Abstract
The 5′ cap structure of trypanosomatid mRNAs, denoted cap 4, is a complex structure that contains unusual modifications on the first four nucleotides. We examined the four eukaryotic initiation factor 4E (eIF4E) homologues found in the Leishmania genome database. These proteins, denoted LeishIF4E-1 to LeishIF4E-4, are located in the cytoplasm. They show only a limited degree of sequence homology with known eIF4E isoforms and among themselves. However, computerized structure prediction suggests that the cap-binding pocket is conserved in each of the homologues, as confirmed by binding assays to m7GTP, cap 4, and its intermediates. LeishIF4E-1 and LeishIF4E-4 each bind m7GTP and cap 4 comparably well, and only these two proteins could interact with the mammalian eIF4E binding protein 4EBP1, though with different efficiencies. 4EBP1 is a translation repressor that competes with eIF4G for the same residues on eIF4E; thus, LeishIF4E-1 and LeishIF4E-4 are reasonable candidates for serving as translation factors. LeishIF4E-1 is more abundant in amastigotes and also contains a typical 3′ untranslated region element that is found in amastigote-specific genes. LeishIF4E-2 bound mainly to cap 4 and comigrated with polysomal fractions on sucrose gradients. Since the consensus eIF4E is usually found in 48S complexes, LeishIF4E-2 could possibly be associated with the stabilization of trypanosomatid polysomes. LeishIF4E-3 bound mainly m7GTP, excluding its involvement in the translation of cap 4-protected mRNAs. It comigrates with 80S complexes which are resistant to micrococcal nuclease, but its function is yet unknown. None of the isoforms can functionally complement the Saccharomyces cerevisiae eIF4E, indicating that despite their structural conservation, they are considerably diverged.
Trypanosomatids are ancient eukaryotes that cycle between invertebrate vectors and mammalian hosts, causing a wide range of diseases. Leishmania parasites exist as extracellular flagellated promastigotes in the alimentary canal of female flies, and upon transfer to the mammalian host, they enter macrophages and cells of the immune system, transforming into amastigotes. Leishmania parasites are exposed to a broad range of environmental conditions, and stage differentiation is triggered by changes in temperature and pH (22, 57).
Trypanosomatids are characterized by a variety of unique molecular features, including polycistronic transcription of protein-coding genes (48, 60) and trans splicing (37), whereby a small leader RNA of 39 nucleotides, denoted spliced leader RNA (SL RNA), is spliced onto the 5′ ends of all mRNAs, providing the cap structure. The trypanosomatid cap is a highly modified structure that, in addition to m7GTP, contains 2′-O-methylations on the ribose moieties of the first four transcribed nucleotides and unusual base methylations on the first adenine and last uridine of the SL RNA (7). We recently reported on the chemical synthesis of the cap 4 analogue m7Gpppm26AmpAmpCmpm3Um (35). Cap 4 formation in trypanosomatids is essential for trans splicing, and drugs that inhibit methylation stop RNA processing (38). Mutagenesis of the capped nucleotides in the SL RNA also has a negative effect on trans splicing, showing that cap 4 formation is essential for RNA processing (39). Similar to other eukaryotes, the trypanosomatid cap structure is expected to play a key role in translation initiation, although the details of how cap 4 functions in this process have not yet been elucidated.
Cap-dependent translation initiation in eukaryotes is a highly regulated rate-limiting step, which involves assembly of eukaryotic initiation factor 4F (eIF4F), a multiprotein complex on the 5′ cap of the mRNA. eIF4F consists of at least three proteins: the cap-binding protein eIF4E, the ATP-dependent RNA helicase eIF4A, and the scaffold protein eIF4G. The last protein interacts with the other eIF4F subunits as well as with the poly(A) binding protein to create a close mRNA circle during translation initiation (24, 58). The three-dimensional structure of eIF4E from mouse (40), human (59), and Saccharomyces cerevisiae (42) shows conservation of its overall tertiary structure, specifically in the amino acids comprising the cap-binding pocket.
While all eukaryotes express eIF4E, which is essential for translation, several eIF4E homologues that can serve as tissue-specific translation factors or as gene-specific repressors have been identified. All three mammalian eIF4E isoforms bind m7GTP but vary in their ability to bind eIF4G. Only one isoform, eIF4E-1, can rescue the growth of a yeast mutant that fails to express its own eIF4E gene (31). Differences in the ability to complement the yeast eIF4E were also reported for the two isoforms from Arabidopsis thaliana. At.eIF4E-1 can fully replace the yeast eIF4E gene, whereas At.eIF4E-2, which encodes eIF(iso)4E, provides only partial complementation and the recovered yeast cells grow very slowly (54). The genome of Drosophila melanogaster also encodes multiple isoforms of eIF4E that vary in their function (28). d4EHP can bind to the cap structure; however, it fails to interact with eIF4G and prevents assembly of the eIF4F complex. A recent study showed that d4EHP functions as a translation repressor that is involved in axis formation during embryonic development. It interacts with Bicoid, a regulatory protein that is expressed in the anterior part of the embryo, and binds a 3′ untranslated region (UTR) element in caudal mRNA. Thus, d4EHP blocks the formation of the translation initiation complex eIF4F by impeding the binding of eIF4E to the cap structure (13). Five eIF4E isoforms were identified in Caenorhabditis elegans. This organism uses both cis and trans splicing and generates transcripts that are capped by m7GTP and trimethyl guanosine (TMG), respectively. IF4E-1, IF4E-2, and IF4E-5 can bind both m7GTP and TMG, whereas IF4E-3 and IF4E-4 can exclusively bind m7GTP (32, 45). Knockout of IF4E-4, the 4EHP homologue in C. elegans, using RNA interference or a null mutation produced a pleiotropic phenotype that included egg-laying defects (17).
Cap-binding proteins in trypanosomatids are expected to have gone through structural adaptations that enable them to interact with the unusual cap 4 structure. Additionally, the nuclear cap-binding protein CBP20 of Trypanosoma brucei was identified and shown to specifically bind cap 4 (36). We formerly described the biochemical and cellular features of a cytoplasmic eIF4E homologue, LeishIF4E-1, and determined its binding affinities to different cap structures (62). Basic features of the other homologues in Leishmania were also reported (16), yet their roles and their binding specificities remain vague. Here, we provide new biochemical information that can shed light on the potential roles of the different LeishIF4E homologues. We describe their binding affinities for a collection of cap analogues, including m7GTP, cap 4, and its intermediates. We also follow their association with high-molecular-weight complexes and assay their interaction with the mammalian translation repressor 4EBP1. Finally, we show that none of the four isoforms can complement the missing function of eIF4E in yeast, suggesting that they have diverged considerably throughout evolution.
MATERIALS AND METHODS
Organisms.
Leishmania major (Friedlin) and Leishmania amazonensis were cultured in Schneider's medium supplemented with 10% fetal calf serum, 4 mM l-glutamine, and 25 μg/ml gentamicin.
Cloning and expression of the Leishmania eIF4E isoforms in bacteria for protein purification.
The open reading frames of eIF4E isoforms from Leishmania were amplified by PCR, using L. major genomic DNA as a template. The primers were derived from the amino and carboxy termini and included anchor sequences that added restriction enzyme sites. The amplified fragments were cloned into the pHis-parallel expression vector, yielding plasmids pHisLeishIF4E-1 through pHisLeishIF4E-4. These plasmids were transformed into Escherichia coli BL21 cells, and expression was induced at 20°C in log-phase cultures by the addition of 0.5 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG). Cells were harvested, resuspended in sonication buffer 1 (SB1) (20 mM Tris-HCl, pH 8, 0.5 mM NaCl, 20 mM imidazole) or sonication buffer 2 (SB2) (20 mM HEPES, pH 7.6, 1 mM dithiothreitol [DTT], 2 mM EDTA, 5% glycerol), and disrupted by sonication. Following sonication, the cell extracts were clarified by centrifugation for 30 min at 20,000 × g. For purification over Ni-nitrilotriacetic acid (Ni-NTA) columns, the cell pellets were disrupted in SB1 and the supernatant was loaded on a HiTrap column using AKTA fast protein liquid chromatography (Amersham Bioscience). Initial washes were done in SB1 containing 20 mM and 100 mM imidazole. The proteins were then eluted with SB1 containing 250 mM imidazole and dialyzed against the fluorescence buffer (20 mM Tris HCl, pH 7.5, 1 mM DTT, 1 mM EDTA, 50 mM NaCl). For purification over m7GTP-Sepharose, the cell pellets were sonicated in SB2 and the supernatant was loaded on m7GTP-Sepharose (Amersham). The protein was eluted by SB2 containing high salt (600 mM NaCl) or the free ligand (m7GTP). The purified proteins were dialyzed against fluorescence buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Antibodies.
Recombinant LeishIF4E-1 through LeishIF4E-4 were affinity purified over m7GTP-Sepharose (LeishIF4E-1, LeishIF4E-3, and LeishIF4E-4) or Ni-NTA (LeishIF4E-2), emulsified with complete Freund's adjuvant, and injected subcutaneously into New Zealand White rabbits (500 μg per rabbit). Two boosts of the protein emulsified in incomplete Freund's adjuvant were given in 2-week intervals. Serum samples from immunized animals were obtained 10 to 20 days after the boost. Where necessary, Western blot analyses were carried with antibodies that were affinity purified over the recombinant protein that was immobilized on nitrocellulose blots. Mouse monoclonal antibodies against Hsp70 were obtained from R. Morimoto, Northwestern University, and antibodies directed against the murine eIF4E were a gift from N. Sonenberg, McGill University.
Sequence alignment and homology modeling.
Four open reading frames encoding homologues of the murine eIF4E were identified in the L. major genome database using a BLAST search. The annotated genes LmjF27.1620, LmjF19.1500, LmjF28.2500, and LmjF30.0450 were denoted LeishIF4E-1 through LeishIF4E-4. The amino acid sequences of all four isoforms were analyzed using the MUSCLE multiple sequence alignment algorithm (20) and viewed with Jalview editor (14).
Homology modeling for each of the predicted structures was performed using SwissPDBViewer, based on the published structure of the mouse eIF4E (Protein Data Bank no. 1EJ1a). The models with the lowest Z score of the overall structure were visualized.
Measurements of binding affinity for the different LeishIF4E isoforms.
Cap 4 and its intermediates were prepared as previously described (35). The equilibrium association constants (Kas) for complexes of Leishmania eIF4E isoforms with different cap analogues were determined by intrinsic protein fluorescence quenching, as described previously (49, 62). Freshly prepared protein samples were filtered through 0.2-μm polyvinylidene difluoride membrane filters (Roth) prior to spectroscopic measurements. Protein concentrations were determined by absorption, assuming the following: ɛ280 = 47,500 cm−1 M−1 for LeishIF4E-1, ɛ280 = 63,660 cm−1 M−1 for LeishIF4E-2, ɛ280 = 54,600 cm−1 M−1 for LeishIF4E-3, and ɛ280 = 67,970 cm−1 M−1 for LeishIF4E-4 (calculated from amino acid composition) (23). Fluorescence titration measurements were carried out on an LS-50B spectrofluorometer (PerkinElmer Co.) in 50 mM HEPES, pH 7.2, 100 mM NaCl, 1 mM EDTA, and 1 mM DTT at 20°C using protein concentrations that ranged between 0.2 μM and 1 μM. The fluorescence intensity (excited at 295 nm and observed at 320 or 345 nm) was corrected, taking into account the sample dilution, the inner filter effect, and the instability of protein fluorescence. The analysis was repeated with proteins that were obtained from multiple preparations and analyzed over SDS-PAGE for their integrity. In all cases, the results were highly reproducible. All measurements were done with freshly prepared proteins that were not exposed to freezing and thawing.
GST-4EBP pull-down assay.
Glutathione S-transferase (GST) was expressed from the pGST parallel vector, and 4EBP1 fused to GST was expressed from pGEX-6p1-h4EBP1 (kindly provided by N. Sonenberg). GST and GST-4EBP1 were expressed in E. coli BL21 cells, and the cells were harvested and disrupted by sonication in phosphate-buffered saline (PBS) containing protease inhibitor cocktail (Sigma). GST or GST-4EBP1 was immobilized on glutathione-agarose beads and washed with binding buffer (40 mM MOPS [morpholinepropanesulfonic acid], pH 7.2, 50 mM KCl, 250 mM NaCl, 7 mM β-mercaptoethanol, 2 mM MgCl2) containing 0.5% Triton X-100, and the beads were incubated with supernatants of lysed bacteria that expressed either the mouse eIF4E or the four different LeishIF4E isoforms in binding buffer. The beads were washed extensively five times with 100 volumes of binding buffer containing 0.5% Triton X-100, and the proteins were eluted with Laemmli's SDS-PAGE sample buffer, separated on 15% SDS-PAGE, and subjected to Western analysis using specific antibodies (33).
Functional complementation in yeast.
p301-eIF4E constructs were transformed into S. cerevisiae derivative strain CWO4#368 (genotype, MATa ade2 trp1 ura3 leu2 his3 tif4E::LEU2 [pVTURA3-4E]). This strain does not grow on media containing 5′-fluoroorotic acid (5′-FOA), as its unique eIF4E gene copy is localized on a plasmid with the gene URA3 as a selectable marker (pVTURA3-4E). Growth on 5′-FOA can be resumed only by plasmid shuffling: a functional eIF4E gene copy is provided from a second plasmid with a different marker, such as TRP1 (i.e., plasmid p301), and the URA3-carrying plasmid is lost through mitotic segregation. This system therefore evaluates the functionality of a protein derived from a heterologous source. Upon transformation, yeast cells were replicated on minimal medium containing 2% galactose (SGal) plus 0.5% 5′-FOA (as eIF4E from p301 constructs are expressed only in the presence of galactose). Growth was monitored for 3 days after transformation. Positive complementation was performed with eIF4E genes from Drosophila melanogaster (p301-DmeIF4E-1) and with the endogenous yeast protein (p301-yIF4E) (28).
Differential expression of LeishIF4E isoforms under different environmental conditions.
L. amazonesis cells were propagated in Schneider's medium at 26°C, and log-phase cultures were shifted to 33°C for 18 h. The cells were washed twice in PBS, resuspended in lysis buffer (50 mM Tris, pH 6.8, 2% SDS, 10% glycerol and protease inhibitor cocktail), and heated to 100°C for 10 min. Protein concentrations were quantified by a bicinchoninic acid protein assay kit (Pierce), and then β-mercatoethanol was added to a final concentration of 2.5%. Similar protein amounts were loaded for SDS-PAGE and subjected to Western analysis using antibodies against LeishIF4E isoforms, Hsp70, and tubulin.
Subcellular fractionation.
For total protein extracts, Leishmania parasites (108 cells) were harvested, washed twice in PBS, and extracted in SDS-PAGE sample buffer (33). Protein concentrations were determined with the bicinchoninic acid reagent kit (Pierce). All fractionation was performed following published protocols (11). The cells were washed twice, and the pellet was frozen in liquid nitrogen and then resuspended in Dignam buffer (10 mM HEPES, pH 7.6, 1.5 mM MgAc2, 10 mM KAc, 2 mM iodoacetamide, 0.5 mM DTT) containing a cocktail of protease inhibitors (Sigma). The mixture was incubated on ice for 15 min, Nonidet P-40 was added to a final concentration of 0.8%, and the mixture was vortexed for 10 seconds and centrifuged at 12,000 × g for 15 min at 4°C. The pellet was resuspended in Dignam buffer, and the protein concentrations of the cytoplasmic fraction and the resuspended pellet were determined. Samples of whole-cell extracts and of the subcellular fractions containing equal protein loads were analyzed over 15% SDS-PAGE. The protein blots were reacted with antibodies raised against LeishIF4E, α-tubulin, and Hsp83.
Sucrose gradients and polysome analysis.
L. major promastigote cells (5 × 108 to 10 × 108 per gradient) were incubated for 5 min in 5 ml of medium containing 100 μg/ml cycloheximide. After centrifugation, the cells were washed twice in PBS containing 100 μg/ml cycloheximide and once with lysis buffer (15 mM Tris-HCl, pH 7.4, 0.3 M KCl, 5 mM MgCl2, 0.5 mM DTT, 100 μg/ml cycloheximide, and 1 mg/ml heparin). The cell pellet was resuspened in lysis buffer and incubated on ice for 10 min. The cells were lysed by the addition of Triton X-100 to a final concentration of 1%. The cell lysates were centrifuged at 12,000 × g at 4°C for 15 min. The cell extracts were loaded on an 11-ml 10 to 50% sucrose step gradient in the polysome buffer (20 mM Tris-HCl, pH 8, 140 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 100 μg/ml cycloheximide, and 0.5 mg/ml heparin and sucrose) and centrifuged for 160 min at 35,000 rpm in a Beckman SW40 rotor. For control gradients, the extracts were incubated with 30 mM EDTA for 10 min on ice before being loaded on the gradient. Alternatively, polysomes were disintegrated by the addition of micrococcal nuclease to a final concentration of 500 U/μl in the presence of 2 mM CaCl2 for 30 min at 20°C. The reaction was stopped by the addition of EGTA to a final concentration of 2.5 mM, and the extracts were loaded on the sucrose gradients. Fractions (300 μl) were collected from the top, and the optical density at 260 nm was monitored. Proteins were recovered by trichloroacetic acid precipitation, separated by SDS-PAGE, and further subjected to immunoblotting using antibodies against LeishIF4E-1 to LeishIF4E-4.
RESULTS
Structural conservation of the cap-binding pocket in the different Leishmania eIF4E isoforms.
We identified in the genome database of Leishmania (LeishDB) four different isoforms of eIF4E, denoted LeishIF4E-1 to LeishIF4E-4, based on their homology with the mouse and yeast translation factor eIF4E. We previously reported on the characterization of LeishIF4E-1, and now we describe in detail all four isoforms.
Sequences of eIF4E isoforms from Leishmania and a variety of other eukaryotes were aligned, showing that all four LeishIF4E isoforms had a relatively low degree of homology to the mouse and yeast proteins (30 to 40%); however, the core region that contains residues encompassing the cap-binding pocket is mostly conserved (see Fig. S1 in the supplemental material). The Leishmania proteins extend to various sizes of 214, 281, 349, and 308 amino acids for LeishIF4E-1 through LeishIF4E-4, respectively. LeishIF4E-1 lacks the region that corresponds to the carboxy terminus of the mouse protein (amino acids 205 to 217), which is known to serve as a phosphorylation site (56, 66). LeishIF4E-1 and LeishIF4E-2 each possess insertions that are adjacent to the conserved cap-binding pocket. LeishIF4E-3 and LeishIF4E-4 have a long extension at their amino terminus that has no corresponding sequences in the mouse homologous proteins. Amino acids at positions that are expected to promote binding to eIF4G are partially conserved, including the mouse Trp73 and Val69 (26, 41). These partial conservations, combined with the variability in the amino terminus, are not sufficient to predict the ability of each isoform to bind eIF4G (61).
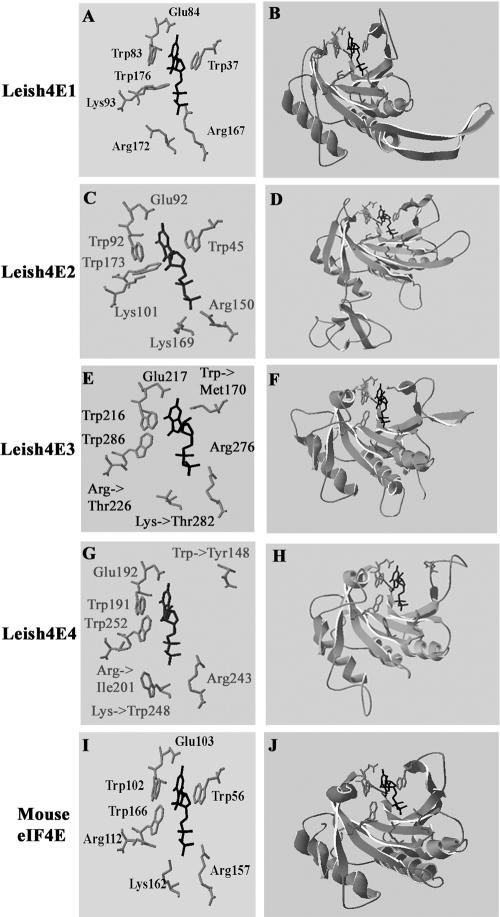
A computerized prediction of the different eIF4E isoforms in Leishmania was carried out, based on the known tertiary structure of the mouse eIF4E (40). Homology modeling suggests that the structure of the cap-binding pocket is basically conserved in each of the four proteins (Fig. 1), including the typical stacking interactions between two Trp residues (corresponding to Trp56 and Trp102 in the mouse protein) and van der Waals contacts between Trp166 and the m7G base. These three Trp residues were conserved in LeishIF4E-1 and LeishIF4E-2. In LeishIF4E-4, the equivalent of Trp56 is replaced by Tyr, but this replacement maintains an aromatic residue in this position and therefore is not expected to affect cap binding. In LeishIF4E-3, however, the position that corresponds to the mouse Trp56 was occupied by a Met residue, and despite this, the protein readily interacts with m7GTP-Sepharose. Only a few substitutions of Trp56 can be tolerated without affecting binding to m7GTP. The replacement of this amino acid with an aromatic residue has little effect on cap binding (1); however, replacement with a Leu residue eliminated completely the ability of the mutated protein to bind m7GTP (46). Interestingly, introducing an amino acid that contains a sulfur atom does not eliminate cap-binding activity (31). The basic residues of the cap-binding pocket, which are expected to interact with the phosphate backbone of the cap nucleotides, are conserved in LeishIF4E-1 and LeishIF4E-2. In LeishIF4E-3 and LeishIF4E-4, the only position that contains a basic residue corresponds to Arg157 (according to mouse eIF4E numbering), whereas the other two basic amino acids are replaced with Thr in LeishIF4E-3 and with Ile and Trp in LeishIF4E-4. We assume that the methylations in cap 4 mask the negative charges of the phosphate backbone, allowing exchange of the basic amino acid residues, which are conserved in most eIF4E homologues in metazoa. However, it should be noted that the Lys and Arg amino acids in the cap-binding pocket interact with the phosphate groups of m7GpppN and that Thr may also form hydrogen bonds as an electron donor.
FIG. 1.
Homology modeling of LeishIF4E isoforms. A computerized prediction of the tertiary structures was performed using PDBView (A to H), based on the mouse eIF4E crystal structure (Protein Data Bank no. 1EJ1a) (I and J). The side residues that comprise the ligand binding pocket are shown in the left panels. The ligand (m7GDP) is marked in black.
Binding affinities between the LeishIF4E isoforms and different cap structures.
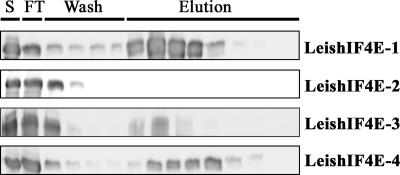
To examine whether the LeishIF4E proteins could indeed bind m7GTP, as suggested by the computerized structure prediction, the recombinant proteins were subjected to affinity purification on m7GTP-Sepharose. Three of the isoforms, LeishIF4E-1, LeishIF4E-3, and LeishIF4E-4, bound to the affinity resin and could be eluted with m7GTP or with high salt buffer (Fig. 2). However, LeishIF4E-2 could not be purified over m7GTP-Sepharose. This result correlated with the Trp fluorescence quenching analysis that showed preferential binding of this protein to cap 4.
FIG. 2.
Affinity purification of LeishIF4E. LeishIF4E isoforms were expressed in E. coli, the cells were lysed by sonication, and the supernatants were loaded on an m7GTP-Sepharose column (S represents 1% of the total input protein). After collection of the flowthrough fractions (FT), the column was washed with equilibrium buffer (wash). The proteins were eluted with the same buffer containing 600 mM NaCl (elution). Samples of equal volume from the different fractions were analyzed on Western blots using specific antibodies.
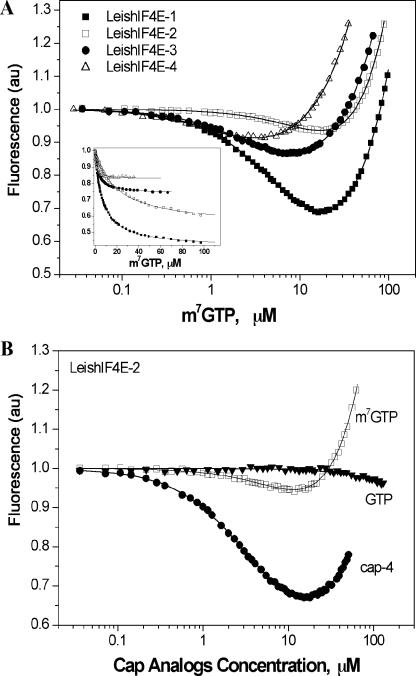
Conservation of Trp residues in the cap-binding pocket of the LeishIF4E proteins enabled us to use the Trp fluorescence time-synchronized titration and to determine the Kas for complexes of eIF4E isoforms and different cap analogues. This method is based on measuring the fluorescence quenching of Trp residues in response to binding of a given ligand (49). The fluorescence spectra of the LeishIF4E isoforms are typical for eIF4E proteins; however, the emission spectra of LeishIF4E-1, LeishIF4E-2, and LeishIF4E-4 are slightly shifted towards the red wavelength compared to that of the mouse eIF4E (data not shown). The fluorescence quenching data are shown in Fig. 3. For LeishIF4E-1 and LeishIF4E-2, in which the sandwich stacking of m7G with two of the Trp residues as well as the Van der Waals contacts with the third Trp in the cap-binding pocket are conserved, the total protein fluorescence was reduced up to approximately 60% and 40% of the original level, respectively (Fig. 3, insert). This range was also typical for the mouse protein (data not shown). The slightly lower level of quenching that was observed for LeishIF4E-2 is possibly due to a masking effect caused by the presence of two additional Trp residues in the sequence outside the cap-binding pocket. For LeishIF4E-3 and LeishIF4E-4, total fluorescence quenching was two- and threefold lower than that observed for LeishIF4E-1. This effect could originate mostly from the replacement of the Trp residue at position 56 (mouse numbering) by either Met or Tyr in LeishIF4E-3 or LeishIF4E-4, respectively.
FIG. 3.
Results of fluorescence binding assays of LeishIF4E isoforms to cap analogues. (A) Comparison of the titration curves for complexes of Leishmania eIF4E isoforms with m7GTP. The insert shows the changes of protein fluorescence (Ft) in response to binding of m7GTP. These were obtained by subtracting the fluorescence of the free cap analogues (Fc), which were calculated from the obtained Kas. (B) Titration curves for binding of LeishIF4E-2 to cap 4 (•), m7GTP (□), and GTP (▾). Protein fluorescence was excited at 295 nm, and emission was observed at 320 nm. Fluorescence is presented as relative values. The increasing fluorescence intensity observed at the higher ligand concentrations originates from emission of the free cap analogues in solution. au, arbitrary units.
Binding affinities for the different cap analogues were tested with each of the LeishIF4E isoforms and the mouse eIF4E. The ligands used were m7GTP, an analogue of the consensus eukaryote cap structure, m32,2,7GTP, the hypermethylated cap structure which is found on U snRNAs, an analogue of cap 4 (m7Gpppm26AmpAmpCmpm3Um) which was recently synthesized (35) along with the nonmethylated tetranucleotide m7GpppAACU (cap 0), and several cap 4 intermediates. The intermediates analyzed were m7Gpppm26Am (cap 1), m7Gpppm26AmpAm (cap 2), and m7Gpppm26AmpAmpCm (cap 3). The results shown in Table 1 reveal that the association constants for complexes of LeishIF4E homologues with different cap structures were two orders of magnitude lower than those of the mouse protein. We assume that their binding may be further stabilized by association with additional components of the cap-binding complex. However, evaluation of the binding of each protein to various cap analogues indicates that there are basic differences between the LeishIF4E isoforms. LeishIF4E-1 and LeishIF4E-4 each bound cap 4 and m7GTP with similar efficiencies. However, their binding to cap 0 was higher than to cap 4, suggesting that hypermethylations on cap 4 have a destabilizing effect on cap binding. LeishIF4E-2 showed clear preferential binding to the methylated cap 4 structure, and the absence of methyl bases (m7GTP or cap 0) decreased its ability to bind this cap analogue. An opposite effect was observed for LeishIF4E-3, which bound specifically to m7GTP (Kas = 0.32 ± 0.07 μM−1) and showed very weak binding to cap 4 (Kas = 0.046 ± 0.002 μM−1) (mean ± standard deviation). A similar range of reduction in the binding affinity caused by hypermethylations was observed for the mouse protein. Among LeishIF4E isoforms, the highest binding affinity for m7GTP and cap 4 was observed for LeishIF4E-4 (Kas = ∼0.8 μM−1 for both ligands). Although the basic amino acids of the cap-binding pocket that stabilize binding to the cap structure are not well conserved in this isoform, these changes may comply with the requirement to interact with the hypermethylated cap 4 structure. It should also be noted that all LeishIF4E isoforms showed low affinity for the TMG cap analogue. However, the highest binding was observed for LeishIF4E-3. Altogether, it appears that the different Leishmania isoforms vary in their binding specificities, suggesting that they are responsible for different functions.
TABLE 1.
Equilibrium association constants for complexes of Leishmania elF4E isoforms and mouse eIF4E (amino acids 28 to 217) with cap analoguesd
| Cap analogue |
Kas (μM−1) for:
|
||||
|---|---|---|---|---|---|
| Mouse eIF4E (28-217) | LeishIF4E-1 | LeishIF4E-2 | LeishIF4E-3 | LeishIF4E-4 | |
| m7GTP | 121.4 ± 3.0a | 0.16 ± 0.02 | 0.037 ± 0.006 | 0.32 ± 0.07 | 0.8 ± 0.08 |
| m7GpppA | 4.4 ± 0.1a | 0.072 ± 0.002b | 0.016 ± 0.010 | 0.020 ± 0.005 | 0.21 ± 0.03 |
| Cap 1 | |||||
| m7Gpppm36,6,2′ A | 1.45 ± 0.03a | 0.065 ± 0.004b | 0.020 ± 0.004 | 0.029 ± 0.007 | 0.075 ± 0.031 |
| Cap 2 | |||||
| m7Gpppm36,6,2′ Apm2′ A | 10.7 ± 1.4 | 0.067 ± 0.005 | 0.092 ± 0.008 | 0.063 ± 0.010 | 0.23 ± 0.06 |
| Cap 3 | |||||
| m7Gpppm36,6,2′ Apm2′ Apm2′ C | 22.2 ± 1.0 | 0.19 ± 0.01 | 0.24 ± 0.02 | ND | ND |
| Cap 4 | |||||
| m7Gpppm36,6,2′ Apm2′ Apm2′ Cpm23,2′ U | 26.1 ± 0.7a | 0.253 ± 0.003b | 0.28 ± 0.04 | 0.046 ± 0.02 | 0.77 ± 0.08 |
| Cap 0 | |||||
| m7GpppApApCpU | 73.3 ± 1.8a | 0.467 ± 0.005c | 0.089 ± 0.013 | 0.12 ± 0.03 | 2.0 ± 0.4 |
| TMG | |||||
| m32,2,7GTP | 0.64 ± 0.06 | <0.01 | <0.003 | <0.04 | <0.006 |
| GTP | 0.032 ± 0.004a | <0.01 | <0.003 | <0.01 | <0.01 |
Interaction of LeishIF4E isoforms with the mammalian 4EBP1.
Different roles can be assigned to homologues of eIF4E that can serve as general translation factors or as repressors (52). The criteria for evaluating whether a given protein serves as a translation factor are often based on its ability to interact with the scaffold protein eIF4G and to complement the missing eIF4E activity in a heterologous organism (28, 53). eIF4G, which in mammals is a large protein, has not yet been identified with certainty in Leishmania, although there are several candidates in the L. major genome database (16; Y. Yoffe et al., unpublished data). As eIF4G interacts with conserved residues in eIF4E and these are also targets for binding of translation regulators that compete with eIF4G (25), we examined the abilities of the different LeishIF4E proteins to interact with mammalian 4EBP1 in pull-down assays.
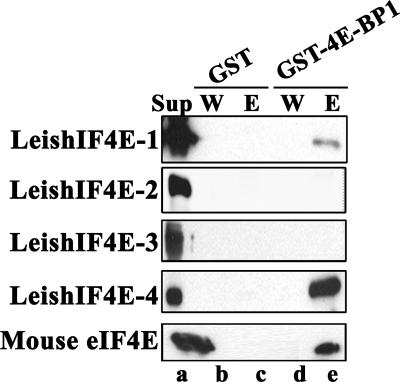
Bacterial extracts that express GST or the 4EBP1-GST fusion protein were immobilized on glutathione beads and further incubated with the soluble fraction of bacterial extracts expressing each of the recombinant LeishIF4E isoforms or the mouse protein. The eluted complexes were subjected to Western analysis with antibodies raised specifically against each LeishIF4E. As can be seen in Fig. 4. LeishIF4E-1 and LeishIF4E-4, as well as the murine eIF4E, were pulled down by GST 4EBP1, though the interaction with LeishIF4E-1 was weaker.
FIG. 4.
LeishIF4E-1 and LeishIF4E-4 interact with h4EBP1. GST or GST-4EBP1 was immobilized on glutathione-agarose beads. The beads were incubated with supernatants of bacteria that express the different LeishIF4E isoforms or mouse eIF4E (Sup represents 1% of the input for the pull-down assay and indicates that equimolar amounts of the recombinant protein were used). After extensive washes (W), the protein was eluted (E) with Laemmli's sample buffer and analyzed with specific antibodies against the different eIF4E isoforms.
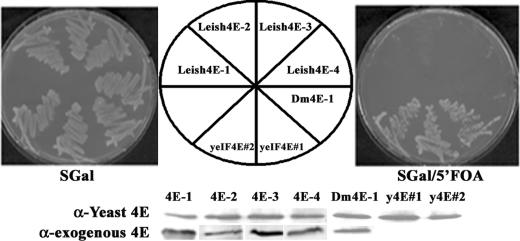
None of the LeishIF4E proteins complement for yeast eIF4E.
The in vivo activity of eIF4E has been evaluated in complementation experiments of a conditionally lethal yeast mutant strain deficient in eIF4E. Each of the four LeishIF4E isoforms was cloned into the yeast expression vector p301, and the constructs were transformed into S. cerevisiae strain CWO4#368 (See Materials and Methods). Upon transformation, yeast cells were replicated on minimal medium containing SGal or SGal plus 0.5% 5′-FOA. None of the four Leishmania eIF4E gene constructs was able to complement for the missing yeast function, except for the positive controls, where complementation was achieved by expressing Drosophila eIF4E-1 or the endogenous yeast protein (28). Proper expression of LeishIF4E-1 to LeishIF4E-4 proteins as well as Drosophila eIF4E was verified by Western blot analysis of total yeast cell extracts using antibodies against the different eIF4E isoforms analyzed (Fig. 5). It therefore appears that the parasite cap-binding translation factor has diverged throughout evolution, presumably to adapt for binding to cap 4. Thus, the conservation of its sequence and structure is not sufficient to rescue a yeast strain lacking endogenous eIF4E.
FIG. 5.
None of the LeishIF4E isoforms complements for yeast eIF4E. p301-TRP1-eIF4E constructs were transformed into S. cerevisiae strain CWO4#368. Upon transformation, yeast cells were replicated on minimal medium containing SGal or SGal plus 0.5% 5′-FOA. After 3 days of growth, none of the four Leishmania eIF4E gene constructs was able to grow on plates containing 5′-FOA, and only p301-DmeIF4E-1 (Drosophila eIF4E-1) and p301-eIF4E (expressing yeast eIF4E [yeIF4E], clones 1 and 2) could complement for the loss of endogenous yeast eIF4E. Expression in yeast of the foreign genes tested is shown at the bottom, using extracts of cells freshly transformed and grown in SGal (in the absence of 5′-FOA). The top row shows expression of yeast eIF4E from the pVTURA3-4E plasmid, and the bottom row shows expression of the exogenous genes LeishIF4E-1 to LeishIF4E-4 and deIF4E (the yeast gene expressed from pVTURA3 cannot be distinguished from that expressed from p301).
Differential expression of LeishIF4E under different environmental conditions.
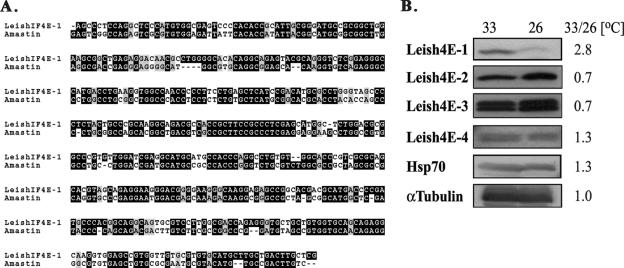
Stage-specific gene expression in Leishmania is controlled by the 3′ UTR (3, 30, 44, 50, 65), and an amastigote-specific element has been identified (44). Such an element (bearing 68% identity) was found in the 3′ UTR of LeishIF4E-1, suggesting that expression of this protein is higher in amastigotes (Fig. 6A). Stage transformation of several Leishmania species can be obtained under axenic conditions (8, 9); thus, a promastigote culture of L. amazonensis that was exposed to 33°C resulted in morphologically altered cells, which changed from flagellated promastigotes to rounded amastigote-like cells. Western analysis of LeishIF4E isoforms isolated from cells exposed to different conditions revealed that the level of LeishIF4E-1 was clearly higher in axenic amastigotes, as expected from the finding of an amastigote-specific element in its 3′ UTR. The other isoforms showed milder and less-significant changes at the different temperatures. Protein loads were adjusted by comparing signal intensities to those obtained with antibodies against α-tubulin and antibodies against Hsp70. Both proteins are expressed at all life stages, with a slight increase in the steady-state level of Hsp70 at elevated temperatures (Fig. 6B) (12).
FIG. 6.
Differential expression of the LeishIF4E isoforms in promastigotes and axenic amastigotes. (A) Sequence alignment of the 450-nucleotide amastin element of Leishmania infantum (GenBank accession no. AF195531) with the 3′ UTR of LeishIF4E-1 from L. major (AAZ09911) performed with ClustalX. (B) L. amazonensis promastigotes were grown at 26°C and transferred overnight to 33°C. Cells were harvested, extracted, and analyzed on Western blots with antibodies against the different eIF4E isoforms, with anti-Hsp70 and antitubulin antibodies as controls.
Subcellular distribution of LeishIF4E isoforms.
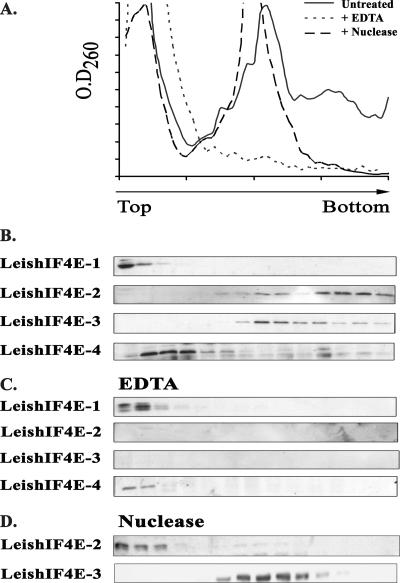
The basal translation factor eIF4E and related homologues that function as translation regulators are located mainly in the cytoplasm, although they can be detected at low levels in the nucleus (34). The eIF4E homologues are distinct from the more distant nuclear cap-binding protein CBC20, which was recently identified in trypanosomes (36). Subcellular fractionation experiments, followed by Western analysis, that were performed on Leishmania promastigotes indicate that all four LeishIF4E isoforms were expressed in the cytoplasm (Fig. 7). Polysome analysis indicated that only LeishIF4E-2 and LeishIF4E-3 comigrated with high-molecular-weight fractions on sucrose gradients (Fig. 8). While the migration profile suggests that LeishIF4E-2 cosediments with polysomes (and with 80S particles), LeishIF4E-3 is found only in the slower-migrating fractions that cosediment with 80S complexes. The addition of EDTA, which dissociates polysomes and other high-molecular-weight complexes, to cell extracts eliminated LeishIF4E-2 and LeishIF4E-3 from the Western blots, most probably due to their degradation. LeishIF4E-1 and LeishIF4E-4 migrated at the top of the sucrose gradient with and without EDTA, indicating that they are not associated with polysomes. Since eliminating Mg2+ can affect many complexes in the cell, polysome disintegration was also performed by digestion with micrococcal nuclease. Under limiting conditions, this nuclease cleaves the mRNA between the ribosomes, thus generating 80S particles (18). As shown in Fig. 8, nuclease treatment eliminated the polysomes and shifted the migration of LeishIF4E-2 towards the top of the gradient, supporting the association between LeishIF4E-2 and polysomes. However, migration of LeishIF4E-3 remained unaltered, suggesting that it associates with nonpolysomal complexes.
FIG. 7.
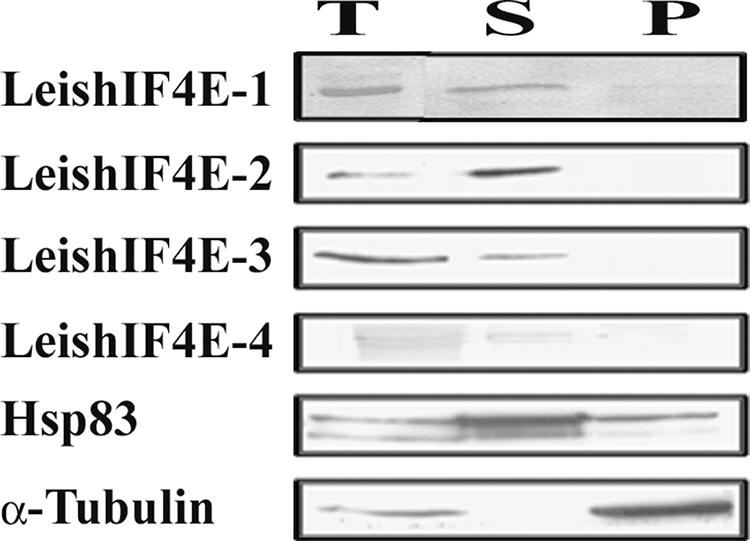
Localization by Western analysis of fractionated cells. Extracts were prepared from whole cells (T) and from subcellular fractions containing the cytoplasm (S) and nucleocytoskeletal (P) fractions and separated over 15% SDS-PAGE. Western blots were reacted with antibodies against LeishIF4E isoforms. Control blots containing equal loads of the same fractions were reacted with monoclonal antibodies against Hsp70 and α-tubulin, representing the cytoplasmic and nonsoluble nucleocytoskeletal fractions, respectively.
FIG. 8.
Polysome distribution of the different LeishIF4E isoforms. L. major cells were lysed, and the extracts were loaded on 10 to 50% sucrose gradients. (A) Fractions were collected from the top, and the optical density at 260 nm (OD260) was monitored. The graphs depict patterns of migration in sucrose gradients of untreated polysomes (continuous line), EDTA-treated polysome extracts (dots), and polysomes treated with micrococcal nuclease (broken line). Proteins from untreated extracts (B) and from EDTA-treated (C) and micrococcal nuclease-treated (D) proteins were recovered by trichloroacetic acid precipitation and further immunoblotted with antibodies that were raised against the different LeishIF4E isoforms.
DISCUSSION
The Leishmania genome encodes four isoforms of eIF4E, all of which are located in the cytoplasm. The different isoforms show a relatively low degree of conservation with eIF4E of other eukaryotes or among themselves, and the genome of Trypanosoma brucei contains four homologues which are highly similar to the Leishmania proteins (see Fig. S1 in the supplemental material). Computerized modeling suggests that all of the LeishIF4E isoforms contain a typical cap-binding pocket. Using fluorescence titration measurements, we show that these isoforms demonstrate differential binding capacities for the various cap analogues. The results were supported in part by affinity chromatography, although slight quantitative differences between the two approaches were apparent, for reasons not fully clear to us. LeishIF4E-1 and LeishIF4E-4 each bind to m7GTP and cap 4 with comparable affinities, unlike the mouse eIF4E, which binds to m7GTP fivefold better than to cap 4. This pattern was less apparent when we compared binding between cap 4 and cap 0, where only a slight preference for cap 4 was monitored with LeishIF4E-1, indicating that the length of the analogue should be taken into account. Despite this, the length of the cap analogue does not change the preference of LeishIF4E-2 to cap 4. Overall, it appears that the LeishIF4E isoforms have diverged from their eukaryotic isoforms, possibly in conformity with the appearance of a hypermodified cap structure. The outcome of these changes is reflected in the reduced binding affinity of the recombinant proteins to both m7GTP and cap 4 compared to that of the mammalian protein. The difference between the binding parameters of the mouse and parasite proteins could also be attributed to variations in the purification protocols, as noted elsewhere (49). While the totally insoluble recombinant mouse protein was subjected to a denaturation-renaturation process, the different LeishIF4E isoforms were purified from the soluble fraction of the corresponding bacterial extracts over m7GTP (or Ni-NTA) columns.
It is also possible that the presence of accompanying factors that bind eIF4E is critical for stabilizing the interaction between this protein and its cap ligand. It was shown that the purified trypanosome nuclear complex could discriminate between cap 4- and m7GTP-protected SL RNAs, with preference for cap 4, and this could be attributed to the use of purified multiprotein complexes in the binding assays (36). Cap-binding activity of the T. brucei CBC20 complex was ∼30-fold higher than that measured for the recombinant LeishIF4E-4. We therefore expect that an eIF4G-like protein is required for stabilizing this interaction, as previously shown for heterologous organisms (61).
The binding efficiency to the dinucleotide analogues consistently drops for all of the eIF4E proteins that were tested, as also noted for the mouse protein (49). In the mononucleotide, there is an additional OH group on the last phosphate that is absent from the dinucleotide. This group is partially ionized and could therefore strengthen the interaction with the positively charged amino acids at the entrance of the cap-binding pocket.
LeishIF4E-3 is a cytoplasmic protein that binds mainly to m7GTP and hardly interacts with cap 4. This most probably excludes it from serving as a basal translation factor in Leishmania, since cap 4 is found at the 5′ end of all trypanosomatid mRNAs. The potential role of LeishIF4E-3 remains elusive. However, it is possible that it is involved in the biogenesis of other small nuclear U RNAs, for example, those carrying a TMG cap structure (4, 63). The hypermethylation of m7GTP that generates TMG-protected snRNAs occurs in the cytoplasm of mammalian cells (43) or in the nucleolus of yeast cells, where snRNPs are not obliged to transit through the cytoplasm during their biogenesis (47). It is not clear at this stage which pathway prevails in kinetoplastids. The possibility that LeishIF4E-3 is involved in the modification process of SL RNA is less feasible. If this were the case, we would expect to find this protein where cap 4 methylations take place. It was reported that the enzymes responsible for the 2′-O-methylations of the ribose residues of the SL RNA are nuclear (5, 6, 64) and that SL RNA modification and Sm assembly take place in the nucleus (10, 38). LeishIF4E-3 is associated with a slow-migrating complex in sucrose gradients but not with polysomes. However, additional characterization of this complex is required.
The observation that LeishIF4E-2 binds mainly to cap 4 is rather intriguing since this cap ligand contains m7GTP, and the computerized modeling of this protein suggests that the cap-binding pocket is conserved. The binding of LeishIF4E-2 to cap 4 intermediates increases with their length; however, the highest Kas values were observed for the hypermethylated cap 3 and cap 4 analogues but not for the nonmethylated cap 0 tetranucleotide. We therefore assume that the originally weak binding of the m7GTP moiety by LeishIF4E-2 may be stabilized by the hypermethylations in cap 4. A recent study of the nuclear CBC20 in T. brucei also shows a stronger binding to RNAs protected by cap 4 than to RNAs protected by cap 2 (5, 6).
LeishIF4E-2 is a cytoplasmic protein that comigrates with polysomal fractions on sucrose gradients. However, this does not indicate that LeishIF4E-2 is a basal translation factor, since in higher eukaryotes, the majority of the eIF4E is found in the postribosomal supernatant and in 48S complexes. A recent report describing the structure of the 80S ribosome from Trypanosoma cruzi revealed that the 40S ribosomal subunit has a unique structure that forms a large helix located in the vicinity of the mRNA exit channel. The authors of that report suggest that this region could serve as a target for direct interaction with the RNA and could contain a distinct factor for stabilizing the interaction between the ribosome and cap 4-protected mRNAs (21). LeishIF4E-2 could therefore function as a mediator between the ribosome and the parasite mRNAs.
A bioinformatics approach did not reveal unequivocal homologues of eIF4G or 4EBP in Leishmania. Five candidates of eIF4G were recently identified in LeishDB, all of which contain the middle HEAT domain of eIF4G, but only LmEIF4G3 could bind eIF4A (15, 16). In the absence of a more detailed analysis of the eIF4G candidates, we tested the capacity of each LeishIF4E to interact with mammalian 4EBP1, a translation repressor that competes with eIF4G on binding to eIF4E (24, 27). A strong binding to h4EBP1 was observed with LeishIF4E-4 and, to a lesser extent, with LeishIF4E-1; thus, if 4EBP1 and eIF4G interact with the same residues in eIF4E, LeishIF4E-4, and LeishIF4E-1 could be part of the eIF4F complex. The eIF4E-eIF4G interaction is promoted by the cap-distal structure of eIF4E. In addition to conservation of specific residues such as Trp73 and Val 69 (26, 41), other regions of the molecule are involved, including the N terminus (61). Thus, conservation of these residues cannot account for the ability to bind 4EBP1, as was also noted for 4EHP, a cap-binding protein that does not interact with 4EBP1, despite sequence conservation of specific amino acids which are dedicated to this interaction (13, 55).
Neither LeishIF4E-1 nor LeishIF4E-4 is found in the polysomal fractions. This is in accordance with the analysis of eIF4E in rabbit reticulocyte lysates, where eIF4E is found in the postribosomal supernatant and in the 48S fraction (29, 51). Indeed, analysis of the sucrose gradient profile shows that LeishIF4E-1 and LeishIF4E-4 are found at the top of the gradient and that LeishIF4E-4 comigrates with 48S particles (Fig. 8).
LeishIF4E-1 contains an RNA sequence element in its 3′ UTR, which bears 68% homology to the amastigote-specific element found in the amastin gene family and Hsp100, which show a stage-specific pattern of expression (11, 44). Indeed, expression of LeishIF4E-1 in axenic amastigotes of L. amazonensis is much higher. Expression of the other three isoforms was subject to much milder changes, (∼30%).
Functional analysis of eIF4E homologues can be deduced from complementation studies. S. cerevisiae contains a single eIF4E isoform which is essential for its survival. eIF4E isoforms from heterologous organisms can replace yeast eIF4E, indicating that these proteins are evolutionary conserved (2, 28, 53, 54). None of the LeishIF4E isoforms could complement yeast eIF4E, possibly due to their high structural divergence. Unfortunately, there is yet no in vitro translation system for any trypanosomatid that can initiate translation. The only cell-free system that was developed for T. brucei can elongate but not initiate protein synthesis (19). Thus, we base the functional evaluation of different eIF4E isoforms in Leishmania mainly on biochemical analysis.
Supplementary Material
Acknowledgments
This work was supported by the German-Israeli Foundation (GIF; grant 728-23.2/2002 to M.S.), the Israel Ministry of Health, (grant 5440 to M.S.), the Howard Hughes Medical Institute (grant 55005604 to E.D.), the Polish Ministry of Science (grant 2P04A 006 28 to E.D.), and the Swiss National Science Foundation (grant 3100A0-104207 to M.A.).
Footnotes
Published ahead of print on 13 October 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Altmann, M., I. Edery, H. Trachsel, and N. Sonenberg. 1988. Site-directed mutagenesis of the tryptophan residues in yeast eukaryotic initiation factor 4E. Effects on cap binding activity. J. Biol. Chem. 263:17229-17232. [PubMed] [Google Scholar]
- 2.Altmann, M., P. P. Muller, J. Pelletier, N. Sonenberg, and H. Trachsel. 1989. A mammalian translation initiation factor can substitute for its yeast homologue in vivo. J. Biol. Chem. 264:12145-12147. [PubMed] [Google Scholar]
- 3.Aly, R., M. Argaman, and M. Shapira. 1994. A regulatory role for the 3′ and 5′ untranslated regions in differential expression of hsp83 in Leishmania. Nucleic Acids Res. 22:2922-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, J., and G. W. Zieve. 1991. Assembly and intracellular transport of snRNP particles. Bioessays 13:57-64. [DOI] [PubMed] [Google Scholar]
- 5.Arhin, G. K., H. Li, E. Ullu, and C. Tschudi. 2006. A protein related to the vaccinia virus cap-specific methyltransferase VP39 is involved in cap 4 modification in Trypanosoma brucei. RNA 12:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arhin, G. K., E. Ullu, and C. Tschudi. 2006. 2′-O-methylation of position 2 of the trypanosome spliced leader cap 4 is mediated by a 48 kDa protein related to vaccinia virus VP39. Mol. Biochem. Parasitol. 147:137-139. [DOI] [PubMed] [Google Scholar]
- 7.Bangs, J. D., P. F. Crain, T. Hashizume, J. A. McCloskey, and J. C. Boothroyd. 1992. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J. Biol. Chem. 267:9805-9815. [PubMed] [Google Scholar]
- 8.Barak, E., S. Amin-Spector, E. Gerliak, S. Goyard, N. Holland, and D. Zilberstein. 2005. Differentiation of Leishmania donovani in host-free system: analysis of signal perception and response. Mol. Biochem. Parasitol. 141:99-108. [DOI] [PubMed] [Google Scholar]
- 9.Bates, P. 1993. Axenic culture of Leishmania amastigotes. Parasitol. Today 9:143-146. [DOI] [PubMed] [Google Scholar]
- 10.Biton, M., M. Mandelboim, G. Avratz, and S. Michaeli. RNAi interference of XPO1 and Sm genes and their effect on the spliced leader RNA in Trypanosoma brucei. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]
- 11.Boucher, N., Y. Wu, C. Dumas, M. Dube, D. Sereno, M. Breton, and B. Papadopoulou. 2002. A common mechanism of stage-regulated gene expression in Leishmania mediated by a conserved 3′-untranslated region element. J. Biol. Chem. 277:19511-19520. [DOI] [PubMed] [Google Scholar]
- 12.Brandau, S., A. Dresel, and J. Clos. 1995. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem. J. 310:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho, P. F., F. Poulin, Y. A. Cho-Park, I. B. Cho-Park, J. D. Chicoine, P. Lasko, and N. Sonenberg. 2005. A new paradigm for translational control: inhibition via 5′-3′ mRNA tethering by Bicoid and the eIF4E cognate 4EHP. Cell 121:411-423. [DOI] [PubMed] [Google Scholar]
- 14.Clamp, M., J. Cuff, S. M. Searle, and G. J. Barton. 2004. The Jalview Java alignment editor. Bioinformatics 20:426-427. [DOI] [PubMed] [Google Scholar]
- 15.Dhalia, R., N. Marinsek, C. R. Reis, R. Katz, J. R. Muniz, N. Standart, M. Carrington, and O. P. de Melo Neto. 2006. The two eIF4A helicases in Trypanosoma brucei are functionally distinct. Nucleic Acids Res. 34:2495-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhalia, R., C. R. Reis, E. R. Freire, P. O. Rocha, R. Katz, J. R. Muniz, N. Standart, and O. P. de Melo Neto. 2005. Translation initiation in Leishmania major: characterisation of multiple eIF4F subunit homologues. Mol. Biochem. Parasitol. 140:23-41. [DOI] [PubMed] [Google Scholar]
- 17.Dinkova, T. D., B. D. Keiper, N. L. Korneeva, E. J. Aamodt, and R. E. Rhoads. 2005. Translation of a small subset of Caenorhabditis elegans mRNAs is dependent on a specific eukaryotic translation initiation factor 4E isoform. Mol. Cell. Biol. 25:100-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djikeng, A., H. Shi, C. Tschudi, S. Shen, and E. Ullu. 2003. An siRNA ribonucleoprotein is found associated with polyribosomes in Trypanosoma brucei. RNA 9:802-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duszenko, M., X. Kang, U. Bohme, R. Homke, and M. Lehner. 1999. In vitro translation in a cell-free system from Trypanosoma brucei yields glycosylated and glycosylphosphatidylinositol-anchored proteins. Eur. J. Biochem. 266:789-797. [DOI] [PubMed] [Google Scholar]
- 20.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, H., M. J. Ayub, M. J. Levin, and J. Frank. 2005. The structure of the 80S ribosome from Trypanosoma cruzi reveals unique rRNA components. Proc. Natl. Acad. Sci. USA 102:10206-10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garlapati, S., E. Dahan, and M. Shapira. 1999. Effect of acidic pH on heat shock gene expression in Leishmania. Mol. Biochem. Parasitol. 100:95-101. [DOI] [PubMed] [Google Scholar]
- 23.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 24.Gingras, A. C., S. P. Gygi, B. Raught, R. D. Polakiewicz, R. T. Abraham, M. F. Hoekstra, R. Aebersold, and N. Sonenberg. 1999. Regulation of 4EBP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13:1422-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gingras, A. C., B. Raught, S. P. Gygi, A. Niedzwiecka, M. Miron, S. K. Burley, R. D. Polakiewicz, A. Wyslouch-Cieszynska, R. Aebersold, and N. Sonenberg. 2001. Hierarchical phosphorylation of the translation inhibitor 4EBP1. Genes Dev. 15:2852-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross, J. D., N. J. Moerke, T. von der Haar, A. A. Lugovskoy, A. B. Sachs, J. E. McCarthy, and G. Wagner. 2003. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 115:739-750. [DOI] [PubMed] [Google Scholar]
- 27.Haghighat, A., S. Mader, A. Pause, and N. Sonenberg. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14:5701-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández, G., M. Altmann, J. M. Sierra, H. Urlaub, R. D. del Corral, P. Schwartz, and R. Rivera-Pomar. 2005. Functional analysis of seven genes encoding eight translation initiation factor 4E (eIF4E) isoforms in Drosophila. Mech. Dev. 122:529-543. [DOI] [PubMed] [Google Scholar]
- 29.Hiremath, L. S., S. T. Hiremath, W. Rychlik, S. Joshi, L. L. Domier, and R. E. Rhoads. 1989. In vitro synthesis, phosphorylation, and localization on 48 S initiation complexes of human protein synthesis initiation factor 4E. J. Biol. Chem. 264:1132-1138. [PubMed] [Google Scholar]
- 30.Hug, M., V. B. Carruthers, C. Hartmann, D. Sherman, G. Cross, and C. Clayton. 1993. A possible role for the 3′-untranslated region in developmental regulation in Trypanosoma brucei. Mol. Biochem. Parasitol. 61:87-96. [DOI] [PubMed] [Google Scholar]
- 31.Joshi, B., A. Cameron, and R. Jagus. 2004. Characterization of mammalian eIF4E-family members. Eur. J. Biochem. 271:2189-2203. [DOI] [PubMed] [Google Scholar]
- 32.Keiper, B. D., B. J. Lamphear, A. M. Deshpande, M. Jankowska-Anyszka, E. J. Aamodt, T. Blumenthal, and R. E. Rhoads. 2000. Functional characterization of five eIF4E isoforms in Caenorhabditis elegans. J. Biol. Chem. 275:10590-10596. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Lejbkowicz, F., C. Goyer, A. Darveau, S. Neron, R. Lemieux, and N. Sonenberg. 1992. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc. Natl. Acad. Sci. USA 89:9612-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewdorowicz, M., Y. Yoffe, J. Zuberek, J. Jemielity1, J. Stepinski1, R. Kierzek, R. Stolarski1, M. Shapira, and E. Darzynkiewicz. 2004. Chemical synthesis and binding activity of the trypanosomatid cap-4 structure. RNA 10:1469-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, H., and C. Tschudi. 2005. Novel and essential subunits in the 300-kilodalton nuclear cap binding complex of Trypanosoma brucei. Mol. Cell. Biol. 25:2216-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang, X.-H., A. Haritan, S. Uliel, and S. Michaeli. 2003. trans and cis splicing in trypanosomatids: mechanism, factors, and regulation. Eukaryot. Cell 2:830-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mair, G., E. Ullu, and C. Tschudi. 2000. Cotranscriptional cap 4 formation on the Trypanosoma brucei spliced leader RNA. J. Biol. Chem. 275:28994-28999. [DOI] [PubMed] [Google Scholar]
- 39.Mandelboim, M., C. L. Estrano, C. Tschudi, E. Ullu, and S. Michaeli. 2002. On the role of exon and intron sequences in trans-splicing utilization and cap 4 modification of the trypanosomatid Leptomonas collosoma SL RNA. J. Biol. Chem. 277:35210-35218. [DOI] [PubMed] [Google Scholar]
- 40.Marcotrigiano, J., A. C. Gingras, N. Sonenberg, and S. K. Burley. 1997. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89:951-961. [DOI] [PubMed] [Google Scholar]
- 41.Marcotrigiano, J., A. C. Gingras, N. Sonenberg, and S. K. Burley. 1999. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell 6:707-716. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo, H., H. Li, A. M. McGuire, C. M. Fletcher, A. C. Gingras, N. Sonenberg, and G. Wagner. 1997. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat. Struct. Biol. 9:717-724. [DOI] [PubMed] [Google Scholar]
- 43.Mattaj, I. W. 1986. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell 46:905-911. [DOI] [PubMed] [Google Scholar]
- 44.McNicoll, F., M. Muller, S. Cloutier, N. Boilard, A. Rochette, M. Dube, and B. Papadopoulou. 2005. Distinct 3′-untranslated region elements regulate stage-specific mRNA accumulation and translation in Leishmania. J. Biol. Chem. 280:35238-35246. [DOI] [PubMed] [Google Scholar]
- 45.Miyoshi, H., D. S. Dwyer, B. D. Keiper, M. Jankowska-Anyszka, E. Darzynkiewicz, and R. E. Rhoads. 2002. Discrimination between mono- and trimethylated cap structures by two isoforms of Caenorhabditis elegans eIF4E. EMBO J. 21:4680-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morino, S., H. Hazama, M. Ozaki, Y. Teraoka, S. Shibata, M. Doi, H. Ueda, T. Ishida, and S. Uesugi. 1996. Analysis of the mRNA cap-binding ability of human eukaryotic initiation factor-4E by use of recombinant wild-type and mutant forms. Eur. J. Biochem. 239:597-601. [DOI] [PubMed] [Google Scholar]
- 47.Mouaikel, J., C. Verheggen, E. Bertrand, J. Tazi, and R. Bordonne. 2002. Hypermethylation of the cap structure of both yeast snRNAs and snoRNAs requires a conserved methyltransferase that is localized to the nucleolus. Mol. Cell 9:891-901. [DOI] [PubMed] [Google Scholar]
- 48.Myler, P. J., and K. D. Stuart. 2000. Recent developments from the Leishmania genome project. Curr. Opin. Microbiol. 3:412-416. [DOI] [PubMed] [Google Scholar]
- 49.Niedzwiecka, A., J. Marcotrigiano, J. Stepinski, M. Jankowska-Anyszka, A. Wyslouch-Cieszynska, M. Dadlez, A. C. Gingras, P. Mak, E. Darzynkiewicz, N. Sonenberg, S. K. Burley, and R. Stolarski. 2002. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4EBP1 proteins. J. Mol. Biol. 319:615-635. [DOI] [PubMed] [Google Scholar]
- 50.Quijada, L., M. Soto, C. Alonso, and J. M. Requena. 2000. Identification of a putative regulatory element in the 3′-untranslated region that controls expression of HSP70 in Leishmania infantum. Mol. Biochem. Parasitol. 110:79-91. [DOI] [PubMed] [Google Scholar]
- 51.Rau, M., T. Ohlmann, S. J. Morley, and V. M. Pain. 1996. A reevaluation of the cap-binding protein, eIF4E, as a rate-limiting factor for initiation of translation in reticulocyte lysate. J. Biol. Chem. 271:8983-8990. [DOI] [PubMed] [Google Scholar]
- 52.Richter, J. D., and N. Sonenberg. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433:477-480. [DOI] [PubMed] [Google Scholar]
- 53.Robalino, J., B. Joshi, S. C. Fahrenkrug, and R. Jagus. 2004. Two zebrafish eIF4E family members are differentially expressed and functionally divergent. J. Biol. Chem. 279:10532-10541. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez, C. M., M. A. Freire, C. Camilleri, and C. Robaglia. 1998. The Arabidopsis thaliana cDNAs coding for eIF4E and eIF(iso)4E are not functionally equivalent for yeast complementation and are differentially expressed during plant development. Plant J. 13:465-473. [DOI] [PubMed] [Google Scholar]
- 55.Rom, E., H. C. Kim, A. C. Gingras, J. Marcotrigiano, D. Favre, H. Olsen, S. K. Burley, and N. Sonenberg. 1998. Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein. J. Biol. Chem. 273:13104-13109. [DOI] [PubMed] [Google Scholar]
- 56.Scheper, G. C., B. van Kollenburg, J. Hu, Y. Luo, D. J. Goss, and C. G. Proud. 2002. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Biol. Chem. 277:3303-3309. [DOI] [PubMed] [Google Scholar]
- 57.Shapira, M., J. G. McEwen, and C. L. Jaffe. 1988. Temperature effects on molecular processes which lead to stage differentiation in Leishmania. EMBO J. 7:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarun, S. Z., Jr., S. E. Wells, J. A. Deardorff, and A. B. Sachs. 1997. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl. Acad. Sci. USA 94:9046-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, M. Sasaki, T. Taniguchi, H. Miyagawa, K. Kitamura, K. Miura, and T. Ishida. 2003. Structural features of human initiation factor 4E, studied by X-ray crystal analyses and molecular dynamics simulations. J. Mol. Biol. 328:365-383. [DOI] [PubMed] [Google Scholar]
- 60.Tschudi, C., and E. Ullu. 1988. Polygene transcripts are precursors to calmodulin mRNAs in trypanosomes. EMBO J. 7:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von der Haar, T., Y. Oku, M. Ptushkina, N. Moerke, G. Wagner, J. D. Gross, and J. E. McCarthy. 2006. Folding transitions during assembly of the eukaryotic mRNA cap-binding complex. J. Mol. Biol. 356:982-992. [DOI] [PubMed] [Google Scholar]
- 62.Yoffe, Y., J. Zuberek, M. Lewdorowicz, Z. Zeira, C. Keasar, I. Orr-Dahan, M. Jankowska-Anyszka, J. Stepinski, E. Darzynkiewicz, and M. Shapira. 2004. Cap-binding activity of an eIF4E homolog from Leishmania. RNA 10:1764-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, Y. T., M. D. Shu, and J. A. Steitz. 1998. Modifications of U2 snRNA are required for snRNP assembly and pre-mRNA splicing. EMBO J. 17:5783-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zamudio, J. R., B. Mittra, G. M. Zeiner, M. Feder, J. M. Bujnicki, N. R. Sturm, and D. A. Campbell. 2006. Complete cap 4 formation is not required for viability in Trypanosoma brucei. Eukaryot. Cell 5:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zilka, A., S. Garlapati, E. Dahan, V. Yaolsky, and M. Shapira. 2001. Developmental regulation of heat shock protein 83 in Leishmania. 3′ processing and mRNA stability control transcript abundance, and translation is directed by a determinant in the 3′-untranslated region. J. Biol. Chem. 276:47922-47929. [DOI] [PubMed] [Google Scholar]
- 66.Zuberek, J., A. Wyslouch-Cieszynska, A. Niedzwiecka, M. Dadlez, J. Stepinski, W. Augustyniak, A. C. Gingras, Z. Zhang, S. K. Burley, N. Sonenberg, R. Stolarski, and E. Darzynkiewicz. 2003. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA 9:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.