Abstract
Recent studies have shown that matrix metalloproteinases (MMPs) are induced by Mycobacterium tuberculosis during pulmonary infection. Here, expression of MMP-9 during pulmonary M. tuberculosis infection was characterized to determine whether its production correlated with disease resistance in vivo and to determine what role, if any, MMP-9 might have in granuloma formation. Following aerosol infection with M. tuberculosis, dissemination of bacilli occurred earlier in the C57BL/6 resistant mouse strain than in the susceptible CBA/J strain, as was evident from an increased number of bacteria in the blood, spleen, and liver at day 14 after infection. In addition, early dissemination of the bacilli was associated with early induction of protective immunity as assessed from gamma interferon levels. Nonspecific blocking of MMPs in C57BL/6 mice early during infection reduced hematogenous spread of the bacilli, suggesting that MMPs indeed play a role in facilitating dissemination, likely via extracellular matrix degradation. The concentration of active MMP-9, specifically, was greater in the lungs of C57BL/6 mice than in those of the CBA/J mice at day 28, thereby suggesting that MMP-9 is not one of the MMPs directly involved in promoting early dissemination of M. tuberculosis. Instead, however, histological lung sections and flow cytometric analysis of lung cells from MMP-9-knockout mice showed that MMP-9 is involved in macrophage recruitment and granuloma development. These combined data support the idea that early MMP activity is an essential component of resistance to pulmonary mycobacterial infection and that MMP-9, specifically, is required for recruitment of macrophages and tissue remodeling to allow for the formation of tight, well-organized granulomas.
Tuberculosis continues to be a major health problem worldwide, and it is estimated that one-third of the earth's population is infected with Mycobacterium tuberculosis (6). It is thought that the majority of people who become infected clear the bacilli before a productive infection is established. In some people, however, the immune response is inefficient at killing the bacteria, thereby resulting in the survival of a small number of bacteria within lung granulomas. Existing in a latent state, the bacilli give rise to a subclinical, chronic infection which can reactivate when the host faces immunosuppressive events such as human immunodeficiency virus infection or aging (6).
Acquired immunity to M. tuberculosis is cell mediated such that T cells producing gamma interferon (IFN-γ) activate bactericidal mechanisms of infected macrophages to kill or, at least in some cases, to contain mycobacterial growth (4, 5, 9, 30). The accumulation of monocytes and lymphocytes from the blood around the infectious focus results in the formation of granulomas (3, 28, 30), in which M. tuberculosis-infected macrophages secrete inflammatory cytokines (interleukin-1 [IL-1], IL-6, IL-12, and tumor necrosis factor alpha [TNF-α]) and chemokines such as macrophage chemoattractant protein 1 (MCP-1) (10, 11, 40). Extensive interstitial fibrosis associated with granulomas consisting mainly of epithelioid and foamy macrophages and lymphocytes is evident during the course of infection (37).
Enzymes of the matrix metalloproteinase (MMP) family play a significant role in many biological activities including many aspects of the immune response such as granuloma formation (17, 32). In general, MMPs are endopeptidases responsible for degrading components of the extracellular matrix (ECM) such as collagen and proteoglycans, and as potent chemokine antagonists, they play an important role in leukocyte migration and tissue remodeling. Of interest in inflammatory models are MMP-2 (gelatinase A) and MMP-9 (gelatinase B), which degrade type IV collagen, a major component of the basement membrane within the lung (31, 44). MMPs are tightly regulated at the posttranslational level by proteolytic activation and interaction with specific inhibitors called tissue inhibitors of metalloproteinases (45). Cytokines control MMP production through positive or negative regulatory elements at the gene level (45), as well as influence the production of certain proteolytic enzymes that activate or inhibit MMPs (12, 38). Thus, in a cytokine-rich environment such as that established during infection with M. tuberculosis, it may be anticipated that cytokines control MMP regulation and activity.
A strong connection between the immune response and MMP activity has recently emerged, and MMPs have been shown to mediate the expression of immunity to infectious pathogens (21, 43), as well as the inflammatory process in general (24, 29, 33, 46). Several studies have shown that MMPs, in particular MMP-9, are expressed during the various manifestations of tuberculosis, including active cavitary tuberculosis (2, 16, 35), meningitis (20, 22, 34, 41), and pleuritis (15, 16, 18). Thus, there are emerging data to suggest a strong role for MMP activity with the various pathological states associated with tuberculosis. The ECM plays an important role in the structure and composition of the granuloma in terms of leukocyte trafficking to and from this dynamic environment and may contribute to the relative location of leukocyte subpopulations (13, 37).
Mice infected via the pulmonary route with M. tuberculosis have been grouped into susceptible and resistant strains based on their survival (26), which correlates with differences in leukocyte recruitment, granuloma structure, cytokine production, and adhesion molecule expression (42). Recently, it has been shown that in response to M. tuberculosis infection in vitro, macrophages from a resistant mouse strain (C57BL/6) produced significantly higher levels of MMP-9 mRNA than did macrophages from a susceptible mouse strain (CBA/J) (19). In this study, we set out to determine if MMP protein expression in vivo correlated with resistance to pulmonary infection with M. tuberculosis. Results of this investigation showed that early dissemination of M. tuberculosis bacilli was reduced when MMPs were blocked with a broad-spectrum MMP inhibitor and that this dissemination was associated with the induction of Th1-type immunity and protection in the resistant C57BL/6 mouse strain compared to the susceptible CBA/J strain. The amount of active MMP-9, specifically, was greater in the resistant C57BL/6 mice than in the susceptible CBA/J mice, and while this particular MMP enzyme does not seem to be involved in facilitating early dissemination of the bacilli, data from MMP-9-knockout mice showed that MMP-9 was essential for recruitment of macrophages to the lungs, as well as for tissue remodeling that facilitated the development of well-formed granulomas.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female, 6- to 8-week-old, CBA/J and C57BL/6 mice were purchased from Taconic (Germantown, NY), and wild-type FVB mice and MMP-9 null (MMP-9KO) breeder mice were purchased from Jackson Laboratory (Bar Harbor, ME). The MMP-9KO breeders were maintained as a colony at Colorado State University. All mice were maintained under barrier conditions with sterile mouse chow and water ad libitum. The specific-pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals, which were shown to be negative for 12 known mouse pathogens. All experimental procedures were approved by the Colorado State University Animal Care and Use Committee.
Infection with M. tuberculosis.
Mycobacterium tuberculosis strain H37Rv, obtained from the Trudeau Mycobacterial Culture Collection, was passaged three times through pellicle and then stored frozen as a seed stock. Working stocks were made by passage through liquid culture of Proskauer-Beck medium containing 0.01% Tween 80, and aliquots of the organism in mid-log-phase growth were stored at −80°C until needed. Mice were infected using procedures described previously (4). Briefly, bacterial stocks were diluted in 5 ml of sterile distilled water to 2 × 106 CFU/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN). Mice were exposed to 40 min of aerosol, during which approximately 50 to 100 bacteria were deposited in the lungs of each animal. For the experiments in which mice were treated with BB-94 (also known as Batimastat), mice were infected with 500 CFU intratracheally (17). Bacterial load was determined by plating whole-organ homogenates onto nutrient 7H11 agar supplemented with oleic acid-albumin-dextrose-catalase. Colonies were enumerated after a 21-day incubation at 37°C.
Inhibition of MMP activity.
BB-94 (Batimastat), a broad-spectrum inhibitor of MMP activity (17, 27) (see the supplemental material), was provided by British Biotech Pharmaceuticals Limited (Oxford, United Kingdom) and prepared for injection according to the manufacturer's instructions. BB-94-treated mice were injected intraperitoneally with 0.2 ml of 20 mg of BB-94 per kg of body weight at the time of intratracheal infection with 500 CFU of M. tuberculosis and daily for the duration of the experiment.
Measurement of MMPs.
The concentration of active MMP-9 was measured in lung homogenate supernatants using an MMP-9 Biotrak Activity Assay System (Biotrak Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Briefly, standards and samples were incubated in microtiter wells coated with anti-MMP-9 antibody. Any MMP-9 present remained bound to the precoated wells during subsequent washing and aspiration. MMP activity was detected through activation of the modified prodetection enzyme and the subsequent cleavage of its chromogenic peptide substrate. The resulting color was read at 405 nm on a spectrophotometer.
Lung cell digestion.
Mice were euthanized by CO2 asphyxiation, and the pulmonary cavity was opened. The lungs were cleared of blood by perfusion through the pulmonary artery with 10 ml of cold phosphate-buffered saline containing 50 U/ml heparin (Sigma-Aldrich, St. Louis, MO). The lungs were removed from the thoracic cavity, teased apart, and treated with a solution of DNase IV (Sigma-Aldrich; 30 μg/ml) and collagenase D (Roche, Nutley, NJ; 0.7 mg/ml) for 30 min at 37°C. To obtain a single-cell suspension, the lungs were passed through cell strainers (BD Biosciences, Mountain View, CA). Remaining red blood cells were then lysed with Gey's solution (0.15 M NH4Cl and 10 mM KHCo3), and the cells were washed with RPMI (Invitrogen, Carlsbad, CA) containing 5% heat-inactivated fetal bovine serum (Atlas Biologicals, Fort Collins, CO). Total viable cell numbers were determined using a Neubauer chamber (IMV International, Minneapolis, MN) and 2% trypan blue solution.
Flow cytometric analysis.
Single-cell lung suspensions were stained with fluorescently labeled, monoclonal antibodies against CD3 and CD4 surface markers on T lymphocytes and CD11b, CD11c, and Gr-1 on macrophages. Antibodies were purchased from BD PharMingen (San Diego, CA) unless otherwise stated and were used at 25 μg/ml. Cells were gated on lymphocytes or macrophages by forward and side scatter according to their characteristic scatter profile. Individual cell populations were identified according to the presence of specific fluorescence-labeled antibody, all analyses were performed with an acquisition of at least 30,000 events on a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed using Cell Quest software (BD Biosciences, San Jose, CA).
Cytometric bead array.
A Cytometric Bead Array Mouse Inflammation kit (BD Biosciences, San Jose, CA) was used to measure TNF-α, IFN-γ, and MCP-1 in the lung homogenate supernatants. The assay procedure was performed according to kit instructions, and the beads were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Immunohistochemistry.
Whole lungs from euthanized C57BL/6 and CBA/J mice were inflated with 10% formalin. The tissues were then paraffin embedded and sectioned. Following dewaxing, endogenous peroxidase was inhibited by incubation in 3% H2O2. Nonspecific staining was blocked with DAKO Protein Block Serum-Free (DakoCytomation, Carpinteria, CA). The cells containing cytokeratin were immunohistochemically stained with a polyclonal rabbit anti-cow cytokeratin antibody for wide-spectrum screening (DakoCytomation). A pretreatment of proteinase K (DakoCytomation) was utilized prior to antibody incubation. After incubation with primary antibody, the tissue sections were sequentially incubated with Dako Envision+ Rabbit System Labeled Polymer and horseradish peroxidase (DakoCytomation). Staining was developed with Liquid DAB+ (DakoCytomation), and cells were counterstained with hematoxylin. The antibody specifically stained epidermal keratin subunits of 58, 56, and 52 kDa; less abundant subunits of 60, 51, and 48 kDa were also found. For macrophage staining, anti-F4/80 antibody was used.
Histological analysis.
Whole lungs from euthanized FVB and MMP-9KO mice (n = 3) were inflated with 10% formalin and embedded in paraffin, and five 4-μm serial sections were cut. Sections 1 and 5 were then stained with hematoxylin and eosin and examined by a veterinary pathologist with no prior knowledge of the experimental design.
Statistical analysis.
Statistical significance was determined with the two-tailed Student t test assuming unequal variances.
RESULTS
Early extrapulmonary dissemination of M. tuberculosis varies between resistant and susceptible mouse strains.
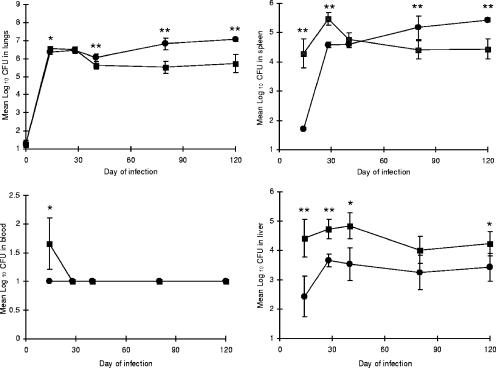
To determine if early dissemination and subsequent growth of the bacilli are associated with resistance or susceptibility to tuberculosis infection, CBA/J and C57BL/6 mice were examined for a period of 120 days of infection with a low-dose aerosol of 100 CFU of M. tuberculosis H37Rv. At days 14, 28, 40, 80, and 120, mice were sacrificed and their lungs, spleens, livers, peripheral blood, and lung-associated lymph nodes (LALN) were harvested to determine the number of viable organisms in each organ. The data in Fig. 1 show that dissemination of M. tuberculosis occurred earlier in the resistant C57BL/6 strain, as was evident from an increased number of bacilli in the spleen, liver, and blood at day 14 after infection, while there was no difference between the two strains of mice in CFU counts in the lungs and LALN (data not shown) early during the infection. Our data support previous studies using C3H (susceptible) and C57BL/6 mice which showed earlier dissemination in the resistant strain of mice (1). As expected, the bacterial burden in the lungs, spleen, and LALN was higher during the chronic infection in the CBA/J mice, which confirmed previous reports that CBA/J mice are susceptible to M. tuberculosis infection (26, 42).
FIG. 1.
Early dissemination of M. tuberculosis following aerosol infection of C57BL/6 and CBA/J mice. Bacterial counts in the spleen, blood, and liver were higher at day 14 in the C57BL/6 mice than in the CBA/J mice but not in the lung or lung-associated lymph nodes (data not shown). As the infection progressed, the number of bacteria in the lungs and spleen increased in the susceptible CBA/J strain. Circles, CBA/J mice; squares, C57BL/6 mice. Data are expressed as the mean (n = 5 mice) ± standard deviation. P values were calculated using Student's t test comparing C57BL/6 and CBA/J mice at each time point; *, P < 0.05, and **, P < 0.01.
Inhibition of MMP activity reduces early dissemination of M. tuberculosis in resistant mice.
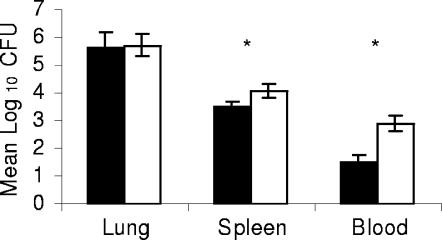
To determine if MMPs play a causative role in facilitating early dissemination of bacilli to extrapulmonary sites in the resistant strain, C57BL/6 mice were infected via the pulmonary route with approximately 500 CFU of M. tuberculosis H37Rv and immediately treated with an inhibitor of MMP activity, BB-94. As shown in Fig. 2, dissemination of viable bacilli to the blood was greater in mice that received the vehicle control than in mice that received the MMP inhibitor, suggesting a role for MMPs in the hematogenous dissemination of M. tuberculosis. While there were statistically significant differences between the groups in CFU counts during the early phase of infection at day 14 in the spleen and blood, there was no effect on the growth of M. tuberculosis later during infection in the lungs of BB-94-treated mice compared to the carrier-treated mice.
FIG. 2.
MMPs are involved in early dissemination of M. tuberculosis. C57BL/6 mice were treated with MMP inhibitor BB-94 at the time of pulmonary infection with 500 CFU of M. tuberculosis. At day 14 postinfection, dissemination of the bacilli from the lungs to the spleen and blood was decreased in mice given BB-94 compared to the mice that received the vehicle control. Black bars, BB-94-treated mice; white bars, vehicle control-treated mice. Data are expressed as the mean (n = 3 mice) ± standard deviation. P values were calculated using Student's t test comparing BB-94-treated mice and vehicle control-treated mice; *, P < 0.05.
Lung cytokine production in resistant and susceptible strains of mice.
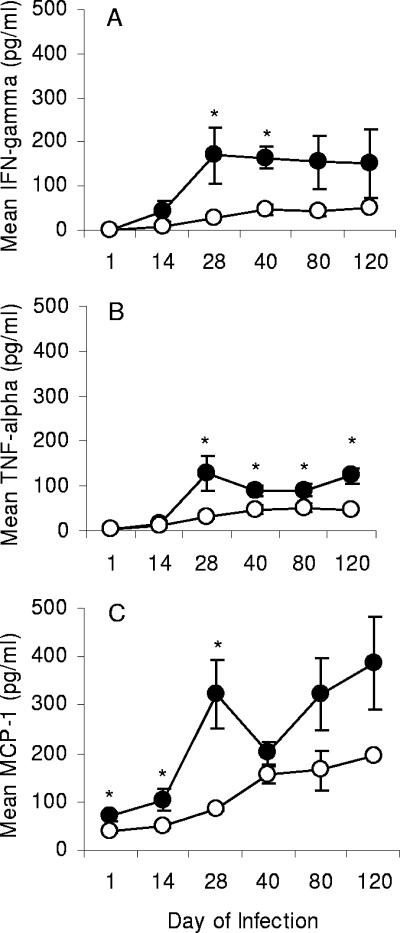
To investigate a potential relationship between MMPs and protective immunity generated during infection with M. tuberculosis, lung supernatants from days 14, 28, 40, 80, and 120 of infection were also analyzed for the presence of IFN-γ, TNF-α, and MCP-1. Both cytokines, IFN-γ and TNF-α (Fig. 3A and B), and the chemokine MCP-1 (Fig. 3C) were significantly elevated in the C57BL/6 mice but not in the CBA/J mice. In the infected C57BL/6 mice, IFN-γ and TNF-α production peaked at day 28 and appeared to level off during the chronic stage of infection. To determine if the cytokine differences between strains were due to differences in recruitment of T cells, lung leukocytes were also analyzed for CD3 and CD4 markers by flow cytometry, and there was no difference in the total number of CD4 T cells between the two mouse strains (data not shown).
FIG. 3.
Production of proinflammatory cytokines in the lungs of susceptible versus resistant mice. Lungs were harvested over a period of 120 days of pulmonary infection with M. tuberculosis and were analyzed for the production of IFN-γ (A), TNF-α (B), and MCP-1 (C) proteins. Open circles, CBA/J mice; closed circles, C57BL/6 mice. Data are expressed as the mean (n = 5 mice) ± standard error of the mean. P values were calculated using Student's t test comparing C57BL/6 with CBA/J mice at each time point; *, P < 0.05.
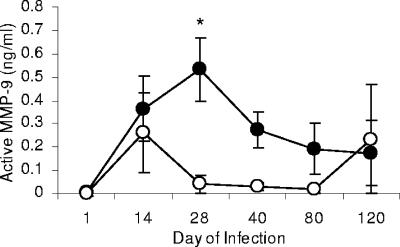
MMP-9 activity is elevated in M. tuberculosis-resistant mice.
We next set out to determine if MMP-9 activity in the lungs differed between strains of mice after M. tuberculosis infection. Supernatants from lung homogenates harvested at days 14, 28, 40, 80, and 120 from C57BL/6 and CBA/J mice after pulmonary infection were analyzed for active MMP-9 protein. The overall trend showed that the concentration of active MMP-9 was consistently greater in the lungs of infected C57BL/6 mice than in the CBA/J mice, with peak production of MMP-9 at day 28 of infection (P < 0.05) in the resistant strain (Fig. 4). We did not test levels of MMP-9 in extrapulmonary organs because our previous studies showed that MMP activity was greatest in the lungs compared to the spleen in C57BL/6 mice (data not shown). Although the concentration of MMP-9 in the lungs of CBA/J mice began to increase from day 1 to day 14 of infection, this increase was not sustained over time. Furthermore, although C57BL/6 mice had either the same number of or fewer organisms in the lung than did the CBA/J mice, the concentration of MMP-9 was greater in the C57BL/6 mice, indicating that MMP concentrations were independent of bacillus numbers.
FIG. 4.
Level of active MMP-9 in the lungs following aerosol infection with M. tuberculosis. The level of active MMP-9 was measured in lung homogenates from infected C57BL/6 and CBA/J mice. The amount of MMP-9 was consistently higher in the resistant C57BL/6 mice. Open circles, CBA/J mice; closed circles, C57BL/6 mice. Data are expressed as the mean (n = 5 mice) ± standard error of the mean and are representative of two similar experiments. P values were calculated using Student's t test comparing C57BL/6 with CBA/J mice at each time point; *, P < 0.05.
Bacterial load in FVB and MMP-9KO mouse strains.
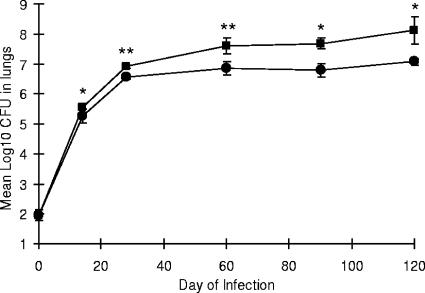
To determine how MMP-9 might be involved during pulmonary infection with M. tuberculosis, mice lacking MMP-9 and wild-type FVB mice were examined for a period of 120 days of infection with a low-dose aerosol of 100 CFU of M. tuberculosis H37Rv. At days 14, 28, 60, 90, and 120, mice were sacrificed and their lungs, spleens, lymph nodes, and livers were harvested to determine the number of viable organisms in each organ. The data in Fig. 5 show that the bacterial load in the lungs of the MMP-9KO mice was significantly lower than that in the lungs of the wild-type FVB mice at each time point after day 14 of infection (P < 0.05). There was no difference in bacterial load between the two mouse strains in the spleen, lymph nodes, and liver (data not shown).
FIG. 5.
Bacterial load in the lungs of MMP-9KO mice. By day 30 and continuing throughout the course of infection, bacterial counts in the lung were decreased in the MMP-9KO mice compared to the wild-type FVB mice. Circles, MMP-9KO mice; squares, FVB mice. Data are expressed as the mean (n = 5 mice) ± standard deviation. P values were calculated using Student's t test comparing FVB with MMP-9KO mice at each time point; *, P < 0.05, and **, P < 0.01.
Increased MMP-9 activity is associated with pulmonary granuloma structure and organization.
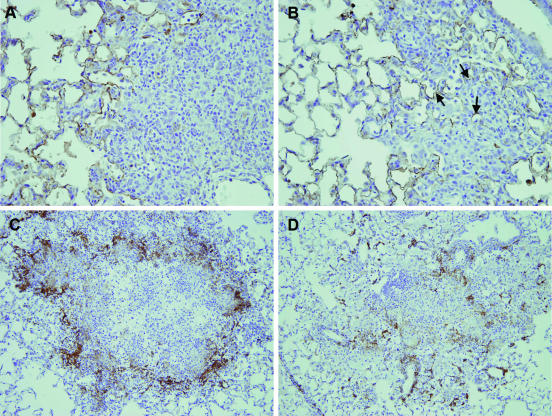
Given the difference in MMP-9 activity in the lungs between the resistant and susceptible mouse strains and the role that this enzyme plays in tissue remodeling, we next wished to determine if this difference also resulted in changes in lung architecture and granuloma structure. Lung sections from C57BL/6 and CBA/J mice were stained to assess the general integrity of the alveolar septa within lesions at day 28 of infection, the time of greatest difference in MMP-9 activity between strains. Serial lung sections were stained with an antibody against cytokeratin to delineate defined parenchymal structures within lesions. C57BL/6 mice showed sparse cytokeratin staining within the tightly formed granulomatous lesions (Fig. 6A), with a loss in alveolar septal wall integrity compared to the CBA/J mice, in which cytokeratin staining was more visible throughout the loosely associated granuloma (Fig. 6B). To assess organization of macrophages within the granuloma, lung sections were taken from C57BL/6 and CBA/J mice at day 40 of infection and stained with macrophage marker F4/80 antibody. Lung granulomas in the resistant mouse strain were well organized with macrophages staining positively for F4/80 forming a discrete border (Fig. 6C). The granuloma structure in the susceptible mouse strain, however, appeared disorganized with sparse, random foci of macrophages (Fig. 6D). In summary, C57BL/6 mice, with significantly greater concentrations of MMP-9, underwent a loss of alveolar integrity but had well-organized granulomas. CBA/J mice, on the other hand, which had less MMP activity, had better alveolar wall integrity but poor granuloma organization and fewer macrophages.
FIG. 6.
Differences in granuloma organization and structure between mouse strains. At day 28 after aerosol infection, lung sections from C57BL/6 mice (A) showed granulomatous lesions with a marked decrease in alveolar septal wall integrity compared to the CBA/J mice (B). The arrows in panel B depict keratin staining within the granuloma where the septa are still present, and the total magnification of sections A and B is ×400. At day 40 after aerosol infection, granulomas in the C57BL/6 mice were well organized with F4/80-positive macrophages bordering focused lymphocytes and macrophages (C) while granulomas in CBA/J mice were loosely organized with F4/80-positive macrophages distributed randomly throughout the granuloma (D). Sections in panels C and D were stained with anti-F4/80 primary antibody, and the total magnification is ×200. Each section was counterstained with hematoxylin. Photomicrographs are representative of five mice per group.
MMP-9 is required for cellular migration within the granuloma.
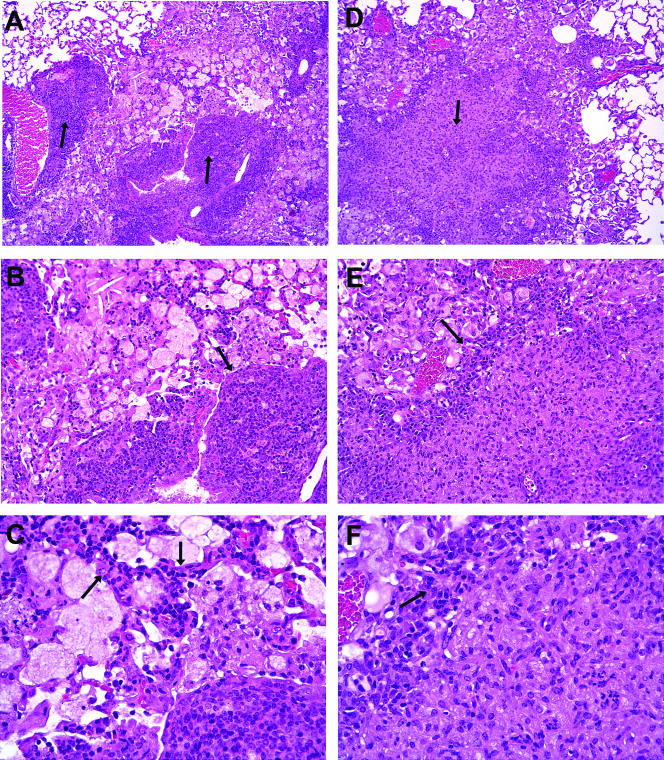
Lung sections from MMP-9KO and FVB mice were examined at day 60 of infection to determine if MMP-9 plays a causative role in granuloma formation. The most striking feature observed throughout the lungs of the MMP-9KO mice was the visible lack of well-formed granulomas (Fig. 7A to C). Instead, in areas of cellular accumulation, the alveolar septa were visibly thickened with the appearance of cells being stuck within the alveolar septa, similar to what was observed in the lungs of the susceptible CBA/J strain of mice. Additionally, cells that were predominantly lymphocytes accumulated around perivascular regions rather than migrating into the lesions. In contrast, the lungs of FVB mice at the same time point consisted of tight, well-formed granulomas with the absence of visible septa (Fig. 7D and E).
FIG. 7.
Representative hematoxylin-and-eosin-stained lung sections from MMP-9KO (A to C) and FVB wild-type (D to F) mice at day 60 of infection with M. tuberculosis. In panel A, arrows point to perivascular accumulations of cells that are predominately lymphocytes. There is trapping of cells in the perivascular connective tissue that compresses the surrounding parenchyma with intact septal walls. The leading edge of the lesion is sharply demarcated from the parenchyma (panel B, arrow) where there is infiltration of intact interalveolar septa by lymphocytes (panel C, arrows). In contrast, the FVB wild-type lesion in panel D is expansive and effaces the architecture to the parenchyma (arrow in panel D) such that epithelioid macrophages, fewer lymphocytes, granulocytes, and cells consistent with dendritic cells replace alveolar septal walls and alveolar spaces. The leading edge of the lesion (panel E, arrow) is composed predominately of lymphocytes that are shown at higher magnification in panel F (arrow). The perilesional parenchyma is attenuated such that alveolar septal walls and alveolar spaces are ill defined. The total magnification of panels A and D is ×89, of panels B and E is ×178, and of panels C and F is ×356.
Macrophage recruitment and migration are dependent on MMP-9.
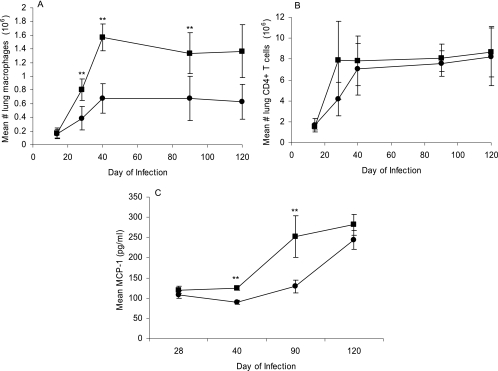
Given the decreased number of bacilli in the lungs of the MMP-9KO mice compared to the wild-type FVB mice, single-cell suspensions from the lung were also examined to determine if these two mouse strains differ in their abilities to recruit macrophages and T cells to the lungs. Over the course of M. tuberculosis infection, the total number CD11b-positive of macrophages staining negatively for CD11c and Gr-1 markers was significantly reduced in the MMP-9KO (P < 0.01) mice compared to the wild-type FVB mice at days 28, 40, and 90 (Fig. 8A), while there was no difference in the number of CD4 T cells in the lungs between the two groups of mice (Fig. 8B). In addition, lung homogenates were analyzed for the presence of inflammatory cytokines and chemokines. While there were no differences in levels of TNF-α and IFN-γ produced between the two strains (data not shown), the MMP-9KO mice produced significantly less MCP-1 (P < 0.001) at days 40 and 90 (Fig. 8C).
FIG. 8.
MMP-9 is required for macrophage recruitment in the lungs of M. tuberculosis-infected mice. (A) By day 30 of the infection and throughout the course of infection, the total number of macrophages was decreased in the lungs of the MMP-9KO mice compared to the wild-type FVB mice. (B) There was no difference in T-cell numbers over the course of infection between the two mouse strains. (C) The level of MCP-1 in the lungs of the FVB mice was significantly increased at days 40 and 90. Circles, MMP-9KO mice; squares, FVB mice. Data are expressed as the mean (n = 5 mice) ± standard deviation. P values were calculated using Student's t test comparing FVB with MMP-9KO mice at each time point; **, P < 0.01.
DISCUSSION
It has been shown previously that mouse strains can be categorized based on their susceptibility to M. tuberculosis (25, 26), which was characterized by differences in survival, recruitment, and localization of T lymphocytes, granuloma formation, cytokine production, and expression of adhesion and memory molecules (14, 42). MMPs play a significant role in the pathology of many diseases, and recent studies have shown that MMPs are induced by mycobacterial infection (2, 22, 34, 36, 39). In the present study, we wished to characterize the expression of MMPs during M. tuberculosis infection in a resistant (C57BL/6) and a susceptible (CBA/J) strain of mice to determine whether the expression of pulmonary MMP activity was related to resistance. The data presented here indicate that early expression of MMPs was associated with resistance to pulmonary M. tuberculosis infection and early dissemination of the bacilli leading to the subsequent induction of Th1-type immunity and efficient granuloma formation in the lungs. While MMP-9, specifically, was not involved in facilitating early dissemination of the bacilli, data from MMP-9-knockout mice showed that MMP-9 was required for recruitment of macrophages to the lungs and for tissue remodeling making way for the development of well-organized granulomas.
We have previously established that MMPs are involved in dissemination of tubercle bacilli to the blood and in the development of pulmonary granulomas (17). In the current study, we show that M. tuberculosis bacilli disseminated significantly more rapidly to the blood, spleen, and liver in the resistant C57BL/6 mouse strain than in the susceptible CBA/J mouse strain (Fig. 1), suggesting that early dissemination of the bacilli may be necessary in resistance against the disease. When MMPs were blocked in the resistant mouse strain with a broad-range MMP inhibitor (BB-94), early dissemination of the bacilli was also blocked, suggesting that these enzymes are involved in degrading the lung ECM such that bacteria can escape into the blood and disseminate to extrapulmonary organs (Fig. 2). Interestingly, the decreased bacterial load of M. tuberculosis in the liver was lower than that in the spleen and may be related to the immune response; however, studies to determine this go beyond the scope of this paper. These combined results suggest that early dissemination of the bacilli is associated with resistance and that MMPs play a role in facilitating this dissemination as early as day 14 of infection.
Increased MMP activity in the resistant strain of mice (Fig. 4) was also associated with the induction of a Th1-type immune response with elevated levels of IFN-γ, TNF-α, and MCP-1 (Fig. 3). These data, together with the dissemination data, suggest that an early increase in MMP activity in the lungs is necessary for early dissemination of the bacilli to secondary lymphoid organs where protective immunity can be generated. A correlation between early dissemination of M. tuberculosis and resistance has been shown previously in C57BL/6 mice compared to C3H mice (1). Flow cytometric analysis of the T lymphocytes present in the lungs of resistant versus susceptible mice showed that the number of CD4 T cells was increased upon infection with M. tuberculosis in both strains of mice, and there was no difference in the number of CD4 T cells between the two strains (data not shown). Thus, given that the CBA/J mice produced little MMP-9, this would indicate that CD4 T cells can migrate into the lungs in an MMP-independent manner and that the cytokine environment may determine MMP production. The CD4 T cells in the lungs of the CBA/J mice, however, were not of the IFN-γ-secreting phenotype since levels of IFN-γ were not elevated in this mouse strain compared to the resistant strain. These combined data, therefore, indicate that the inability of the CBA/J mice to produce active MMP-9 during infection precluded adequate production of protective cytokines such as IFN-γ and TNF-α, consequently rendering the strain incapable of forming tight, well-formed granulomas.
Having established that MMPs facilitate early dissemination of M. tuberculosis to extraulmonary sites where protective immunity can be generated, we next wished to determine if MMP-9 specifically might be involved in facilitating early dissemination of the bacilli out of the lungs. Figure 4 shows that the resistant C57BL/6 mouse strain produced increased levels of active MMP-9 compared to the susceptible CBA/J mouse strain, suggesting that this enzyme may be a component associated with resistance to M. tuberculosis. However, this increased level of MMP-9 was not evident until day 28 of infection, therefore indicating that MMP-9 is not responsible for the early dissemination of M. tuberculosis observed in the resistant strain. Assessment of the bacterial load in extrapulmonary organs at day 14 confirmed that MMP-9 is not involved in early dissemination of the bacilli since no differences in CFU in the spleen, LALN, or liver were observed between the MMP-9KO mice and the FVB wild-type mice (data not shown).
While MMP-9, specifically, does not appear to be involved in facilitating early dissemination of the M. tuberculosis bacilli, the data presented here suggest that this degradative enzyme plays a causative role in granuloma development. As shown in Fig. 7A to C, in the absence of MMP-9 cells appeared trapped in visibly condensed alveolar septa at day 60 of infection, a phenomenon observed by other investigators using MMP-2KO (7) and MMP-9KO (23) mice in allergen challenge models. In addition, lymphocytes accumulated around the perivascular region, suggesting that they were unable to migrate into and around the granuloma. In contrast, visible septa were absent in the FVB wild-type lung, and granulomas were well formed as in Fig. 7D and E. Interestingly, similar to lung sections from the MMP-9KO mice, the susceptible CBA/J mouse strain also exhibited visibly thickened alveolar septa within loosely associated cellular accumulations at day 28 of infection, whereas granuloma formation in resistant C57BL/6 mice was marked by a decrease in alveolar septal wall integrity (Fig. 6A and 6B). As granuloma formation progressed to day 40, it was evident that the susceptible strain of mouse was unable to form well-organized granulomas compared to the resistant strain, which formed tight granulomas (Fig. 6C and 6D). The failure by the susceptible mouse strain to form tight, well-organized granulomas may be the result of an inability to remodel tissue sufficiently, due to reduced MMP-9 activity. In fact, when MMP-9 was absent, macrophage recruitment into the lungs was significantly reduced compared to that observed in the FVB wild-type lungs (Fig. 8A), and there were fewer F4/80-positive cells within the lung lesions of the knockout strain (data not shown). With fewer host cells present in which to multiply, the reduced number of macrophages in the lungs of the MMP-9KO mice may have accounted for the decreased bacterial load in the mice lacking MMP-9 compared to the FVB wild-type strain (Fig. 5). Interestingly, although there were fewer macrophages in the lungs of the MMP-9KO mice, there were similar levels of TNF-α and IFN-γ in the lungs between the FVB wild-type and the knockout mice (data not shown), indicating that the reduced CFU numbers in the knockout strain were not the result of reduced production of these two cytokines. The knockout mice, however, produced significantly less MCP-1 (Fig. 8C), and they recruited fewer macrophages to the lungs (Fig. 8A), suggesting that MMP-9 may play a role with MCP-1 in recruiting macrophages to the lungs during granuloma development. In addition, it seems likely that macrophage-derived MCP-1, previously shown to strongly recruit monocytes and Th1-type lymphocytes in response to products of M. tuberculosis (8), played a role in facilitating cellular organization within the granuloma since this chemokine was up regulated in the resistant C57BL/6 mice compared to the susceptible CBA mice (Fig. 3C). Based on these data together with the histologic findings, it is probable that the inability of the susceptible strain to form tight granulomas was based on a lack of MMP-9 activity in the lungs and therefore a lack of tissue remodeling and cellular recruitment that must occur in order for protective granulomas to form.
In summary, the data presented here indicate that expression of active MMP-9 is greater in resistant mice than in susceptible mice and that MMPs may facilitate early dissemination of M. tuberculosis and subsequent immunity in the resistant strain, most likely by degrading the lung tissue ECM. The loss of alveolar septa in the resistant mouse strain with the coordinated up regulation of MMPs and chemokine MCP-1 further supports the idea that MMPs contribute to the formation of tight granulomas, mediating tissue remodeling that must occur in order for granulomas to form properly. In fact, granuloma formation was impaired in the MMP-9KO mice due in part to the inability of these mice to recruit macrophages to the lungs. Future studies designed to determine the mechanisms that control MMP production and activation will lend insight into the process of granuloma formation and control of this pathological response, thus leading to better ways of preventing and possibly treating tuberculosis (45).
Supplementary Material
Acknowledgments
Funding for this research was provided by The American Lung Association (Illinois) and NIH AI52040 (A.A.I.).
Immunohistochemistry was performed by Premier Histology Laboratory, LLC, Boulder, CO. We thank Phillip Chapman of the Statistics Department at Colorado State University for providing advice on the statistical analysis for this paper.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 18 September 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Chackerian, A. A., J. M. Alt, T. V. Perera, C. C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, J. C., A. Wysocki, K. M. Tchou-Wong, N. Moskowitz, Y. Zhang, and W. N. Rom. 1996. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 51:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, F. M., and G. B. Mackaness. 1970. The relationship of delayed type hypersensitivity to acquired antituberculosis immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell. Immunol. 1:253-265. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 7.Corry, D. B., K. Rishi, J. Kanellis, A. Kiss, L. Song, J. Xu, L. Feng, Z. Werb, and F. Kheradmand. 2002. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 3:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrero, E., P. Biswas, K. Vettoretto, M. Ferrarini, M. Uguccioni, L. Piali, B. E. Leone, B. Moser, C. Rugarli, and R. Pardi. 2003. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on gd cells. Immunology 108:365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 12.Galboiz, Y., S. Shapiro, N. Lahat, and A. Miller. 2002. Modulation of monocytes matrix metalloproteinase-2, MT1-MMP and TIMP-2 by interferon-gamma and -beta: implications to multiple sclerosis. J. Neuroimmunol. 131:191-200. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruppo, V., O. C. Turner, I. M. Orme, and J. Turner. 2002. Reduced up-regulation of memory and adhesion/integrin molecules in susceptible mice and poor expression of immunity to pulmonary tuberculosis. Microbiology 148:2959-2966. [DOI] [PubMed] [Google Scholar]
- 15.Hoheisel, G., U. Sack, D. S. Hui, K. Huse, K. S. Chan, K. K. Chan, K. Hartwig, E. Schuster, G. H. Scholz, and J. Schauer. 2001. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculous pleuritis. Tuberculosis (Edinburgh) 81:203-209. [DOI] [PubMed] [Google Scholar]
- 16.Hrabec, E., M. Strek, M. Zieba, S. Kwiatkowska, and Z. Hrabec. 2002. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int. J. Tuberc. Lung Dis. 6:713-719. [PubMed] [Google Scholar]
- 17.Izzo, A. A., L. S. Izzo, J. Kasimos, and S. Majka. 2004. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis (Edinburgh) 84:387-396. [DOI] [PubMed] [Google Scholar]
- 18.Jin, H. Y., K. S. Lee, S. M. Jin, and Y. C. Lee. 2004. Vascular endothelial growth factor correlates with matrix metalloproteinase-9 in the pleural effusion. Respir. Med. 98:115-122. [DOI] [PubMed] [Google Scholar]
- 19.Keller, K., J. Lauber, A. Blumenthal, J. Buer, and S. Ehlers. 2004. Resistance and susceptibility to tuberculosis analysed at the transcriptome level: lessons from mouse macrophages. Tuberculosis 84:144-158. [DOI] [PubMed] [Google Scholar]
- 20.Lee, K. Y., E. H. Kim, W. S. Yang, H. Ryu, S. N. Cho, B. I. Lee, and J. H. Heo. 2004. Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J. Neurol. Sci. 220:73-78. [DOI] [PubMed] [Google Scholar]
- 21.Lee, M. M., B. J. Yoon, K. Osiewicz, M. Preston, B. Bundy, A. M. van Heeckeren, Z. Werb, and P. D. Soloway. 2005. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect. Immun. 73:661-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura, E., F. Umehara, T. Hashiguchi, N. Fujimoto, Y. Okada, and M. Osame. 2000. Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J. Neurol. Sci. 173:45-52. [DOI] [PubMed] [Google Scholar]
- 23.McMillan, S. J., J. Kearley, J. D. Campbell, X. W. Zhu, K. Y. Larbi, J. M. Shipley, R. M. Senior, S. Nourshargh, and C. M. Lloyd. 2004. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J. Immunol. 172:2586-2594. [DOI] [PubMed] [Google Scholar]
- 24.McQuibban, G. A., J. H. Gong, J. P. Wong, J. L. Wallace, I. Clark-Lewis, and C. M. Overall. 2002. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100:1160-1167. [PubMed] [Google Scholar]
- 25.Medina, E., and R. J. North. 1999. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology 96:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morphy, J. R., T. A. Millican, and J. R. Porter. 1995. Matrix metalloproteinase inhibitors: current status. Curr. Med. Chem. 2:743-762. [Google Scholar]
- 28.North, R. J. 1970. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J. Exp. Med. 132:521-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 30.Orme, I. M., P. Andersen, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 31.Parks, W. C., and S. D. Shapiro. 2001. Matrix metalloproteinases in lung biology. Respir. Res. 2:10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parks, W. C., C. L. Wilson, and Y. S. Lopez-Boado. 2004. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4:617-629. [DOI] [PubMed] [Google Scholar]
- 33.Preece, G., G. Murphy, and A. Ager. 1996. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J. Biol. Chem. 271:11634-11640. [DOI] [PubMed] [Google Scholar]
- 34.Price, N. M., J. Farrar, T. T. Tran, T. H. Nguyen, T. H. Tran, and J. S. Friedland. 2001. Identification of a matrix-degrading phenotype in human tuberculosis in vitro and in vivo. J. Immunol. 166:4223-4230. [DOI] [PubMed] [Google Scholar]
- 35.Price, N. M., R. H. Gilman, J. Uddin, S. Recavarren, and J. S. Friedland. 2003. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J. Immunol. 171:5579-5586. [DOI] [PubMed] [Google Scholar]
- 36.Quiding-Jarbrink, M., D. A. Smith, and G. J. Bancroft. 2001. Production of matrix metalloproteinases in response to mycobacterial infection. Infect. Immun. 69:5661-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhoades, E. R., A. A. Frank, and I. M. Orme. 1997. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung Dis. 78:57-66. [DOI] [PubMed] [Google Scholar]
- 38.Ries, C., and P. E. Petrides. 1995. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol. Chem. Hoppe-Seyler. 376:345-355. [PubMed] [Google Scholar]
- 39.Rivera-Marrero, C. A., W. Schuyler, S. Roser, and J. Roman. 2000. Induction of MMP-9 mediated gelatinolytic activity in human monocytic cells by cell wall components of Mycobacterium tuberculosis. Microb. Pathog. 29:231-244. [DOI] [PubMed] [Google Scholar]
- 40.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 41.Thwaites, G. E., C. P. Simmons, N. T. H. Quyen, T. T. H. Chau, P. P. Mai, N. T. Dung, N. H. Phu, N. P. White, T. T. Hien, and J. J. Farrar. 2003. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J. Infect. Dis. 188:1105-1115. [DOI] [PubMed] [Google Scholar]
- 42.Turner, J., M. Gonzalez-Juarrero, B. M. Saunders, J. V. Brooks, P. Marietta, D. L. Ellis, A. A. Frank, A. M. Cooper, and I. M. Orme. 2001. Immunological basis for reactivation of tuberculosis in mice. Infect. Immun. 69:3264-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 44.Woessner, J. F., Jr. 1998. The matrix metalloproteinase family, p. 1-14. In R. P. Mecham and W. C. Parks (ed.), Matrix metalloproteinases. Academic Press, Inc., New York, N.Y.
- 45.Woessner, J. F., Jr. 1991. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 5:2145-2154. [PubMed] [Google Scholar]
- 46.Wright, K. M., and J. S. Friedland. 2004. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J. Leukoc. Biol. 75:1086-1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.