Southern and Northern blot hybridizations with [α-32P]dCTP-labeled probes and washes were done following standard procedures (57). In order to detect sense and antisense stage-specific ncRNA gene transcripts in Northern blot hybridizations, end labeling of oligonucleotides was undertaken with [γ-32P]dCTP. The following oligonucleotides were used to detect sense transcripts: 5′-TATATAGGGAGATGAGAGACGAATGGGAGTAGATGTAAAAGACGAGGGCGAAAGAAAGAAAGATATAGTCGACGAAAAGAGGT GTCTGCAACCAGCCGA-3′ and 5′-CGGAAGGCGTGCAAACACACACGCTTGATTTTCGAAAAAACAATTTGAACGAGGCGACAACTACAGGAATC GACAAGAGAGGCGAACCACCACCACAC-3′. The following oligonucleotides were used to detect antisense transcripts: 5′-CACTCCCTCGGTGCGTGTGGTGGTGGTTCGCCTCTCTTGTCGATTCCTGTAGTTGTCGCCTCGTTTCAAATTGTTTTTTCGAAAATCAAGCGTGTGTGTT-3′ and 5′-CCGTGGTTGCTGTCGTTACTGCGTCGGCTGGTTGCAGACACCTCTTTTCGTCGACTATATC TTTCTTTCTTTCGCCCTCGTCTTTTACATCTACTCCCAT-3′. Screening of the L. infantum cosmid library was undertaken as described previously (29). The library was hybridized independently to [α-32P]dCTP-labeled cDNA probes which were obtained from total RNA from L. infantum amastigotes or promastigotes. The DNA from cosmid clones of interest was obtained by phenol-chloroform extraction and ethanol precipitations under standard conditions. The 270-bp SacII genomic fragment derived from cosmid D6-46 was cloned into the pSP72 vector (Promega) in both orientations using standard conditions and sequenced. RNA transcripts were produced from the above vectors in vitro using the Maxiscript transcription kit (Ambion). Both orientations of the 270-bp SacII fragment were blunt ended and inserted into the EcoRV site of vector pSPαNEOα (75). This vector contains the neomycin phosphotransferase (NEO) selection marker, and maturation of the NEO transcript in this cassette occurs by sequences within the intergenic region of the α-tubulin gene (αIR) (28). Approximately 10- to 20-μg samples of the plasmid constructs were used to transfect L. infantum cells by electroporation as described previously (48). Transfectants were selected with 40 mg/ml G418 (Sigma). Transfected cells were plated on SDM-79 (2×) medium with 1.5% agar and 0.01 mg/ml of G418 (Sigma), and individual clones were obtained after 2 to 3 weeks. The sites for the addition of the miniexon and polyadenylation signals in the amastigote-specific ncRNAs were determined by cloning and sequencing of reverse transcriptase PCR (RT-PCR) products. A cDNA source that was expected to represent all poly(A)+ RNAs was synthesized from 5 μg of RNA by using an oligo(dT)18 primer and SuperScript II reverse transcriptase (Invitrogen). A 2-μl aliquot of cDNA was used as the template in PCR amplification reaction mixtures that contained the following pairs of oligonucleotides: 5′-TTCCTCTCCCTCACTCCATCGGT-3′ (see Fig. 5A) and oligo(dT) (5′-GGGCGCCTTTTTTTTTTTTT-3′) and an oligonucleotide recognizing part of the 39-nt miniexon sequence (5′-AACTAACGCTATATAAGTATC-3′) and the complementary ss ncRNA oligonucleotide (5′-GGGAGATGAGAGAACGAATGGG-3′) (see Fig. 5A). PCR products were blunt end cloned into a TA vector (Invitrogen) and sequenced. The oligonucleotide 5′-GGGAGATGAGAGAACGAATG-3′ (see Fig. 8C) was used with the primer oligo(dT) in PCR amplification of cDNAs in order to identify the presence of polyadenylated antisense ss ncRNAs in S150 fractions.
FIG. 5.
5′- and 3′-end processing of stage-specific noncoding RNAs by trans splicing and polyadenylation. (A) Genomic sequence of three, arbitrarily chosen, ∼270-bp repeats organized in tandem on chromosome 22 of L. infantum. An asterisk above a nucleotide indicates the first nucleotide in each repeat. Two putative splice donor sites for the addition of the SL sequence (miniexon) are present in each of the repeats. The putative splice donor sites (invariant dinucleotide AG) are in bold type. Putative polypyrimidine tracts are indicated by gray boxes. The AG dinucleotide at the 5′ splice site and its associated upstream polypyrimidine tracts are the most prominent and highly conserved cis-acting sequences in trypanosomatid pre-mRNA substrates (24, 29, 33, 37, 58). Underlined sequences represent the primers used in RT-PCRs to map the cDNA ends in panel B. (B) Sequence comparison of five cDNA clones that represent the full-length ss ncRNAs in L. infantum. The longest sequence in this group was arbitrarily chosen to act as a reference for sequence alignments. The 39-nt miniexon sequence in each of the cDNAs is underlined. Sites for the SacII restriction enzyme are shown in bold type. The poly(A) tail is represented by x(>20) and was found to be longer than 20 nucleotides for all cDNA clones. Nucleotide differences are shown in bold lowercase type. Dashes were used to maintain sequence alignment. Nucleotide deletions are symbolized with asterisks. Nucleotide insertions are shown by dots. The number of nucleotides is indicated at the end of each sequence.
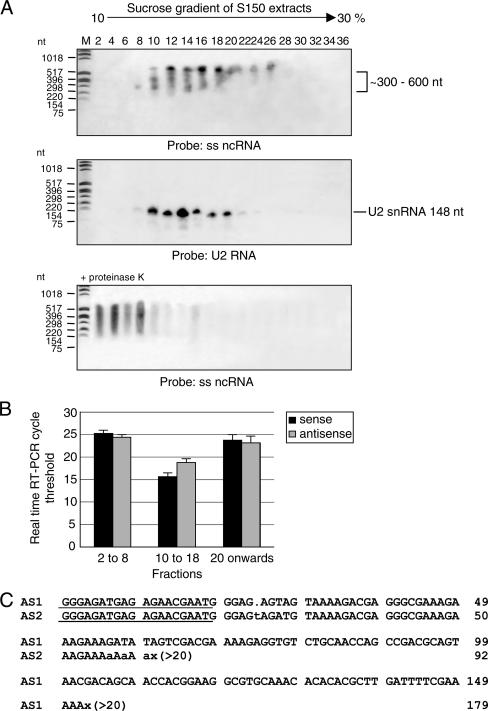
FIG. 8.
The stage-specific noncoding RNAs sediment at sucrose gradient coefficients similar to those of other small RNAs that form small RNP complexes in Leishmania. Thirty-six fractions were collected from a S150 extract of L. infantum amastigote lysates that had been centrifuged through a continuous 10 to 30% sucrose gradient. (A) Total RNA was obtained only from the even-numbered fractions and resolved by electrophoresis through a denaturing 6% acrylamide gel. A Northern blot with this gel was hybridized with the DNA probe corresponding to the ss ncRNA repeat and the U2 snRNA gene as a control. The fractions showing hybridization with the U2 probe are consistent with previously published data of Michaeli et al. (41). The bottom gel represents a Northern blot hybridization in which samples had been treated with proteinase K to assess whether the ss ncRNA-protein complexes could be disassociated. Lanes M contain the molecular size standards. The positions of molecular size markers are shown to the right of the gels. The numbers above the top gel correspond to the even-numbered fractions that were obtained from the sucrose gradient. (B) Real-time RT-PCR threshold cycle values for the levels of sense and antisense ss ncRNAs in an independent source of S150 extract that was fractionated by a 10 to 30% sucrose gradient. Data represent the average cycle threshold values plus standard deviations (error bars) that were obtained from multiple real-time RT-PCR experiments. Fractions 2 to 8 and 20 onwards produced a cycle threshold value relatively similar to that of the negative control without reverse transcriptase or without template (28.40 ± 0.05). Real-time RT-PCR experiments suggest that the sense and antisense ncRNAs are present in the same fractions. (C) Polyadenylated antisense ss ncRNAs are present in the S150 fractions. The cDNA clones of these polyadenylated antisense transcripts fell into two groups on the basis of their size like their sense counterparts. For this reason, the representatives of the two groups (AS1 and AS2) are shown aligned. Underlined sequence represents the internal ss ncRNA-specific primer that was used with an oligo(dT) primer to obtain the RT-PCR products from the S150 fractions. Numbers on the right represent the numbers of nucleotides. The poly(A) tail is denoted by a series of A nucleotides followed by the symbol x(>20). Matching nucleotides are shown in uppercase. A nucleotide insertion is represented by a dot.
Flow cytometry.
After 4 and 7 days of axenic culture, amastigotes were stained with 0.1% propidium iodide (Coulter Immunology, Hialeah, FL) for 5 min at room temperature. A minimum of 105 amastigotes were analyzed immediately on a cytofluorometer (EPICS Elite ESP; Coulter Electronics) and analyzed with WinMDI.
Nuclear run-on and RNA stability assays.
Axenically cultured L. infantum parasites were used for the nuclear run-on experiments and RNA stability assays. Fresh nuclei from promastigotes in exponential phase and amastigotes that were obtained after 7 days of differentiation under axenic conditions of growth were isolated, and nascent transcription was performed as described previously (51). For the nuclear run-on experiments, nuclei were obtained from the same promastigotes which were left untreated or incubated with 20, 50, and 200 μg/ml α-amanitin. Hybridization of these different samples of nuclei were undertaken with the following blotted double-stranded DNA templates: ss ncRNA, α-tubulin, 18S rRNA, 5S rRNA, and the empty vector. The phosphor autoradiography for each band after hybridization was quantitated with the Typhoon 9200 phosphorimager (GE Healthcare). Experiments assessing the stability of the stage-specific ncRNAs used amastigotes that had been differentiating for 7 days. Actinomycin D (Sigma) at 10 μg/ml was added to these amastigotes. Aliquots of this amastigote culture were removed after the addition of this inhibitor of transcription (1, 3, and 5 h after its addition) so that RNA could be extracted from these parasites.
FISH.
In vitro-transcribed sense and antisense stage-specific ncRNA sequences were generated by standard protocols and labeled with fluorescein isothiocyanate Alexa Fluor 488-5-UTP (Molecular Probes) by following the manufacturer's instructions. The Alexa Fluor 488-5-UTP-labeled RNA probes were purified with an RNeasy kit (QIAGEN) and quantified by standard agarose gel electrophoresis. The probes were stored at −80°C before use in fluorescence in situ hybridization (FISH). The protocol used for FISH was similar to the method of Zeiner et al. (78); their method was used with some modifications which are shown here. A total of 1 × 108 promastigotes or amastigotes that had been differentiating for 6 to 7 days were washed twice in 1× phosphate-buffered saline and fixed in 1 ml of fixation buffer (4% electron microscopy-grade formaldehyde [Sigma] and 5% acetic acid in 1× phosphate-buffered saline) for 20 min at room temperature. Formaldehyde traces were eliminated by three washes with 5 ml of 70% ethanol, and the pellet of cells was resuspended in 1 ml of 70% ethanol. Ten microliters of this suspension containing 1 × 106 parasites was spotted onto a microscope slide, dried at room temperature for 10 min, and incubated at 80°C for 10 min. Before hybridization with the RNA probes, the slides were treated twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min each time and then treated twice in 40% formamide-2× SSC solution for 5 min each time. Ten nanograms of Alexa Fluor 488-5-UTP-labeled RNA in a volume of 10 μl was mixed with 4 μl of a 5-mg/ml 1:1 solution of denatured salmon sperm DNA (Ambion) and Escherichia coli tRNA (Sigma), lyophilized, and resuspended in 12 μl of 80% formamide-10 mM sodium phosphate (pH 7.0) solution. The 12-μl solution of probes was incubated at 95°C for 3 min and mixed with a 12-μl solution containing 4× SSC, 4 μg/μl RNase-free bovine serum albumin (Sigma), and 50 U of RNAGuard RNase inhibitor (Amersham). This mixture was dropped immediately onto the fixed cells, and the slide was preheated at 72°C for 5 min. The slides were incubated at 37°C overnight in a humidity chamber. Unbound probe on the slide was removed by a series of washes as follows: once in 30 ml of 50% formamide-2× SSC solution at 50°C for 15 min, once in 2× SSC at room temperature, and twice in 1× SSC at room temperature for 15 min each wash. Finally, nuclei were counterstained with 1 ng/ml 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) according to the manufacturer's instructions. A 10-μl volume of immuno-fluor mounting medium (ICN Biomedical) was dropped onto each slide in order to preserve the fluorescence for a longer period of time. Fluorescence was visualized with a Nikon C80i microscope that was equipped with a CF160 Plan-Fluor 100×/0.5-1.3 (numerical aperture) oil iris objective and the following filter sets: for DAPI, excitation wavelength, 340 to 380 nm; dichromatic mirror (DM), 400 nm; barrier filter (BA), 435 to 485 nm; for FITC, 468 to 495 nm; DM, 505 nm; BA, 515 to 555 nm. Images were obtained with a cooled mono 12-bit Retiga Exi Fast charge-coupled device camera (Quantitative Imaging Corporation) which was coupled with Qcapture version 2.68.6 software (Quantitative Imaging Corporation). Color images were produced with Adobe version 6.0 software.
Sucrose gradient analysis.
The S150 fractionation protocol was used with extracts obtained from approximately 2 × 1010 to 3 × 1010 L. infantum amastigotes as described previously (41). The S150 extracts were left untreated or treated with 20 μl of proteinase K (20 mg/ml) for 1 h at 37°C in order to dissociate the RNA-protein complexes before being layered on the gradient. Untreated and treated Leishmania S150 fractionated extracts were pelleted by centrifugation at 12,000 rpm for 15 min at 4°C, and the supernatant (40 OD260 [optical density at 260 nm] units) was layered on top of a 10% to 30% linear sucrose gradient (10 ml) in gradient buffer (50 mM Tris-HCl, pH 7.4, 50 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, 3 U/ml RNAGuard). The gradient was made with the gradient maker (catalog no. GM-100; CBS Scientific Co.). After centrifugation, approximately 35 to 36 0.3-ml fractions were collected at 4°C using an ISCO density gradient fractionation system under constant monitoring of absorption at 254 nm. RNA was extracted from each fraction by phenol-chloroform, followed by ethanol precipitation, and analyzed by Northern blot hybridization.
Real-time PCR studies.
Real-time RT-PCR studies were undertaken with the same RNA isolated from the gradient fractions. An aliquot representing 5% of the RNA in each sucrose gradient fraction was converted to cDNA by incubation at 42°C for 50 min with 50 U SuperScript reverse transcriptase (Invitrogen) and a gene-specific primer 5′-TTC CTC TCC CTC ACT CCC TTC GGT-3′ for the sense strand and 5′-ATG GGA GTA GAT GTA AAA GAC GAG GGC GAA AGA AAG-3′ for the antisense strand. The cDNA was used to perform fluorescence-based real-time PCR quantification using the Rotor-gene (Corbett; MBI). The SYBR green dye diluted 1/10,000 (catalog no. S7563; Molecular Probe) was used in the PCRs as described by the manufacturer. The thermal cycling protocol was as follows: 5 min at 95°C for the initial denaturation and then 35 cycles, with each cycle consisting of three steps, denaturation (45 s at 95°C), primer annealing (45 s at 95°C), and extension (45 s at 72°C).
RESULTS
Conserved tandem head-to-tail subtelomeric repeats in Leishmania infantum are expressed in a stage-specific manner.
In order to identify genes and DNA sequences that are regulated during the life cycle of L. infantum, a genomic DNA library of this parasite was constructed and screened with radiolabeled cDNA that was synthesized from total RNA of promastigote or amastigote origin. Several cosmid clones were discovered to produce a stronger hybridization signal when they were probed with amastigote cDNA than when they were probed with promastigote cDNA. One of these clones, designated D6-46, was arbitrarily chosen for further analyses. In Southern blot analyses, the D6-46 DNA was digested with SacII and hybridized with either the amastigote or promastigote cDNA probe, which revealed a band of ∼270 bp with specificity only to the amastigote cDNA probe (Fig. 1A). The 270-bp SacII DNA fragment was cloned and used as a probe in Northern blot hybridizations of total RNA isolated from both promastigotes and amastigotes. A smear of RNA fragments with lengths ranging from approximately 300 nt to approximately 600 nt in which there are two major transcripts of ∼300 and ∼600 nucleotides was detected by Northern blot hybridization only in the amastigote RNA derived either from L. infantum axenic cultures (Fig. 1B) or from L. donovani LV39 amastigotes which were isolated from the spleens of infected golden Syrian hamsters (Fig. 1C). A time course of amastigote growth under axenic conditions demonstrated that an increased expression of these RNAs is typically observable from day 7 onward, which corresponds to at least 15 amastigote cell divisions, and the accumulation of these RNAs increases significantly by day 8 (Fig. 1D, top blot). Expression of these RNAs has been detected as early as day 5 of axenic amastigote culture (see Fig. S1A in the supplemental material). In addition, flow cytometry analyses of parasites that had been stained with propidium iodide indicated that the majority of the axenic amastigotes after 4 and 7 days of culture were still viable (see Fig. S1B in the supplemental material). Late expression of these small RNAs was not due to a delay in parasite differentiation, as the amastin amastigote-specific gene that was used here as a control was already expressed at day 5 (Fig. 1D, middle blot). These data suggest that the 270-bp D6-46-derived sequence is expressed specifically in the intracellular form of the parasite after several rounds of amastigote division.
FIG. 1.
Identification of small noncoding developmentally regulated RNAs in Leishmania infantum by differential cDNA screening. (A) Southern blot hybridization of SacII-digested D6-46 cosmid DNA with cDNA probes which were prepared from total RNA of axenically grown promastigotes (p) or amastigotes (a). A ∼270-bp fragment hybridized only with the amastigote cDNA probe. (B) Total RNA from axenically grown promastigotes or amastigotes of L. infantum was Northern blotted and hybridized with the 270-bp SacII fragment or A2 gene. Hybridization of the 270-bp DNA probe resulted in a smear of hybridizing fragments in which there are two major transcripts with approximate sizes of 300 nt and 600 nt. (C) Hybridization of the 270-bp DNA probe to Northern blots of total RNA obtained from L. donovani promastigotes or amastigotes that had been isolated from the spleens of infected golden Syrian hamsters. (D) Hybridization of probes for 270-bp DNA, amastin gene, and 18S rRNA to Northern blots of total RNA obtained from L. infantum amastigotes grown under axenic conditions for 5 to 8 days. To evaluate the efficiency of axenic differentiation in culture from promastigotes into amastigotes, the expression of the A2 gene (9) (middle blot of panel B) or amastin (75) amastigote-specific gene (middle blot of panel D) was tested by Northern blot hybridization. RNA loading was monitored either by ethidium bromide staining of the agarose gels prior to Northern blotting or by hybridization with the 18S rRNA probe.
DNA sequencing of the 270-bp SacII genomic fragment and comparison to the existing Leishmania databases revealed that it was nearly identical to the previously reported 272-bp and 274-bp subtelomeric repeats of L. major Friedlin and L. major LV39 present predominantly on chromosomes 1 and 22 and in substantially lower numbers on chromosome 5 (18, 66). The L. infantum genome contains a much higher number of these repeats (∼162) which have 85 to 100% sequence identity with the 270-bp SacII fragment and are mainly found on chromosomes 1, 19, and 22 (http://www.genedb.org/genedb/linfantum/). As shown in Fig. 2A, there are 10 tandem repeats on chromosome 1, 38 tandem repeats on chromosome 19, and ∼113 repeats on chromosome 22, and these tandem repeats are all organized in a head-to-tail manner. On chromosomes 1 and 19, these repeats are subtelomeric. However, on chromosome 22, these repeats are organized in several clusters within the same polycistronic unit that spans a ∼133-kb region that ends at ∼11 kb from the right telomere (Fig. 2A). These clusters are interrupted by genomic sequences which are for the most part noncoding. The developmentally regulated 270-bp repeats that we have identified as part of the cosmid D6-46 are located on chromosome 22 and correspond to the first cluster of 12 repeats (Fig. 2A). As shown in experiments where chromosomes were separated by pulsed-field gel electrophoresis, blotted, and hybridized to the 270-bp repeat probe, sequences homologous to these repeats are found in several other Leishmania strains or species and seem to have a relatively similar chromosomal organization (Fig. 2B). Although these repeats are present in L. major, it is not known whether they are expressed, as no hybridization signal was detected in Northern blots of L. major total RNA that had been isolated from the footpad lesions of infected mice and hybridized with the 270-bp SacII fragment (data not shown). Interestingly, L. tarentolae, which is a nonpathogenic species, does not contain these expressed repetitive sequences, as no hybridization signal was detected, even under less stringent hybridization conditions (Fig. 2B and data not shown).
FIG. 2.
Genomic organization of the developmentally regulated ∼270-bp tandem subtelomeric repeats in L. infantum. (A) Schematic representations of the tandem organization of the ∼270-bp repeats found on L. infantum chromosomes (Chr) 1, 19, and 22. The sequence organization and the orientation of these repeats were deduced from L. infantum genome database version 2.0 (http://www.genedb.org/genedb/linfantum/). A single repeat is depicted as a filled triangle. Black arrows represent tandem head-to-tail repeats (from 10 to 53) present on the different chromosomes. On chromosomes 1 and 19, the ∼270-bp repeats are located close to the right telomeres. However, on chromosome 22, 113 repeats are presented in different clusters that cover a region of ∼133 kb that is mainly nonprotein coding on the upper DNA strand. At ∼11 kb from the right telomere, there is a bicistronic transcription unit on the lower DNA strand and in the opposite orientation. (B) Hybridization of the L. infantum 270-bp repeat probe to Leishmania chromosomes. Chromosomes of Leishmania tarentolae (Lta), L. donovani 1S2D strain (Ld IS), L. infantum (Lin), L. major LV39 strain (Lm LV39), L. major Friedlin strain (LmF), and L. tropica (Ltr) were subjected to pulsed-field gel electrophoresis. In most Leishmania species, chromosomes (Chr) 1, 5, 19, and 22 appear to contain homologs of the developmentally regulated ∼270-bp repeats but have a variable number of copies.
This is the first time that repetitive sequences found close to the chromosomal ends are reported to be expressed in a stage-specific manner. These repeats are likely to be noncoding, as suggested by several algorithms (26) and by BLAST analyses of the L. major and L. infantum genome databases (http://www.genedb.org). Moreover, no putative initiation methionine codons that would result in expressed sequence conforming to the sizes of the ∼300- to 600-nt RNA species (Fig. 1) were found. Due to their specific expression in the amastigote stage of L. infantum, we refer to these RNAs as stage-specific noncoding RNAs (ss ncRNAs) henceforth.
The stage-specific ncRNA genes are transcribed by RNA Pol II.
Noncoding RNAs which have been characterized in trypanosomatids utilize one of three RNA polymerases for their transcription (reviewed in reference 8). The rRNA genes are transcribed by RNA polymerase (Pol) I, and transcription of the 5S rRNA and the tRNAs occurs with RNA polymerase III (44, 77). In contrast, transcription of the protein-coding genes, snoRNAs and the spliced leader RNA is performed by RNA polymerase II (19, 76). To determine which of the three RNA polymerases is responsible for the transcription of the ss ncRNA genes, nuclear run-on experiments were undertaken with L. infantum promastigotes which had been incubated with 0, 20, 50, and 200 μg/ml of α-amanitin. The α-amanitin drug inhibits the activity of RNA polymerase II (reviewed in reference 8). Nuclear run-on analyses suggest that the ss ncRNA genes are transcribed by RNA polymerase II (Fig. 3A and B), as their transcription was greatly inhibited in the presence of 200 μg/ml α-amanitin, similar to the α-tubulin-RNA Pol II-transcribed gene. Moreover, the hybridization signals of the 18S rRNA and 5S rRNA controls with respect to the different drug concentrations in the nuclear run-on assays are consistent with these two genes being transcribed by RNA Pol I and III, respectively (Fig. 3A and B).
FIG. 3.
The stage-specific noncoding RNAs are transcribed by RNA polymerase II, and RNA transcripts are present in both developmental stages of Leishmania. (A) Nuclear run-on experiments were undertaken with L. infantum amastigotes that had been incubated in the absence or presence of various concentrations of α-amanitin (20, 50, and 200 μg/ml). Nascent [32P]dUTP-labeled RNA transcripts were obtained from nuclei of L. infantum left untreated or treated with α-amanitin and were hybridized with sequences (5 μg each) representing the ss ncRNA genomic repeat (∼270 bp), α-tubulin, and 18S and 5S rRNA genes which had been blotted onto a nylon membrane. The α-tubulin gene is known to be transcribed by RNA Pol II, the 18S rRNA is transcribed by RNA Pol I, and the 5S rRNA is transcribed by RNA Pol III (19, 76). (B) Quantitation by phosphorimaging of the hybridization signals from the nuclear run-on experiments in panel A. The relative activity for each gene was determined by using the value of the hybridizing band in the sample that had not been treated with α-amanitin as a basis for comparison. Values are expressed as a percentage. (C) Nuclear run-on studies with L. infantum promastigotes (p) or amastigotes (a) were done to determine whether there is any change in the transcription of the ss ncRNA genes in both stages of the parasite. Nascent [32P]UTP-labeled RNA transcripts from nuclei were hybridized to the ss ncRNA gene probe and to the α-tubulin gene which had been blotted onto a membrane. The α-tubulin gene is constitutively expressed in Leishmania. In this experiment, the hybridization signals for the α-tubulin gene were the same for promastigotes and amastigotes, suggesting that equivalent amounts of nuclear extracts had been used. In the experiments performed in panels A and C, use of the empty vector as a negative control resulted in little or no signal (not shown).
Stage-specific accumulation of the L. infantum ncRNAs is likely to be the result of an increase in RNA stability.
Expression of the ss ncRNAs has been demonstrated here to occur in intracellular L. infantum and L. donovani amastigotes but not in extracellular promastigotes (Fig. 1). As reported for several stage-specific transcripts in Leishmania, regulation takes place at the level of mRNA stability and/or translation (3, 7, 10, 75). Because the ss ncRNAs are not translated to a protein, their stage-specific regulation should occur at the level of RNA stability. It is possible that the ss ncRNA repeats are transcribed at levels in amastigotes that are similar to the levels in promastigotes but are highly unstable in promastigotes. To test this possibility, nuclear run-on experiments were undertaken using fresh nuclei isolated from both life stages of L. infantum. No differences in the density of RNA polymerase were indeed observed between promastigotes and amastigotes (Fig. 3C), suggesting that regulation of the ss ncRNAs occurs at the level of RNA stability. To further prove this, we looked at the half-life of the ss ncRNAs following treatment of amastigotes with 10 μg/ml of actinomycin D, which stalls transcription, hence facilitating the measurement of RNA decay. The ss ncRNAs were found to be very stable in amastigotes, with a half-life exceeding 5 h, which is far longer than that of the amastin amastigote-specific transcript (Fig. 4).
FIG. 4.
An increased stability of the stage-specific noncoding RNAs is responsible for their specific accumulation in Leishmania amastigotes. Total RNA was obtained from axenically cultured amastigotes prior to the addition of actinomycin D and at 1, 3, and 5 h after their exposure to this inhibitor of transcription. These RNA samples were Northern blotted and hybridized to a DNA probe corresponding to the ss ncRNA repeat (∼270 bp), the amastigote-specific amastin gene, and 18S rRNA gene. RNA loading was monitored by ethidium bromide staining of the agarose gels prior to Northern blotting.
The stage-specific Leishmania ncRNAs are processed by trans splicing and polyadenylation.
In Leishmania, mature mRNAs are generated by processing of the polycistronic precursors, which involves a trans splicing reaction in which a capped spliced leader of 39 to 41 nt is added to the 5′ end of each mRNA followed by polyadenylation of the 3′ ends of the RNAs (reviewed in reference 33). It is not yet known whether the ss ncRNAs are synthesized from discrete transcription units, but given their chromosomal organization in multiple tandem repeats (Fig. 2A), these RNAs are likely to be trans spliced to generate the smaller RNA species seen in Northern blot hybridizations (Fig. 1 and 4). To determine the splicing sites and map the 3′ ends of the ss ncRNAs, total RNA from L. infantum amastigotes was reverse transcribed and the cDNA from the first cDNA strand synthesis was used in PCRs to amplify the 5′ and 3′ ends of the ss ncRNAs (see Materials and Methods). Cloning and sequencing of PCR amplification products revealed that the 39-nt miniexon sequence had been added predominantly at the first AG splice acceptor site (Fig. 5). Within the 270-bp repeat, there are two putative splice acceptor sites flanked by upstream polypyrimidine tracts that could be used for accurate trans splicing (Fig. 5A). The addition of the miniexon to the second splice acceptor site has also been observed in a few of the cDNA clones sequenced (clone 3 in Fig. 5B and data not shown), indicating that all putative splice acceptor sites can be used. Surprisingly, for noncoding RNAs, all cDNA clones were found to be polyadenylated. Polyadenylation occurred in all cases at the same position, an adenosine 18 nucleotides downstream of the first AG splice acceptor site from the start of the repeat (Fig. 5B). There is no consensus polyadenylation signal in trypanosomatids such as the 5′-AATAAA-3′ sequence in higher eukaryotes (6). Instead, polyadenylation occurs within a short region 100 to 400 nt upstream of the next polypyrimidine trans splice signal (32, 37, 58, 71), and polypyrimidine tracts seem to play a major role in the coupling of these processes (25, 37). Sequence comparison between the five cDNA clones (clones 1 to 5 in Fig. 5B) indicated the presence of three types of polyadenylated transcripts of ∼330 nt, ∼360 nt, and ∼630 nt, which is consistent with the smear of RNA fragments ranging from ∼300 to 600 nt seen in Northern blot hybridizations (Fig. 1 and 4). However, we cannot exclude the possibility that nonpolyadenylated forms of the ss ncRNAs exist, as the first cDNA strand for these studies was synthesized using an oligo(dT) primer. Among the cDNA clones analyzed, three (clones 1 to 3 in Fig. 5B) contain approximately two copies of the 270-bp repeat and two carry one tandem repeat (Fig. 5B, clones 4 and 5).
The stage-specific ncRNAs are localized in the cytosol.
To gain better insight into the function of the ss ncRNAs, we first looked at their subcellular localization. This was achieved by using fluorescence in situ hybridization analyses with axenically cultured amastigotes that had undergone differentiation for ∼7 days (Fig. 6). In the FISH experiments, the antisense strand of the ss ncRNA was labeled with Alexa Fluor 488-5-UTP to detect the sense transcripts (Fig. 6). A discrete signal was detected in the majority of the amastigote cells (Fig. 6, left panel). For a control, the sense orientation of the ss ncRNA that should detect antisense transcripts was labeled with Alexa Fluor 488 and used in FISH. To our surprise, a similar signal localization was obtained with the sense probe as well (Fig. 6, right panel), indicating that antisense ss ncRNAs are also expressed in amastigote cells. Amastigotes were hybridized with labeled sense or antisense ss ncRNA transcripts and then counterstained with 4′,6′-diamidino-2-phenylindole to determine where the ss ncRNAs are localized with respect to the nucleus (pale blue) and the kinetoplast (intense blue) (Fig. 6). Merging the signals revealed that both orientations of ncRNAs localized as one to three discrete spots in the cytosol of amastigote cells (Fig. 6). This experiment was repeated several times in order to confirm the location of the ss ncRNAs and the number of spots detected per cell due to the difficulty in obtaining single cells for axenically cultured amastigotes (data not shown). In the majority of the cells analyzed, sense and antisense ss ncRNAs consistently localized as one discrete spot in the cytosol; however, two or three spots were observed in a few cases only (Fig. 6). In the FISH studies undertaken here, the signal observed for the ss ncRNAs in the amastigotes was not due to the labeled probes binding to their cDNA strand in the genome, because no signal was detected with promastigotes in multiple experiments (data not shown). For this same reason, we are able to exclude the possibility that the signals for the ss ncRNAs correspond to the subtelomeres carrying the ss ncRNA repeats that congregate at the nuclear boundary, as has been seen in the different life cycle stages of Plasmodium falciparum (15, 17). Moreover, no signal in the nucleus was observed (Fig. 6). Control FISH experiments without a probe did not produce any specific signals (data not shown).
FIG. 6.
The amastigote-specific noncoding RNAs are localized at discrete positions within the cytosol. L. infantum amastigotes that had been grown under axenic conditions for more than 6 days were used for fluorescence in situ hybridization analyses to determine the cellular localization of the ss ncRNAs. Differential interference contrast (DIC) microscopy was used to visualize individual amastigote cells that were used for FISH experiments. Nuclei and kinetoplast DNA were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), which fluoresces blue. Sense and antisense ss ncRNAs were synthesized by in vitro transcription and labeled with Alexa Fluor 488-5-UTP, a green fluorescing compound, before their hybridization to the amastigotes. The DAPI and Alexa Fluor 488-5-UTP images were merged to determine the subcellular localization of the ss ncRNAs.
The stage-specific ncRNAs are expressed in sense and antisense orientations of transcription.
Data from the FISH analyses above indicate that both orientations of the ss ncRNAs are expressed in Leishmania amastigotes. This was an intriguing result, and additional experiments were undertaken to determine the levels of expression of sense and antisense ncRNAs in the amastigote stage. The presence of sense and antisense ss ncRNAs are indeed present as determined by Northern blot hybridizations with end-labeled oligonucleotide probes that would detect either the sense or antisense transcript of the ss ncRNA gene (Fig. 7A). Promastigotes show no hybridization signal for probes detecting transcripts pertaining to either orientation of the ss ncRNA gene, which is consistent with what was observed when using the double-stranded DNA probe for this gene (Fig. 7A). Sense and antisense transcripts were detected only in the amastigote life cycle stage. The amastigote-specific hybridization signals for both the sense and antisense ss ncRNA probes revealed a smear of fragments with lengths ranging from 300 to 600 nt (Fig. 7A), which indicates that both orientations of the ss ncRNA gene are expressed. In these Northern blot hybridizations, the specific activities of the end-labeled oligonucleotide probes for both the sense and antisense orientations were not equalized, so for this reason it was not possible to make a quantitative statement about the number of transcripts present for either orientation of the ss ncRNA gene in the amastigote life cycle stage. Semiquantitative real-time RT-PCR experiments with amastigote total RNA using specific primers capable of distinguishing between sense and antisense transcripts (see Materials and Methods) indeed confirmed that antisense ss ncRNAs are expressed in amastigotes, albeit at a level approximately 10-fold lower than that of the sense ncRNAs (Fig. 7B). Preliminary results from cDNA sequencing indicate that the sizes of the antisense transcripts are similar to those of the sense transcripts (data not shown). To investigate whether the underrepresentation of the antisense ss ncRNAs is subjected to a tight regulation in amastigotes, we overexpressed the sense and antisense ncRNAs as part of the pSPαNEOα episomal vector in L. infantum amastigotes (Fig. 7C). As shown by Northern blot hybridizations with total RNA isolated from the above recombinant Leishmania, overexpression of the sense ss ncRNAs in amastigotes resulted in higher levels of transcript compared to the levels in wild-type parasites (Fig. 7D). However, overexpression of the antisense ss ncRNA construct did not alter the levels of the endogenous ss ncRNAs (Fig. 7D). These data are in agreement with the real-time RT-PCR results (Fig. 7B) and suggest that the expression of the antisense ss ncRNAs in L. infantum amastigotes is tightly regulated and that antisense transcript has to be present at a level lower than that of the sense transcript. Although the ss ncRNAs are transcribed similarly in promastigotes and amastigotes (Fig. 3C), these RNAs do not accumulate in promastigotes (Fig. 1). Interestingly, the overexpression of either the sense or antisense ncRNAs in L. infantum promastigotes did not increase transcript accumulation, despite the fact that the neomycin phosphotransferase gene on the same vector was indeed expressed (Fig. 7D), suggesting that ss ncRNAs may be rapidly degraded in this stage of the parasite. The lower hybridization signal with the NEO mRNA in promastigotes is not due to less RNA loading (Fig. 7D). The lower hybridization signal with the NEO probe in promastigotes suggests that the promastigotes have a lower copy number of the NEO-containing vector potentially due to the toxicity of the ss ncRNA to this parasite life stage. Longer exposures of the Northern blots did not reveal ss ncRNA expression in promastigotes (data not shown). These data further emphasize the exclusive expression of the ncRNAs in Leishmania amastigotes and the important role that these RNAs may play in the intracellular development of the parasite.
FIG. 7.
The antisense stage-specific noncoding RNAs are expressed at levels significantly lower than those of the sense ss ncRNAs. (A) Northern blot hybridization of L. infantum total RNA with end-labeled oligonucleotide probes specific for the sense and antisense transcripts of the ss ncRNA gene. Total RNA from promastigotes (p) and amastigotes (a) was used. Amastigote-specific transcripts are expressed for both orientations of the ss ncRNA gene. Double-stranded DNA (ds DNA) of the cloned ss ncRNA gene was used as a probe, and it was used as a positive control. RNA loading was checked by ethidium bromide staining of the agarose gels prior to Northern blotting. oligo, oligonucleotide. (B) Real-time RT-PCR was undertaken with total RNA isolated from amastigotes to semiquantitatively measure the levels of the antisense ncRNAs and to determine the ratio of sense/antisense transcripts. Real-time RT-PCR cycle threshold values revealed that the levels of antisense ss ncRNAs are more than 10-fold lower than those of sense ss ncRNAs. (C) Both orientations of the ss ncRNAs were cloned into the expression vector pSP72-αIRNEOαIR (see Materials and Methods), transfected into L. infantum, and grown as axenic amastigotes. Parasites containing the construct with the ss ncRNA cloned in the sense or antisense orientation are numbered 1 and 2, respectively. (D) Total RNA was obtained from L. infantum promastigotes and amastigotes and Northern blotted onto the same membrane. The identical membrane was hybridized to DNA probes corresponding to the ss ncRNA repeat and the neomycin phosphotransferase gene (NEO). RNA loading was monitored by ethidium bromide staining of the agarose gels prior to Northern blotting to visualize rRNA. WT, wild type. 1 and 2 are as in panel C.
Polyadenylated sense and antisense ss ncRNAs form a RNP complex.
Noncoding RNAs, such as SL RNA, snoRNAs, and BC1 RNA, can form a ribonucleoprotein (RNP) complex with splicing factors or RNA-modifying enzymes and/or translation initiation factors, respectively (41, 72, 74). Hence, a preliminary investigation was undertaken to determine whether the ss ncRNAs could be part of a ribonucleoprotein complex by subjecting amastigote extracts to S150 centrifugation (41) and fractionation over a 10 to 30% sucrose gradient. Hybridization of total RNA isolated from each fraction with a probe recognizing the ncRNA 270-bp repeat revealed that these RNAs are present mainly in fractions 12 to 18 (Fig. 8A, top panel) . Northern blot hybridizations with the U2 snRNA gene probe which has been previously reported to complex to proteins involved in RNA splicing and have known S150 extract sedimentation coefficients between 4S to 11S (41) indicated that the ss ncRNAs could form RNA-protein complexes with sizes similar to that of the RNP splicing complex (Fig. 8A, middle panel). The ss ncRNAs in the above fractions are associated with proteins, as proteinase K treatment of the S150 extracts abolished the formation of these complexes (Fig. 8A, lower panel). However, the ss ncRNAs have a cytoplasmic localization (Fig. 6), which excludes them as having a role in trans splicing and suggests that these ncRNAs probably form a distinct RNP complex.
The Northern blot hybridization experiments in Fig. 8A were undertaken with a probe that was not capable of distinguishing the sense ss ncRNAs from antisense ss ncRNAs. In order to determine whether both orientations of the ss ncRNA could form RNP complexes, semiquantitative real-time RT-PCR was utilized with independent sources of RNA that had been fractionated in the same manner as described above. The sense and antisense ncRNAs were detected in fractions 10 to 18 at levels ∼40-fold higher than those detected in the other fractions of the gradient (Fig. 8B), which is consistent with the Northern blot hybridization results (Fig. 8A). These data suggest that both the sense and antisense ss ncRNAs are likely to be found within the same RNP complex. The levels of the antisense transcripts in fractions 10 to 18 are approximately 10-fold lower than the levels of the sense ss ncRNAs (Fig. 8B), which is consistent with the real-time RT-PCR results obtained for both orientations when unfractionated total RNA was used (Fig. 7B).
The possibility that nonpolyadenylated ss ncRNAs are present in RNP complexes cannot be excluded at this time. However, it is very difficult to experimentally identify these nonpolyadenylated transcripts. Nevertheless, at the very least, it was possible to determine whether polyadenylated forms of the sense and antisense RNAs are present in the S150 fractions by sequencing cloned RT-PCR products from these S150 fractions. Polyadenylated sense and antisense ss ncRNAs were identified (Fig. 8C). The polyadenylated sense ss ncRNA transcripts corresponded to sequence already shown in Fig. 5 and for this reason are not shown with the polyadenylated antisense cDNAs in Fig. 8C.
DISCUSSION
Here, we have presented data on the characterization of a novel class of developmentally regulated noncoding RNAs that seem to be unique to Leishmania. We show that these ncRNAs have several interesting properties, including RNA Pol II transcription from subtelomeric tandem repeats, 3′-end processing by polyadenylation for sense and antisense strands, expression of sense and antisense transcripts in a tightly regulated manner, discrete cytosolic localization, and potential association with a small RNP complex. This is the first report of developmentally regulated noncoding RNAs in protozoan parasites.
Parasites of the L. donovani complex express a novel class of noncoding RNAs specifically in the intracellular life cycle stage.
The investigations undertaken here have resulted in the identification of a novel class of noncoding RNAs that are expressed specifically in the amastigote life cycle stage of L. infantum and L. donovani (Fig. 1). Expression of these ss ncRNAs occurs as early as day 5 and increases thereafter in axenic amastigote cultures, which typically contain only a small population of dead parasites (see Fig. S1 in the supplemental material). Moreover, the ss ncRNAs were expressed in amastigotes that had been grown in hamsters (Fig. 1C), which suggests that these ss ncRNAs are not likely to appear at the onset of apoptosis. These ncRNAs seem to be unique to Leishmania, and although they are present in different species, they seem to be mainly expressed by the L. donovani complex. No expression of these ncRNAs has been seen in L. major, at least under our experimental conditions, which leads us to hypothesize that these RNAs are expressed mainly by the L. donovani complex. Interestingly, only amastigotes that have undergone several rounds of cell divisions express these ncRNAs (Fig. 1D), which suggests that ss ncRNAs are not required for parasite differentiation but are likely to be important for the intracellular development of the parasite. Specific accumulation of these ncRNAs in Leishmania amastigotes is due to increased stability, whereas a rapid degradation of these RNAs is likely to occur in promastigotes (Fig. 4 and 7D). The latter is better illustrated by the fact that even episomal overexpression of the ss ncRNAs in L. infantum promastigotes failed to yield any transcript accumulation (Fig. 7D). It is even possible that these noncoding RNAs may be toxic in the promastigote life cycle stage.
At the completion of the genome sequencing project for L. major Friedlin, 911 RNA genes have been identified, and these RNA genes fall into the categories of tRNAs, rRNAs, SL RNAs, snRNAs, snoRNAs, and signal recognition particle RNAs (26). To the best of our knowledge, none of these RNA genes has been reported to be regulated in a stage-specific manner. On the basis of this stage-specific expression alone, the ss ncRNAs represent a new class of RNA genes in Leishmania. We investigated whether these RNAs have any sequence identity with other reported eukaryotic and prokaryotic noncoding RNAs. This led us to screen the RNAdb database (http://research.imb.uq.edu.au/RNAdb) (47) that contains approximately 20,000 noncoding RNAs of which there are more than 1,100 putative antisense RNAs. Currently, the ss ncRNAs show no identity to any reported noncoding RNAs and hence, represent a novel class of noncoding RNAs in eukaryotes and prokaryotes.
Noncoding RNAs are a major class of RNAs that vary in size from a few nucleotides to several thousand nucleotides and have been identified and characterized both in prokaryotes and eukaryotes (reviewed in reference 63). The ncRNAs other than tRNAs, rRNAs, and spliceosomal RNAs participate in a variety of cellular functions, including transcription, RNA processing or stability, RNA modification, RNA interference (RNAi), mRNA translation, imprinting, DNA methylation, and X-chromosome dosage compensation (reviewed in references 13 and 39). Several of these ncRNAs in humans have links to a number of diseases (reviewed in reference 39).
The stage-specific ncRNAs are transcribed by RNA polymerase II and processed by trans splicing and polyadenylation.
Similar to other small ncRNA genes in trypanosomatids (12, 45, 52, 53), the ss ncRNA genes in L. infantum are organized in clusters of tandem head-to-tail repeats on chromosomes 1, 19, and 22, which are mainly subtelomeric (Fig. 2A and data not shown) (18, 66). The ss ncRNA genes are transcribed by RNA polymerase II (Fig. 3), as is the case for other trypanosomatid small ncRNA genes, such as the SL RNA (19) and snoRNAs (76). Given that the majority of small ncRNAs in plants (31), Saccharomyces cerevisiae (35) and trypanosomatids (reviewed in reference 8) are transcribed from independent promoters, it is possible that this could be the case for the ss ncRNAs. However, this remains to be demonstrated.
The stage-specific ncRNAs exist as a population of stable RNAs that are heterogeneous in size (300 to 600 nt), and in this mixed RNA population, there are at least two predominant sizes for the polyadenylated sense and antisense transcripts (Fig. 1, 4, 5, 7, and 8). This size heterogeneity can be explained by the variable number of repeats (one to three), splice acceptor sites, and levels of polyadenylation that can be found within these ss ncRNAs (Fig. 5). Remarkably, the ss ncRNAs can be polyadenylated (Fig. 5 and 8) in contrast to other small RNAs, which usually utilize polyadenylation-independent pathways. In the case of snRNAs and snoRNAs, cleavage must be uncoupled from polyadenylation (14, 43, 62) to produce entry sites for 3′-5′ trimming performed by the exosome (2, 70) and to allow correct 3′-end maturation. The 3′-maturation pathways of most kinetoplastid small RNAs are not well defined, and it is yet not known whether they involve the concerted action of the endonucleolytic and/or exosome function (23). It is not yet understood why the ss ncRNAs are polyadenylated. We have previously reported that the noncoding SL RNA can be polyadenylated specifically in Leishmania amastigotes (29). This type of regulation may be related to the adenylation and deadenylation control that takes place in the maturation of several snRNAs and 7SL RNA (50). The spliced leader addition at the 5′ end of the ss ncRNAs may facilitate polyadenylation, as it has been reported that trans splicing and polyadenylation are possibly coupled in trypanosomatids (32, 37, 71). It is generally assumed that polyadenylation stabilizes the 3′ end of the mRNA (4), facilitates transport of the mRNA to the cytoplasm (24), and increases the efficiency of translation initiation (67). The RNA stability data (Fig. 4) and the cytosolic localization of these RNAs (Fig. 6) appear to be consistent with these hypotheses.
The stage-specific ncRNAs are expressed in both the sense and antisense orientations.
As shown by FISH and real-time PCR studies, both the sense and antisense ss ncRNAs are expressed in L. infantum amastigotes (Fig. 6 and 7). These findings add further insights into the biology of antisense RNA transcripts. Work undertaken with episomally expressed DNA by Curotto de Lafaille et al. provided early insights into sense and antisense transcription in Leishmania enriettii (11). Endogenously expressed antisense RNAs are known to exist in Leishmania (5, 27, 42). At the chromosomal level, strand-specific nuclear run-on assays showed that a low level of nonspecific antisense transcription probably takes place over the entire chromosome 1 of L. major Friedlin (36). However, an approximately 10-fold-higher level of coding strand-specific RNA polymerase II-mediated transcription initiates within the strand switch region (36). Moreover, investigations performed on the histone His-1.2 gene by Belli et al. showed that the complementary strand of DNA also contains sequences that could drive expression of open reading frames from the antisense strand of DNA (5). Nevertheless, the reasons for the existence of these antisense transcripts are puzzling in the absence of any RNAi machinery in Leishmania (54). The lack of RNAi in this parasite could possibly be explained by its tolerance to the presence of natural levels of double-stranded RNA due to some background transcription of the noncoding strand (36). In the absence of RNAi in Leishmania, it is very unlikely that the antisense ss ncRNAs could silence the sense transcripts through RNA degradation after base pairing. Moreover, the investigations undertaken here revealed that overexpressed antisense transcripts had no effect on the accumulation of the sense transcripts (Fig. 7D and data not shown).
The results presented in this study indicate that the antisense ss ncRNAs are at least 10-fold less abundant than the sense transcripts and that both orientations are expressed simultaneously in amastigotes and appear to form protein complexes that localize at similar subcellular positions (Fig. 6, 7, and 8). The levels of antisense ss ncRNAs in Leishmania amastigotes are tightly regulated and have to remain low, as overexpression of these RNAs did not change overall transcript accumulation, whereas the overexpression of the sense transcripts did (Fig. 7D). On the basis of these findings, it is conceivable that the antisense ss ncRNAs may act either to buffer against the sense RNA levels, for example, by competing for binding to similar factors or to base pair the sense RNA, hence changing, for example, the secondary structure of the latter and rendering it ineffective for complexing to its effector proteins. With the exception of small interfering RNAs (34), only a limited number of chromosomal cis-encoded antisense RNAs in eukaryotic cells have been shown to exert a regulatory effect on the cis-encoded mRNA by base pairing interactions (reviewed in reference 63). Many examples from bacteria for mechanisms that base pair the sense with antisense RNAs to regulate the function of the former are emerging (reviewed in reference 63). In E. coli, base pairing between the sense and antisense small RNAs has a requirement for the Hfq chaperone protein, a homolog of the Sm-like proteins (reviewed in reference 63) which are found in many eukaryotes and are also present in trypanosomatids (20, 46). Interestingly, sequence analysis of the sense ss ncRNAs revealed the presence of three putative U-rich Sm-like binding sites (data not shown). Experiments to characterize the RNA-protein interactions involving sense and antisense ss ncRNAs are planned.
Both the polyadenylated sense and antisense ss ncRNAs can form a RNP complex.
Our preliminary data suggest that the ss ncRNAs are part of a small ribonucleoprotein complex that has a sedimentation coefficient similar to that of the U2 RNP complex (Fig. 8A) (41). Interestingly, both sense and antisense ncRNAs are found within the same S150 fractions and at the same ∼10/1 ratio as observed in total RNA (Fig. 7 and 8), suggesting that they may interact with the same RNP complex. The results of FISH studies are consistent with this possibility, as the sense and antisense ss ncRNAs localize at the same positions within the amastigote cell (Fig. 6).
So far, only protein-coding stage-specific genes have been identified in Leishmania (9, 55, 75). Regulation of these transcripts is mediated by sequences in the 3′ untranslated region and is often controlled at the level of translation initiation (7, 16, 30, 40, 79). The possibility that developmentally regulated noncoding RNAs in Leishmania could influence the synthesis of specific proteins, as is the case in several eukaryotes but also in bacteria, is very intriguing. Interestingly, preliminary data from polysome profiling studies indicated that both the sense and antisense ncRNAs are associated with the small 40S ribosomal subunit, which suggests a role for these RNAs in the regulation of gene expression mainly at the level of translation (data not shown). Experiments are now under way to study the putative regulatory roles of these ncRNAs. Further studies characterizing the molecular interactions of these stage-specific noncoding Leishmania RNAs with proteins or other RNA molecules should provide new insights into the molecular mechanisms that control stage-specific gene regulation in protozoan parasites.
Supplementary Material
Acknowledgments
We thank Nathalie Boucher, Ying Wu, and Isabelle Pelletier for their initial involvement in the subcloning of the ncRNA gene from the L. infantum cosmid library. In addition, we thank Marc Ouellette for critical reading of the manuscript.
This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant (MOP-12182) awarded to B.P. C.C. is a postdoc fellow of the CIHR STP-53924 Strategic Training Program. B.P. is a Burroughs Wellcome Fund New Investigator in Molecular Parasitology and a member of a CIHR group on host-pathogen interactions.
Footnotes
Published ahead of print on 27 October 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Agami, R., R. Aly, S. Halman, and M. Shapira. 1994. Functional analysis of cis-acting DNA elements required for expression of the SL RNA gene in the parasitic protozoan Leishmania amazonensis. Nucleic Acids Res. 22:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allmang, C., J. Kufel, G. Chanfreau, P. Mitchell, E. Petfalski, and D. Tollervey. 1999. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 18:5399-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aly, R., M. Argaman, S. Halman, and M. Shapira. 1994. A regulatory role for the 5′ and 3′ untranslated regions in differential expression of hsp83 in Leishmania. Nucleic Acids Res. 22:2922-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beelman, C. A., and R. Parker. 1995. Degradation of mRNA in eukaryotes. Cell 81:179-183. [DOI] [PubMed] [Google Scholar]
- 5.Belli, S. I., S. Monnerat, C. Schaff, S. Masina, T. Noll, P. J. Myler, K. Stuart, and N. Fasel. 2003. Sense and antisense transcripts in the histone H1 (HIS-1) locus of Leishmania major. Int. J. Parasitol. 33:965-975. [DOI] [PubMed] [Google Scholar]
- 6.Borst, P. 1986. Discontinuous transcription and antigenic variation in trypanosomes. Annu. Rev. Biochem. 55:701-732. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, N., Y. Wu, C. Dumas, M. Dube, D. Sereno, M. Breton, and B. Papadopoulou. 2002. A common mechanism of stage-regulated gene expression in Leishmania mediated by a conserved 3′-untranslated region element. J. Biol. Chem. 277:19511-19520. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, D. A., S. Thomas, and N. R. Sturm. 2003. Transcription in kinetoplastid protozoa: why be normal? Microbes Infect. 5:1231-1240. [DOI] [PubMed] [Google Scholar]
- 9.Charest, H., and G. Matlashewski. 1994. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol. Cell. Biol. 14:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charest, H., W. W. Zhang, and G. Matlashewski. 1996. The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3′-untranslated region. J. Biol. Chem. 271:17081-17090. [DOI] [PubMed] [Google Scholar]
- 11.Curotto de Lafaille, M. A., A. Laban, and D. F. Wirth. 1992. Gene expression in Leishmania: analysis of essential 5′ DNA sequences. Proc. Natl. Acad. Sci. USA 89:2703-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar, D. A., A. A. Chen, S. Wormsley, and S. J. Baserga. 2000. The genes for small nucleolar RNAs in Trypanosoma brucei are organized in clusters and are transcribed as a polycistronic RNA. Nucleic Acids Res. 28:2855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddy, S. R. 2001. Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2:919-929. [DOI] [PubMed] [Google Scholar]
- 14.Fatica, A., M. Morlando, and I. Bozzoni. 2000. Yeast snoRNA accumulation relies on a cleavage-dependent/polyadenylation-independent 3′-processing apparatus. EMBO J. 19:6218-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueiredo, L. M., L. H. Freitas-Junior, E. Bottius, J. C. Olivo-Marin, and A. Scherf. 2002. A central role for Plasmodium falciparum subtelomeric regions in spatial positioning and telomere length regulation. EMBO J. 21:815-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folgueira, C., L. Quijada, M. Soto, D. R. Abanades, C. Alonso, and J. M. Requena. 2005. The translational efficiencies of the two Leishmania infantum HSP70 mRNAs, differing in their 3′-untranslated regions, are affected by shifts in the temperature of growth through different mechanisms. J. Biol. Chem. 280:35172-35183. [DOI] [PubMed] [Google Scholar]
- 17.Freitas-Junior, L. H., E. Bottius, L. A. Pirrit, K. W. Deitsch, C. Scheidig, F. Guinet, U. Nehrbass, T. E. Wellems, and A. Scherf. 2000. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407:1018-1022. [DOI] [PubMed] [Google Scholar]
- 18.Fu, G., and D. C. Barker. 1998. Characterisation of Leishmania telomeres reveals unusual telomeric repeats and conserved telomere-associated sequence. Nucleic Acids Res. 26:2161-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilinger, G., and V. Bellofatto. 2001. Trypanosome spliced leader RNA genes contain the first identified RNA polymerase II gene promoter in these organisms. Nucleic Acids Res. 29:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncharov, I., Z. Palfi, A. Bindereif, and S. Michaeli. 1999. Purification of the spliced leader ribonucleoprotein particle from Leptomonas collosoma revealed the existence of an Sm protein in trypanosomes. Cloning the SmE homologue. J. Biol. Chem. 274:12217-12221. [DOI] [PubMed] [Google Scholar]
- 21.Grishok, A., A. E. Pasquinelli, D. Conte, N. Li, S. Parrish, I. Ha, D. L. Baillie, A. Fire, G. Ruvkun, and C. C. Mello. 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106:23-34. [DOI] [PubMed] [Google Scholar]
- 22.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 23.Hitchcock, R. A., G. M. Zeiner, N. R. Sturm, and D. A. Campbell. 2004. The 3′ termini of small RNAs in Trypanosoma brucei. FEMS Microbiol. Lett. 236:73-78. [DOI] [PubMed] [Google Scholar]
- 24.Huang, Y., and G. C. Carmichael. 1996. Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol. Cell. Biol. 16:1534-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hug, M., H. R. Hotz, C. Hartmann, and C. Clayton. 1994. Hierarchies of RNA-processing signals in a trypanosome surface antigen mRNA precursor. Mol. Cell. Biol. 14:7428-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivens, A. C., C. S. Peacock, E. A. Worthey, L. Murphy, G. Aggarwal, M. Berriman, E. Sisk, M. A. Rajandream, E. Adlem, R. Aert, et al. 2005. The genome of the kinetoplastid parasite, Leishmania major. Science 309:436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapler, G. M., and S. M. Beverley. 1989. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol. Cell. Biol. 9:3959-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laban, A., J. F. Tobin, M. A. Curotto de Lafaille, and D. F. Wirth. 1990. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature 343:572-574. [DOI] [PubMed] [Google Scholar]
- 29.Lamontagne, J., and B. Papadopoulou. 1999. Developmental regulation of spliced leader RNA gene in Leishmania donovani amastigotes is mediated by specific polyadenylation. J. Biol. Chem. 274:6602-6609. [DOI] [PubMed] [Google Scholar]
- 30.Larreta, R., M. Soto, L. Quijada, C. Folgueira, D. R. Abanades, C. Alonso, and J. M. Requena. 2004. The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation. BMC Mol. Biol. 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leader, D. J., G. P. Clark, J. Watters, A. F. Beven, P. J. Shaw, and J. W. Brown. 1997. Clusters of multiple different small nucleolar RNA genes in plants are expressed as and processed from polycistronic pre-snoRNAs. EMBO J. 16:5742-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeBowitz, J. H., H. Q. Smith, L. Rusche, and S. M. Beverley. 1993. Coupling of poly(A) site selection and trans-splicing in Leishmania. Genes Dev. 7:996-1007. [DOI] [PubMed] [Google Scholar]
- 33.Liang, X.-H., A. Haritan, S. Uliel, and S. Michaeli. 2003. trans and cis splicing in trypanosomatids: mechanism, factors, and regulation. Eukaryot. Cell 2:830-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippman, Z., and R. Martienssen. 2004. The role of RNA interference in heterochromatic silencing. Nature 431:364-370. [DOI] [PubMed] [Google Scholar]
- 35.Lowe, T. M., and S. R. Eddy. 1999. A computational screen for methylation guide snoRNAs in yeast. Science 283:1168-1171. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Calvillo, S., S. Yan, D. Nguyen, M. Fox, K. Stuart, and P. J. Myler. 2003. Transcription of Leishmania major Friedlin chromosome 1 initiates in both directions within a single region. Mol. Cell 11:1291-1299. [DOI] [PubMed] [Google Scholar]
- 37.Matthews, K. R., C. Tschudi, and E. Ullu. 1994. A common pyrimidine-rich motif governs trans-splicing and polyadenylation of tubulin polycistronic pre-mRNA in trypanosomes. Genes Dev. 8:491-501. [DOI] [PubMed] [Google Scholar]
- 38.Mattick, J. S. 2001. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2:986-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattick, J. S. 2005. The functional genomics of noncoding RNA. Science 309:1527-1528. [DOI] [PubMed] [Google Scholar]
- 40.McNicoll, F., M. Muller, S. Cloutier, N. Boilard, A. Rochette, M. Dube, and B. Papadopoulou. 2005. Distinct 3′-untranslated region elements regulate stage-specific mRNA accumulation and translation in Leishmania. J. Biol. Chem. 280:35238-35246. [DOI] [PubMed] [Google Scholar]
- 41.Michaeli, S., T. G. Roberts, K. P. Watkins, and N. Agabian. 1990. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J. Biol. Chem. 265:10582-10588. [PubMed] [Google Scholar]
- 42.Monnerat, S., S. Martinez-Calvillo, E. Worthey, P. J. Myler, K. D. Stuart, and N. Fasel. 2004. Genomic organization and gene expression in a chromosomal region of Leishmania major. Mol. Biochem. Parasitol. 134:233-243. [DOI] [PubMed] [Google Scholar]
- 43.Morlando, M., P. Greco, B. Dichtl, A. Fatica, W. Keller, and I. Bozzoni. 2002. Functional analysis of yeast snoRNA and snRNA 3′-end formation mediated by uncoupling of cleavage and polyadenylation. Mol. Cell. Biol. 22:1379-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakaar, V., A. O. Dare, D. Hong, E. Ullu, and C. Tschudi. 1994. Upstream tRNA genes are essential for expression of small nuclear and cytoplasmic RNA genes in trypanosomes. Mol. Cell. Biol. 14:6736-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson, R. G., M. Parsons, M. Selkirk, G. Newport, P. J. Barr, and N. Agabian. 1984. Sequences homologous to variant antigen mRNA spliced leader in Trypanosomatidae which do not undergo antigenic variation. Nature 308:665-667. [DOI] [PubMed] [Google Scholar]
- 46.Palfi, Z., S. Lucke, H. W. Lahm, W. S. Lane, V. Kruft, E. Bragado-Nilsson, B. Seraphin, and A. Bindereif. 2000. The spliceosomal snRNP core complex of Trypanosoma brucei: cloning and functional analysis reveals seven Sm protein constituents. Proc. Natl. Acad. Sci. USA 97:8967-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang, K. C., S. Stephen, P. G. Engstrom, K. Tajul-Arifin, W. Chen, C. Wahlestedt, B. Lenhard, Y. Hayashizaki, and J. S. Mattick. 2005. RNAdb—a comprehensive mammalian noncoding RNA database. Nucleic Acids Res. 33:D125-D130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papadopoulou, B., G. Roy, and M. Ouellette. 1992. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 11:3601-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadopoulou, B., G. Roy, and M. Ouellette. 1993. Frequent amplification of a short chain dehydrogenase gene as part of circular and linear amplicons in methotrexate resistant Leishmania. Nucleic Acids Res. 21:4305-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perumal, K., and R. Reddy. 2002. The 3′ end formation in small RNAs. Gene Expr. 10:59-78. [PMC free article] [PubMed] [Google Scholar]
- 51.Quijada, L., M. Soto, C. Alonso, and J. M. Requena. 1997. Analysis of post-transcriptional regulation operating on transcription products of the tandemly linked Leishmania infantum hsp70 genes. J. Biol. Chem. 272:4493-4499. [DOI] [PubMed] [Google Scholar]
- 52.Roberts, T. G., J. M. Dungan, K. P. Watkins, and N. Agabian. 1996. The SLA RNA gene of Trypanosoma brucei is organized in a tandem array which encodes several small RNAs. Mol. Biochem. Parasitol. 83:163-174. [DOI] [PubMed] [Google Scholar]
- 53.Roberts, T. G., N. R. Sturm, B. K. Yee, M. C. Yu, T. Hartshorne, N. Agabian, and D. A. Campbell. 1998. Three small nucleolar RNAs identified from the spliced leader-associated RNA locus in kinetoplastid protozoans. Mol. Cell. Biol. 18:4409-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson, K. A., and S. M. Beverley. 2003. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128:217-228. [DOI] [PubMed] [Google Scholar]
- 55.Rochette, A., F. McNicoll, J. Girard, M. Breton, E. Leblanc, M. G. Bergeron, and B. Papadopoulou. 2005. Characterization and developmental gene regulation of a large gene family encoding amastin surface proteins in Leishmania spp. Mol. Biochem. Parasitol. 140:205-220. [DOI] [PubMed] [Google Scholar]
- 56.Saito, R. M., M. G. Elgort, and D. A. Campbell. 1994. A conserved upstream element is essential for transcription of the Leishmania tarentolae mini-exon gene. EMBO J. 13:5460-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Schürch, N., A. Hehl, E. Vassella, R. Braun, and I. Roditi. 1994. Accurate polyadenylation of procyclin mRNAs in Trypanosoma brucei is determined by pyrimidine-rich elements in the intergenic regions. Mol. Cell. Biol. 14:3668-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sereno, D., M. Cavaleyra, K. Zemzoumi, S. Maquaire, A. Ouaissi, and J. L. Lemesre. 1998. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob. Agents Chemother. 42:3097-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sereno, D., and J. L. Lemesre. 1997. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob. Agents Chemother. 41:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simpson, L., and O. H. Thiemann. 1995. Sense from nonsense: RNA editing in mitochondria of kinetoplastid protozoa and slime molds. Cell 81:837-840. [DOI] [PubMed] [Google Scholar]
- 62.Steinmetz, E. J., N. K. Conrad, D. A. Brow, and J. L. Corden. 2001. RNA-binding protein Nrd1 directs poly(A)-independent 3′-end formation of RNA polymerase II transcripts. Nature 413:327-331. [DOI] [PubMed] [Google Scholar]
- 63.Storz, G., S. Altuvia, and K. M. Wassarman. 2005. An abundance of RNA regulators. Annu. Rev. Biochem. 74:199-217. [DOI] [PubMed] [Google Scholar]
- 64.Sturm, N. R., and L. Simpson. 1990. Kinetoplast DNA minicircles encode guide RNAs for editing of cytochrome oxidase subunit III mRNA. Cell 61:879-884. [DOI] [PubMed] [Google Scholar]
- 65.Sundar, S., K. Pai, R. Kumar, K. Pathak-Tripathi, A. A. Gam, M. Ray, and R. T. Kenney. 2001. Resistance to treatment in kala-azar: speciation of isolates from northeast India. Am. J. Trop. Med. Hyg. 65:193-196. [DOI] [PubMed] [Google Scholar]
- 66.Sunkin, S. M., P. Kiser, P. J. Myler, and K. Stuart. 2000. The size difference between Leishmania major Friedlin chromosome one homologues is localized to sub-telomeric repeats at one chromosomal end. Mol. Biochem. Parasitol. 109:1-15. [DOI] [PubMed] [Google Scholar]
- 67.Tarun, S. Z., Jr., and A. B. Sachs. 1996. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 15:7168-7177. [PMC free article] [PubMed] [Google Scholar]
- 68.Uliel, S., X. H. Liang, R. Unger, and S. Michaeli. 2004. Small nucleolar RNAs that guide modification in trypanosomatids: repertoire, targets, genome organisation, and unique functions. Int. J. Parasitol. 34:445-454. [DOI] [PubMed] [Google Scholar]
- 69.Ullu, E., and C. Tschudi. 1991. Trans splicing in trypanosomes requires methylation of the 5′ end of the spliced leader RNA. Proc. Natl. Acad. Sci. USA 88:10074-10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Hoof, A., P. Lennertz, and R. Parker. 2000. Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol. Cell. Biol. 20:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vassella, E., R. Braun, and I. Roditi. 1994. Control of polyadenylation and alternative splicing of transcripts from adjacent genes in a procyclin expression site: a dual role for polypyrimidine tracts in trypanosomes? Nucleic Acids Res. 22:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, H., A. Iacoangeli, S. Popp, I. A. Muslimov, H. Imataka, N. Sonenberg, I. B. Lomakin, and H. Tiedge. 2002. Dendritic BC1 RNA: functional role in regulation of translation initiation. J. Neurosci. 22:10232-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watkins, K. P., and N. Agabian. 1991. In vivo UV cross-linking of U snRNAs that participate in trypanosome trans-splicing. Genes Dev. 5:1859-1869. [DOI] [PubMed] [Google Scholar]
- 74.Weinstein, L. B., and J. A. Steitz. 1999. Guided tours: from precursor snoRNA to functional snoRNP. Curr. Opin. Cell Biol. 11:378-384. [DOI] [PubMed] [Google Scholar]
- 75.Wu, Y., Y. El Fakhry, D. Sereno, S. Tamar, and B. Papadopoulou. 2000. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol. Biochem. Parasitol. 110:345-357. [DOI] [PubMed] [Google Scholar]
- 76.Xu, Y., L. Liu, C. Lopez-Estrano, and S. Michaeli. 2001. Expression studies on clustered trypanosomatid box C/D small nucleolar RNAs. J. Biol. Chem. 276:14289-14298. [DOI] [PubMed] [Google Scholar]
- 77.Yan, Q., R. J. Moreland, J. W. Conaway, and R. C. Conaway. 1999. Dual roles for transcription factor IIF in promoter escape by RNA polymerase II. J. Biol. Chem. 274:35668-35675. [DOI] [PubMed] [Google Scholar]
- 78.Zeiner, G. M., N. R. Sturm, and D. A. Campbell. 2003. Exportin 1 mediates nuclear export of the kinetoplastid spliced leader RNA. Eukaryot. Cell 2:222-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zilka, A., S. Garlapati, E. Dahan, V. Yaolsky, and M. Shapira. 2001. Developmental regulation of heat shock protein 83 in Leishmania. 3′ processing and mRNA stability control transcript abundance, and translation is directed by a determinant in the 3′-untranslated region. J. Biol. Chem. 276:47922-47929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.