Abstract
Yersinia pseudotuberculosis mutants deficient for the adhesins invasin and/or YadA were injected intravenously into BALB/c mice. Invasin expression inhibited colonization of the liver and spleen. YadA decreased liver colonization but promoted growth within the lung. The persistence of leukocytes within liver microabscesses correlated with enhanced colonization and lack of adhesin expression.
The Yersinia pseudotuberculosis outer membrane adhesins invasin and YadA play an important role in adherence and invasion of host cells and tissue (8, 15). After entry into the intestinal lumen, Y. pseudotuberculosis penetrates the epithelium via interactions between these adhesins and integrin receptors on the surface of specialized M cells that overlie Peyer's patches (11). Systemic infections, marked by dissemination to the liver, spleen, and lung, can ensue under conditions that favor extracellular growth of bacteria and avoidance of phagocytosis by innate immune cells (4). The Yersinia outer proteins (Yops), which are encoded on the virulence plasmid pYV, contribute to the inhibition of innate immunity. Despite these mechanisms, it has been estimated that Yops are only about 50% effective in preventing uptake by host phagocytes (19) and that even in the presence of these molecules, invasin and YadA function to promote uptake by mammalian cells (10).
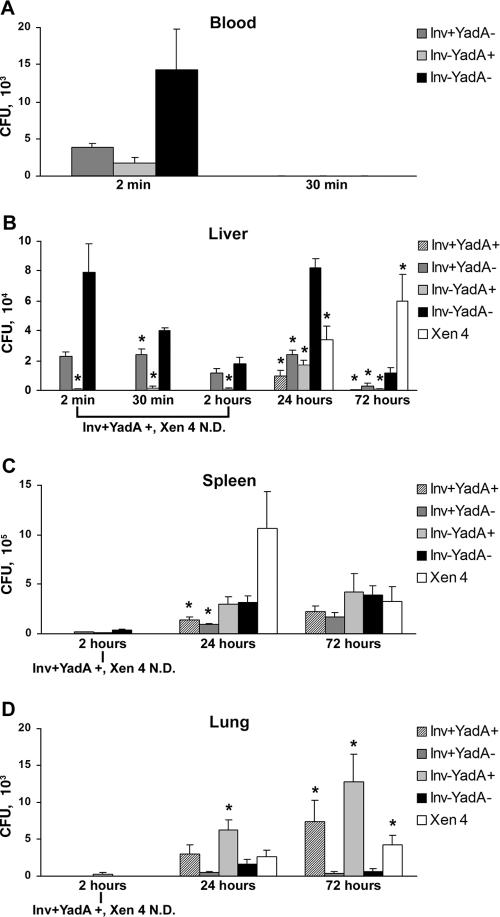
To determine whether invasin and/or YadA expression contribute to tissue colonization during a systemic Y. pseudotuberculosis infection, 6- to 8-week-old BALB/c male mice (Jackson Laboratories, Bar Harbor, ME) were injected via the tail vein with a sublethal dose (5 × 104) of Y. pseudotuberculosis inv and/or yadA YPIII mutants that were cultured as described previously (14). These strains all lacked the pYV virulence plasmid, providing a means to investigate the contributions of invasin and YadA to tissue colonization without interference from the plasmid-encoded type III secretion apparatus and antiphagocytic Yop effector molecules. They were generated from Y. pseudotuberculosis strains YP137 or YP202 (an inv transposon insertion mutant of YP137 kindly provided by Ralph Isberg, Tufts University, Boston, MA [see reference 15]) by the introduction of either a control vector (pMMB67EH) or the related multicopy plasmid (pYadA) encoding YadA under the control of a lactose-inducible promoter (3). All mice were fed water containing 10 mM IPTG (isopropyl-β-d-thiogalactopyranoside) beginning 3 days prior to infection to induce in vivo expression of YadA (see Fig. S1 in the supplemental material). As a positive control for infection, mice were infected in parallel with Xen 4 (Xenogen Corp., Alameda, CA), a YPIII strain that carries the pYV virulence plasmid and expresses both invasin and YadA. Levels of bacteremia were determined by measuring the number of CFU in blood obtained via cardiac puncture. The mutant that was deficient for the expression of both invasin and YadA (Inv−YadA−) demonstrated reproducibly higher levels of bacteremia 2 min postinfection compared to otherwise-isogenic strains expressing invasin (Fig. 1A, left group). It is important to note that all strains were detected at equivalent levels after direct dilution in whole mouse blood (data not shown), indicating that the number of bacteria isolated from infected mouse blood was not affected by differences between the strains in survival or oligomerization. However, while reproducible, the difference between Inv−YadA− levels in the blood and the other strains was not found to be statistically significant. This may be due to the inherent variability in sampling at short times following infection and the rapidity of bacterial clearance from the blood. At 30 min following tail vein injection, all strains were largely cleared from the blood (Fig. 1A, right group) and were not detected at subsequent time periods postinfection (data not shown).
FIG. 1.
Bacteremia and tissue colonization by Y. pseudotuberculosis adhesin mutants. CFU per ml of blood (A) or gram of tissue (B to D) are shown for each strain of bacteria. Infections with strains Inv+YadA+ and Xen 4 were performed at 24 and 72 h only. N.D., not done. The data shown represent means from three to four mice for infections lasting from 2 min through 2 h and five to eight mice for infections of 24 and 72 h. Statistical analysis was performed using raw, non-normalized data. Standard errors from the mean are indicated by error bars. A two-sample t test, assuming unequal variance, was used to determine the statistical significance between condition means, with a significance level of ≤0.05. Asterisks represent a statistically significant deviation from the means relative to the Inv−YadA− strain.
We next investigated whether the presence or absence of invasin and/or YadA affected bacterial colonization, proliferation, or survival in the liver. Tissues were removed aseptically from mice sacrificed between 2 min and 72 h postinfection, weighed, and homogenized with a TissueMaster 240 homogenizer (Omni International, Marietta, GA) in phosphate-buffered saline. Relative to the strain that was deficient for adhesin expression (Inv−YadA−; Fig. 1B, black bar), invasin and/or YadA expression was associated with decreased colonization of the liver throughout the time course of the present study (2 min to 72 h). All strains maintained expression of invasin and/or YadA in the mouse (see Fig. S1 in the supplemental material), and there was no evidence of pMMB67EH plasmid loss in the absence of antibiotic selection over the 72-h period of infection (data not shown). In addition, although it was not possible to measure proliferation rates in the mouse, the growth rate of these strains in culture was identical (data not shown). These findings thus suggest that the Inv−YadA− strain was more effective at reaching the liver and/or evading phagocytosis within this tissue during a systemic infection compared to adhesin-expressing strains. Interestingly, colonization of the liver by the Inv−YadA− strain was also elevated compared to the wild-type strain at 24 h (Xen 4; Fig. 1B, white bar), despite the fact that Xen 4 expresses the Yop effector molecules.
Earlier tissue culture studies suggested that the contribution of invasin and YadA to cellular adherence and uptake is dependent on the content of the extracellular environment (12, 14). Therefore, we assessed the levels of bacterial colonization in the spleen and lung in addition to the liver to determine whether the functions of these adhesins are affected by different tissue microenvironments. All strains demonstrated a net gain (5- to 36-fold) in colonization of the spleen from 2 to 24 h postinfection (Fig. 1C). At 24 h, colonization by the Inv−YadA− (Fig. 1C, black bar) and Inv−YadA+ (Fig. 1C, light gray bar) strains was significantly elevated relative to invasin-expressing strains (Inv+YadA+ and Inv+YadA−). Colonization of the spleen over the next 48 h remained relatively constant for all of the Yop-deficient adhesin mutants. However, the Xen 4 strain showed a significant reduction in colonization during the period from 24 to 72 h postinfection. This may be explained by the robust immune response mounted against this strain in the spleen, coincident with the appearance of microabscesses (see Fig. S2F, L, and R in the supplemental material).
Lung tissue includes a complex array of matrix proteins that support the biomechanics of respiration (5, 18, 21). We, along with others, have demonstrated that several of these extracellular matrix proteins enhance YadA-mediated, but inhibit invasin-mediated, adherence of Y. pseudotuberculosis to host cells (8, 12, 14). In the lung, YadA-expressing strains (Inv+YadA+, Inv−YadA+, and Xen 4) demonstrated an advantage in colonization compared to strains deficient for YadA (Fig. 1D).
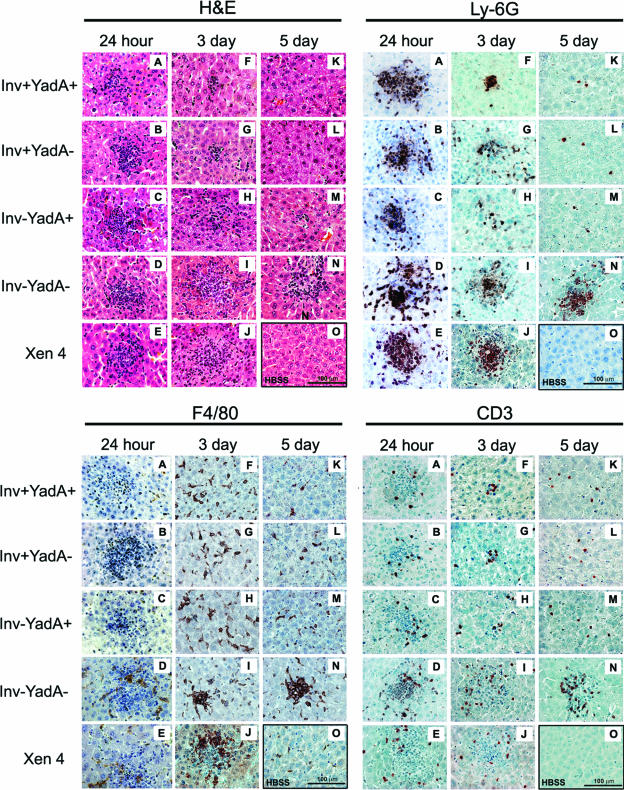
To examine whether bacterial colonization correlated with disease pathology and immune responses, sections of hepatic tissues that had previously been fixed in 4% paraformaldehyde and embedded in paraffin were processed for staining with hematoxylin and eosin (H&E) or immunohistochemistry (IHC). Staining was performed according to the manufacturer's recommendations (Vectastain ELITE ABC, DAB peroxidase substrate; Vector Laboratories, Burlingame, CA). We focused on the liver because, in the course of performing the mouse infections, it became clear that enhanced bacterial colonization of liver tissue correlated with increased morbidity of the mice. In addition, both mouse (1, 2, 16, 20) and human (7, 9, 13, 22) Yersinia infections are associated with infiltrating phagocytes and the formation of microabscesses in the liver. Multiple microabscesses and increased cellularity in interstitial spaces, populated with neutrophils (Ly-6G-positive), macrophages (F4/80-positive), and T cells (CD3-positive), were observed in the liver 24 h postinoculation (Fig. 2 and see Fig. S3A to E in the supplemental material). These features were not observed in tissue from mock-infected mice (panel O). Between 24 h and 5 days after infection, microabscesses that had formed in response to strains expressing invasin and/or YadA were reduced in both number and area, whereas lesions formed in response to the Inv−YadA− and the Xen 4 wild-type strains remained large, abundant, and densely populated with immune cells (Fig. 2 and S3F to N in the supplemental material). Quantitation of cellular infiltration of the liver by flow cytometry demonstrated an increase in the total number of leukocytes, including neutrophils, macrophages/monocytes, and T cells, in response to infection by all strains of Y. pseudotuberculosis (data not shown). There were, however, no significant strain-dependent differences in recruitment of these cell types to the liver. Therefore, the increased bacterial colonization that was observed in the presence of Y. pseudotuberculosis strains lacking invasin and YadA (Fig. 1B) correlated with more persistent localization of immune cells within liver microabscesses rather than differences in total cellular infiltration to the liver.
FIG. 2.
Pathology and recruitment of leukocytes to the liver. (A to O) H&E staining and IHC analysis of control liver tissue isolated from mice injected with Hanks balanced salt solution (O) or infected by the designated strains of Y. pseudotuberculosis (A to N). Representative images (magnification, ×400) were captured with a light microscope controlled by Image Pro plus software (EPIX, Inc., Buffalo Grove, IL). IHC staining of neutrophils (LY-6G-positive cells, detected by monoclonal antibody [MAb] NIMP-R14; Hycult Biotechnology, Uden, The Netherlands), macrophages (F4/80-positive cells, detected by rat anti-mouse F4/80 MAbs; Serotec, Raleigh, NC), and T cells (CD3-positive cells, detected by MAb MCA 1477; Serotec) is shown. All experiments were repeated three times; the data shown are from one representative experiment.
Because the adhesin mutant strains were isogenic with the exception of invasin and/or YadA expression (14), disparities in bacterial colonization, tissue pathology, and localization of leukocytes within microabscesses can be attributed to altered adhesin expression. Bacteremia and tissue colonization at early time points after infection (2 min to 2 h) likely involve adhesin functions associated with promoting tissue adherence and perhaps interaction with capillary vessels and/or professional phagocytes. In contrast, differences in colonization and persistence of infection over longer periods of time (2 h to 5 days) are likely to be governed both by bacterial proliferation within the tissue and by avoidance of clearance by the innate immune response of the host. The results of the present study support a model whereby the tissue-specific microenvironment, including the extracellular matrix content and immune cell repertoire, plays an important role in determining whether invasin and YadA promote bacterial clearance or persistence of infection at any given time. These findings also lend support for the theory that mutations resulting in the loss of functional inv and yadA genes during the evolution of Y. pestis (6, 17) might contribute to the increased pathogenicity of this organism by facilitating dissemination and/or evasion of the immune response in selected host tissues. However, it is important to note that the bacterial strains utilized in the present study are distinct from naturally occurring Y. pseudotuberculosis strains with respect to the absence of the pYV virulence plasmid, the route of infection, and the expression of YadA from a non-native promoter. Further studies are therefore needed to address how the presence of the pYV virulence plasmid affects the role of these adhesins in determining tissue colonization and the ensuing immune response.
Supplementary Material
Acknowledgments
We thank members of the laboratory for their contributions to this work. We thank James Bliska (SUNY, Stony Brook, NY) for kindly providing the adhesin mutant strains of Y. pseudotuberculosis used in this study. We thank Sherri Vanhoose and the Histology Core lab at UVA for their help in processing tissue samples. We thank Mark H. Stoler for his assistance and expertise in analyzing tissue samples. We thank especially Lisa Gross and the laboratory of Tom Obrig for their guidance in performing IHC on tissue samples, and we thank Joanne Lannigan and Michael Solga in the flow cytometry core at UVA for help with flow cytometry. We value the intellectual contributions to this study provided by Kodi Ravichandran, Robert J. Kadner, James Casanova, Anne Sutherland, Jay Brown, and Tom Obrig.
K.J.H. has been supported by the Infectious Disease Training Grant (University of Virginia School of Medicine; T32 AI 07046). This work was supported by the National Institutes of Health grant AI 050733 to A.H.B.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 18 August 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Autenrieth, I. B., P. Hantschmann, B. Heymer, and J. Heesemann. 1993. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology 187:1-16. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska, J. B., K. L. Guan, J. E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 88:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcante, F. S., S. Ito, K. Brewer, H. Sakai, A. M. Alencar, M. P. Almeida, J. S. Andrade, Jr., A. Majumdar, E. P. Ingenito, and B. Suki. 2005. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J. Appl. Physiol. 98:672-679. [DOI] [PubMed] [Google Scholar]
- 6.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosbie, J., J. Varma, and J. Mansfield. 2005. Yersinia enterocolitica infection in a patient with hemachromatosis masquerading as proximal colon cancer with liver metastases: report of a case. Dis. Colon Rectum. 48:390-392. [DOI] [PubMed] [Google Scholar]
- 8.Eitel, J., and P. Dersch. 2002. The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect. Immun. 70:4880-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrer, W., P. Kloser, and S. Ketyer. 1988. Yersinia pseudotuberculosis sepsis presenting as multiple liver abscesses. Am. J. Med. Sci. 295:129-132. [DOI] [PubMed] [Google Scholar]
- 10.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grutzkau, A., C. Hanski, H. Hahn, and E. O. Riecken. 1990. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heise, T., and P. Dersch. 2006. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc. Natl. Acad. Sci. USA 103:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopfner, M., R. Nitsche, A. Rohr, D. Harms, S. Schubert, and U. R. Folsch. 2001. Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis. Scand. J. Gastroenterol. 36:220-224. [DOI] [PubMed] [Google Scholar]
- 14.Hudson, K. J., J. B. Bliska, and A. H. Bouton. 2005. Distinct mechanisms of integrin binding by Yersinia pseudotuberculosis adhesins determine the phagocytic response of host macrophages. Cell Microbiol. 7:1474-1489. [DOI] [PubMed] [Google Scholar]
- 15.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 16.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 18.Roman, J. 1996. Extracellular matrix and lung inflammation. Immunol. Res. 15:163-178. [DOI] [PubMed] [Google Scholar]
- 19.Rosqvist, R., I. Bolin, and H. Wolf-Watz. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonet, M., S. Richard, and P. Berche. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobin, S. S., Y. C. Fung, and H. M. Tremer. 1988. Collagen and elastin fibers in human pulmonary alveolar walls. J. Appl. Physiol. 64:1659-1675. [DOI] [PubMed] [Google Scholar]
- 22.Strungs, I., D. J. Farrell, L. D. Matar, L. Dekker, and R. J. Franz. 1995. Multiple hepatic abscesses due to Yersinia enterocolitica. Pathology 27:374-377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.