Abstract
Clostridium difficile is the leading cause of nosocomial antibiotic-associated diarrhea, and recent outbreaks of strains with increased virulence underscore the importance of identifying novel approaches to treat and prevent relapse of Clostridium difficile-associated diarrhea (CDAD). CDAD pathology is induced by two exotoxins, toxin A and toxin B, which have been shown to be cytotoxic and, in the case of toxin A, enterotoxic. In this report we describe fully human monoclonal antibodies (HuMAbs) that neutralize these toxins and prevent disease in hamsters. Transgenic mice carrying human immunoglobulin genes were used to isolate HuMAbs that neutralize the cytotoxic effects of either toxin A or toxin B in cell-based in vitro neutralization assays. Three anti-toxin A HuMAbs (3H2, CDA1, and 1B11) could all inhibit the enterotoxicity of toxin A in mouse intestinal loops and the in vivo toxicity in a systemic mouse model. Four anti-toxin B HuMAbs (MDX-1388, 103-174, 1G10, and 2A11) could neutralize cytotoxicity in vitro, although systemic toxicity in the mouse could not be neutralized. Anti-toxin A HuMAb CDA1 and anti-toxin B HuMAb MDX-1388 were tested in the well-established hamster model of C. difficile disease. CDA1 alone resulted in a statistically significant reduction of mortality in hamsters; however, the combination treatment offered enhanced protection. Compared to controls, combination therapy reduced mortality from 100% to 45% (P < 0.0001) in the primary disease hamster model and from 78% to 32% (P < 0.0001) in the less stringent relapse model.
Clostridium difficile is a gram-positive spore-forming bacillus and is the leading cause of nosocomial antibiotic-associated diarrhea (4, 21). This disease is induced by the disruption of the colonic flora through the administration of antibiotics such as clindamycin, ampicillin, or cephalosporins (1). This perturbation in the colonic microenvironment along with exposure to C. difficile spores leads to colonization. Approximately one-third of all patients that become colonized develop C. difficile-associated diarrhea (CDAD) (28), and it has been estimated that CDAD affects more than 300,000 patients per year in the United States (15, 28, 31). Some studies have speculated that CDAD increases the length of hospital stay by as much as 2 weeks for the average patient (30). Current treatment consists of the discontinuation of the offending antibiotic as well as the administration of metronidazole or vancomycin. This treatment is typically successful, but approximately 10 to 20% of all CDAD patients relapse when antibiotic therapy is halted (9). Recent outbreaks of C. difficile strains with increased virulence or antibiotic resistance have led to treatment failures, more-frequent relapses, and increased mortality rates (8, 26, 27, 29). In addition, the widespread use of vancomycin is commonly restricted to prevent the emergence of vancomycin-resistant enterococci.
C. difficile disease is mediated by two exotoxins, toxin A and toxin B (2, 3, 5, 34, 35). Both are high-molecular-mass proteins (280 to 310 kDa) that possess multiple functional domains. The N-terminal domains of both toxins contain glucosyltransferase activity that modifies Rho-like GTPases (14, 16, 17). This modification leads to cytoskeletal dysregulation in the toxified cells and the disruption of colonic epithelial tight junctions. The central domain is predicted to be involved in membrane transport given the presence of hydrophobic regions and caveolin binding sites (39). The C-terminal third of the toxins contains repeating subunits believed to interact with carbohydrate receptors expressed on the target cell surface (38). The interaction of toxin A with carbohydrates also induces the hemagglutination of rabbit erythrocytes (6) and provides a model for the study of toxin A receptor binding. Both toxins are cytotoxic, with toxin B being 1,000 times more potent than toxin A when tested in in vitro cytotoxicity assays, and both are lethal when injected intravenously or intraperitoneally (i.p.) into a mouse. Toxin A is also a potent enterotoxin, as demonstrated by the induction of fluid accumulation in the mouse ligated intestinal loop diarrhea model (12).
For humans, a variety of studies have suggested the importance of antibody in affecting disease outcome. Case series of passive administration of intravenous immune globulin containing anti-toxin A and B antibodies suggested the resolution of symptoms for patients with CDAD (24, 32, 40). Immunization of long-term relapsing humans with a combination toxoid A-toxoid B vaccine has also been pursued to prevent additional relapses (33). Finally, in a prospective controlled blinded study, serum anti-toxin A immunoglobulin G (IgG) concentrations were shown to significantly correlate with protection from CDAD (23). A second study also demonstrated that early development of serum anti-toxin A antibody following primary disease was significantly correlated with protection from relapse (22). These data support the role of antibody in mitigating both primary disease and relapse associated with C. difficile.
Studies with animal models have been conducted that demonstrate the importance of the immune response in mitigating CDAD. Hamsters, when challenged with C. difficile spores following antibiotic treatment, develop fatal diarrhea and colitis, and the hamster model has been employed for the study of CDAD. Immunization of hamsters with toxin A and toxin B in combination, either actively or passively, has been shown to prevent CDAD. The titers of serum antibodies to toxin A and toxin B achieved following immunization correlated with levels of protection from mortality induced by C. difficile (10, 13, 18, 19, 25, 36). Orogastric administration of chicken IgY directed against toxin A could protect hamsters from CDAD (20). In other animals, such as gnotobiotic mice, passive administration of monoclonal antibody to toxin A could prevent disease (7). These human and animal studies, taken together, demonstrate the relevance of toxin-reactive antibodies in disease outcomes.
Here we describe the characterization of a panel of neutralizing, fully human monoclonal antibodies (HuMAbs) directed against either toxin A or toxin B. Based on initial in vitro and in vivo analysis, HuMAb CDA1 (against toxin A) and MDX-1388 (against toxin B) were selected for further study in two separate models of disease in hamsters. CDA1 alone could protect hamsters from mortality, but significantly enhanced protection was observed when the antibodies were administered as a combination therapy.
MATERIALS AND METHODS
Cells and cell culture.
IMR-90 and P3X-AG8.653 cells were obtained from the ATCC. IMR-90 cells were cultured in minimal essential media supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin (P/S). P3X-AG8.653 cells were cultured in RPMI supplemented with 10% FCS, 4 mM glutamine, 1 mM pyruvate, 5 mM HEPES, 1% P/S, 55 μM 2-mercaptoethanol. Hybridomas were cultured in Dulbecco's modified Eagle Medium (DMEM) containing 10% FCS, nonessential amino acids, 1× oxaloacetate-pyruvate-insulin (Sigma), 4 mM glutamine, 1× hypoxanthine-aminopterin-thymidine (Cellgro), 1× P/S, 1× hybridoma cloning factor (Bioveris), 2-mercaptoethanol (GIBCO), and 10% NCTC-109 (GIBCO). All cells were propagated at 37°C in air supplemented with 5% CO2.
Mouse immunization and splenic fusions.
HuMAb mice (Medarex Inc., Bloomsbury, New Jersey) are transgenic for human immunoglobulin genes; mouse heavy chain and kappa light chain immunoglobulin genes are inactivated. HuMAb mice were injected weekly with 10 μg of antigen using RIBI (Corixa) as the adjuvant for 6 to 12 weeks. When toxin A or toxin B (both from Techlab) was used as the antigen, toxin was inactivated prior to injection by the UDP-dialdehyde method described elsewhere (11). Enzyme-linked immunosorbent assay (ELISA) was employed to measure serum responses to antigen, and animals were sacrificed when serum responses reached a plateau. Hybridomas were generated by the standard polyethylene glycol method with P3X63-AG8.653 mouse myeloma cells used as the fusion partner.
Identification of antigen-reactive hybridomas.
Culture supernatants of hybridomas and immunized mouse serum were assessed for antigen binding using ELISA. Ninety-six-well microtiter plates were coated with 2 μg/ml either of toxin A or of toxin B in phosphate-buffered saline (PBS) overnight at 4°C. Two hundred microliters of culture supernatant at various dilutions was added to the wells and incubated at 22°C for 2 h. Antibody binding was detected using anti-human IgG-alkaline phosphatase conjugate (1:5,000; Jackson ImmunoResearch), and plates were analyzed using a Molecular Devices Emax plate reader (405 nm) with Softmax software.
Antibody purification.
Hybridoma cell cultures were expanded, and supernatant was harvested by centrifugation. Small-scale culture supernatants were incubated with protein A- or protein G-Sepharose (Amersham) for 2 h at room temperature while rocking. Beads were removed by column filtration, and antibody was eluted with 100 mM glycine, pH 2.8. The eluate was dialyzed against PBS and concentrated using an Amicon Centriprep YM-30 concentrator as described by the manufacturer. Purified antibody was filter sterilized and protein concentration quantitated by spectrophotometry. For large-scale antibody purification, HiTrap (Amersham) protein A column chromatography was employed and purified antibody was concentrated as described above.
Toxin neutralization assays.
IMR-90 cells were seeded at 1 × 105 cells per well in a 96-well microtiter plate and incubated for 12 h at 37°C with 5% CO2. Toxin A (6 ng/ml) or toxin B (20 pg/ml) was incubated with various concentrations of antibody for 1 h, added to the cell culture, and incubated for 18 h at 37°C. Cytopathic effect was determined microscopically and scored as 0 (0% rounded cells), 1 (25% rounded cells), 2 (50% rounded cells), 3 (75% rounded cells), or 4 (100% rounded cells).
Hemagglutination assay.
Various concentrations of purified antibody were incubated with toxin A (0.5 μg) for 1 h at 4°C in a 96-well microtiter plate in a total volume of 25 μl of PBS. Rabbit erythrocytes (R-RBC; Colorado Serum Co.) were washed with PBS and resuspended to 2% (vol/vol) in PBS. Twenty-five microliters of 2% R-RBC was added to the wells of the 96-well plate and incubated for 4 h at 4°C. Plates were scored visually, with hemagglutination presenting as diffuse red cells in the well and the absence of hemagglutination observed as all red blood cells settled in the well.
Synthesis and purification of recombinant proteins.
C. difficile strain VPI 10463 was grown for 20 h in thioglycolate medium (BBL), and 1 ml of bacterial suspension was centrifuged at 5,000 rpm for 5 min. The bacterial pellet was solubilized and DNA purified using a DNeasy kit (QIAGEN) as described by the manufacturer. Purified DNA was resuspended in 500 μl for subsequent use in PCR. The DNA sequences for C. difficile (strain VPI 10463) toxin A and toxin B were obtained from NCBI (#X92982). Primers were designed to amplify four various fragments from each of the toxin A and toxin B genes (Table 1). 5′ oligonucleotides were modified to contain a BamHI restriction site, and the 3′ oligonucleotides were modified with a 5′ SacI restriction site. PCR was performed in a reaction mixture containing 1 μl genomic DNA, 20 μM deoxynucleoside triphosphates, 20 pmol of each oligonucleotide, 1× cloned Pfu reaction buffer (Stratagene), and 1 U Turbo Pfu (Stratagene). Forty cycles of thermocycling (95°C for 15 s, 55°C for 30 s, and 68°C for 1 to 10 min) were performed, and the PCR products were resolved on 1% agarose gels. PCR products were purified using a gel extraction kit (QIAGEN) and subsequently digested with BamHI and SacI. Digested PCR products were ligated into pET32+ (Novagen), which contains an upstream gene encoding bacterial thioredoxin and a downstream sequence encoding the His6 epitope tag. All cloned genes were sequenced to confirm that no errors had accumulated during the PCR process.
TABLE 1.
Oligonucleotides used for the construction of recombinant toxin fragments
| Toxin | Fragment | Oligonucleotidea
|
bp amplified | Amino acids | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| A | 1 | GGTTGCTGGATCCATGTCTTTAATATCTAAAGAAGAGTTAATAAAACTCGCATATAGC | CCATGCTGAGCTCGCTTCATCTTTACCATGTCCAATAAAGGTTACTTTTACTTTTTCC | 1-1977 | 1-659 |
| A | 2 | GGTTGCTGGATCCTTCAACACAAGCGAATTTGCTAGATTAAGTGTAGATTCAC | CCATGCTGAGCTCGCTGAATCAAGTAATCTAGTTCCGTCATTTTCCAATGATC | 1978-3768 | 660-1256 |
| A | 3 | GGTTGCTGGATCCATAAGAGATTTATACCCAGGTAAATTTTACTGGAGATTCTATGC | CCATGCTGAGCTCGCATTATTTATATTGATTAATCCTTTAACTAATTTACTATCTTCATCATAG | 3769-5556 | 1257-1852 |
| A | 4 | GGTTGCTGGATCCTCATTATTCTATTTTGATCCTATAGAATTTAACTTAGTAACTGGATGG | CCATGCTGAGCTCGCGCCATATATCCCAGGGGCTTTTACTCCATCAAC | 5557-8130 | 1853-2710 |
| B | 1 | GCTTGCTGGATCCAGTTTAGTTAATAGAAAACAGTTAGAAAAAATGG | CCATGCTGAGCTCGCTTCATAACTAATTTTATCTCCTTGTAACTG | 1-1638 | 1-546 |
| B | 2 | GCTTGCTGGATCCGCAGCATGTAACTTATTTGCAAAG | CCATGCTGAGCTCGCTATATCATCAGTTACAGTATGACCTGAAC | 1639-3553 | 547-1184 |
| B | 3 | GCTTGCTGGATCCGATCACTTCTTTTCAGCACCATC | CCATGCTGAGCTCGCCAAAGTCTTATCTTTAAAAACATTAACGAATC | 3553-5328 | 1185-1776 |
| B | 4 | GGTTGCTGGATCCGCAAATAAGCTATCTTTTAACTTTAGTGATAAACAAGATGTACC | CCATGCTGAGCTCGCTTCACTAATCACTAATTGAGCTGTATCAGGATCAAAATAATAC | 5329-7098 | 1777-2366 |
Underlining denotes the introduction of restriction sites to facilitate molecular cloning.
BL21Star Escherichia coli cells (Invitrogen) were transformed with pET32+ toxin fragment vector and grown overnight at 37°C in 20 ml of Luria broth containing 100 μg/ml ampicillin (LB-ampicillin). The culture was diluted 1:20 into 200 ml of LB-ampicillin and grown for 2.5 h at 37°C, and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added. For toxin A fragments, the culture was shifted to 22°C and incubated for 2 h. For toxin B fragments, cultures were incubated for 2 h at 37°C. Bacteria were harvested by centrifugation and frozen at −80°C for 30 min. Pellets were resuspended in 10 ml of PBS supplemented with 350 mM NaCl and 1 mg/ml lysozyme. The suspension was incubated for 30 min on ice followed by 10 min at 22°C while rocking. CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, DNase, and RNase were added to give final concentrations of 1%, 10 μg/ml, and 10 μg/ml, respectively. The mixture was rocked at 22°C for 30 min and the insoluble material removed by centrifugation at 5,000 × g for 15 min. Cleared lysate was incubated with Ni-nitrilotriacetic acid agarose for 2 h at 22°C, and resin was removed by column filtration. Proteins were eluted with 250 mM imidazole, dialyzed against PBS, and concentrated with an Amicon Centriprep YM-30 concentrator as described by the manufacturer.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blotting.
Various concentrations of purified toxin fragments were mixed with 2× reducing Laemmli sample buffer and incubated at 37°C for 45 min. Samples were resolved using 10% or 12% Novex gels (Invitrogen) for 1.5 h at 200 V. Gels were transferred to Immobilon P (Millipore) as described by the manufacturer, and Western blot analysis was performed. Proteins were detected using either purified HuMAbs (1 μg/ml) or anti-His6 antibody (0.1 μg/ml; Invitrogen) followed by an anti-human or anti-mouse IgG-horseradish peroxidase conjugate (1:5,000; Jackson ImmunoResearch). Membranes were incubated with ECL reagent (Amersham) for 1 min and exposed to XOMAT-AR film for various periods of time.
Neutralization of mouse toxicity.
Swiss Webster mice were injected intraperitoneally with 100 to 250 μg of HuMAbs and rested for 24 h. One hundred nanograms of either toxin A or toxin B was injected intraperitoneally, and the mice were observed for up to 72 h. The Fisher exact test was used for all statistical analyses.
Inhibition of intestinal fluid accumulation.
CD1 mice were injected intraperitoneally with various concentrations of purified HuMAbs directed against toxin A or PBS (untreated positive control). Animals were rested for 16 h, during which time food was withheld. Animals were anesthetized with 100 mg ketamine/kg of body weight and 10 mg xylazine/kg administered intraperitoneally. A 1-cm incision was made in the abdomen of the mouse, and the ileum was isolated. A vascularized section of ileum approximately 3 cm in length was doubly ligated as to separate it from the general intestinal tract. Toxin A (0.45 μg; 150 μl) was carefully injected into the lumen of the ligated section of ileum. The ileum was returned into the abdomen of the mouse and the incision closed with surgical staples. The animal was allowed to recover after the surgery was completed and was sacrificed 4 h later. The ligated ileum was removed from the animal with surgical scissors, and its weight and length were determined.
C. difficile spore preparation.
C. difficile strain B1 (gift of Dale Gerding) was grown for 24 h in thioglycolate medium and subsequently streaked on blood agar plates. A total of 40 blood agar plates were incubated anaerobically for 7 days, and the bacterial lawn was scraped into DMEM. Spores were centrifuged at 500 × g for 30 min at 4°C and resuspended in 100 ml of DMEM. Spore suspensions were frozen and stored at −80°C. To assess the spore number in the bank, one vial of spores was thawed and plated in limiting dilution on cycloserine-cefoxitin-fructose agar and incubated anaerobically for 3 days at 37°C. CFU were counted, and the number of spores/ml was calculated.
Hamster primary challenge model.
Syrian golden hamsters (70 to 80 g) were given four total doses of HuMAb (MDX1388 and CDA1) or 1 ml anti-toxin B goat serum intraperitoneally for 4 days beginning 3 days prior to the administration of C. difficile spores. Clindamycin (10 mg/kg) was administered intraperitoneally 24 h prior to C. difficile spore challenge, and the hamsters were challenged with 140 B1 spores orogastrically using a standard small animal feeding needle. Animals were observed for morbidity and mortality, and the experiment terminated when all remaining hamsters were considered healthy. The Fisher exact test was used for all statistical analyses.
Hamster relapse model.
Syrian golden hamsters (70 to 80 g) were given 10 mg/kg clindamycin intraperitoneally and 24 h later challenged with 140,000 C. difficile B1 spores orogastrically. At the time of spore administration, vancomycin treatment (10 mg/kg/day orogastrically) was begun and continued daily for a total of 3 days. Beginning 48 h following spore challenge, HuMAbs or 1 ml goat serum was injected intraperitoneally twice per day for a total of 5 days. Animals were observed for morbidity and mortality, and the experiment terminated when all remaining hamsters were considered healthy. The Fisher exact test was used for all statistical analyses.
RESULTS
Isolation of human monoclonal antibodies directed against Clostridium difficile toxins A and B.
To generate HuMAbs reactive with Clostridium difficile toxin A, transgenic mice containing active human immunoglobulin genes (HuMAb mice; Medarex Inc.) were immunized with toxoid A (inactivated toxin A) for 6 to 12 weeks. Serum titers directed against toxin A were measured by antigen-specific ELISA, and splenic fusions were performed on mice that produced a robust anti-toxin A immune response. Screening yielded 16 toxin A-reactive hybridomas from a total of seven mice. Supernatants from all 16 hybridomas contained human γ heavy chains as well as human κ light chains. Fifteen of the reactive supernatants contained human IgG1, whereas one clone (clone 3H2) produced human IgG3.
To generate HuMAbs directed against toxin B, two immunogens were used. A recombinant fragment of toxin B that includes the C-terminal 590 amino acids of toxin B (toxin B fragment 4) was cloned and expressed. This domain contains repeated sequences and is believed to be involved in the binding of toxin B to cellular receptor. HuMAb mice were immunized with either whole C. difficile toxoid B (inactivated toxin B) or the recombinant toxin B fragment 4 for 6 to 12 weeks. Splenic fusions were performed on mice with a potent immune response to toxin B as measured by ELISA. From animals immunized with toxoid B, a total of 17 toxin B-reactive hybridomas were identified from four distinct animals. Fusion of toxin B fragment 4-immunized animals (eight HuMAb mice) yielded 55 hybridomas reactive to toxin B as measured by ELISA. All 72 distinct toxin B-reactive hybridomas secreted human IgG1 with κ light chains.
Identification of HuMAb antibodies that neutralize toxin A and toxin B in vitro.
The in vitro cytotoxicity induced by both toxin A and toxin B was measured in the IMR-90 cytotoxicity assay. In this assay, monolayers of IMR-90 cells are exposed to toxin for 24 h, and dramatic morphological changes can easily be observed. Cultures are scored on a scale of 0 to 4, in which 0 indicates no toxic effect and 4 signifies that 100% of the cells have lost their typical morphology and are rounded. Notably, toxin A is much less potent than toxin B in this assay and requires approximately 1,000-fold-higher concentrations to induce a similar cytotoxicity level.
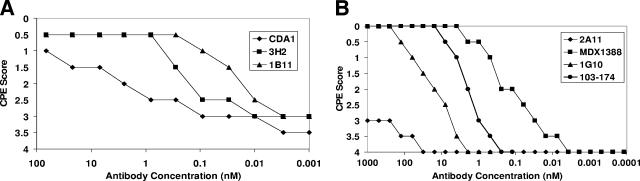
Cell cultures for the 16 toxin A-reactive HuMAbs were scaled to larger volumes, and antibody was purified by protein A/G chromatography. All purified HuMAbs were tested for the ability to neutralize toxin activity in vitro. IMR-90 cells were plated and incubated for 24 h with toxin A (6 ng/ml) in the presence of various concentrations of purified antitoxin HuMAb antibodies. Three purified anti-toxin A HuMAbs, clones CDA1, 1B11, and 3H2, showed neutralizing activity against toxin A, and the results of a representative experiment are shown in Fig. 1A. A total of five comparative experiments were performed, and the hierarchy of toxin A neutralization potency was found to be 1B11 > 3H2 > CDA1.
FIG. 1.
In vitro neutralization of cytotoxicity. (A) C. difficile toxin A (6 ng/well) was mixed with either HuMAb CDA1, 3H2, or 1B11 at various antibody concentrations from 100 to 0.001 nM. IMR-90 cells were plated at 1 × 105 cells/well in a 96-well microtiter plate, and toxin A-antibody mixtures were applied to the cells. IMR-90 cells with a toxin A-antibody mixture applied were incubated for 24 h and scored for cytopathic effect (CPE) visually on a scale of 0 to 4, where 0 represents no observed toxicity and 4 indicates that 100% of the monolayer was effected by toxin A. (B) The experiment was performed as described for panel A, with the exception that C. difficile toxin B (20 pg/well) was mixed with either HuMAb 2A11, MDX1388, 1G10, or 103-174 at various antibody concentrations from 1,000 to 0.0001 nM prior to being applied to IMR-90 cells.
Given the large numbers of hybridomas reactive to toxin B in ELISA (a total of 72), supernatants were first screened for the presence of neutralization activity in the IMR-90 assay. Four supernatants contained detectable activity in this assay (clones MDX1388, 1G10, 103-174, and 2A11; data not shown), and antibodies for each were purified by protein A chromatography. Clones MDX1388 and 1G10 were derived from animals immunized with toxin B fragment 4, and 103-174 and 2A11 arose from animals immunized with toxoid B. All four purified HuMAbs were incubated at various concentrations with 20 pg/ml of toxin B and applied to IMR-90 cells to assess the neutralization of toxin B-mediated cytotoxicity. Cultures were scored as described above, and the results of a representative experiment are shown in Fig. 1B. MDX1388 and 103-174 showed potent toxin B-neutralizing activity, with half-maximal activity levels of 0.1 nM and 1 nM, respectively (average of four comparative experiments). 1G10 possessed moderate neutralization capacity, and 2A11 was a very weak neutralizing HuMAb. Overall, these data demonstrate that three of our toxin A-reactive HuMAbs and four of our toxin B-reactive HuMAbs can neutralize their respective toxins in the in vitro cytotoxicity assay.
CDA1, but not 3H2 or 1B11, inhibits hemagglutination induced by toxin A.
The direct interaction of toxin A with its cellular receptor is difficult to study. Staining hamster colonic epithelium with toxin A is laborious (13). Toxin A does, however, hemagglutinate rabbit erythrocytes. The repeating subunits of the toxin A receptor-binding domain are thought to interact with specific carbohydrate residues of the rabbit erythrocytes, leading to cross-linking and subsequent hemagglutination. We used hemagglutination as a surrogate for studying the interaction of toxin A with its cellular receptor. Various concentrations of toxin A (from 2 to 0.001 μg/well) and PBS as the negative control were mixed with rabbit erythrocytes, and the mixtures were seeded to microtiter plates and incubated at 4°C for 4 h. Plates were scored for toxin A-induced hemagglutination as demonstrated by a diffuse distribution of erythrocytes in the well (as opposed to a typical button of erythrocytes in the negative control). Concentrations of 0.25 μg/well could completely induce hemagglutination, and 0.5 μg/well was selected as the standard concentration to test for hemagglutination inhibition. Toxin A (0.5 μg/well) was incubated with various concentrations (12.5 to 0.006 μg/well) of either CDA1, 3H2, or 1B11 HuMAb and subsequently mixed with rabbit erythrocytes and incubated for 4 h at 4°C. HuMAb CDA1 could inhibit hemagglutination at concentrations as low as 0.4 μg/well, whereas neither 1B11 nor 3H2 could block hemagglutination, even at the highest concentrations tested. These data suggest that CDA1, but not 1B11 or 3H2, can disrupt the interaction between toxin A and its cellular receptor.
HuMAbs to toxin A but not toxin B can neutralize in vivo mouse toxicity.
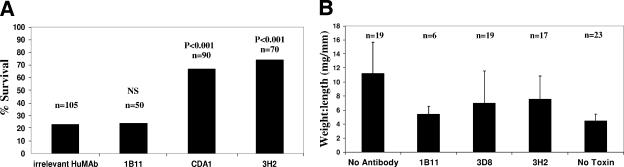
Toxins A and B, when administered i.p. to a mouse, induce lethality in a significant number of animals in less than 72 h. The mechanism of death is presumably related to the cytotoxic effects of the toxins. Although this model does not mimic enteric disease, it is a measure of the ability to neutralize circulating toxin in an animal. Swiss Webster mice were injected intraperitoneally with 1B11, CDA1, or 3H2 (100 to 250 μg/dose) 24 h prior to the administration of toxin A. Toxin A (100 ng) was injected i.p., and animals were observed for 48 to 72 h for mortality. Results of multiple experiments are shown in Fig. 2A. Animals treated with 100 to 250 μg of an irrelevant HuMAb had a survival rate of 23% (24/105). The survival rates for 1B11, CDA1, and 3H2 were 24% (12/50), 67% (60/90), and 74% (52/70), respectively. 1B11 showed no reduction in mortality compared to controls; however, both 3H2 and CDA1 provided significant protection from mortality compared to the irrelevant HuMAb.
FIG. 2.
Neutralization of in vivo toxicity in mice. (A) Mice were treated with 100 to 250 μg either of an irrelevant HuMAb or of 1B11, CDA1, or 3H2 24 h prior to intraperitoneal administration of 100 ng of toxin A. Animals were observed for up to 72 h, and the percentages of surviving animals were plotted. The number of animals tested in each group is shown above the respective column in the bar graph, with P values indicated (the Fisher exact test). NS, not significant. (B) Mice were injected i.p. with 500 μg to 2 mg of 1B11, CDA1, or 3H2 24 h prior to ileal loop surgery. As an untreated positive control, mice were injected i.p. with PBS rather than antibody (no antibody). As a negative control for fluid accumulation due to surgery, PBS was injected into the ligated loop in place of toxin A (no toxin). The number of animals in each group is shown above the respective column in the bar graph. Toxin A (0.45 μg) was injected into ligated ileal loops of treated animals, and loops were excised 4 hours later. Weight-to-length ratios were calculated and plotted.
In vivo mouse toxicity assays were also done using toxin B to test the neutralizing activity of the anti-toxin B HuMAbs. Swiss Webster mice were injected i.p. with 100 to 250 μg of either MDX1388, 1G10, 2A11, 103-174, irrelevant HuMAb, or goat anti-toxin B 24 h prior to toxin B administration. Toxin B (100 ng) was injected i.p., the mice were observed for 72 h, and survival rates were determined. Six percent (3/50) of mice treated with an irrelevant HuMAb survived the challenge within 72 h. Survival rates for animals treated with MDX1388, 2A11, 1G10, and 103-174 were 8% (4/50), 10% (2/20), 10% (2/20), and 20% (4/20), respectively (data not shown). Animals treated systemically with goat anti-toxin B had a survival rate of 98% (49/50). These data demonstrate that none of the anti-toxin B HuMAbs could individually protect mice from lethality associated with toxin B. Polyclonal goat serum, however, showed potent neutralization of toxin B in this model.
Neutralizing HuMAbs directed against toxin A can prevent fluid accumulation in mouse intestinal loops.
Toxin A is a potent enterotoxin and has been shown to induce fluid accumulation in the mouse intestinal tract in a model utilizing an isolated section of mouse ileum (37). Briefly, surgery was performed on the mouse, and the ileum, proximal to the cecum, was doubly ligated, creating a section of ileum isolated from the general intestinal tract. Toxin A (0.45 μg) was then injected into the ligated region, and the animal was allowed to recover from surgery. Approximately 4 h after the completion of surgery, mice were euthanized, and the ligated sections of ilea were removed. The excised ileum was weighed and measured to calculate a weight-to-length ratio. Ileum that accumulated fluid had a measurable increase in weight, causing an increase in the weight-to-length ratio.
Mice were injected i.p. with 500 μg to 2 mg of either 1B11 (6 mice), CDA1 (19 mice), 3H2 (17 mice), or PBS (19 mice [untreated controls]) approximately 24 h prior to surgery. Surgery was performed, toxin A was administered, and animals were euthanized after 4 h. Weight-to-length ratios of the excised ileal loops were calculated; the average ratios are shown in Fig. 2B. Mice that did not receive antibody treatment had a mean weight-to-length ratio of 11.2 mg/mm (range = 4.8 to 22 mg/mm). Weight-to-length ratios for 1B11-, CDA1-, and 3H2-treated mice were calculated to be 5.4 mg/mm (range = 2.9 to 7.2 mg/mm), 7.0 mg/mm (range = 3.3 to 12.4), and 7.6 mg/mm (range = 1.7 to 11.3), respectively. As control for fluid accumulation due to injection trauma, PBS was injected into the ligated ileal loop in place of toxin A. Weight-to-length ratios for the PBS (no toxin) control ilea had a mean of 4.5 mg/mm (range = 2.7 to 6.1). These data demonstrate that all three HuMAbs can neutralize enterotoxicity in the mouse ileum relative to what is seen for untreated controls.
Domain mapping of toxin A- and toxin B-neutralizing HuMAbs.
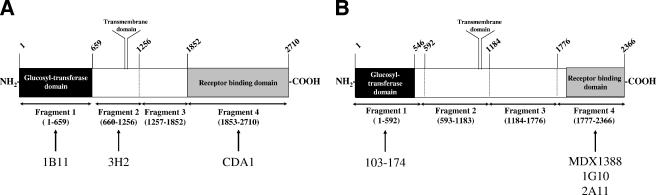
The toxin A protein contains several different functional domains. The N-terminal 25% of the protein contains the enzymatic domain (Rho glucosylation), the central domain contains a putative transmembrane domain, and the C-terminal 30% contains the repeating subunits thought to be required for receptor binding (Fig. 3A). We generated four recombinant fragments of toxin A containing the N- and C-terminal domains as well as two fragments spanning the central region of the protein (Fig. 3A). The recombinant proteins were expressed and resolved by SDS-polyacrylamide gel electrophoresis and detected by Western blotting using mouse anti-His6 (epitope tag), 3H2, CDA1, or 1B11 as detection reagents (data not shown). All four recombinant proteins were detected by anti-His6 antibody. HuMAb 3H2 bound only to toxin A fragment 2. HuMAb CDA1 recognized toxin A fragment 4 (receptor-binding domain) but not fragments 1, 2, or 3. HuMAb 1B11 interacted with toxin A fragment 1 (enzymatic domain) but not fragments 2, 3, or 4. These data demonstrate that the three neutralizing HuMAbs recognize three distinct epitopes (Fig. 3A) within toxin A and suggest that multiple mechanisms for neutralization are possible. The recognition by Western blotting further suggests that the epitopes recognized by all three HuMAbs are not irreversibly sensitive to denaturation with SDS and may be linear in nature.
FIG. 3.
Domain recognition of toxin A- and toxin B-reactive HuMAbs. (A) Schematic representation of recombinant fragments cloned and expressed in E. coli, with amino acid numbers listed above and below the figure. Specific antibodies are listed below the recognized domain. (B) Schematic of recombinant toxin B fragments that were cloned and expressed in E. coli, with amino acid numbers listed above and below the figure. Specific antibodies are listed below the recognized domain.
To domain map our toxin B-reactive HuMAbs, we focused on the most well-defined domains of toxin B. The domain structure of toxin B is similar to that of toxin A, with fragment 1 representing the enzymatic domain and fragment 4 representing the ligand-binding domain (Fig. 3B). MDX1388 and 1G10 were isolated from mice immunized with recombinant toxin B fragment 4, and not surprisingly, these HuMAbs are shown by Western blotting to recognize toxin B fragment 4 (data not shown). However, 103-174 and 2A11 were isolated from animals immunized with whole toxoid B and thus could presumably recognize any domain of toxin B. When assayed with Western blotting for the ability to bind to the two recombinant fragments of toxin B, 103-174 was found to bind strongly to fragment 1, and 2A11 interacted with fragment 4. None of these antibodies recognized toxin B fragments 2 or 3 in Western blot analysis (data not shown). Thus, three of our in vitro neutralizing HuMAbs recognize the receptor-binding domain, whereas one interacts with the enzymatic domain of toxin B (Fig. 3B). All of these HuMAbs recognize denatured antigen, as shown by recognition by Western blotting, and thus may recognize linear epitopes.
Antitoxin HuMAbs protect hamsters from mortality associated with C. difficile disease.
Given that the anti-toxin A HuMAb CDA1 interacts with the receptor-binding domain and demonstrates activity in all in vitro and in vivo assays, we selected this monoclonal antibody as our lead anti-toxin A candidate for use in hamsters. Also, MDX1388 was selected as the lead candidate anti-toxin B HuMAb, given its potent neutralization activity in in vitro studies and its recognition of the toxin B receptor-binding domain. Hybridoma cultures of the two lead HuMAbs were scaled to larger volumes in both spinners and bioreactors for large-scale production of HuMAb. Antibodies were purified from culture supernatants using protein A affinity chromatography and quantitated by spectrophotometry.
The hamster model of C. difficile disease is a well-established model for the study of toxin-induced antibiotic-associated diarrhea. The most extensively studied model of C. difficile infection in hamsters is the primary challenge model. Briefly, hamsters were treated with clindamycin 24 h prior to the administration of C. difficile spores to disrupt the normal colonic flora in the hamster. C. difficile spores (140 spores, B1 strain) were administered orogastrically, and the hamsters were observed. Typically, 100% of hamsters succumb to disease between 36 and 72 h after spore administration. Prior to mortality, symptoms of disease include diarrhea, weight loss, loss of muscle control, and difficulty in breathing. In general, the symptoms are much more severe than those seen in humans, but the hamster model responds to therapeutic maneuvers used in clinical disease and is therefore widely used in the study of C. difficile disease.
For the study of the antitoxin monoclonal antibody combination therapy, hamsters were treated intraperitoneally with CDA1 (50 mg/kg/day), MDX1388 (10 to 50 mg/kg/day), or a combination of the two for a total of 4 days (72, 48, 24, and 0 h prior to the administration of C. difficile spores). Hamsters were injected intraperitoneally with clindamycin 24 h prior to the orogastric delivery of 140 C. difficile strain B1 spores. Hamsters were observed for mortality daily until all hamsters had either succumbed to disease or become free of disease symptoms.
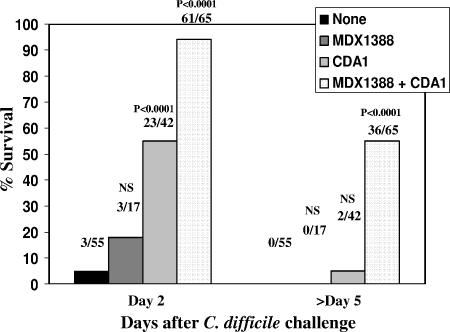
We conducted a total of six experiments to test the abilities of our HuMAb antibodies directed against toxins A and B to protect hamsters from primary challenge with C. difficile, and the results are summarized in Fig. 4. CDA1 alone offered significant early protection compared to no treatment (day 2, 23/42 [surviving hamsters/total hamsters]; P < 0.0001), although this protection waned at later time points (day >5, 2/42). However, combination therapy with CDA1 and MDX1388 resulted in increased protection at day 2 (61/65; P < 0.0001) as well as significant protection throughout the study period (36/65; P < 0.0001). Finally, MDX1388 administered as a single agent did not offer protection at any time point with this model.
FIG. 4.
Protection of hamsters with HuMAbs in the primary challenge model. Hamsters were treated with CDA1 (50 mg/kg/day) alone or in combination with MDX1388 (10 to 50 mg/kg/day) for 4 days beginning 3 days prior to spore administration. Clindamycin (10 mg/kg) was injected intraperitoneally 24 h prior to spore challenge. Spores were delivered orogastrically, and animals were observed for up to 11 days. A total of six experiments were performed, and a summary of all hamster primary challenge experiments using percent survival at either 2 or >5 days after challenge is shown. The fractions above the bar graphs represent the numbers of surviving animals (numerators) and the total numbers of animals (denominators), with P values indicated (the Fisher exact test). NS, not significant.
We modified the primary disease hamster model to simulate the effects of CDA1 and MDX1388 to control relapse. Vancomycin protects hamsters from C. difficile disease, as it does in humans. When vancomycin treatment is discontinued, hamsters relapse with severe disease, but the attack rate varies. Hamsters were given a single dose of clindamycin followed by the orogastric administration of C. difficile strain B1 spores (140,000 CFU) 1 day later. Vancomycin treatment began on the day of spore challenge and continued daily for two subsequent days (three total doses of 10 mg/kg/day).
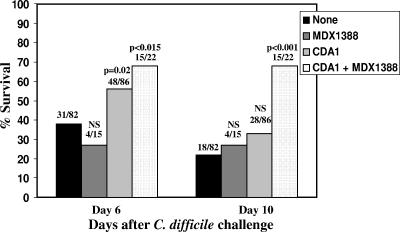
We conducted multiple experiments to assess the therapeutic benefit of MDX1388 and CDA1 (60 mg/kg/day and 20 mg/kg/day, respectively, days 2 to 6 following spore challenge) in preventing mortality in the relapse model of infection in the hamster. In the summary data analysis (Fig. 5), CDA1 administered in addition to vancomycin prevented relapse at day 6 after challenge in 56% (48/86) of hamsters compared to 38% (31/82) of those who received vancomycin alone (P = 0.02); by day 10 after challenge, the rates for the groups were similar. However, combination treatment with CDA1 and MDX1388 offered significant protection at both early and late time points, with 68% survival at day 6 (15/22; P < 0.015) and 68% at day 10 (15/22; P < 0.0001), compared to what was seen for vancomycin-treated controls, which showed 38% and 22% survival at these time points. It is noteworthy that no protection was seen for animals treated with MDX1388 alone at either time point, with only 27% survival (4/15) being observed.
FIG. 5.
Protection of hamsters with HuMAbs in the relapse model. Clindamycin (10 mg/kg) was injected intraperitoneally 24 h prior to spore challenge. Spores were administered orogastrically, at which time vancomycin treatment (10 mg/kg/day) was initiated and continued for a subsequent 2 days. Hamsters were treated with CDA1 (60 mg/kg/day) in combination with MDX1388 (20 mg/kg/day) for 4 days beginning 2 days following spore administration. Animals were observed for mortality until day 10. A summary of all hamster relapse model experiments using percent survival at either 6 or 10 days following challenge was graphed. The fractions above the bar graphs represent the numbers of surviving animals (numerators) and the total numbers of animals (denominators), with P values indicated (the Fisher exact test). NS, not significant.
DISCUSSION
A growing body of evidence suggests that antibodies directed against toxins A and B may provide additional benefit to the standard treatment regimens for CDAD. The current treatment for nosocomial antibiotic-associated diarrhea induced by C. difficile is the discontinuation of the offending antibiotic followed by the administration of vancomycin or metronidazole. This treatment is generally effective, yet relapse of symptoms occurs in a significant number of patients (up to 20%). The development of strains with increased virulence (thought to be mediated by excess toxin production) or with antibiotic resistance as well as the need to restrict vancomycin use to prevent the emergence of vancomycin-resistant enterococci underscores the need for alternative therapeutics. Antibody therapy that directly neutralizes toxins and interferes with the mechanism of C. difficile pathogenesis may also be expected to lead to more-rapid and more-durable therapeutic effects.
Here we describe the identification and characterization of a panel of HuMAbs directed against both toxins A and B of C. difficile. Toxin A-directed HuMAbs CDA1, 3H2, and 1B11 could neutralize in vitro cytotoxicity and prevent fluid accumulation in mouse intestinal loops. However, only CDA1 and 3H2 could neutralize systemic toxicity in the mouse, and only CDA1 had the capacity to prevent toxin A-induced hemagglutination of rabbit erythrocytes. Epitope mapping demonstrated that these three HuMAbs recognized very diverse regions of the toxin, with CDA1 recognizing the C-terminal third of toxin A, 3H2 interacting with the central domain, and 1B11 binding to the N terminus. We speculate that 1B11 neutralizes the enzymatic activity of toxin A and that CDA1 prevents the binding of toxin A to a cognate receptor found on the surface of the target cell. The function of 3H2 is difficult to surmise, but it may prevent the internalization of toxin A by binding to the transmembrane domain or caveolin binding sites. Since CDA1 possessed the broadest range of neutralization activity in these various assays and is presumed to act extracellularly to block toxin A interaction with cells (in contrast to the need to remain associated with toxin to prevent intracellular glucosyltransferase activity), we chose CDA1 as our lead candidate anti-toxin A HuMAb.
Fewer methods exist for assessing the neutralization of toxin B. The IMR-90 cytotoxicity assay with toxin B is the lone in vitro procedure that can be performed. Our four anti-toxin B HuMAbs, MDX1388, 1G10, 103-174, and 2A11, all had the capacity to neutralize toxin B in this assay, albeit at very different concentrations. MDX1388 had the most potent neutralizing activity, with a half-maximal activity level of 0.1 nM. It was demonstrated that three of the HuMAbs interacted with the receptor-binding domain of toxin B (2A11, 1G10, and MDX1388), whereas one, 103-174, bound to the enzymatic domain, suggesting that these two groups neutralize different functional activities of toxin B.
Interestingly, none of the four toxin B-specific HuMAbs could protect mice from a lethal challenge with toxin B. In in vitro assays using IMR-90 fibroblasts, toxin B is much more cytotoxic than toxin A (∼1,000 times) and this challenge (100 ng) in mice may be too severe for our monoclonal antibodies to provide protection. Unfortunately, this level of toxin B is required to induce consistent lethality in the model. It is important to note, however, that goat anti-toxin B polyclonal antibody could protect mice from toxin B challenge. The potency of the goat serum against toxin B in the in vitro cytotoxicity assay is much greater than that of MDX1388, and careful comparisons based on in vitro neutralization data could not be made in the mouse model. Perhaps the optimal neutralization of toxin B in the in vivo mouse intoxification model depends on the presence of more than one epitope specificity. To address this point, we used a cocktail of all four toxin B-reactive HuMAbs in the mouse model yet still saw no protection from mortality (data not shown). MDX1388 was chosen as our lead candidate anti-toxin B HuMAb due to its potent in vitro neutralizing activity and its presumed capacity to block the interaction of toxin B with cellular receptors.
Because the hamster provides a well-characterized model of C. difficile infection, we chose this animal model to test our lead antitoxin HuMAbs in both the standard primary disease and relapse models. Hamsters treated with antibiotics to disrupt colonic flora and then exposed to C. difficile spores succumb to diarrheal disease in less than 48 h. Treatment of hamsters with a combination of HuMAbs CDA1 and MDX1388 offered protection from mortality both in the primary disease model and in the relapse model of infection. In the primary disease model, which consistently demonstrates 100% mortality, the combination treatment protected 55% of challenged hamsters from death associated with C. difficile. In the relapse model, with various mortality rates, significant protection was also observed. These data demonstrate that the combination treatment of CDA1 and MDX1388 provides benefit for untreated hamsters as well as for hamsters receiving vancomycin as a therapeutic. Since these HuMAbs demonstrated protection in such a severe disease model, we speculate that a combination HuMAb cocktail of CDA1 and MDX1388 could also be a useful therapeutic either standing alone or in combination with antibiotics for the treatment of CDAD in humans.
Studies have suggested that C. difficile disease outcome in humans is correlated with circulating titers of toxin A-directed antibody. Once colonized with C. difficile, individuals with high levels of serum IgG against toxin A developed disease at a rate lower than that seen for controls with low anti-toxin A serum titers. Also, anti-toxin A antibody titers correlated with protection from relapse. In our hamster primary disease and relapse models, CDA1 treatment alone delayed the onset of mortality and disease symptoms but did not protect from mortality by the culmination of the experiments. Given that the outcome of CDAD in humans in much less severe than that in the hamster, it is also possible that CDA1 treatment, in the absence of antibody to toxin B, may still provide some benefit to humans with CDAD.
. We noted that protection of hamsters from C. difficile disease required a very high dose of CDA1 (50 mg/kg/day for 4 days). However, circulating titers of CDA1 were measured in some hamsters treated with the above-described regimen, and surprisingly, serum titers were very similar to those that have been seen for human volunteers receiving a single dose of 10 mg/kg (data not shown). Given the administration of such a high dose, it is unclear why the serum titers achieved in the hamsters are so low. In fact, we have found that approximately 10% of all hamsters tested have no measurable titer of HuMAb in the circulation even after a total of 200 mg of antibody is administered intraperitoneally (for both CDA1 and other HuMAbs tested) (unpublished data). Lower-than-anticipated titers in the hamster sera could be the result of the inefficient transport of human antibodies from the peritoneum into the bloodstream. It is also possible that some hamsters generate an immune response to the human antibody and clear it quickly from the circulation. Given the measured levels of circulating protective titers in the hamster sera, we believe a single dose of 10 mg/kg of CDA1 may be beneficial in the treatment of CDAD in humans and achieve serum levels conferring both therapeutic activity and protection from relapse.
Based on the data presented herein, CDA1 and MDX1388 have been chosen for testing in clinical trials for treatment of CDAD or prevention of relapses. Phase I clinical trials of CDA1 in healthy subjects have been completed, and phase II clinical trials are currently being conducted. Phase I trials with the combination of CDA1 and MDX1388 have been initiated, with a phase II trial of combination therapy planned to begin soon. Results of phase II trials will determine if single or combination therapy will be studied in a phase III efficacy trial.
Acknowledgments
We thank Susan Sloan for insightful scientific discussions. We also thank Daniel Carraher, Jennifer Coccia, Diana Esshaki, Christina Hector, Amy Hill, and Heather Walker for technical assistance.
Editor: D. L. Burns
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Bartlett, J. G. 1981. Antimicrobial agents implicated in Clostridium difficile toxin-associated diarrhea of colitis. Johns Hopkins Med. J. 149:6-9. [PubMed] [Google Scholar]
- 2.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334-339. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., T. Chang, N. S. Taylor, and A. B. Onderdonk. 1979. Colitis induced by Clostridium difficile. Rev. Infect. Dis. 1:370-378. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, J. G., T. W. Chang, M. Gurwith, S. L. Gorbach, and A. B. Onderdonk. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298:531-534. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett, J. G., A. B. Onderdonk, R. L. Cisneros, and D. L. Kasper. 1977. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 136:701-705. [DOI] [PubMed] [Google Scholar]
- 6.Clark, G. F., H. C. Krivan, T. D. Wilkins, and D. F. Smith. 1987. Toxin A from Clostridium difficile binds to rabbit erythrocyte glycolipids with terminal Gal alpha 1-3Gal beta 1-4GlcNAc sequences. Arch. Biochem. Biophys. 257:217-229. [DOI] [PubMed] [Google Scholar]
- 7.Corthier, G., M. C. Muller, T. D. Wilkins, D. Lyerly, and R. L'Haridon. 1991. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect. Immun. 59:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallal, R. M., B. G. Harbrecht, A. J. Boujoukas, C. A. Sirio, L. M. Farkas, K. K. Lee, and R. L. Simmons. 2002. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann. Surg. 235:363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fekety, R., et al. 1997. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am. J. Gastroenterol. 92:739-750. [PubMed] [Google Scholar]
- 10.Fernie, D. S., R. O. Thomson, I. Batty, and P. D. Walker. 1983. Active and passive immunization to protect against antibiotic associated caecitis in hamsters. Dev. Biol. Stand. 53:325-332. [PubMed] [Google Scholar]
- 11.Genth, H., J. Selzer, C. Busch, J. Dumbach, F. Hofmann, K. Aktories, and I. Just. 2000. New method to generate enzymatically deficient Clostridium difficile toxin B as an antigen for immunization. Infect. Immun. 68:1094-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianfrilli, P., I. Luzzi, A. Pantosti, M. Occhionero, G. Gentile, and G. Panichi. 1984. Cytotoxin and enterotoxin production by Clostridium difficile. Microbiologica 7:375-379. [PubMed] [Google Scholar]
- 13.Giannasca, P. J., Z. X. Zhang, W. D. Lei, J. A. Boden, M. A. Giel, T. P. Monath, and W. D. Thomas, Jr. 1999. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect. Immun. 67:527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, F., C. Busch, U. Prepens, I. Just, and K. Aktories. 1997. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 272:11074-11078. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, S., C. R. Clabots, F. V. Linn, M. M. Olson, L. R. Peterson, and D. N. Gerding. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97-100. [DOI] [PubMed] [Google Scholar]
- 16.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 17.Just, I., M. Wilm, J. Selzer, G. Rex, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270:13932-13936. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, C. P., C. Pothoulakis, F. Vavva, I. Castagliuolo, E. F. Bostwick, J. C. O'Keane, S. Keates, and J. T. LaMont. 1996. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob. Agents Chemother. 40:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, P. H., J. P. Iaconis, and R. D. Rolfe. 1987. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect. Immun. 55:2984-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kink, J. A., and J. A. Williams. 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 66:2018-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyne, L., R. J. Farrell, and C. P. Kelly. 2001. Clostridium difficile. Gastroenterol. Clin. N. Am. 30:753-777, ix-x. [DOI] [PubMed] [Google Scholar]
- 22.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189-193. [DOI] [PubMed] [Google Scholar]
- 23.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390-397. [DOI] [PubMed] [Google Scholar]
- 24.Leung, D. Y., C. P. Kelly, M. Boguniewicz, C. Pothoulakis, J. T. LaMont, and A. Flores. 1991. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J. Pediatr. 118:633-637. [DOI] [PubMed] [Google Scholar]
- 25.Lyerly, D. M., E. F. Bostwick, S. B. Binion, and T. D. Wilkins. 1991. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect. Immun. 59:2215-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [Epub December 1, 2005.] [DOI] [PubMed] [Google Scholar]
- 27.McEllistrem, M. C., R. J. Carman, D. N. Gerding, C. W. Genheimer, and L. Zheng. 2005. A hospital outbreak of Clostridium difficile disease associated with isolates carrying binary toxin genes. Clin. Infect. Dis. 40:265-272. Epub 2004 Dec 15. [DOI] [PubMed] [Google Scholar]
- 28.McFarland, L. V., M. E. Mulligan, R. Y. Kwok, and W. Stamm. 1989. Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320:204-210. (Comment, 321:190.) [DOI] [PubMed] [Google Scholar]
- 29.Muto, C. A., M. Pokrywka, K. Shutt, A. B. Mendelsohn, K. Nouri, K. Posey, T. Roberts, K. Croyle, S. Krystofiak, S. Patel-Brown, A. W. Pasculle, D. L. Paterson, M. Saul, and L. H. Harrison. 2005. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 26:273-280. [DOI] [PubMed] [Google Scholar]
- 30.Riley, T. V., J. P. Codde, and I. L. Rouse. 1995. Increased length of hospital stay due to Clostridium difficile associated diarrhoea. Lancet 345:455-456. [DOI] [PubMed] [Google Scholar]
- 31.Riley, T. V., G. L. O'Neill, R. A. Bowman, and C. L. Golledge. 1994. Clostridium difficile-associated diarrhoea: epidemiological data from Western Australia. Epidemiol. Infect. 113:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salcedo, J., S. Keates, C. Pothoulakis, M. Warny, I. Castagliuolo, J. T. LaMont, and C. P. Kelly. 1997. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut 41:366-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sougioultzis, S., L. Kyne, D. Drudy, S. Keates, S. Maroo, C. Pothoulakis, P. J. Giannasca, C. K. Lee, M. Warny, T. P. Monath, and C. P. Kelly. 2005. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology 128:764-770. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, N. S., and J. G. Bartlett. 1979. Partial purification and characterization of a cytotoxin from Clostridium difficile. Rev. Infect. Dis. 1:379-385. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, N. S., G. M. Thorne, and J. G. Bartlett. 1981. Comparison of two toxins produced by Clostridium difficile. Infect. Immun. 34:1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres, J. F., D. M. Lyerly, J. E. Hill, and T. P. Monath. 1995. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect. Immun. 63:4619-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triadafilopoulos, G., C. Pothoulakis, M. J. O'Brien, and J. T. LaMont. 1987. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology 93:273-279. [DOI] [PubMed] [Google Scholar]
- 38.von Eichel-Streiber, C., R. Laufenberg-Feldmann, S. Sartingen, J. Schulze, and M. Sauerborn. 1992. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol. Gen. Genet. 233:260-268. [DOI] [PubMed] [Google Scholar]
- 39.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilcox, M. H. 2004. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J. Antimicrob. Chemother. 53:882-884. [DOI] [PubMed] [Google Scholar]