Abstract
The human pathogenic fungus Cryptococcus neoformans can form biofilms on polystyrene plates and medical devices in a process that requires capsular polysaccharide release. Although biofilms are known to be less susceptible to antimicrobial drugs, little is known about their susceptibility to antimicrobial molecules produced by the innate immune system. In this study, we investigated the susceptibility of C. neoformans cells in biofilm and planktonic states to oxidative and nonoxidative antimicrobial molecules produced by phagocytic cells. The effects of various immune effector molecules on the fungal mass, metabolic activity, and architecture of C. neoformans biofilms were measured by colony counts, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide reduction, and confocal microscopy, respectively. Biofilms were more resistant than planktonic cells to oxidative stress but remained vulnerable to cationic antimicrobial peptides. However, melanized biofilms were significantly less susceptible to antimicrobial peptides than nonmelanized biofilms. These results suggest that the biofilm phenotype increases resistance against host immune mechanisms, a phenomenon that could contribute to the ability of biofilm-forming microbes to establish persistent infections.
Cryptococcus neoformans is an encapsulated opportunistic yeast-like fungus that preferentially invades the brain of immunocompromised individuals, causing life-threatening meningoencephalitis. Glucuronoxylomannan (GXM) is the major component of the capsular polysaccharide. GXM is released during cryptococcal infection and accumulates in tissue, where it is believed to mediate many detrimental effects on the immune system (3, 25). GXM release was recently associated with the phenomenon of cryptococcal biofilm formation (13).
C. neoformans can form biofilms on polystyrene plates (13, 24) and medical devices after GXM release. For instance, Walsh et al. reported that C. neoformans could form biofilms in ventriculoatrial shunt catheters (27). The increasing use of ventriculoperitoneal shunts to manage intracranial hypertension associated with cryptococcal meningoencephalitis highlights the importance of investigating the biofilm-forming properties of this organism (2). Moreover, biofilm formation has profound consequences in the establishment of fungal infection and is associated with persistent infection since biofilms increase resistance to host immune mechanisms and antimicrobial therapy (7). For instance, we recently showed that C. neoformans biofilms were significantly more resistant to amphotericin B and caspofungin (14).
The efficacy of antimicrobial host defenses can be attributed to the ability of the immune system to recognize microbial invaders and reduce host damage. The innate immune system has multiple mechanisms to provide a layered defense against pathogenic microbes. For instance, phagocytic cells such as macrophages and neutrophils, respond to any microbial invasion by engulfing microbes and generating microbicidal reactive oxygen-, nitrogen-, and chlorine-derived oxidants (17). These phagocytic cells also possess nonoxidative antimicrobial mechanisms such as defensins, antimicrobial peptides that can kill a variety of microorganisms (21).
We have previously shown that specific antibodies to C. neoformans GXM inhibited biofilm formation by interference with capsular polysaccharide release from the fungal cell (13). In contrast, lactoferrin, an effector molecule of innate immune mechanisms, did not prevent fungal biofilm formation despite its efficacy against bacterial biofilms (13, 22). Apart from the studies with lactoferrin, the effect of other innate immune effector molecules on the susceptibility of microbial biofilms has not been investigated. Consequently, we investigated the susceptibility of cryptococcal biofilms to oxidative and nonoxidative antimicrobial molecules produced by the innate immune system. The results indicate that C. neoformans cells in biofilms are significantly less susceptible to oxidants than planktonic cells but remain vulnerable to microbicidal peptides. Understanding of the mechanisms of host immunity evasion by microorganisms is necessary for the development of novel strategies to combat biofilm-related diseases and to acquire more knowledge about C. neoformans biofilms biology.
MATERIALS AND METHODS
C. neoformans.
C. neoformans strain B3501 (serotypes D) was acquired from the American Type Culture Collection (Rockville, MD) and used in the course of the present study. This strain was selected because it forms strong biofilms (13). Yeasts were grown in Sabouraud dextrose broth (Difco Laboratories, Detroit, Mich.) for 24 h at 30°C in a rotary shaker at 150 rpm (to early stationary phase).
Biofilm formation.
C. neoformans cells were collected by centrifugation, washed twice with phosphate-buffered saline (PBS), counted by using a hemacytometer, and suspended at 107 cells/ml in minimal medium (20 mg of thiamine/ml, 30 mM glucose, 26 mM glycine, 20 mM MgSO4 · 7H2O, 58.8 mM KH2PO4). Then, 100 μl of the suspension was added into individual wells of polystyrene 96-well plates (Fisher, Massachusetts) and incubated at 37°C without shaking. Biofilms were formed for 48 h. After the adhesion stage, the wells containing C. neoformans biofilms were washed three times with 0.05% Tween 20 in Tris-buffered saline to remove nonadhered cryptococcal cells using a microtiter plate washer (Skan Washer 400; Molecular Devices, Virginia). Fungal cells that remained attached to the plastic surface were considered true biofilms.
Measurement of biofilm metabolic activity by the XTT reduction assay and CFU counting.
A semiquantitative measurement of C. neoformans biofilm formation was obtained from the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay. For C. neoformans strains, 50 μl of XTT salt solution (1 mg/ml in PBS) and 4 μl of menadione solution (1 mM in acetone; Sigma) were added to each well. Microtiter plates were incubated at 37°C for 5 h. Fungal mitochondrial dehydrogenase activity reduces XTT tetrazolium salt to XTT formazan, resulting in a colorimetric change that correlates with cell viability (16). The colorimetric change was measured by using a microtiter reader (Labsystem Multiskan MS, Finland) at 492 nm. The percentage of metabolic activity was determined by measuring the optical density of biofilms and planktonic cells exposed to antimicrobial molecules relative to unexposed biofilms or planktonic cells. In all of the experiments, microtiter wells containing heat-killed C. neoformans, minimal medium alone, or minimal medium and the antimicrobial molecules but not C. neoformans cells were included as negative controls.
To determine the density of C. neoformans planktonic cells used for comparison to biofilms, we estimated the cell numbers from the XTT reduction signal by using a dose-response curve. Briefly, cells of C. neoformans B3501 were grown in minimal medium for 48 h 30°C in a rotary shaker at 150 rpm, collected by centrifugation, washed twice with PBS, counted by using a hemacytometer, and suspended at various densities (5 × 106, 1 × 107, and 5 × 107 cells/ml) in minimal medium. Then, 100 μl of each suspension was added into individual wells of polystyrene 96-well plates to final densities of 5 × 105, 1 × 106, and 5 × 106 cells/ml. The viability was measured by XTT reduction.
The effect of antimicrobial molecules for C. neoformans biofilms and planktonic cells was compared by the CFU killing assay. After incubation with antimicrobial molecules, C. neoformans biofilms were scraped from the bottom of the wells with a sterile 200-μl micropipette tip to dissociate yeast cells. A volume of 100 μl of suspension containing dissociated cells was aspirated from the wells, transferred to an Eppendorf tube with 900 μl of PBS, and vortexed gently for 3 min. Serial dilutions were then performed, and 100 μl of diluted suspension was plated on Sabouraud dextrose agar plates. The percentage of CFU survival was determined by comparing C. neoformans biofilms and planktonic cells treated with antimicrobial molecules relative to untreated fungal cells.
Melanized fungal biofilms.
Melanization was induced by growing the biofilms on defined minimal medium broth with 1 mM l-DOPA (l-3,4-dihydroxyphenylalanine) for 7 days. Nonmelanized biofilm controls were obtained by growing the yeast cells on defined minimal medium broth without l-DOPA for 7 days.
Susceptibility of C. neoformans biofilms to defensins. (i) Comparison of biofilm and planktonic cryptococcal cell susceptibility to defensins.
The defensins used are described in Table 1. PG-1 was acquired from R. Lehrer (Los Angeles, CA). This peptide is known to be fungicidal to C. neoformans (10). α-Defensin-3, β-defensin-1, β-defensin-3, and magainin-1 were obtained from Peptides International (Louisville, KY). Susceptibility to defensins was determined by exposing C. neoformans biofilms and planktonic cells to various concentrations of these peptides (2, 4, or 8 μM) for 30 min at 37°C in microtiter plates. After treatment, the metabolic activity and fungal mass of biofilms and planktonic cells were measured by using the XTT reduction and CFU killing assays.
TABLE 1.
Description of the peptides used against C. neoformans B3501 biofilms
| Peptide | Source | Mass units | Sequence | Charge |
|---|---|---|---|---|
| PG-1 | Porcine leukocytes | 2,155 | RGGRLCYCRRRFCVCVGR | +6 |
| α-Defensin-3 | Human neutrophils | 3,442 | DCYCRIPACIAGERRYGTCIYQGRLWAFCC | +2 |
| β-Defensin-1 | Human plasma | 3,928.6 | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | +7 |
| β-Defensin-3 | Human epithelial cells | 5,155.1 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | +11 |
| Magainin-1 | Frog, Xenopus laevis | 2,409.8 | GIGKFLHSAGKFGKAFVGEIMKS | +4 |
(ii) Comparison of melanized and nonmelanized fungal biofilm susceptibilities to PG-1.
C. neoformans biofilms were incubated with 200 μl of PBS containing PG-1 (2, 4, or 8 μM). Melanized or nonmelanized biofilms and PG-1 were mixed to ensure a uniform distribution and were incubated at 37°C for 30 min. Wells containing melanized and nonmelanized biofilms treated with PBS alone were used as a control. The XTT reduction assay was used to determine viability.
Susceptibility of C. neoformans biofilms to antimicrobial molecules. (i) Oxygen-derived oxidant assays.
Oxygen-derived oxidants were generated by using the epinephrine oxidative system (EOS) described by Polacheck et al. (19). In this system, epinephrine serves as an electron donor, Fe3+ is a transition metal catalyst, and H2O2 serves as an electron acceptor; the reaction of these elements results in the production of oxygen-derived free radicals. Briefly, ferric ammonium sulfate (Sigma), H2O2 (Sigma), and epinephrine bitartrate (Sigma) were mixed in that order to final concentrations of 0.5 mM, 0.00021%, and 1.0 mM, respectively. C. neoformans biofilms and planktonic cells were exposed to the products of the EOS in 96-well microtiter plates at 37°C. After various time intervals (0.5 and 1 h) of exposure to the EOS, the viability of biofilms and planktonic cells was measured by XTT reduction and CFU killing assays.
(ii) Nitric oxide assays.
Nitric oxide (NO·) and reactive nitrogen intermediates were generated in a solution of 0.5 mM NaNO2 (Sigma, St. Louis, MO) and 25 mM succinic acid (pH 4; Sigma) as previously described (1). In acidic solutions, nitrite salts react with H+ to produce NO· and a variety of reactive nitrogen species with fungicidal activity against C. neoformans. C. neoformans biofilms and planktonic cells were exposed to chemically generated NO· for various time intervals (2 and 4 h) at 37°C in 96-well microtiter plates. XTT reduction and CFU killing assays were utilized to determine the metabolic activity and fungal mass, respectively.
(iii) Hypochlorite assays.
C. neoformans biofilms and planktonic cells were exposed to 0.0001 mM NaOCl for 30 or 60 min at 37°C. After NaOCl exposure, the metabolic activity and fungal mass of biofilms and planktonic cells were measured.
Confocal microscopy.
C. neoformans biofilms were incubated for 45 min at 37°C in 75 μl of PBS containing the fluorescent stains FUN-1 (10 μM) and concanavalin A-Alexa Fluor 488 conjugate (ConA; 25 μM) (Molecular Probes, Eugene, OR). FUN-1 (excitation wavelength, 470 nm; emission wavelength, 590 nm) is converted to orange-red cylindrical intravacuolar structures by metabolically active cells, whereas ConA (excitation wavelength, 488 nm; emission wavelength, 505 nm) binds to glycan residues of mannoproteins in capsular polysaccharide and fluoresces green (12). Microscopic examinations of biofilms formed in microtiter plates were performed with confocal microscopy using an Axiovert 200 M inverted microscope (Carl Zeiss MicroImaging, NY). The objective used was a ×40 (numerical aperture of 0.6). Depth measurements were taken at regular intervals across the width of the device. To determine the structure of the biofilms, a series of horizontal (xy) optical sections with a thickness of 1.175 μm were taken throughout the full length of the biofilm. Confocal images of green (ConA) and red (FUN-1) fluorescence were recorded simultaneously using a multichannel mode. Z-stack images and measurements were corrected by utilizing Axio Vision 4.4 software-deconvolution mode (Carl Zeiss MicroImaging, New York).
Statistical analysis.
All data were subjected to statistical analysis using Origin 7.0 (Origin Lab Corp., Northampton, MA). P values were calculated by the Student t test. P values of <0.05 were considered significant.
RESULTS
C. neoformans cells in biofilms are less susceptible than planktonic cells to defensins.
Defensins are peptidic products of the innate immune system that play an important role in the host defense properties of granulocytic leukocytes, mucosal surfaces, skin, and other epithelia (21). C. neoformans B3501 biofilms and planktonic cells were incubated with different doses of PG-1, α-defensin-3, β-defensin-1, β-defensin-3, and magainin-1 or without it. C. neoformans biofilms were significantly less susceptible to the antimicrobial activity of PG-1, β-defensin-1 and β-defensin-3 than their planktonic counterparts as measured by XTT reduction and CFU killing assays (Table 2). However, after 30 min we observed that PG-1, β-defensin-1, and β-defensin-3 demonstrated significant activity in reducing the metabolic activity of both biofilm and planktonic cells. For instance, the metabolic activity of cryptococcal biofilms was reduced by approximately only 50% when biofilms were treated with 8 μM PG-1. In contrast, the metabolic activity of planktonic cells was significantly reduced after treatment with 2 μM PG-1. The fungal mass of planktonic cryptococci decreased significantly relative to biofilms after incubation of the fungal cells in the presence of PG-1, β-defensin-1, and β-defensin-3. Conversely, α-defensin-3 and magainin-1 did not significantly damage cryptococcal cells.
TABLE 2.
Susceptibility of C. neoformans B3501 biofilms to defensins
| Defensin (concn [μM]) | % Metabolic activity (SD)a
|
% Survival (SD)b
|
||
|---|---|---|---|---|
| Biofilm | Planktonic | Biofilm | Planktonic | |
| PG-1 (2) | 63 (0.2) | 47 (0.5)* | 72 (1.8) | 60 (1)* |
| PG-1 (4) | 59 (0.2) | 38 (0.3)* | 70 (1.6) | 35 (0.7)* |
| PG-1 (8) | 52 (0.2) | 20 (0.1)* | 59 (1.2) | 2 (0.2)* |
| α-Defensin-3 (2) | 100 (0.4) | 97 (0.7) | 88 (1.7) | 83 (0.8) |
| α-Defensin-3 (4) | 100 (0.1) | 94 (1.1) | 76 (0.4) | 76 (0.9) |
| α-Defensin-3 (8) | 98 (1) | 92 (3.2) | 80 (1.2) | 72 (0.8) |
| β-Defensin-1 (2) | 89 (0.2) | 84 (1.5) | 88 (1) | 88 (0.8) |
| β-Defensin-1 (4) | 62 (0.5) | 45 (0.4)* | 58 (0.1) | 47 (0.7)* |
| β-Defensin-1 (8) | 51 (0.3) | 22 (1.3)* | 40 (0.7) | 27 (3)* |
| β-Defensin-3 (2) | 87 (1.8) | 88 (0.2) | 89 (3) | 88 (2) |
| β-Defensin-3 (4) | 76 (0.9) | 63 (0.1)* | 75 (0.5) | 65 (0.1)* |
| β-Defensin-3 (8) | 71 (0.5) | 38 (0.2)* | 72 (2) | 27 (1.4)* |
| Magainin-1 (2) | 100 (0.2) | 99 (0.4) | 99 (0.6) | 98 (1.2) |
| Magainin-1 (4) | 100 (0.7) | 94 (0.3) | 99 (0.5) | 98 (0.3) |
| Magainin-1 (8) | 98 (1) | 92 (1.3) | 97 (1) | 93 (1.5) |
The metabolic activity of age-matched biofilms and planktonic cells was measured by using the XTT reduction assay. The values are the averages of three XTT measurements. Asterisks indicate P value significance as calculated by the Student t test for the comparison between the biofilm and planktonic susceptibilities.
The percentage of survival of age-matched biofilms and planktonic cells was determined by CFU counts. The values are averages of three CFU counts. Asterisks indicate P value significance as calculated by the Student t test for the comparison between the biofilm and planktonic susceptibilities.
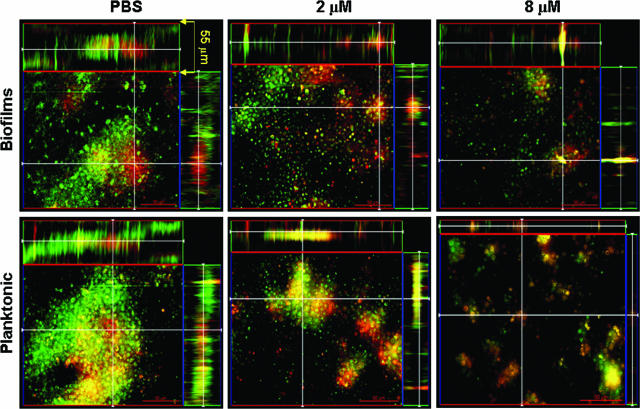
Confocal microscopic examination was used to correlate the XTT reduction and CFU killing assay results with visual effects on biofilm architecture. Regions of red fluorescence (FUN-1) represent metabolically active cells; the green fluorescence (ConA) labels the biofilm matrix, possibly by binding to mannoproteins in capsular polysaccharide; and yellow-brownish areas represent metabolically inactive or nonviable cells. C. neoformans biofilms and planktonic cells manifested regions of high metabolic activity in the absence of PG-1 (Fig. 1). Biofilms treated with 2 μM PG-1 showed distinguishable viable and nonviable cells with partial disruption and decreased in thickness of the exopolymeric matrix. Biofilms treated with 8 μM PG-1 showed a monolayer arrangement of clustered metabolically active cells and disruption of the exopolymeric architecture. Planktonic cells treated with 2 and 8 μM PG-1 were severely damaged, manifesting low metabolic activity and a lack of capsular polysaccharide.
FIG. 1.
C. neoformans biofilms are less susceptible than planktonic cells to the antimicrobial activity of defensins. Confocal microscopy of C. neoformans B3501 biofilms and planktonic cells exposed to PG-1 was performed. Orthogonal images of mature C. neoformans biofilms and planktonic cells showed metabolically active (red, FUN-1-stained) cells embedded in the polysaccharide extracellular material (green, ConA stained), whereas the yellowish brown areas represent metabolically inactive or nonviable cells. Images were obtained after 30 min of exposure of fungal cells to PG-1 and compared to yeast cells incubated in the presence of PBS. Pictures were taken using a ×40 power field. Scale bar, 50 μm.
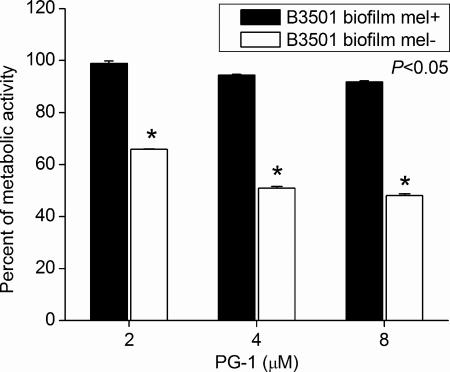
Moreover, melanized and nonmelanized C. neoformans strain B3501 biofilms were compared to examine whether melanin production enhance biofilm resistance to PG-1. Melanized cryptococcal biofilms were significantly less susceptible to PG-1 than nonmelanized biofilms (Fig. 2). For example, the metabolic activity of nonmelanized biofilms was significantly reduced 35% after treatment with 2 μM PG-1.
FIG. 2.
Melanized C. neoformans biofilms were less susceptible to PG-1. Percentage of metabolic activity of melanized and nonmelanized C. neoformans strain B3501 biofilms as measured by the XTT reduction assay. The cells in biofilms were exposed to various concentrations (2, 4, or 8 μM) of PG-1 for 30 min, and their metabolic activities were compared to those of fungal cells incubated in PBS.
C. neoformans cells in biofilms are less susceptible than planktonic cells to oxidative stress.
To compare the susceptibility of fungal cells in mature biofilms and in suspension to oxidative stress, cryptococcal cells were incubated with chemically generated oxygen-, nitrogen-, and chlorine-derived species. The metabolic activity and fungal mass were determined by using the XTT reduction and CFU killing assays, respectively. Oxygen-derived oxidants produced in the EOS (19) are similar to some products of the oxidative burst of immune effector cells (9). C. neoformans cells in biofilms were less susceptible to oxidative stress than planktonic cells as measured by the XTT reduction and CFU killing assays. In both assays, planktonic cells displayed significant reduction in viability (∼50%) and fungal mass (∼90%) after 30 min of treatment with reactive oxygen species (Table 3).
TABLE 3.
Susceptibility of C. neoformans B3501 biofilms to oxidative activity of antimicrobial molecules produced by innate immunity
| Antimicrobial molecule (treatment time [h]) | % Metabolic activity (SD)a
|
% Survival (SD)b
|
||
|---|---|---|---|---|
| Biofilm | Planktonic | Biofilm | Planktonic | |
| EOS (0.5) | 71 (0.8) | 51 (2.3)* | 62 (0.2) | 8 (0.4)* |
| EOS (1) | 63 (0.6) | 37 (0.5)* | 42 (0.4) | 1 (0.2)* |
| NOS (2) | 66 (1.1) | 40 (0.9)* | 92 (0.5) | 68 (1.8)* |
| NOS (4) | 55 (0.7) | 21 (0.2)* | 63 (1.2) | 34 (3.2)* |
| NaOCl (0.5) | 97 (0.8) | 39 (0.2)* | 100 (3.5) | 76 (2)* |
| NaOCl (1) | 88 (1.7) | 19 (0.8)* | 100 (4.2) | 39 (0.4)* |
The metabolic activity of age-matched biofilms and planktonic cells was measured by using the XTT reduction assay. The values are the averages of three XTT measurements. Asterisks indicate P value significance as calculated by the Student t test for the comparison between the biofilm and planktonic susceptibilities.
The percentage of survival of age-matched biofilms and planktonic cells was determined by CFU counts. The values are the averages of three CFU counts. Asterisks indicate P value significance as calculated by the Student t test for the comparison between the biofilm and planktonic susceptibilities.
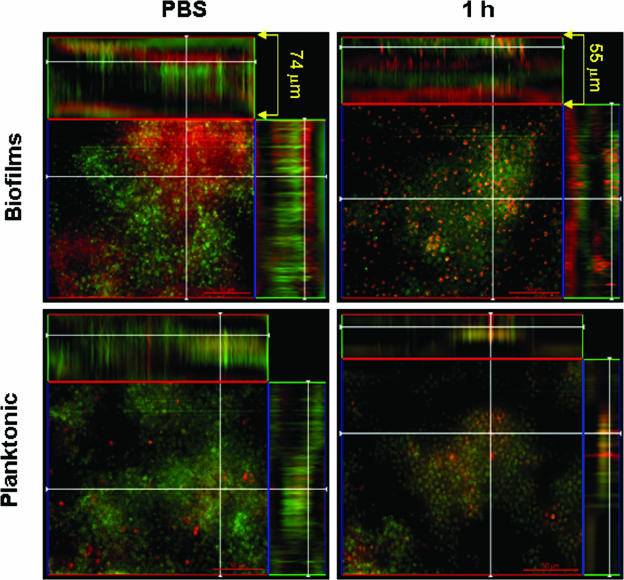
Confocal microscopic examination of planktonic cells and biofilms incubated with EOS products revealed regions of high metabolic activity (Fig. 3). After 1 h, biofilms exposed to EOS showed distinguishable viable and nonviable cells due to partial disruption and decreased in thickness of the exopolymeric matrix from 74 to 55 μm compared to untreated biofilms. Planktonic cells exposed to oxidative stress for a similar period of time appeared to be damaged, with decreased metabolic activity, compared to untreated fungal cells.
FIG. 3.
C. neoformans biofilms are less susceptible than planktonic cells to oxygen-derived oxidants generated in the EOS. Confocal microscopy of C. neoformans B3501 biofilms and planktonic cells exposed to oxygen-derived oxidants was performed. Orthogonal images of mature C. neoformans biofilms and planktonic cells showed metabolically active (red, FUN-1-stained) cells embedded in the polysaccharide extracellular material (green, ConA stained), whereas the yellowish brown areas represent metabolically inactive or nonviable cells. Images were obtained after 1 h of exposure of fungal cells to EOS and compared to yeast cells incubated in the presence of PBS. Pictures were taken using a ×40 power field. Scale bar, 50 μm.
Nitric oxide and related products produced by murine immune effector cells are fungistatic and fungicidal for C. neoformans (17), depending on their concentration. C. neoformans biofilms were significantly less susceptible to nitrosative stress than planktonic cells when viability was measured by the XTT reduction assay (Table 3). Similar results were observed when the experiment was repeated using the CFU count as the indication for fungal cell survival.
Leukocytes can kill pathogenic microbes with a flux of chlorine-derived oxidants (6). Hence, the susceptibility of C. neoformans biofilms to oxidation by hypochlorite was investigated. Biofilms displayed significantly greater metabolic activity than planktonic cells after incubation in 0.0001 mM hypochlorite (Table 3). A reduction to 60% in the metabolic activity and fungal mass of C. neoformans planktonic cells was observed after 0.5 and 1 h of exposure to hypochlorite, respectively. In contrast, cryptococcal biofilms were not significantly affected by chlorine oxidation.
DISCUSSION
Despite intense interest in microbial biofilms in recent years, there is a dearth of information on the activity of innate immune system antimicrobial molecules against cells in biofilms. Singh et al. had demonstrated that lactoferrin could prevent the development of bacterial biofilms (22), but this protein was ineffective against cryptococcal biofilms (13). Lactoferrin alone or in combination with lysozymes was not effective in decreasing the viability of mature cryptococcal biofilms (15). Jesaitis et al. reported that human neutrophils failed to eliminate Pseudomonas aeruginosa biofilms, possibly as a consequence of not generating an effective oxidative response (8). Therefore, we assessed the susceptibility of C. neoformans cells in biofilm and planktonic states to oxygen-, nitrogen-, and chlorine-derived oxidants such as those produced by the phagocytic cells of innate immune system. C. neoformans biofilms were consistently less susceptible than their planktonic counterparts to the microbicidal effects of chemically generated oxidants.
Fungal biofilms have a complex and well-organized architecture (4, 13). The biofilm state provides microbial cells an advantage in vivo because the exopolymeric matrix impedes the direct interaction of phagocytic cells with fungal cells inside of the biofilm. The presence of internal channels may allow accessibility for phagocytic cells and their effector molecules without affecting yeast cells surrounded by the exopolymeric matrix. Consequently, cells in a biofilm are protected against phagocytosis and are relatively resistant to host immune mechanisms (11). For instance, Walker et al. have recently shown that when the host fails to eradicate the infection, neutrophils can undergo necrosis, serving as a biological matrix that may further facilitate microbial biofilm formation (26).
Microorganisms in the environment are often found in biofilms attached to surfaces (20) to prevent predation by other organisms. It has been proposed that C. neoformans virulence factors for mammals such as the capsule and melanin formation are a result of environmental selection pressures that include predation by phagocytic microorganisms such as amoebae and slime mold (23). The ability of microbes to communicate, form biofilms, and resist oxidative attack by the cells of innate immunity during infection establishment may help explain the mechanisms by which fungal pathogens establish persistent infections with a propensity for latency.
Defensins are antimicrobial peptides found in many animal species that contribute to host defense against bacterial, fungal, and viral infections (21). In humans, they are produced by cells of the skin and mucous membranes of the respiratory, genitourinary, and gastrointestinal tracts. The secondary structure of these cationic peptides is usually composed of both a hydrophobic surface and a hydrophilic surface. This amphipathic structural feature is believed to play a key role in the antimicrobial mechanism of action. The hydrophilic property is proposed to initiate peptide interaction with the negatively charged fungal surface and the negatively charged headgroups of bilayer phospholipids. The hydrophobic property would permit the peptides to enter the membrane interior, forming transient channels or pores, leading to the leakage of cell contents and cell death. These peptides are also involved, in addition to permeabilization of the target cell membrane of microorganisms, in a variety of cellular processes, including chemotaxis of inflammatory cells release of histamine from mast cells, opsonization, interaction with complement, and wound repair. In contrast to the various oxidants studied, defensins with higher positive charge such as PG-1, β-defensin-1, and β-defensin-3 significantly reduced the metabolic activity of biofilms. In this regard, the two microbicidal peptides with the lowest positive charges, α-defensin-3 and magainin-1, were significantly less effective against biofilms. This observation suggests that microbicidal peptides retain considerable activity against biofilms and that the net positive charge may be an important variable in their relative efficacy. Given that C. neoformans capsules are highly negatively charged (18) and that the cryptococcal exopolymeric matrix contains the negatively charged polysaccharide glucuronoxylmannan, we speculate that the higher activity of the more positively charged peptides reflects greater affinity for biofilms based on charged interactions. Consistent with prior observations showing that melanized planktonic cells are less susceptible to PG-1 (5), melanized biofilms were significantly less susceptible to the microbicidal peptide.
In conclusion, C. neoformans biofilms were more resistant than planktonic cells to microbicidal oxidants and peptides. Even though our study system relied on chemically generated oxidants and biofilms formed in vitro, these findings provide strong evidence for the reduced efficacy of antimicrobial molecules produced by the innate immune cells. Consequently, the biofilm phenotype may increase microbial resistance to host immune mechanisms by providing a state of reduced susceptibility to host antimicrobial molecules. Nevertheless, our observation that fungal biofilms remained susceptible to certain microbicidal peptides implies a potential usefulness of these compounds in therapy and host defense.
Acknowledgments
This study was supported by National Institute of Health grants AI033142-11, AI033774-11, and HL059842-08.
Editor: J. L. Flynn
REFERENCES
- 1.Alspaugh, J. A., and D. L. Granger. 1991. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect. Immun. 59:2291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach, M. C., P. W. Tally, and E. W. Godofsky. 1997. Use of cerebrospinal fluid shunts in patients having acquired immunodeficiency syndrome with cryptococcal meningitis and uncontrollable intracranial hypertension. Neurosurgery 41:1280-1282. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 4.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doering, T. L., J. D. Nosanchuk, W. K. Roberts, and A. Casadevall. 1998. Melanin as a potential cryptococcal defense against microbicidal proteins. Med. Mycol. 37:175-181. [PubMed] [Google Scholar]
- 6.Gaut, J. P., G. C. Yeh, H. D. Tran, J. Byun, J. P. Henderson, G. M. Richter, M. L. Brennan, A. J. Lusis, A. Belaaouaj, R. S. Hotchkiss, and J. W. Heinecke. 2001. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. USA 98:11961-11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 8.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff, S. J. 1980. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 93:480-489. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer, R. I., and T. Ganz. 1996. Endogenous vertebrate antibiotics: defensins, protegrins, and other cysteine-rick antimicrobial peptides. Ann. N. Y. Acad. Sci. 797:228-239. [DOI] [PubMed] [Google Scholar]
- 11.Leid, J. G., M. E. Shirtliff, J. W. Costerton, and A. P. Stoodley. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70:6339-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour, M. K., L. E. Yauch, J. B. Rottman, and S. M. Levitz. 2004. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infect. Immun. 72:1746-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez, L. R., and A. Casadevall. 2005. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73:6350-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez, L. R., and A. Casadevall. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50:1021-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez, L. R. 2006. Biofilm formation by Cryptococcus neoformans: effect of specific antibody and consequences for drug therapy and immune responses. Ph.D. thesis. Albert Einstein College of Medicine of Yeshiva University, Bronx, New York.
- 16.Meshulam, T., S. M. Levitz, L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 17.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosanchuk, J. D., and A. Casadevall. 1997. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 65:1836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polacheck, I., Y. Platt, and J. Aronovitch. 1990. Catecholamines and virulence of Cryptococcus neoformans. Infect. Immun. 58:2919-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramage, G., S. P. Saville, D. P. Thomas, and J. L. Lopez-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 22.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 23.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theraud, M. B., Y. Guiguen, C., and J.-P. Gangneux. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 53:1013-1018. [DOI] [PubMed] [Google Scholar]
- 25.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 26.Walker, T. S., K. L. Tomlin, G. S. Worthen, K. R. Poch, J. G. Lieber, M. T. Saavedra, M. B. Fessler, K. C. Malcolm, M. L. Vasil, and J. A. Nick. 2005. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect. Immun. 73:3693-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh, T. J., R. Schlegel, M. M. Moody, J. W. Costerton, and M. Salcman. 1986. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery 18:373-375. [DOI] [PubMed] [Google Scholar]