Abstract
Mycobacterium tuberculosis is a highly successful human pathogen, with ∼2 × 109 individuals infected globally. To understand the responses of M. tuberculosis to the in vivo environment, we studied the in vivo regulation of M. tuberculosis genes whose M. marinum homologs are induced in chronically infected frog tissues. The expression of 16S rRNA was shown to remain constant in M. tuberculosis under in vivo and in vitro conditions and therefore could be used for internal normalization in quantitative reverse transcription-PCR assays. We found whiB3, a putative transcriptional regulator implicated in mediating tissue damage, to be maximally induced at 2 weeks postinfection in the lungs of wild-type and immunodeficient (gamma interferon receptor−/−, Rag1−/−, and tumor necrosis factor alpha−/−) mice. At later time points in wild-type mice, whiB3 induction was decreased and gradually declined over the course of infection. In immunodeficient mice, whiB3 induction declined rapidly and was completely abolished in moribund animals. whiB3 was also found to be induced in naïve bone marrow-derived macrophages after 6 h of infection. whiB3 expression in vivo and in vitro was found to be inversely correlated with bacterial density. These results indicate that M. tuberculosis regulates the expression of whiB3 in response to environmental signals present in vivo and are consistent with a model of regulation by quorum sensing.
Mycobacterium tuberculosis is a successful human pathogen that has infected one-third of the world's population (19). Although the global human immunodeficiency virus pandemic has created an expanded host population for this pathogen, the majority of individuals with tuberculosis have intact immune systems without any identifiable risk factors. Based on studies in mice, upon infection with aerosolized M. tuberculosis the development of acquired cellular immunity halts the progression of bacterial multiplication and tissue pathology and transforms the process into a chronic persistent infection (16, 25). Unlike immunity to other bacterial pathogens, “sterilizing immunity” is not achieved after infection with M. tuberculosis and the bacteria remain capable of reactivating and causing progressive disease (22). Furthermore, although the outcome of infection in humans is a result of complex interactions between the host and the pathogen, it is unclear whether the host or bacterial responses are dominant in determining the outcome (11). The latter is supported by microarray studies showing that M. tuberculosis has a signature transcriptional response in naïve and gamma interferon (IFN-γ)-activated intraphagosomal environments (23). It has also been shown that like other bacteria, M. tuberculosis transcriptionally responds and adapts to in vivo conditions such as hypoxia and low iron (21, 30, 32). Thus, transcriptional responses likely play important roles in the adaptation of M. tuberculosis to the host environments it encounters during the course of infection, disease, and transmission.
In this report we studied the in vivo regulation of 22 M. tuberculosis genes whose homologs were previously shown to be induced in M. marinum during chronic frog infection (4, 18). We used quantitative reverse transcription-PCR (qRT-PCR) to first validate the use of 16S rRNA for normalization and then compared M. tuberculosis gene expression in the mouse lung to that of broth culture. Among the 22 genes studied, M. tuberculosis whiB3, a putative transcriptional regulator implicated in causing gross and microscopic lesions (26), was found to be maximally induced in the lungs of wild-type and immunodeficient (IFN-γR−/−, Rag1−/−, and tumor necrosis factor alpha−/− [TNF-α−/−]) mice at 2 weeks after infection. Induction of whiB3 gradually declined at later time points in wild-type mice and was completely abolished in lungs of terminally ill immunodeficient mice. whiB3 was also found to be induced in naïve macrophages. Experiments to characterize the regulation of whiB3 revealed that expression of whiB3 in vivo, ex vivo, and in vitro is inversely correlated with bacterial density. In contrast to findings by Steyn et al. (26), we did not find a difference in histopathology in the lungs of wild-type mice infected with H37Rv and whiB3 knockout.
MATERIALS AND METHODS
Mice.
C57BL/6, IFN-γR−/−, Rag1−/−, and TNF-α−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the New York University (NYU) Berg Animal Facility under specific-pathogen-free conditions. Mice were utilized at 6 to 8 weeks of age for obtaining bone marrow-derived macrophage (BMMφ) and 8 to 12 weeks for in vivo infections. Mice were used in compliance with NYU institutional policies.
Bacteria.
M. tuberculosis cultures were grown in 30 ml Nalgene square bottles in a shaking incubator at 80 rpm in Middlebrook 7H9 broth (Difco) supplemented with 0.2% glycerol, 10% oleic acid-albumin-dextrose-catalase (Becton Dickinson), and 0.05% Tween 80. Cultures were seeded with 5 to 10 ml of M. tuberculosis at a starting optical density (A580) of 0.05.
BMMφ.
Bone marrow was removed from the femur and tibia of euthanized mice, and macrophage precursors were differentiated in tissue culture Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum, 20% L929 cell-conditioned medium, 0.2 M l-glutamine, and 0.1 M sodium pyruvate. Seven days after differentiation, cells were replated at a density of 1.4 × 106 cells/T25 flask in the above medium except with 10% L929 cell-conditioned medium (BMMφ medium) and allowed to adhere overnight.
Ex vivo infection.
Mid-logarithmic broth cultures of M. tuberculosis were sedimented, and the bacteria were resuspended in BMMφ medium, vortexed with glass beads for 3 min, and passed through a 5-μm sterile filter by gravity. Bacterial counts were estimated with a Petroff-Hausser counting chamber and also determined by serial dilution and plating on 7H11 agar. Bacteria were added to BMMφ monolayers at the indicated multiplicities of infection (MOIs) (bacteria:macrophage). At 10 h postinfection, extracellular bacteria were treated with 200 μg/ml of streptomycin or amikacin for 45 min, and the cell monolayer was washed twice with phosphate-buffered saline (PBS) and activated with recombinant IFN-γ at 20 ng/ml or left unactivated. At designated times, the monolayer was washed twice with PBS and lysed with Trizol, and bacterial RNA was isolated and processed as described for broth culture.
In vivo infection.
Mice were infected with an aerosolized dose of M. tuberculosis (∼200 CFU/mouse) using an inhalation exposure device (Glas-Col, Terre Haute, IN). Day 1 CFU were enumerated from lung homogenates of four mice on Middlebrook 7H11 agar. On designated dates, mice were sacrificed and lungs were removed and immediately frozen in liquid nitrogen and stored at −70°C.
Nucleic acid isolation and quantitative PCR (qPCR).
M. tuberculosis pellets from broth culture or homogenized mouse lung sediments were resuspended in TRI reagent and mechanically disrupted with 0.1-mm zirconia/silica beads with two 1-min bursts in a BioSpec Products Bead Beater. RNA was extracted from the aqueous layer and precipitated and treated with DNase I. DNA was isolated from the interphase and dissolved in 5 mM sodium hydroxide. RT reactions with gene-specific primers (Table 1) were performed with ThermoScript transcriptase according to the manufacturer's instructions (Invitrogen). Identical reactions not treated with reverse transcriptase (−RT) were included as controls for genomic DNA carryover. Quantitative PCR with SYBR Green (Invitrogen) was performed in an MJ Research DNA Engine Opticon 2 Continuous Fluorescence Detection System in duplicates, and the specificity of PCR products was confirmed by sequencing (data not shown) and melting point analysis. When the amplification curve differences between +RT and −RT reactions were <10 cycles, genomic RNA samples were reprocessed as stated above. The copy number of genes were extrapolated from a standard curve prepared using 10-fold dilutions of estimated copies (104 to 108) of M. tuberculosis H37Rv genomic DNA. cDNA copy numbers were normalized to 16S rRNA in the same sample, and the fold change of target genes was calculated according to the following equation: fold change = (Rv genecopy number/16S rRNA copy number)in vivo or ex vivo/(Rv genecopy number/16S rRNAcopy number)in vitro.
TABLE 1.
List of primers used in this studya
| Target and purpose | Forward | Reverse |
|---|---|---|
| RT-PCR | ||
| Rv0012 | ||
| RT | GCATAGCCGACTATCTGCAAG | |
| PCR | GTTGTGTCGGAAACACGTTG | TGGACTTCTTCGCAGTACCC |
| Rv0014c (pknB) | ||
| RT | TGCCCTTGGACACCTGTAGT | |
| PCR | GACGTCTCCACGCTGACATA | CATCGGGAATGTCTTTGGTC |
| Rv0133 | ||
| RT | AGAGCGATCTCACGGGTCA | |
| PCR | CGAAGAACCCCACTGGTATC | GGAAACCGAACCGTTGATAG |
| Rv0265c (fecB2) | ||
| RT | CTGATCTGTGGCGGCAGT | |
| PCR | GGCGAACTGACTTCCTCAAC | TCCTTGGAAGTGAAGATGTGG |
| Rv0321 (dcd) | ||
| RT | CGCTGACCCTGGTATTTCG | |
| PCR | GACGCTGGAGCTTTTCACTC | ATGCACAGCTGACCGATTTT |
| Rv0467 (icl) | ||
| RT | CAGCTCCTTCTGGAACTTGG | |
| PCR | GCTTCTACCGCACCAAGAAC | TCGAGGTGCTTTTTCCAGTT |
| Rv0575c | ||
| RT | TGGCGAAAAACCAGATGAAC | |
| PCR | GCGCAGCTATGTCCTCTACA | GCTGACCACGTCGAAGTACA |
| Rv0631c (recC) | ||
| RT | CTGTTGGTCTGCCAGCATTC | |
| PCR | GGTGTCGGTGACCTACTCAAA | TCGTACAGCAACACCAGCTC |
| Rv0811c | ||
| RT | GATGCAACAACACCAGCATC | |
| PCR | AAATCGAACTGGACGTGGTG | CTCGGTAACAGCCCTTGTTC |
| Rv0826 | ||
| RT | ATCGTAGAGTCCGACGGTCA | |
| PCR | GCCAGCTATTTGACGAGCAC | TTGGGTAGTTCGGTCAGGTC |
| Rv1106c | ||
| RT | CACGTAGTACGGCAGGCATT | |
| PCR | CCGGCTGGATAACTCTTACG | CCGAGCGAACTCGAACATA |
| Rv1200 | ||
| RT | CCCGTGTATCGGTAGCTAGT | |
| PCR | CATCCTGTTCGACAGTGTGC | AGTTCTGGGATGAACGATGC |
| Rv1205 | ||
| RT | GTAACCGGTGTCGAGCAATC | |
| PCR | GATTCCCAAGATGCTGGTGT | TCACAATGGGTTTGTCATGG |
| Rv1645c | ||
| RT | GGCTATTAGCGGCTTGAACA | |
| PCR | CATCAAGGTCGCCGAATACT | CGGTCCCAATAGTCCTGAAA |
| Rv1651c | ||
| RT | CACCGTTGCCCCATATCAG | |
| PCR | AGTAGCTTCATCGGCAATGG | AGAGGGAGCCAGAGTTAGCC |
| Rv1874 | ||
| RT | CATCCGGCTTATCCGATTC | |
| PCR | GAGTCCGGTTCTCGTACACC | CGAAGAAGGCTTTCAGATCG |
| Rv2031c (acr) | ||
| RT | GAATGCCCTTGTCGTAGGTG | |
| PCR | AGATGAAAGAGGGGCGCTAC | TAATGTCGTCCTCGTCAGCA |
| Rv2179c | ||
| RT | CGGGCCGCGCCGCGGCCCG | |
| PCR | CCATGTAGCGCTGTGTCAAT | ATCGTCTGTGGACGTGATGA |
| Rv2780 (ald) | ||
| RT | GGTTCGCATCAGGTGGTAAG | |
| PCR | CGACGCTGATTTATTGCTCA | TCTCGTAGGCAATTGACGTG |
| Rv3106 (fprA) | ||
| RT | TTCTTGTTGGTCCCGATCAC | |
| PCR | CTCTCCGATCGAGATCAAGG | GATGGTCCCGCTCTGGTC |
| Rv3416 (whiB3) | ||
| RT | GGTGCCCTTGAGGAGTAGGT | |
| PCR | AACGCAGACATCTGGAACTG | TAGGGCTCACCGACCTCTAA |
| Rv3451 | ||
| RT | ATGTGTCCGCTGAATTCGTT | |
| PCR | TGGCATGGAGATCGGAGTAT | CACGATATCGATCACGGTTG |
| Rv3709c (ask) | ||
| RT | GATACCTTGCCGATGTGGTC | |
| PCR | CAAGGACGTACCCATGGAAG | GGTGAAGGTGATGTCGGTCT |
| Rv3812 | ||
| RT | ATTCCCATGAAGGGTGTGC | |
| PCR | TAGACGGCTTCCTCAACAGC | GAAAACGGTTACGGTGGTTG |
| 16S rRNA | ||
| RT | ATTACGTGCTGGCAACATGA | |
| PCR | GCCGTAAACGGTGGGTACTA | TGCATGTCAAACCCAGGTAA |
| whiB3 allelic exchange substrate | ||
| Upper | CTTAAGCGCCAGCGTGTGCCAACCAA | CTCAGAGCTGCTCCGGCTGTGGCATT |
| Lower | AAGCTTGGCATCCGCCGCACAGCTTA | ACTAGTCGCACTCAAGGAGCGCAAGG |
| whiB3 complement | TCTAGACAACCACCGGAGCGTGAGGA | AAGCTTGACAAGCCGGCCAAGGCAGA |
Restriction enzyme sites are underlined.
whiB3 knockout mutant and complement.
A whiB3 knockout mutant was created with the conditionally replicating mycobacteriophages as previously described (1). Upper (671 bp) and lower (570 bp) allelic exchange substrates (AES) were PCR amplified (see Table 1 for primers) from H37Rv genomic DNA and ligated into pCR2.1-TOPO (Invitrogen). The AES were sequenced and subcloned into pYUB854. Following transduction of H37Rv and plating on Middlebrook 7H9 agar with albumin-dextrose-catalase (ADC) and hygromycin (50 mg/ml), four colonies were picked and screened for the absence of whiB3 transcript by real-time RT-PCR. A PCR fragment encoding the entire whiB3 open reading frame and 401 bp of 5′-flanking sequence was cloned into pMV306 integrating vector (27) for complementation of the whiB3 knockout. Transformed bacteria were plated on Middlebrook 7H9 agar supplemented with ADC and 25 mg/ml of kanamycin, and four colonies were picked and confirmed for complementation by real-time RT-PCR.
Statistical analysis.
Student's t test was used to determine significant different between samples. The regression module of SPSS (version 12) was used to perform multiple-regression analysis of 16S rRNA regressed on DNA.
RESULTS
The majority of M. tuberculosis putative granuloma activated genes are expressed in vitro and in vivo.
Prior studies by Chan et al. and Ramakrishnan and colleagues identified 24 M. marinum granuloma-activated genes whose promoters were shown to be activated in chronically infected frogs but not in bacteria in broth cultures (4, 18). The M. tuberculosis homologs of 22 of these genes, herein referred to as putative granuloma-activated genes (P-GAGs), were the subjects of this study. Our aim was to identify and characterize M. tuberculosis P-GAGs that are induced in the lungs of mice. To determine which of the 22 M. tuberculosis P-GAGs are expressed in broth culture and in the mouse lung, RT-PCR was performed on RNA isolated from M. tuberculosis in mid-log broth cultures and in the lungs of mice at 7 weeks postinfection. Transcripts for 19 of the 22 M. tuberculosis P-GAGs were detected in broth culture, and 17 of the 19 were also detected in the mouse lung (data not shown). All 22 M. tuberculosis P-GAGs could be PCR amplified from M. tuberculosis chromosomal DNA, thus ruling out the possibility of primer failures for the genes for which expression was undetectable.
M. tuberculosis 16S rRNA:DNA ratio remains constant in vitro and in vivo.
In order to perform quantitative RT-PCR (qRT-PCR) to compare M. tuberculosis gene expression in the mouse lung to broth culture, we first identified and validated a gene whose expression remains constant under both conditions and therefore could be used for internal normalization. Previous studies had shown that M. tuberculosis 16S rRNA remains constant per genome during various phases of growth in broth culture and per CFU during the course of infection in the mouse lung (7, 25). In order to determine whether 16S rRNA remains constant under both of these conditions, we simultaneously isolated RNA and DNA from M. tuberculosis in mid-log broth cultures and in the lungs of infected mice and quantified 16S rRNA and DNA (genomes) copies with qRT-PCR and qPCR, respectively. Multiple-regression analysis showed that 16S rRNA is maintained at a constant level of ∼103 copies per genome in bacteria growing in broth culture and during the course of infection in the mouse lung (Fig. 1). There was no statistically significant difference either between the slopes or the y intercepts of the lines representing in vitro and in vivo data (alpha [type 1 error] = 0.05). Although we demonstrated that 16S rRNA remains constant per genome, our results are fully consistent with that reported per CFU (∼103 copies) during the course of mouse lung infection (25). This is not unexpected, given that M. tuberculosis replication in the mouse lung reaches a static equilibrium where CFU and genome counts do not diverge (16). Thus, these results validate the use of 16S rRNA for internal normalization of qRT-PCR data in subsequent experiments.
FIG. 1.
M. tuberculosis 16S rRNA:DNA ratio in the mouse lung and in broth culture. RNA and genomic DNA were simultaneously isolated from M. tuberculosis growing in aerated broth culture (mid-log phase) and in lungs of infected mice at 2, 4, and 12 weeks postinfection. 16S rRNA and DNA copy numbers were quantified with qPCR, and the log copy number of 16S rRNA was regressed on DNA for data from in vitro and in vivo bacteria. Three models were compared by multiple regression analysis (SPSS version 12): (i) a model with two separate slopes and intercepts, (ii) a model with parallel lines but differing intercepts, and (iii) a single line for both data sets. Comparison of the three models indicated that the regression lines of in vitro and in vivo data did not differ statistically either in slope (0.9025 and 0.9736) or intercept (3.4815 and 3.3362) at an alpha of 0.05, and thus the third model was accepted to best describe the data. Four mice were included per time point. Data are representative of two independent experiments. wk, week.
M. tuberculosis whiB3 is induced maximally during the early phase of mouse lung infection.
To compare the normalized expression of M. tuberculosis P-GAGs in the lungs of wild-type mice to that of broth culture, qRT-PCR was performed on RNA samples isolated from bacteria in mid-log broth cultures and in the lungs of infected mice at various time points. The results show that of the 17 M. tuberculosis P-GAGs examined, whiB3 was most markedly induced during the course of mouse lung infection (Fig. 2a). Of the other M. tuberculosis P-GAGs, ald (Rv2780) and ask (Rv3709) were induced two- to fivefold at the examined time points (data not shown). Induction of whiB3 was at its peak (∼111-fold) at 2 weeks postinfection during the unrestricted growth phase of acute mouse lung infection (Fig. 2a). At 3 weeks postinfection, at the onset of acquired cellular immunity and thus the development of a static bacterial burden, whiB3 induction dropped (to ∼40-fold over that in broth culture) and gradually declined (to ∼30-fold) by 8 weeks postinfection (Fig. 2a; data not shown).
FIG. 2.
Regulation of M. tuberculosis whiB3 and acr in wild-type C57BL/6 and immunodeficient mice. RNA was isolated from M. tuberculosis in lungs of infected mice at the indicated time points. Quantitative RT-PCR was performed for whiB3 (a) and acr (b) and normalized to 16S rRNA in the same sample. Fold induction, defined as a ratio of normalized expression in mouse lung to mid-log broth culture, was calculated. The inset shows CFU equivalents calculated based on the 16S rRNA copy numbers. TNF-α−/− mice did not survive beyond week 4. Bars show mean induction plus standard deviations of quadruplicates. Wild-type and IFN-γR−/− data are representative of three independent experiments. The difference in whiB3 induction at 2 weeks was not reproducible. *, P < 0.05 compared to same time point in the wild-type; **, P < 0.0001 compared to same time point in the wild-type. wk, week; WT, wild type; eq., equivalent.
To determine whether the decrease in whiB3 induction, which coincided with development of cell-mediated immunity, was mediated by cues from the adaptive immune response, we examined whiB3 expression in mice with absent or delayed cell-mediated acquired immunity (IFN-γR−/−, Rag1−/−, and TNF-α−/−) (9, 10). We reasoned that if whiB3 induction is down-regulated in response to adaptive cellular immunity, then we would expect to see sustained induction of whiB3 in the lungs of these mice. We found that as in wild-type mice, whiB3 was also induced maximally at 2 weeks postinfection in the lungs of immunodeficient mice; however, not only did whiB3 induction subside in the ensuing weeks but it also subsided to a significantly greater extent compared to wild-type mice (P < 0.05 and P < 0.0001) (Fig. 2a). The loss of whiB3 induction was complete in moribund mice.
M. tuberculosis whiB3 is induced in resting macrophages and repressed after activation with IFN-γ.
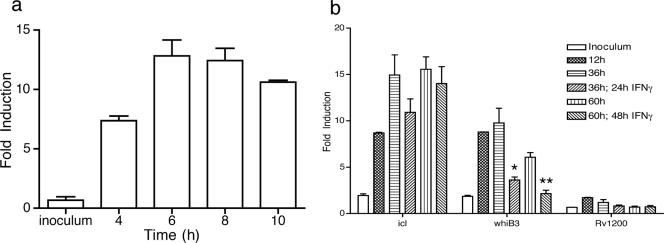
Prior studies have shown that M. marinum whiB3 is induced in macrophages in vitro and in vivo as well as in chronic granulomas (4, 6, 18). To determine whether the induction of M. tuberculosis whiB3 is restricted to the in vivo environment or whether the intracellular environment of a macrophage is sufficient for its induction, we studied the expression of whiB3 in bone marrow-derived macrophages (BMMφ). We found that, compared to bacteria in mid-log broth culture, whiB3 is induced ∼13-fold by 6 h postinfection of resting BMMφ and remains induced over the next 54 h (Fig. 3a). Next, to determine the effect of macrophage activation on the expression of whiB3, BMMφ were activated with IFN-γ at 12 h postinfection, and whiB3 expression was quantified at 24 and 48 h after activation. As shown in Fig. 3b, upon activation of BMMφ with IFN-γ, whiB3 induction gradually declined back to baseline levels (that of the inoculum) over a 48-h period. In contrast, whiB3 remained induced in BMMφ not treated with IFN-γ. We considered the possibility that the down-regulation of whiB3 by IFN-γ was mediated by nitric oxide (NO), but attempts to substantiate this in broth culture with an NO donor (diethylenetriamine [DETA]/NO) were inconclusive (data not shown).
FIG. 3.
Regulation of M. tuberculosis whiB3 in naïve and IFN-γ-activated macrophages. Induction of whiB3 in resting (a) and activated (b) BMMφ after infection with an MOI of 5:1. Quantitative RT-PCR was performed and normalized to 16S rRNA from the same sample. Fold induction is defined as the ratio of normalized expression in BMMφ to broth culture. Bars show means plus standard deviations of biological triplicates. icl and Rv1200 represent positive and negative controls, respectively. icl induction in naïve macrophages is consistent with that reported previously (23). Data are representative of two to four independent experiments. *, P < 0.05 compared to untreated macrophages; **, P < 0.005 compared to untreated macrophages.
Expression of whiB3 in vivo inversely correlates with bacterial density.
Given that whiB3 induction in the lungs of immunodeficient mice was at its lowest at the time when bacterial counts were at their peak, we considered the possibility that whiB3 expression is regulated by bacterial density in vivo. As shown in Fig. 4a, an inverse correlation was found between whiB3 expression and bacterial burden in the lungs of wild-type and immunodeficient mice (R2, 0.73). The correlation was strongest in wild-type, IFN-γR−/−, and TNF-α−/− mice (R2, 0.82, 0.91, and 0.97, respectively). The effect of bacterial density on the expression of whiB3 was also studied in BMMφ. We found that infection of BMMφ with different multiplicities of infection results in an inverse correlation between whiB3 expression and bacterial counts (R2, 0.83) (Fig. 4b). A similar finding was also observed in broth culture where incubation of M. tuberculosis at different bacterial densities inversely correlated with whiB3 expression (Fig. 4c).
FIG. 4.
Correlation of whiB3 expression with bacterial density. Correlation curve for the expression of whiB3 and CFU equivalents in bacteria from the lungs of wild-type (WT) and immunodeficient mice at 2, 3, 4, and 5 weeks postinfection (a) and resting BMMφ infected with MOIs ranging from 1:6 to 8:1 (b). (c) Expression of whiB3 at the indicated bacterial densities in broth culture for 10 h. Bars show means plus standard deviations. Data are representative of two to three independent experiments done in quadruplicates (a), triplicates (b), and duplicates (c). WT, wild type; eq., equivalent.
We also considered the possibility that repression of whiB3 in the necrotic lung lesions of immunodeficient mice (9) is mediated by tissue hypoxia. To investigate the hypoxic status of M. tuberculosis in vivo, we measured the expression of acr, a gene induced by hypoxia (24). Although expression of acr gradually increased in the lungs of immunodeficient mice (Fig. 2b), the inverse correlation between the induction of acr and whiB3 was absent (R2, 0.01, 0.07, and 0.13 for IFN-γR−/−, Rag1−/−, and TNF-α−/−, respectively). Furthermore, hypoxia is unlikely to be the inhibitory stimulus in vivo, given that whiB3 induction in immunodeficient mice declines during a period when the bacteria are replicating and thus do not appear to be under a hypoxic stress.
Identifying a cell-free whiB3-inducing condition.
The observation that whiB3 is maximally induced in vivo during the phase of infection that precedes appearance of an adaptive immune response together with the result that whiB3 is induced in resting macrophages suggest that conditions within macrophage phagosomes are sufficient for maximum induction of whiB3. We therefore sought to identify a condition that stimulates M. tuberculosis to induce whiB3 in vitro. A review of published reports on the transcriptional response of M. tuberculosis to conditions believed to be present in phagosomes did not identify a whiB3-inducing stimulus (2, 5, 8, 13, 14, 17, 21, 23, 24, 30, 31). These conditions included acidic pH (pH 5.5) (8), detergent stress (0.05% sodium dodecyl sulfate) (14), low iron (Chelex 100 treatment) (21), oxidative (5 mM diamide or 5 mM H2O2) and nitrosative (50 μM DETA/NO or 100 μM spermine/NO) (13, 17, 23, 30) stress, hypoxia (0.2% O2) (24, 31), and starvation (PBS culture) (2). We also confirmed the lack of induction of whiB3 under the latter three conditions by qRT-PCR (data not shown). In addition, we determined the effect of low divalent cations on the expression of whiB3 and found that treatment of M. tuberculosis with dipyridyl (250 μM), phenanthroline (10 mM), or EDTA (5 mM) had no effect on the expression of whiB3 in vitro (data not shown).
Phenotype of M. tuberculosis whiB3 knockout in wild-type and IFN-γR−/− mice.
A previous study found that an H37Rv whiB3 mutant is attenuated in C57BL/6 mice in terms of histopathology and time to death of mice but not by bacterial CFU counts (26). To confirm these results, we constructed a whiB3 knockout (Fig. 5) and evaluated its phenotype in wild-type C57BL/6 mice at 154 days after infection. Although we also did not see a difference in bacterial CFU counts between H37Rv and whiB3 knockout mutants (Fig. 6a), we did not see a difference on histopathological lung sections from the same mice (Fig. 6b and c). Furthermore, given that whiB3 expression is suppressed in IFN-γ-activated BMMφ, we sought to determine whether the whiB3 knockout is attenuated in IFN-γR−/− mice. Aerosol infection of IFN-γR−/− mice revealed a small difference in survival between mice infected with parental M. tuberculosis (H37Rv) and the whiB3 knockout, but this difference did not achieve significance (Fig. 6d).
FIG. 5.
Disruption of M. tuberculosis whiB3. (a) Genomic map of whiB3 and flanking genes in M. tuberculosis H37Rv. (b) AES used to make a whiB3 knockout mutant. The shaded box with a hyg cassette represents the region deleted from whiB3. (c) Genomic fragment PCR amplified to create a ΔwhiB3attB::whiB3 complement. (d) Phenotypic confirmation of ΔwhiB3 by real-time RT-PCR. The expression of whiB3 transcript in H37Rv, ΔwhiB3 (whiB3 mutant), and ΔwhiB3attB::whiB3 (whiB3 complement) is shown.
FIG. 6.
Phenotype of whiB3 knockout in wild-type and IFN-γR−/− mice. (a) Bacterial counts from the lungs of C57BL/6 mice infected with H37Rv, whiB3 knockout (ΔwhiB3), and the complement (+whiB3). Bars show means plus standard deviations of four mice on day 1 and two mice on day 154. (b and c) Images of lungs and sections taken from C57BL/6 mice infected with H37Rv (Rv) and ΔwhiB3 at 154 days postinfection. Magnification, ×40. (d) Survival of IFN-γR−/− mice (13 per group) infected with aerosolized M. tuberculosis. Day 1 CFU counts were 100 and 65 for H37Rv and ΔwhiB3, respectively.
DISCUSSION
Mycobacterium tuberculosis is a highly successful human pathogen, as indicated by the number of individuals infected and afflicted with disease globally (19). The success of M. tuberculosis is at least partly rooted in its ability to adapt and persist in diverse host environments. These environments range from phagocytic vacuoles and granulomas to oxygen-rich alveolar airspaces. At each step along its pathogenic course, M. tuberculosis must sense environmental cues and respond to them or modify them in a regulated and coordinated fashion. Previous studies have provided evidence on the transcriptional responses and adaptations of M. tuberculosis to several in vitro and in vivo environments (13, 15, 21, 23, 30). Although a recent study suggests that M. tuberculosis persistence in mouse macrophages depends predominantly on constitutively expressed genes, the relevance of this to the heterogeneous and complex environments encountered in vivo remains to be determined (20).
Of the genetic strategies developed for the identification of conditionally expressed genes, whole-genome microarrays offer the most comprehensive analysis. However, the use of microarrays for profiling of bacterial transcriptomes within the respective host has been limited by the paucity of bacterial mRNA in samples containing a preponderance of mammalian RNA. Based on our analyses using qRT-PCR with 16S rRNA, we have determined that M. tuberculosis total RNA represents ∼0.24% and ∼0.045% of total RNA extracted from infected mouse lungs at 4 and 8 weeks postinfection, respectively (data not shown). Although genome-derived primers were used to resolve the issue of transcript scarcity for microarray profiling of M. tuberculosis genes in vivo (28, 29), we were unable to show a selective enrichment of M. tuberculosis RNA by genome-derived primers as assessed by qRT-PCR. Thus, we used qRT-PCR to study the in vivo regulation of a subset of M. tuberculosis genes whose homologs were previously shown by a promoter trap gfp reporter to be induced in M. marinum in frog granulomas (4, 18). Of the 22 M. tuberculosis genes we studied, whiB3 was the one that was most markedly induced in the mouse lung (Fig. 2). Like its homolog in M. marinum, M. tuberculosis whiB3 was also found to be induced in BMMφ, though to a lesser intensity than in mouse lung. The finding that most of the other genes were not induced in the mouse lung is surprising but may be attributed to (i) the inherent differences between the biology of M. tuberculosis and M. marinum, (ii) the differences in the animal models (mouse versus leopard frog), and (iii) the differences in the methods (qRT-PCR versus gfp reporter) used in our study and that by Chan et al. (4). It has been shown that mutation of whiB3 in M. tuberculosis and M. bovis results in two different phenotypes in guinea pigs (26). Furthermore, recent work has demonstrated that the same strain of M. tuberculosis can have different in vivo phenotypes depending on which animal model (mice or guinea pigs) is used as the host (12). Thus, based on these studies alone, the differences in the biology of mycobacterial species and animal models may explain the differences we found for M. tuberculosis gene expression compared to that reported for M. marinum.
The expression pattern of M. tuberculosis whiB3 is distinct compared to that of other M. tuberculosis genes that have been characterized. In the lungs of wild-type mice and in bone marrow-derived macrophages (BMMφ), whiB3 was maximally induced early after infection (Fig. 2a and 3a). At later time points, after the development of acquired immunity in mice, whiB3 induction was repressed. We tested the hypothesis that M. tuberculosis represses whiB3 in response to activation of acquired immunity but found instead that whiB3 is repressed in the lungs of mice with impaired abilities to raise an acquired immune response; the repression was more intense and also complete in moribund animals (Fig. 2a). Repression of whiB3 in mice may either be due to loss of a whiB3-inducing stimulus or due to the presence of a repressing stimulus that is present in both wild-type and immunodeficient mice. One potential cause of repression may be due to increasing bacterial density through quorum sensing. Quorum sensing is bacterial cell-cell communication which allows bacteria to detect each other and therefore respond or adapt as a population (3). We showed that expression of whiB3 inversely correlated with bacterial density in the mouse lung, BMMφ, and broth culture (Fig. 4). This pattern of expression is consistent with quorum sensing and may allow us to further study this system in M. tuberculosis.
M. tuberculosis WhiB3, a member of the WhiB family of transcriptional regulators, has been previously shown to interact with SigA to cause gross and microscopic pathological lesions in guinea pigs and mice, respectively, and reduced survival in C57BL/6 mice (26). Although we found that induction of whiB3 coincides with the period in which gross and microscopic lung lesions form in wild-type mice, we did not note a difference on histopathological lung sections from C57BL/6 mice infected with H37Rv and whiB3 knockout mutants (Fig. 6b and c). We also did not find a significant difference in survival in IFN-γR−/− mice infected with whiB3 knockout (Fig. 6d). The reason for the difference between our findings and those reported by Steyn et al. (26) could be due to route of infection (aerosol versus intravenous), dose (100 versus 106 CFU), duration of infection (154 versus 126 days), and differences in mouse and bacterial knockout strains.
In summary, a survey of 22 M. tuberculosis genes showed that whiB3 is induced maximally during the early phase of infection in the mouse lung and cultured macrophages. Regulation of whiB3 appears to be complex and a consequence of signals distinct from those that have been identified in macrophages or in vivo. The pattern of whiB3 repression in vivo and in vitro is most consistent with regulation by population density, i.e., quorum sensing.
Acknowledgments
We thank Robert S. Holzman for his assistance with statistical analysis, John McKinney for RNA isolation protocol, and anonymous reviewers for their insightful comments on an earlier version of the manuscript.
This study was supported by grants from the National Institutes of Health (R01AI51242, R01AI26170, and K08AI061105).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. [DOI] [PubMed] [Google Scholar]
- 2.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 3.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 311:1113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, K., T. Knaak, L. Satkamp, O. Humbert, S. Falkow, and L. Ramakrishnan. 2002. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc. Natl. Acad. Sci. USA 99:3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl, J. L., C. N. Kraus, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of M. tuberculosis-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, J. M., H. Clay, J. L. Lewis, N. Ghori, P. Herbomel, and L. Ramakrishnan. 2002. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17:693-702. [DOI] [PubMed] [Google Scholar]
- 7.Desjardin, L. E., L. G. Hayes, C. D. Sohaskey, L. G. Wayne, and K. D. Eisenach. 2001. Microaerophilic induction of the alpha-crystallin chaperone protein homologue (hspX) mRNA of Mycobacterium tuberculosis. J. Bacteriol. 183:5311-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher, M. A., B. B. Plikaytis, and T. M. Shinnick. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 184:4025-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 11.Jung, Y. J., L. Ryan, R. Lacourse, and R. J. North. 2005. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 201:1915-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H., S. T. Cole, V. Mizrahi, E. Rubin, and C. Nathan. 2005. Mycobacterium tuberculosis and the host response. J. Exp. Med. 201:1693-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, E. Dubnau, M. Gomez, and I. Smith. 2002. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 45:365-374. [DOI] [PubMed] [Google Scholar]
- 14.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 15.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Elias, E. J., J. Timm, T. Botha, W. T. Chan, J. E. Gomez, and J. D. McKinney. 2005. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect. Immun. 73:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno, H., G. Zhu, V. P. Mohan, D. Chu, S. Kohno, W. R. Jacobs, Jr., and J. Chan. 2003. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell. Microbiol. 5:637-648. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 19.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis (Edinburgh) 83:4-14. [DOI] [PubMed] [Google Scholar]
- 20.Rengarajan, J., B. R. Bloom, and E. J. Rubin. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. USA 102:8327-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez, G. M., M. I. Voskuil, B. Gold, G. K. Schoolnik, and I. Smith. 2002. ideR, an essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70:3371-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steyn, A. J., D. M. Collins, M. K. Hondalus, W. R. Jacobs, Jr., R. P. Kawakami, and B. R. Bloom. 2002. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl. Acad. Sci. USA 99:3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 28.Talaat, A. M., P. Hunter, and S. A. Johnston. 2000. Genome-directed primers for selective labeling of bacterial transcripts for DNA microarray analysis. Nat. Biotechnol. 18:679-682. [DOI] [PubMed] [Google Scholar]
- 29.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskuil, M. I., K. C. Visconti, and G. K. Schoolnik. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinburgh) 84:218-227. [DOI] [PubMed] [Google Scholar]
- 32.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]