Abstract
The role of dendritic cells (DC) in urinary tract infections (UTI) is unknown. These cells contribute directly to the innate defense against various viral and bacterial infections. Here, we studied their role in UTI using an experimental model induced by transurethral instillation of the uropathogenic Escherichia coli (UPEC) strain 536 into C57BL/6 mice. While few DC were found in the uninfected bladder, many had been recruited after 24 h, mostly to the submucosa and uroepithelium. They expressed markers of activation and maturation and exhibited the CD11b+ F4/80+ CD8− Gr-1− myeloid subtype. Also, tumor necrosis factor alpha (TNF-α)- and inducible nitric oxide synthase (iNOS)-producing CD11bINT DC (Tip-DC) were detected, which recently were proposed to be critical in the defense against bacterial infections. However, Tip-DC-deficient CCR2−/− mice did not show reduced clearance of UPEC from the infected bladder. Moreover, clearance was also unimpaired in CD11c-DTR mice depleted of all DC by injection of diphtheria toxin. This may be explained by the abundance of granulocytes and of iNOS- and TNF-α-producing non-DC that were able to replace Tip-DC functionality. These findings demonstrate that some of the abundant DC recruited in UTI contributed innate immune effector functions, which were, however, dispensable in the microenvironment of the bladder.
Urinary tract infections (UTI), such as cystitis and pyelonephritis, are among the most prevalent infections and account for significant morbidity and medical costs in developed countries (13). Most of these infections are caused by uropathogenic Escherichia coli (UPEC) bearing distinct virulence factors, such as fimbriae and pili that facilitate bacterial attachment to uroepithelial cells, bacterial ascension from the bladder to the kidney, and organ infiltration (23). Various defense mechanisms protect the body from UTI. Uroepithelial cells secrete antimicrobial agents (38) or are exfoliated when infected by UPEC (23). Among the immunocytes involved in the defense against UTI, granulocytes play a crucial role (9). These nonresident cells are attracted by mediators, such as CXCL8 (interleukin-8) (11) and tumor necrosis factor alpha (TNF-α) (20), released from cells that have sensed the presence of UPEC. Sensing could occur by Toll-like receptors (TLR) (40). In particular, TLR4 recognizes lipopolysaccharide in gram-negative bacteria (31), while TLR11 responded to unidentified molecular patterns unique to UPEC (42). Recently, it was shown that TLR4 expression both in uroepithelial cells and in hematopoietic cells within the bladder wall was important for optimal defense against UTI (34). The identity of the TLR4-expressing hematopoietic cells remained unresolved. Possible candidates known to express TLR are macrophages and dendritic cells (DC) (25). In models of bacterial respiratory tract and skin infections, recruitment of both cell types from the circulation critically depended on the chemokine receptor CCR2 (21, 28, 33). The contribution of this receptor to the defense against UTI is unknown. Such a role is supported by a study demonstrating release of the CCR2 ligand, MCP-1 (CCL2), in UTI (10).
DC represent the most effective inducers of adaptive immunity (22). Recent studies have also demonstrated a role of these cells in innate immunity (32). For example, plasmacytoid DC stimulated antiviral defense by secretion of type I interferons (6). In bacterial infections, a newly discovered DC subpopulation termed TNF-α/inducible nitric oxide synthase (iNOS)-producing DC (Tip-DC) has been reported to be critical, as demonstrated in a model of infection with Listeria monocytogenes (36), a gram-positive rod that targets the murine spleen and liver. These cells were absent in CCR2-deficient mice, and the resulting lack of splenic iNOS- and TNF-α-producing cells led to extreme susceptibility to these gram-positive bacteria. Although these two mediators are known to generally be involved in antibacterial defense, their roles in UTI, like those of CCR2 and of Tip-DC, are unknown. In the present study, we have addressed these questions in a murine model of this infection.
MATERIALS AND METHODS
Mice and reagents.
All mice used had been backcrossed >10 times to the C57BL/6 background and were bred and kept under specific-pathogen-free conditions. CCR2−/− ApoE−/− double knockout mice (18, 35) were crossed with C57BL/6 mice to yield CCR2 single knockout mice in the F2 generation. CD11c-DTR mice (14) expressing green fluorescent protein (GFP) and the diphtheria toxin (DT) receptor in CD11c+ cells had been backcrossed 10 times to the C57BL/6 background. To deplete DC, 4 ng DT per gram of body weight was injected intraperitoneally 72 h and 24 h before infection. Animal experiments had been approved by a local animal ethics reviewing board. Unless indicated otherwise, all reagents were obtained from Sigma-Aldrich (Taufkirchen, Germany).
UPEC and UTI model.
The UPEC strain 536 (O6:K15:H31) originated from a UTI patient (1, 3). Tagging of E. coli 536 with a stable fluorescence marker was achieved by λ Red recombinase-mediated chromosomal insertion of a Ptetp/o::gfpmut3.1 fusion into the attB site of the bacteriophage λ (7). The promoterless gfpmut3.1 gene was amplified from pGFPmut3.1 (Clontech) by PCR, using primers gfpfus1 (5′-GAATTAAAGAGGAGAAATTAAG-3′) and gfpfus2 (5′-CGCGGCAGCAAACGCCAGCCTGGCGATTCTCGAATCTGGCGACTGGCAGCGACTAGTAGGTCAGCTAATTAAGC-3′). The 98-bp tetp/o region was amplified from pASK75 (37) with primers tetfus1 (5′-TGAAATAGAAAAATGAATCCGTTGAAGCCTGCTTTTTTATACTAACTTGACCATCGAAT GGCCAGATG-3′) and tetfus2 (5′-CTTAATTTCTCCTCTTTAATATTTCACTTTTCTCTATCACTGATAG-3′). Both PCR products were fused in a recombinant PCR with the primers tetfus1 and gfpfus2. The PCR resulted in amplification of an 850-bp Ptetp/o::gfpmut3.1 DNA fragment with flanking 50-bp overhangs homologous to the chromosomal bacteriophage λ attB attachment site, which was subsequently cloned into pGEM-T Easy (Promega, Mannheim, Germany). This plasmid was used as a template for further amplification of the 850-bp PCR fragment flanked by 50-bp overhangs homologous to the λ attB site with the primer pair tetfus1 and gfpfus2. The resulting PCR product was electroporated into E. coli 536/pKD46. Fluorescent derivatives of strain 536 with a chromosomally inserted Ptetp/o::gfpmut3.1 fusion were then selected using a Typhoon 8600 variable mode imager (Molecular Dynamics, Krefeld, Germany).
For infection, UPEC strains were grown overnight in LB medium, then harvested by centrifugation at 1,200 × g for 20 min, and resuspended in phosphate-buffered saline (PBS) to a concentration of 1 × 1010 CFU per ml. Anesthetized female mice of 8 to 10 weeks of age were infected by transurethral inoculation of 5 × 108 E. coli 536 cells (0.05 ml) into the bladder by using a soft polyethylene catheter (outer diameter, 0.6 mm; BD, Heidelberg, Germany) (9, 23). For analysis, the bladders were rinsed extensively with PBS in situ, then removed under sterile conditions, and homogenized mechanically. The number of bacteria was quantified by scoring CFU after overnight culture at 37°C on E. coli-Proteus-Streptococcus ID plates (bioMérieux, Nürtingen, Germany) as described previously (16).
Immunohistochemistry and electron microscopy.
For immunohistology, organs were fixed with 0.1 M Tris, pH 7.4, 0.05% zinc-acetate, and 0.5% zinc-chloride and embedded in Steedman's wax (39). Blocks were cut into 5-μm sections and mounted on poly-l-lysine-coated glass slides. For identification of CD11c+ cells, biotinylated anti-CD11c (clone HL-3; Pharmingen, Heidelberg, Germany) was revealed with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine and counterstained with methyl green. For immunofluorescence, antibodies were revealed with streptavidin-Alexa 568 and counterstained with Hoechst 33258. This technique specifically revealed DC, as isotype controls did not yield positive signals (17).
For electron microscopy, bladder tissue was fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) followed by 2% osmium tetroxide (OSO4). Tissues were embedded in Epon 812 embedding resin (Serva, Heidelberg, Germany), and 40- to 50-nm sections were cut with an LKB ultramicrotome UM IV (Leika, Frankfurt/Main, Germany) and analyzed using a CM10 electron microscope (Philips, Hamburg, Germany).
Isolation and analysis of leukocytes from the bladder.
A protocol for DC isolation from the kidney (17) was adapted for application to bladder tissue. Briefly, bladders were sliced with a scalpel and digested for 30 min at 37°C with 0.5 mg/ml collagenase and 100 μg/ml DNase I in RPMI 1640 medium (Invitrogen, Karlsruhe, Germany) containing 0.5% heat-inactivated fetal calf serum (PAA Laboratories, Pasching, Austria) and 20 mM HEPES. Cell suspensions were filtered through 100-μm nylon mesh and washed with Hanks balanced salt solution, without Ca2+ and Mg2+, containing 10 mM EDTA, 0.1% bovine serum albumin, and 20 mM HEPES. The number of viable cells was determined by trypan blue staining. Fc receptors were always blocked with 24G2 culture supernatant. Titrated amounts of the following labeled antibodies from Pharmingen were used for staining of 1 × 106 cell samples: anti-Iab-fluorescein isothiocyanate (FITC) (clone 25-9-3), anti-iNOS-FITC (BD6), anti-TNF-α-phycoerythrin (PE), anti-CD80-biotin (16-10A1), anti-CD86-FITC (GL1), anti-CD40-allophycocyanin (3/23), anti-CD11b-PerCP/Cy5.5 (M1/70), anti-Gr-1-PE/Cy7 (RB6-8C5), and anti-CD11c-allophycocyanin, -PE, and -PerCP (HL-3). Anti-F4/80-biotin was obtained from Caltag (clone CI:A3-1), and anti-CCR2 was kindly provided by M. Mack, Regensburg, Germany (19). Cells were analyzed on an LSR II cytometer (BD, Heidelberg, Germany) using Flow Jo software (Tristar, Phoenix, AZ). Forward and side scatter gating was adapted to include macrophages and granulocytes. The abundance of different cell populations was calculated by adding 10 μm PerCP/Cy5.5-labeled microbeads (BD).
RESULTS
DC are recruited to the bladder in UTI.
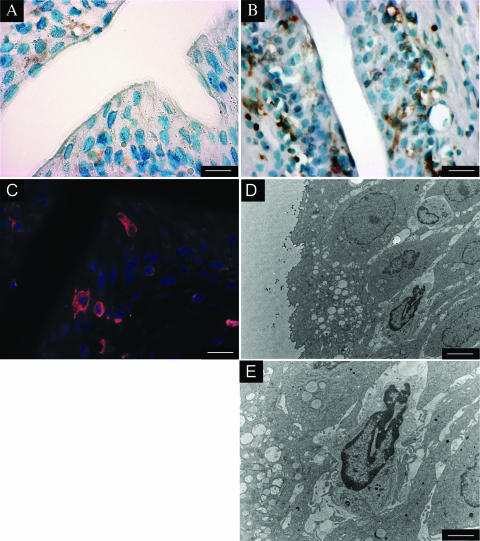
To study the role of DC in the innate defense against bacterial UTI, we instilled E. coli of the uropathogenic strain 536 transurethrally into the bladders of C57BL/6 mice and stained bladder sections after 24 h for the murine DC marker CD11c. This approach demonstrated CD11c+ mononuclear leukocytes with dendritic morphology in the uroepithelium and bladder submucosa (Fig. 1B and C). These vesical leukocytes were absent from the bladders of noninfected mice (Fig. 1A). Electron microscopy revealed leukocytes in the uroepithelium (Fig. 1D and E) and bladder submucosa, which contained neither the segmented nuclei of granulocytes nor large lysosomes typical of macrophages. These cells showed abundant perinuclear organelles and protrusions devoid of organelles (Fig. 1E). These features were compatible with those of DC in nonlymphoid tissue (15).
FIG. 1.
Recruitment of DC in UTI to the bladder wall. C57BL/6 mice were injected transurethrally with 5 × 108 E. coli 536 cells. After 24 h, sections of the bladder were stained for CD11c and analyzed by immunohistochemistry (A and B), by immunofluorescence (C), or by electron microscopy (D and E). CD11c+ cells were brown (A and B) or red (C). Counterstaining was performed with methyl green (A and B) or Hoechst 33258 (C). The images are representative of more than 10 mice analyzed. The bars in the lower right corners indicate 20 μm (A to C), 4 μm (D), and 10 μm (E).
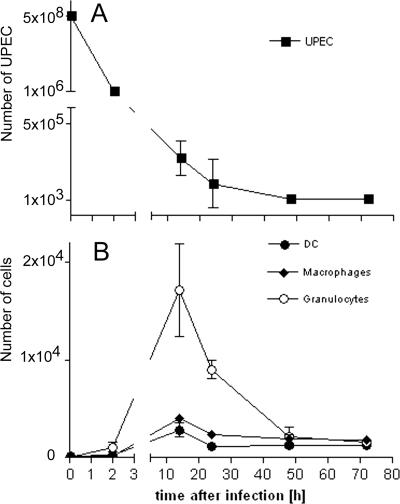
To quantitatively assess the extent of infection, we determined the numbers of bacterial CFU in the bladders of infected mice. CFU numbers rapidly decreased after instillation of UPEC (Fig. 2A). After 3 days, few bacteria remained detectable in the bladder.
FIG. 2.
Kinetics of bacterial clearance and leukocyte infiltration in UTI. (A) C57BL/6 mice were injected transurethrally with 5 × 108 E. coli 536 cells. At various time points, bladders were rinsed extensively with PBS in situ and removed for analysis. To determine the bacterial load, the bladders were mechanically homogenized. Aliquots were dispersed on E. coli-Proteus-Streptococcus plates and incubated at 37°C. The CFU of UPEC strains were counted after 14 h. For the time point of infection, the number of instilled UPEC strains was given. (B) To determine the number of infiltrating leukocytes, bladders were digested with collagenase, filtered on 100-μm mesh, Fc receptors were blocked, and cells were stained for flow cytometrical analysis. The numbers of CD11c+ dendritic cells, F4/80+ CD11c− macrophages, and Gr1+ CD11c− F4/80− MHC-II− granulocytes were determined by adding standardized numbers of latex particles. Dead cells were excluded using Hoechst 33258. Separate mouse groups were used for determining bacterial and cell counts. Shown are the means ± standard deviations from one of three experiments with groups of five mice.
We next correlated the bacterial load with the extent of vesical leukocyte infiltration by quantifying the numbers of F4/80+ CD11c− macrophages, Gr1+ CD11c− F4/80− major histocompatibility complex class II− (MHC-II−) granulocytes, and CD11c+ DC in the bladder. This staining protocol was chosen to avoid the inclusion of F4/80+ CD11c+ DC (17) into the macrophage count and to prevent the inclusion of Gr-1+ F4/80+ monocytes (8) or CD11c+ Gr-1+ MHC-II+ plasmacytoid DC (6) into the granulocyte count. In noninfected animals, very few leukocytes were detected. In infected mice, the numbers of all vesical leukocyte populations peaked after about 14 h (Fig. 2B and Table 1). The numbers of all leukocyte populations were greatly reduced already after 3 days but had not yet reached normal values (Fig. 2B and Table 1).
TABLE 1.
Numbers of DC, granulocytes, and macrophages in the bladders of C57BL/6 mice at various time points after transurethral instillation of UPECa
| Time after infection (h) | No. of DC | No. of macrophages | No. of granulocytes |
|---|---|---|---|
| 0 | 144 ± 36 | 109 ± 34 | 41 ± 26 |
| 2 | 323 ± 75 | 149 ± 27 | 1,053 ± 700 |
| 14 | 2,845 ± 976 | 4,035 ± 586 | 17,096 ± 6,625 |
| 24 | 1,199 ± 296 | 2,408 ± 471 | 9,001 ± 1,310 |
| 48 | 1,255 ± 144 | 1,928 ± 453 | 2,126 ± 1,061 |
| 72 | 1,311 ± 83 | 1,822 ± 502 | 1,622 ± 87 |
Given are the means ± standard deviations from groups of five mice.
Characterization of DC infiltrating the bladder in UTI.
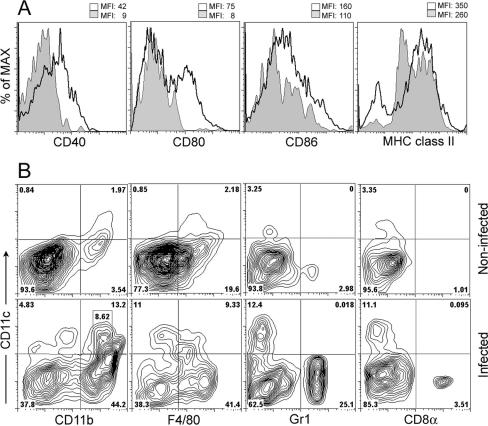
To further characterize the DC infiltrating the bladder, we determined expression of activation and maturation markers. The few vesical DC in noninfected control mice expressed low to intermediate levels of CD40, CD80, CD86, and MHC-II (Fig. 3A). All costimulatory molecules and MHC-II cells were markedly upregulated 24 h after infection (Fig. 3A), indicating DC maturation. For CD80, a DC subpopulation showed no upregulation. A smaller subset of CD11c+ cells devoid of surface expression of MHC-II cells was evident in infected mice, which may represent immature DC newly recruited from the circulation that had not yet expressed MHC-II cells on their surface (17). Activated DC were not detectable after infection in the spleen or in subcutaneous lymph nodes, indicating that UPEC had not spread systemically (data not shown).
FIG. 3.
Characterization of vesical DC subpopulations in UTI. (A) C57BL/6 mice were infected with 5 × 108 E. coli 536 cells. After 24 h, vesical CD11c+ cells were isolated by collagenase digestion, Fc receptors were blocked, and expressions of the costimulatory molecules CD40, CD80, and CD86 and of MHC-II were determined. Expression profiles of noninfected (gray area) versus infected (transparent area with thick line) mice were overlaid in histograms. Numbers indicate the mean fluorescence intensities (MFI) of these two cell populations. (B) Viable CD11c+ cells from infected and noninfected mice were stained for the DC subtype markers CD11b, F4/80, Gr-1, and CD8α and analyzed by flow cytometry. Numbers indicate the cellular proportions in each quadrant. The area in the lower left dot plot indicates CD11bINT DC. Data are representative for >10 (A and B) individual experiments.
Next, we determined the subtype of infiltrating DC. The few CD11c+ cells in noninfected bladders expressed F4/80 and the myeloid marker CD11b (Fig. 3B and Table 2). At 24 h after infection, only some DC devoid of these markers were detectable, while most were of the CD11b+ myeloid phenotype (Fig. 3B and Table 2). CD11c+ cells expressing CD8α, Gr-1, or B220 were absent (Fig. 3B and Table 2; data not shown), indicating that neither lymphoid nor plasmacytoid DC had been recruited. In addition to DC, F4/80+ CD11c− macrophages and Gr1+ CD11c− F4/80− granulocytes were evident (Fig. 3B and Table 1).
TABLE 2.
Numbers of different DC subtypes 24 h after transurethral instillation of UPECa
| DC subtype | No. of DC in noninfected bladders | No. of DC in infected bladders |
|---|---|---|
| CD11b+ myeloid DC | 98 ± 13 | 821 ± 91 |
| CD11bINT Tip-DC | 0 ± 0 | 422 ± 21 |
| F4/80+ DC | 61 ± 13 | 621 ± 78 |
| Gr1+ plasmacytoid DC | 0 ± 0 | 2 ± 0.2 |
| CD8α+ lymphoid DC | 0 ± 0 | 9 ± 1 |
Given are the means ± standard deviations from groups of five mice.
Closer examination of the CD11b+ DC from infected mice revealed cell populations that expressed low, intermediate (Fig. 3B, row 2, column 1), or high levels of CD11b. CD11bINT CD11c+ cells were absent from the spleen of infected animals (data not shown) and were not found in the bladders of noninfected mice (Fig. 3B).
Tip-DC are recruited to the bladder in UTI.
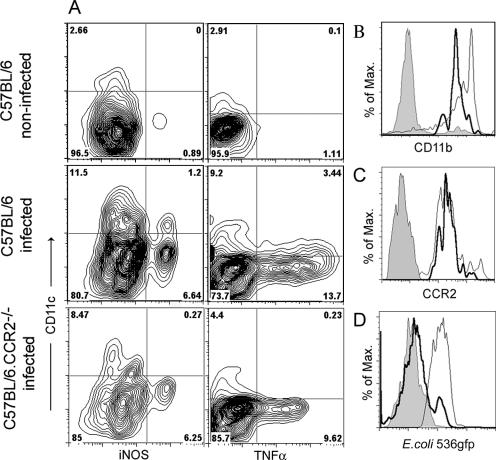
CD11bINT CD11c+ DC producing TNF-α and iNOS were recently shown to be critical in the innate defense against bacterial infection with Listeria monocytogenes (36). We wished to determine whether the vesical CD11bINT DC population identified in UTI (Fig. 3B) matched the criteria of Tip-DC. Indeed, about 8 to 15% of the vesical DC contained iNOS, in contrast to less than 0.1% in noninfected bladders (Fig. 4A). TNF-α was produced by about 20 to 30% of the vesical DC, in contrast to less than 0.1% in noninfected bladders (Fig. 4A). All iNOS+ DC (Fig. 4B) and all TNF-α+ DC (data not shown) expressed intermediate CD11b levels, as described for Tip-DC (36). Another characteristic of Tip-DC was their absence from CCR2-deficient mice. Indeed, CCR2−/− mice with UTI lacked these DC, as evidenced by the absence of iNOS- and TNF-α-producing CD11c+ cells (Fig. 4A). All vesical DC in UTI expressed CCR2 (Fig. 4C), which implies that the CD11bINT DC must have also been positive. Finally, as opposed to CD11bHI myeloid DC, CD11bINT DC did not take up UPEC-derived antigens (Fig. 4D), consistent with the previous demonstration that Tip-DC were not involved in adaptive immune responses against bacterial pathogens (36). In summary, vesical CD11bINT DC in UTI displayed the characteristics of Tip-DC (36).
FIG. 4.
Tip-DC are recruited to the bladder in UTI. (A) C57BL/6 or CCR2−/− mice were injected transurethrally with 5 × 108 E. coli 536 cells. After 24 h, the bladders of infected mice and noninfected controls were digested with collagenase and stained for CD11c and for intracellular expression of iNOS and TNF-α without in vitro restimulation. (B and C) Vesical DC from infected mice were analyzed for CD11b (B) and CCR2 (C) expression. The thick line indicates expression by iNOS+/TNF-α+ CD11c+ Tip-DC, and the thin line indicates expression by iNOS−/TNF-α− CD11c+ DC. The gray area represents the isotype control. (D) C57BL/6 mice were infected with 5 × 108 E. coli 536.gfp cells. After 24 h, vesical DC were isolated and fluorescence uptake was determined on CD11bINT Tip-DC (thick line) and CD11bHI myeloid DC (thin line). The gray area shows background fluorescence after infection with nonfluorescent UPEC.
Neither DC nor CCR2-deficient cells are required for bacterial clearance in UTI.
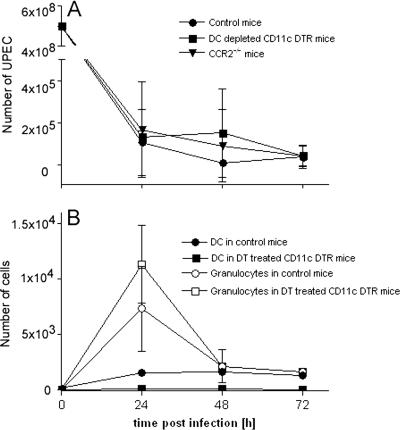
To investigate the requirement of Tip-DC for the defense against UTI, we determined UPEC clearance from CCR2-deficient mice. These mice cleared infection as efficiently as wild-type controls (Fig. 5A), which implies that Tip-DC were dispensable for bacterial clearance from the bladder in UTI.
FIG. 5.
Neither CD11c+ nor other CCR2-dependent cells are required for clearance of UPEC in UTI. (A) CD11c-DTR mice, CCR2−/− mice, and C57BL/6 wild-type controls were injected transurethrally with 5 × 108 E. coli 536 cells. At various time points, the number of CFU per bladder was determined. Bladder weights did not significantly differ at 72 h after infection (wild type, 20.4 ± 1.8 mg; CCR2−/−, 21.2 ± 1.7 mg). (B) The numbers of CD11c+ DC (▪, •) and Gr1+ F4/80− MHC class II− granulocytes (□, ○) in DT-treated CD11c-DTR/GFP mice (▪, □) and in wild-type controls (•, ○) are given as means ± standard deviations from groups of five mice. Results are representative of four individual experiments.
To directly address the requirement of DC for the clearance of UPEC, we infected CD11c-DTR mice that expressed the DT receptor under the influence of the CD11c promoter in DC (14). Intraperitoneal injection of DT resulted in depletion of more than 95% of CD11c+ cells in the circulation (data not shown) and about 90% in the bladder (Fig. 5B). The number of granulocytes was not diminished but reproducibly increased, albeit not to a statistically significant extent (Fig. 5B). Bacterial clearance in DC-depleted CD11c-DTR mice was not different from that in nondepleted controls (Fig. 5A).
CCR2-independent immune effector cells replace the functionality of Tip-DC in UTI.
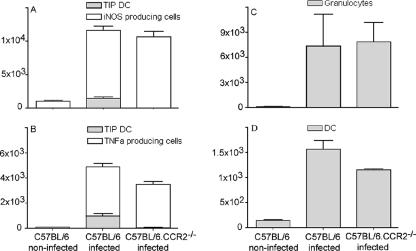
To elucidate the discrepancy between the requirement of Tip-DC in listeriosis and UTI, we investigated whether other immune effectors may have substituted for the absence of Tip-DC. Indeed, the bladders of CCR2−/− mice contained abundant iNOS-producing CD11c− cells. These cells represented about 85% of the total number of vesical iNOS+ cells in wild-type mice with UTI, so that the loss of Tip-DC in CCR2-deficient animals caused only a minor decrease (Fig. 6A). iNOS-producing non-DC expressed CD11b and Gr-1 (data not shown) and thus represented granulocytes and/or macrophages.
FIG. 6.
CCR2-independent immune effector cells replace the functionality of Tip-DC in UTI. C57BL/6 or CCR2−/− mice were infected with 5 × 108 E. coli 536 cells. After 24 h, the bladders of infected mice and noninfected controls were digested with collagenase and stained for surface molecules. (A and B) The numbers of iNOS+ (A) and TNF-α+ (B) CD11c+ cells (gray bars) and those of iNOS+ (A) and TNF-α+ (B) CD11c− cells (white bars) in single-cell suspensions from the bladder were determined by flow cytometry. Bars were stacked to yield the total number of iNOS+ (A) or TNF-α+ (B) cells. (C and D) The numbers of granulocytes (C) and DC (D) in single-cell suspensions from the bladder were determined by flow cytometry. Shown are the means ± standard deviations from groups of five mice. Results are representative of three individual experiments.
We next determined the quantitative contribution of Tip-DC to TNF-α production in the bladder. These cells constituted no more than 15% of the total vesical TNF-α-producing cells in UTI (Fig. 6B). Consequently, their absence in CCR2−/− mice diminished the total number of vesical TNF-α-producing cells only slightly (Fig. 6B). The production of TNF-α per cell, however, was reduced in CCR2-deficient mice (Fig. 4a).
Also, the total number of DC in CCR2−/− mice was barely reduced by the absence of the Tip-DC subpopulation (Fig. 6D), indicating that molecular mechanisms other than MCP-1 had mediated DC recruitment. Finally, the number of granulocytes was unchanged in CCR2−/− mice (Fig. 6C), consistent with previous reports showing that granulocyte recruitment in UTI was mediated by CXCL8 (11).
DISCUSSION
The early defense against bacterial infections is thought to depend mainly on resident tissue cells and innate immune effectors, such as macrophages and granulocytes (11, 24). A role of DC as innate effectors was recently demonstrated in several experimental models (6, 27). In particular, a DC subpopulation termed Tip-DC was critical for defense against infection with the gram-positive bacterium Listeria monocytogenes. Its absence in CCR2-deficient mice resulted in high susceptibility to this infection (36). These recent findings encouraged us to study the role of DC in bacterial UTI, one of the most prevalent infections worldwide. We demonstrated that DC appeared in the bladder as fast as granulocytes and macrophages, which implies recruitment from the circulation. Infiltrating DC displayed markers of the myeloid subtype. Also, expression of F4/80 at intermediate levels was noted, consistent with previous studies examining the phenotype of DC in other nonlymphoid tissues (17, 28). In addition, a considerable population of DC with phenotypical characteristics of Tip-DC was found, including their absence in CCR2−/− mice. To our knowledge, this finding represents the first evidence of Tip-DC in an infection model other than listeriosis.
The CCR2 ligand, MCP-1 (CCL-2), has been shown to be secreted in cystitis and pyelonephritis (2, 12), suggesting a functional role in these infections. However, bacterial clearance from the bladders of infected CCR2-deficient mice was unchanged. The independence of CCR2-dependent cells indicated that Tip-DC were dispensable for the innate defense against UTI. This discrepancy to the situation in listeriosis may be explained by differences in the immune response elicited by infections with gram-positive Listeria and gram-negative E. coli. Alternatively, immune mediators that could replace the functionality of Tip-DC may reside in the bladder but not in the spleen. In support of the latter possibility, vesical TNF-α- and iNOS-producing non-DC leukocytes were abundant in the absence of CCR2, so that the lack of Tip-DC did not significantly deplete the supply of these immune mediators. TNF-α and iNOS have been shown to be crucial in experimental models of other bacterial infections (20, 36). In UTI, both mediators were produced in large amounts (5, 30). However, a functional role in this infection has not been proven, although some studies reported partial dependence on iNOS in mice deficient for this enzyme or upon chemical inhibition of NO (26, 29). Apart from a possible effector role in the innate defense, it is possible that NO inhibits UTI recurrence by containing UPEC within the urinary tract long term, as observed with tuberculosis (4). The role of TNF-α in UTI has not been experimentally addressed yet. This cytokine performed pleiotropic functions relevant in infections, such as macrophage activation and recruitment of granulocytes (20). Granulocytes are known to be essential for defense against UTI (11, 24). Their numbers were not reduced in the bladders of CCR2−/− mice, which implies that the factors crucial in their recruitment, such as CXCL8 and TNF-α, must have been functionally available without Tip-DC. Indeed, vesical TNF-α production was detectable in these animals, albeit at lower levels. Clarification of the exact roles of TNF-α and iNOS in UTI was beyond the scope of the present study. However, the demonstration of unaltered clearance of UPEC in Tip-DC-deficient CCR2−/− mice indicated dispensability of TNF-α and iNOS produced by this DC subtype in UTI.
These findings fundamentally questioned the necessity of DC for the innate defense against UTI. Indeed, in mice depleted of CD11c+ cells, UPEC strains were cleared as efficiently as in nondepleted controls. The dispensability of all CD11c+ cells for bacterial clearance did not exclude a supportive role of DC in the innate defense against UTI, for example, by secretion of proinflammatory cytokines. Nevertheless, the efficient recruitment of large numbers of DC bearing an activated and mature phenotype into the bladder uroepithelium was remarkable and suggests a role in the induction of adaptive immunity. This notion is further supported by the observed uptake of bacterial antigens by vesical myeloid DC, which is required for subsequent antigen presentation to T cells. T- and B-cell-dependent immunity was recently shown to be able to confer protection in experimental reinfection with UPEC (41). Those and the present observations warrant future studies to elucidate the role of vesical DC in adaptive immunity against UTI.
Acknowledgments
We thank C. Weber (Department of Cardiology, University of Aachen, Germany) for providing CCR2−/− ApoE−/− mice, M. Mack (Department of Nephrology, University of Regensburg, Germany) for providing anti-CCR2 antibody, H.-G. Sahl (Department of Microbiology, University of Bonn, Germany) for advice on microbiological work, and J. Bedorf for help with electron microscopy and acknowledge technical support from the flow cytometry core facility of the IMMEI and from the central animal facilities of the University of Bonn.
C.K. was supported by a junior research group grant of the German state of Nordrhein-Westfalen. D.E. was supported by grant Ku1036/5-1 of the Deutsche Forschungsgemeinschaft.
Editor: F. C. Fang
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Berger, H., J. Hacker, A. Juarez, C. Hughes, and W. Goebel. 1982. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J. Bacteriol. 152:1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchelouche, K., L. Andresen, S. Alvarez, J. Nordling, O. H. Nielsen, and P. Bouchelouche. 2006. Interleukin-4 and 13 induce the expression and release of monocyte chemoattractant protein 1, interleukin-6 and stem cell factor from human detrusor smooth muscle cells: synergy with interleukin-1beta and tumor necrosis factor-alpha. J. Urol. 175:760-765. [DOI] [PubMed] [Google Scholar]
- 3.Brzuszkiewicz, E., H. Brüggemann, H. Liesegang, M. Emmerth, T. Oelschlaeger, G. Nagy, K. Albermann, C. Wagner, C. Buchrieser, L. Emödy, G. Gottschalk, J. Hacker, and U. Dobrindt. 2006. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl. Acad. Sci. USA 103:12879-12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, E. D., J. Chan, and N. W. Schluger. 2001. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am. J. Respir. Cell Mol. Biol. 25:606-612. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. C., C. S. Mudge, and D. Klumpp. 2006. Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am. J. Physiol. Renal Physiol. 291:F741-F749. [DOI] [PubMed] [Google Scholar]
- 6.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 9.Godaly, G., A. E. Proudfoot, R. E. Offord, C. Svanborg, and W. W. Agace. 1997. Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect. Immun. 65:3451-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandaliano, G., L. Gesualdo, F. Bartoli, E. Ranieri, R. Monno, A. Leggio, G. Paradies, E. Caldarulo, B. Infante, and F. P. Schena. 2000. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 58:182-192. [DOI] [PubMed] [Google Scholar]
- 11.Haraoka, M., L. Hang, B. Frendeus, G. Godaly, M. Burdick, R. Strieter, and C. Svanborg. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180:1220-1229. [DOI] [PubMed] [Google Scholar]
- 12.Hertting, O., A. Khalil, G. Jaremko, M. Chromek, Y. H. Li, M. Bakhiet, T. Bartfai, K. Tullus, and A. Brauner. 2003. Enhanced chemokine response in experimental acute Escherichia coli pyelonephritis in IL-1beta-deficient mice. Clin. Exp. Immunol. 131:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooton, T. M., and W. E. Stamm. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. N. Am. 11:551-581. [DOI] [PubMed] [Google Scholar]
- 14.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaissling, B., I. Hegyi, J. Loffing, and M. Le Hir. 1996. Morphology of interstitial cells in the healthy kidney. Anat. Embryol. (Berlin) 193:303-318. [DOI] [PubMed] [Google Scholar]
- 16.Kerrn, M. B., N. Frimodt-Møller, and F. Espersen. 2003. Effects of sulfamethizole and amdinocillin against Escherichia coli strains (with various susceptibilities) in an ascending urinary tract infection mouse model. Antimicrob. Agents Chemother. 47:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruger, T., D. Benke, F. Eitner, A. Lang, M. Wirtz, E. E. Hamilton-Williams, D. Engel, B. Giese, G. Muller-Newen, J. Floege, and C. Kurts. 2004. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J. Am. Soc. Nephrol. 15:613-621. [DOI] [PubMed] [Google Scholar]
- 18.Kuziel, W. A., S. J. Morgan, T. C. Dawson, S. Griffin, O. Smithies, K. Ley, and N. Maeda. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94:12053-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack, M., J. Cihak, C. Simonis, B. Luckow, A. E. Proudfoot, J. Plachy, H. Bruhl, M. Frink, H. J. Anders, V. Vielhauer, J. Pfirstinger, M. Stangassinger, and D. Schlondorff. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697-4704. [DOI] [PubMed] [Google Scholar]
- 20.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 21.Maus, U. A., K. Waelsch, W. A. Kuziel, T. Delbeck, M. Mack, T. S. Blackwell, J. W. Christman, D. Schlondorff, W. Seeger, and J. Lohmeyer. 2003. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J. Immunol. 170:3273-3278. [DOI] [PubMed] [Google Scholar]
- 22.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 23.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 24.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 97:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munz, C., R. M. Steinman, and S. Fujii. 2005. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 202:203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki, B., J. Singhal, L. Fang, S. Nowicki, and C. Yallampalli. 1999. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/HeJ mice. Infect. Immun. 67:2421-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812-823. [DOI] [PubMed] [Google Scholar]
- 28.Peters, W., J. G. Cyster, M. Mack, D. Schlondorff, A. J. Wolf, J. D. Ernst, and I. F. Charo. 2004. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J. Immunol. 172:7647-7653. [DOI] [PubMed] [Google Scholar]
- 29.Poljakovic, M., and K. Persson. 2003. Urinary tract infection in iNOS-deficient mice with focus on bacterial sensitivity to nitric oxide. Am. J. Physiol. Renal Physiol. 284:F22-31. [DOI] [PubMed] [Google Scholar]
- 30.Poljakovic, M., M. L. Svensson, C. Svanborg, K. Johansson, B. Larsson, and K. Persson. 2001. Escherichia coli-induced inducible nitric oxide synthase and cyclooxygenase expression in the mouse bladder and kidney. Kidney Int. 59:893-904. [DOI] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21-25. [DOI] [PubMed] [Google Scholar]
- 33.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling, J. D., S. M. Martin, C. S. Hung, R. G. Lorenz, and S. J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schober, A., A. Zernecke, E. A. Liehn, P. von Hundelshausen, S. Knarren, W. A. Kuziel, and C. Weber. 2004. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ. Res. 95:1125-1133. [DOI] [PubMed] [Google Scholar]
- 36.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 37.Skerra, A. 1994. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151:131-135. [DOI] [PubMed] [Google Scholar]
- 38.Sobel, J. D. 1997. Pathogenesis of urinary tract infection. Role of host defenses. Infect. Dis. Clin. N. Am. 11:531-549. [DOI] [PubMed] [Google Scholar]
- 39.Steedman, H. F. 1949. An ester wax for use in the tropics. Nature 164:1084. [DOI] [PubMed] [Google Scholar]
- 40.Svanborg, C., B. Frendeus, G. Godaly, L. Hang, M. Hedlund, and C. Wachtler. 2001. Toll-like receptor signaling and chemokine receptor expression influence the severity of urinary tract infection. J. Infect. Dis. 183(Suppl. 1):S61-S65. [DOI] [PubMed] [Google Scholar]
- 41.Thumbikat, P., C. Waltenbaugh, A. J. Schaeffer, and D. J. Klumpp. 2006. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 176:3080-3086. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522-1526. [DOI] [PubMed] [Google Scholar]