Abstract
Haemophilus influenzae requires an exogenous heme source for aerobic growth in vitro. Hemoglobin or hemoglobin-haptoglobin satisfies this requirement. Heme acquisition from hemoglobin-haptoglobin is mediated by proteins encoded by hgp genes. Both Hgps and additional proteins, including those encoded by the hxu operon, provide independent pathways for hemoglobin utilization. Recently we showed that deletion of the set of three hgp genes from a nontypeable strain (86-028NP) of H. influenzae attenuated virulence in the chinchilla otitis media model of noninvasive disease. The present study was undertaken to investigate the role of the hgp genes in virulence of the wild-type serotype b clinical isolate HI689 in the infant rat model of hematogenous meningitis, an established model of invasive disease requiring aerobic growth. Bacteremia of high titer and long duration (>14 days) and histopathologically confirmed meningitis occurred in >95% of infant rats challenged at 5 days of age with strain HI689. While mutations disrupting either the Hgp- or Hxu-mediated pathway of heme acquisition had no effect on virulence in infant rats, an isogenic mutant deficient for both pathways was unable to sustain bacteremia or produce meningitis. In contrast, mutations disrupting either pathway decreased the limited ability of H. influenzae to initiate and sustain bacteremia in weanling rats. Biochemical and growth studies also indicated that infant rat plasma contains multiple heme sources that change with age. Taken together, these data indicate that both the hgp genes and the hxuC gene are virulence determinants in the rat model of human invasive disease.
Haemophilus influenzae is a fastidious, gram-negative, facultatively anaerobic, opportunistic pathogen. Humans constitute its only known natural host. At any given time, the majority of healthy adults and children over 5 years of age harbor this microorganism as a commensal in the nasopharynx (17, 64). H. influenzae is a frequent cause of noninvasive disease, including respiratory infections (48, 64) and otitis media (4, 56, 64). Infection by H. influenzae also produces invasive disease, including epiglottitis, pneumonia, bacteremia, meningitis (54, 64), and occasionally endophthalmitis, leading to blindness (2). Invasive disease caused by H. influenzae is often associated with encapsulation. Of the six biochemically and antigenically distinct capsular types (a to f), serotype b strains accounted for the vast majority of bacteremia and meningitis cases prior to the introduction of vaccines based on the type b capsule (54). While such vaccines have nearly eradicated meningitis caused by type b strains in the developed world (54, 67), they lack effectiveness against other capsular types (3, 58) and against nontypeable strains (47, 56). The identification of outer membrane proteins with indispensable functions in vivo could provide new potential targets for development of a vaccine with a broader range of efficacy against H. influenzae infections.
H. influenzae requires iron for growth, as do most bacteria; unlike most bacterial pathogens, it also requires heme or protoporphyrin IX (PPIX) plus iron to grow aerobically (53). (Heme is correctly identified as ferrous PPIX, while ferric PPIX is referred to as hemin; however, throughout this paper heme is used as a general term that is not meant to indicate a particular valence state of the chelated iron.) This requirement reflects the lack of an intact biosynthetic pathway for the endogenous production of PPIX (15, 53). H. influenzae retains the terminal step in heme biosynthesis, the incorporation of Fe2+ into PPIX by ferrochelatase to form heme. However, no significant extracellular source of free PPIX is believed to occur in vivo. Since the ability to grow aerobically is crucial for the establishment of invasive disease by H. influenzae (26), this bacterium must acquire heme from host sources to sustain an invasive infection. Heme is localized intracellularly in hemoglobin and heme-containing enzymes, where it is unavailable to many invading bacteria (18, 59). Hemoglobin released into plasma upon spontaneous lysis of red blood cells is recognized as a principal source of extracellular heme and iron in vivo in normal, uninfected individuals (33). Free hemoglobin is bound to the acute-phase serum protein, haptoglobin, with high affinity and rapidly cleared by the reticuloendothelial system (16, 33). Heme released from methemoglobin is bound by two other acute-phase serum proteins, hemopexin and albumin, and also is cleared from the circulation (18). H. influenzae has evolved multiple molecular mechanisms to counter and to exploit these host mechanisms of iron and heme sequestration (see review by Morton and Stull [40]).
In addition to free heme, H. influenzae can utilize a number of heme- or iron-containing proteins to meet its heme and iron requirements. These include hemoglobin, hemoglobin-haptoglobin complexes, myoglobin, hemopexin- and albumin-bound heme, and transferrin-bound iron of human origin (39, 42, 55, 63). Utilization of iron bound to lactoferrin occurs only in some H. influenzae strains (44, 65). H. influenzae does not produce siderophores (44, 55, 69). Instead this bacterium produces an array of receptors that selectively bind heme- or iron-containing proteins. Receptors binding hemoglobin and hemoglobin-haptoglobin complexes (5, 31, 39, 43, 57), heme-hemopexin (8, 70), transferrin (60), and lactoferrin (60, 65) have been identified. Because of the importance of iron and heme acquisition to the aerobic growth of H. influenzae, receptors in the outer membrane that selectively bind iron- or heme-containing proteins are potential targets for vaccine development.
The premise that hemoglobin and hemoglobin-haptoglobin complexes are likely to be the quantitatively most significant extracellular sources of heme in vivo has led us to undertake the systematic characterization of the hemoglobin and hemoglobin-haptoglobin binding proteins (Hgps) of H. influenzae. We have established that the genome of the serotype b clinical isolate HI689 contains three conserved genes, hgpA, hgpB, and hgpC, encoding structurally and functionally nonidentical outer membrane proteins that bind hemoglobin and hemoglobin-haptoglobin complexes (31, 41, 43, 57). Isogenic mutant strains carrying deletions of one or two of these hgp genes retain the ability to grow in vitro in medium containing hemoglobin or hemoglobin-haptoglobin. However, the triple-mutant strain HI1717, lacking all three genes, cannot utilize hemoglobin-haptoglobin as a heme source for aerobic growth in vitro (43). This mutant also is impaired in its ability to utilize low concentrations of hemoglobin (43). These findings established that the hgp genes have a dual function, utilization of both hemoglobin and hemoglobin-haptoglobin complexes as heme sources supporting aerobic growth. The occurrence of two to four hgp genes in each typeable and nontypeable H. influenzae strain examined to date (5, 25, 40, 43) implies that these genes play an important role in colonization and/or infection.
Interactions between bacteria and the microenvironments of their hosts are complex and cannot be determined solely from in vitro studies (39, 45, 52). Little is known about the importance of the hgp genes (or other genes whose proteins are involved in heme acquisition) during H. influenzae infection, their roles in bacterial proliferation in different niches in the body, or their contributions to virulence. Previously we have shown that the hgpA gene and other iron acquisition genes are expressed in vivo during acute human otitis media infection (68). More recently we showed that deletion of the complement of three hgp genes from a nontypeable strain of H. influenzae attenuated virulence in the chinchilla model of otitis media (38). The present study was undertaken to characterize the contribution of the hgp genes to virulence of H. influenzae in the rat model of invasive disease.
MATERIALS AND METHODS
H. influenzae strains.
The H. influenzae strains used in this study are listed in Table 1. All were derived from a serotype b clinical isolate (strain HI689) obtained from a patient with bacteremia (49). Isolation and properties of the wild-type strain (HI689) as well as its double- and triple-mutant Δhgp derivatives (respectively, strains HI1714, HI1715, HI1716, and HI1717) have been described previously (43). The hxuC deletion mutant for strains HI689 and HI1717 was constructed as follows. Design of two primer pairs to amplify regions upstream and downstream of hxuC was based on the H. influenzae Rd KW20 genomic sequence. Sequences of the two primer pairs are as follows: HXUCUSA, forward, 5′-GAATTCGCATTTCGTGCATCAATCC-3′; HXUCUSB, reverse, 5′-GGATCCGTAATAATTAAAAAGAGG-3′; HXUCDSA, forward, 5′-GGATCCTTACTTTGGAAAACGCCC-3′; HXUCDSB, reverse, 5′-CTGCAGGATAGTTAGCAGTTACACC-3′. The primer pair consisting of HXUCUSA and HXUCUSB was designed to amplify a 1,073-bp product upstream of hxuC and add EcoRI and BamHI sites to the ends of the amplicon to allow for directional cloning. The second primer pair, consisting of HXUCDSA and HXUCDSB, was designed to amplify a 966-bp product downstream of hxuC and similarly add PstI and BamHI sites to the ends of the amplicon. PCRs were performed in a 50-μl reaction mixture containing 1.5 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphates, 10 pM of each primer, and 2 U of Taq DNA polymerase (Roche Diagnostics Corp., Indianapolis, Ind.). Thirty cycles of PCR were performed. Each cycle consisted of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min. This followed an initial denaturation step at 95°C for 5 min; a final extension was carried out at 72°C for 30 min. Approximately 500 ng of H. influenzae strain HI689 chromosomal DNA was used as a template. PCR products of the correct size were initially cloned into pCRTOPO 2.1 and then sequentially subcloned into pUC19N to yield the plasmid pDJM337 that contained upstream and downstream sequences of hxuC abutting each other with a unique BamHI site between them. This unique BamHI site was employed in the insertion of a ∼1,200-bp BamHI-excised kanamycin resistance-encoding marker from pKANR to yield pDJM343. Plasmid pKANR was constructed in this laboratory and consisted of a Tn903-derived aminoglycoside resistance cassette flanked by multiple paired restriction sites (excisable by EcoRI, BamHI, XbaI, SalI, SphI, and HindIII).
TABLE 1.
H. influenzae strains used in this studya
| H. influenzae strain | Relevant genotype | Relevant phenotype | Resistance | Reference |
|---|---|---|---|---|
| HI689 | hgpA+hgpB+hgpC+hxuC+ | Growth on Hb, Hb-Hp, heme, heme-Hpx, heme-Alb | Rbs Tets Specs Kans | 30, 49 |
| HI1714 | ΔhgpA ΔhgpB hgpC+hxuC+ | Like HI689 | Rbr Tetr Specs Kans | 43 |
| HI1715 | ΔhgpA hgpB+ ΔhgpC hxuC+ | Like HI689 | Rbr Tets Specr Kans | 43 |
| HI1716 | hgpA+ ΔhgpB ΔhgpC hxuC+ | Like HI689 | Rbs Tetr Specr Kans | 43 |
| HI1717 | ΔhgpA ΔhgpB ΔhgpC hxuC+ | No growth on Hb-Hp | Rbr Tetr Specr Kans | 43 |
| HI1787 | hgpA+hgpB+hgpC+ ΔhxuC | No growth on low heme, heme-Hpx, heme-Alb | Rbr Tets Specs Kanr | This study |
| HI1810 | ΔhgpA ΔhgpB ΔhgpC ΔhxuC | No growth on Hb-Hp, low heme, heme-Hpx, heme-Alb | Rbr Tetr Specr Kanr | This study |
Each of the mutant strains is isogenic to strain HI689. The designations hgpA, hgpB, and hgpC identify the three genes encoding outer membrane proteins that bind hemoglobin (Hb) and hemoglobin-haptoglobin complexes (Hb-Hp) in the wild-type strain HI689. The hxuC gene encodes a different outer membrane protein that has been shown to be important in the utilization of heme-hemopexin (heme-Hpx) and heme-albumin (heme-Alb) complexes for growth. Antibiotic resistance markers associated with the cassettes utilized to generate the specific deletion mutations include resistance to ribostamycin (Rbr; 15 μg/ml), tetracycline (Tetr; 3 μg/ml), spectinomycin (Specr; 200 μg/ml), and kanamycin (Kanr; 25 μg/ml). The kanamycin resistance mutation also causes coincident resistance to ribostamycin.
H. influenzae strains HI689 and HI1717 were transformed to kanamycin resistance (25 μg/ml with plasmid pDJM343 DNA using a modification of the static aerobic method of Gromkova et al. (22) as previously described (38). Appropriate chromosomal insertions were confirmed by the size of a PCR product, and hxuC deletion mutants of strains HI689 and HI1717 were identified, phenotypically confirmed by growth characteristics in vitro, and designated strains HI1787 and HI1810, respectively.
Growth conditions.
Stock cultures were maintained frozen in 2% nonfat powdered milk solution at −70°C. Strains were routinely cultured at 37°C on plates containing chocolate II agar with bacitracin (16,500 U/liter) (BBL prepared media; Becton Dickinson and Co., Sparks, Md.). Broth cultures of H. influenzae were grown in brain heart infusion (BHI) (Difco, Detroit, Mich.) broth supplemented with β-NAD and heme (10 μg/ml each) (Sigma-Aldrich Chemical Co., St. Louis, Mo.). To augment growth on BHI agar, the β-NAD and heme concentrations were doubled (heme-supplemented BHI [sBHI]).
Plate bioassays were used to assess the dosage-dependent utilization of hemoglobin or hemoglobin-haptoglobin complexes for growth, to compare the abilities of human and rat hemoglobin and hemoglobin-haptoglobin complexes to support growth, and to assess the growth effects of the various mutations. Human hemoglobin, rat hemoglobin, and human haptoglobin type 1-1 were purchased from Sigma-Aldrich, and rat haptoglobin was purchased from Life Diagnostics, Inc. (West Chester, Pa.). To prepare inocula for the plate bioassays, bacteria grown at 37°C overnight on plates containing chocolate agar plus bacitracin were used to inoculate 15-ml culture tubes containing 5 ml BHI broth supplemented with β-NAD but without added heme (heme-deficient BHI [hdBHI]). The turbid cultures obtained after 4 h of growth in this heme-deficient medium were used to streak plates containing hdBHI agar (20 ml) to produce lawns. Hemoglobin and hemoglobin-haptoglobin solutions were prepared in water, filter sterilized, and serially diluted. Wells were made with a sterile no. 5 cork borer (5-mm diameter), and 40 μl of each test solution was added to individual wells. The bioassay plates were then incubated for 24 h, and the zone of growth was scored visually. Bioassays were replicated at least three times.
Animals.
Specified-pathogen-free (SPF), timed-pregnant Sprague-Dawley female rats (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) were received approximately 7 days prior to giving birth. These pregnant females were singly housed on hardwood litter with ad libitum access to water and a standard pelleted food (Purina Lab Rodent Diet 5001). They were maintained on a 12-h light-dark cycle in separate forced-air cubicles in a biocontainment facility to prevent cross-contamination. Newborn pups from different mothers were pooled and randomly reassigned to a given mother (n = 10/dam). Pups were housed continuously with that dam prior to weaning at 21 days of age. In experiments assessing the ability of H. influenzae to infect rats that were 30 days of age, pups were weaned at 21 days of age and group housed (n = 3/cage) prior to infection. Rats were maintained in a biosafety level 2 room within the Animal Facility of the University of Oklahoma Health Sciences Center. The protocol for usage of animals in these studies was reviewed and approved by the Institutional Animal Use and Care Committee of the University of Oklahoma Health Sciences Center.
Procedures for infection, obtaining blood specimens, and quantifying bacteremia.
The infant rat model for hematogenous meningitis following intraperitoneal (i.p.) infection with H. influenzae (61) was used to compare the abilities of strain HI689, strain HI1717, and their respective ΔhxuC mutant derivatives (strains HI1787 and HI1810) to produce invasive disease. Infant rat pups were challenged at 5 days of age in the first part of this study. In subsequent studies we evaluated the abilities of these strains to produce bacteremia after challenge of weanling rat pups at 30 days of age.
Inocula were prepared from broth cultures incubated as described above. Bacterial suspensions were pelleted by centrifugation, washed once with phosphate-buffered saline (PBS) containing 0.1% gelatin, and then resuspended in the same buffer. The suspension was adjusted to an A605 of 0.50 and then diluted serially in the same solution to provide the standard inoculum (200 CFU in 100 μl) that was injected intraperitoneally into the rat pups. The actual infective dosage was confirmed in each experiment by triplicate platings on sBHI agar. Colony counts were made after the plates were incubated at 37°C for 24 h. At 24-h intervals, pups were examined for signs of infection (neurological symptoms: tremor, loss of righting ability, coma, rigidity; systemic symptoms: immobility/lethargy, anorexia, poor grooming/ruffled fur, hypothermia). Blood specimens (50 μl) were obtained from anesthetized animals (gaseous halothane; Halocarbon Laboratories, River Edge, N.J.) by cardiac puncture (61).
The method used for quantifying bacteremia caused by the infection of infant rats with H. influenzae was based on the track-dilution procedure of Jett et al. (29). Serial dilutions (0 to 10−5) of whole-blood specimens freshly drawn in heparinized syringes were made with PBS containing 0.1% gelatin. Aliquots of 10 μl from each dilution were plated in triplicate on sBHI agar in square polystyrene plates (100 by 15 mm) with grids (Fisher Scientific). Colonies were counted after incubation at 37°C for 24 h. Clearance or failure to establish bacteremia was confirmed by plating five 10-μl aliquots of heparinized blood on at least two subsequent days. The sensitivity limit for the detection of bacteremia was 20 CFU/ml blood. To provide assurance (in addition to colony morphology and appearance as well as characteristic odor) that the colonies counted in the blood specimens were indeed H. influenzae and of the appropriate strain, colonies from at least half of the bacteremic pups infected with each strain were routinely screened for their antibiotic resistance profiles and occasionally for their serotype (positive response to H. influenzae antiserum type b [Difco Laboratories]). All bacteremic blood specimens were found to contain only H. influenzae of the appropriate phenotype.
Histopathology.
In addition to the occurrence of tremor and mild seizure activity as indices of meningitis in infant rats, we confirmed the occurrence of meningitis histologically in a cohort of pups. For histopathological identification of meningitis, rat pups (n = 10/strain) 5 days of age were challenged with 200 CFU of either strain HI689 or strain HI1717 and sacrificed 48 h later (61). Specimens from individual animals were fixed in 10% buffered neutral formalin at room temperature. The skulls were decalcified and imbedded in paraffin, and coronal sections 6 μm thick were cut and stained with hematoxylin and eosin. The specimens were evaluated by a veterinary pathologist blinded to the infecting strain. Meningitis was defined as the presence of inflammatory cells within the meninges or in the subarachnoid space.
Immunoblotting to identify free haptoglobin and hemoglobin-haptoglobin complexes.
At the time these experiments were conducted, no enzyme-linked immunosorbent assay (ELISA) kits for quantification of rodent haptoglobins were commercially available. Our method was based upon general immunoblotting methods described by Harlow and Lane (23). The primary antihaptoglobin antibody, rabbit anti-human haptoglobin antibody (AXL153 lot 124; Accurate Chemicals and Scientific Corp., Westbury, N.Y.), was selected for use in our studies because it cross-reacts with haptoglobins from a number of mammalian species and with both free haptoglobin and hemoglobin-haptoglobin complexes. Human haptoglobin type 1-1 at a concentration of 1 mg/ml was used as a standard on each blot. All solutions were prepared immediately prior to use. For dot blots, samples (1 μl diluted in 4 μl of PBS) were applied to a nitrocellulose membrane (0.45 μm; Osmonics, Inc., Westborough, Mass.) prewetted with H2O and were adsorbed by wicking rather than vacuum filtration. Purified human haptoglobin (type 1-1; Sigma-Aldrich, St. Louis, Mo.) served as a positive control. The membrane was washed three times for 10 min with 50 ml PBS and then was blocked for 2 h with 50 ml of 2% dried nonfat cow's milk in PBS. After washing with PBS three times for 10 min, the membrane was incubated for 1 h with the primary antibody diluted 1:10,000 (5 μl antibody in 50 ml PBS). The membrane was washed four times at 10-min intervals in PBS and incubated with a 1:16,000 dilution in PBS of the secondary antibody, goat antirabbit immunoglobulin G conjugated to horseradish peroxidase (Sigma-Aldrich), for 1 h. The membrane was again washed four times at 10-min intervals with PBS and placed on Saran Wrap, and antibody binding was detected by enhanced chemiluminescence (ECL detection reagents; Amersham Biosciences Corp., Piscataway, N.J.). The blot wrapped in Saran Wrap was placed on Cl-Xposure X-ray film (Pierce Biotechnology, Inc., Rockford, Ill.) in a cassette and exposed for 10 to 60 seconds prior to developing. Plasma samples used to evaluate changes in the haptoglobin concentration with developmental age of rat pups (n = 5 for each age) were prepared from nonhemolyzed blood specimens with sodium citrate as the anticoagulant. These samples were stored at −70°C until use and thawed only a single time.
Isolation and purification of rat haptoglobin by affinity chromatography.
Rat haptoglobin also was purified by us for in vivo experiments requiring a large amount of this plasma protein. Haptoglobin was purified from citrated plasma from adult rats (≥350 g) by the affinity chromatography method of Delers et al. (11) employing chicken hemoglobin bound to Sepharose as a selective ligand. To prepare the affinity column, cyanmet chicken hemoglobin (250 mg) was coupled to CNBr-activated Sepharose 4B (Amersham Pharmacia Biosciences Corp.) at room temperature for 60 min according to the procedure recommended in the manufacturer's package insert. Active groups remaining on the Sepharose beads were then blocked by incubation with 0.1 M Tris-HCl buffer, pH 8.0, overnight at 4°C. The gel suspension was poured into a column (bed, 1 by 22 cm) and washed extensively with 0.1 M Tris-HCl buffer, pH 8.0, containing 0.5 M NaCl to remove noncovalently bound hemoglobin, and the column was equilibrated with 150 ml of 0.05 M Tris-HCl buffer, pH 7.0, containing 0.15 M NaCl. Then, rat plasma (20 ml diluted 1:1 with 0.05 M Tris-HCl buffer, pH 7.0, containing 0.15 M NaCl) was incubated with the column gel for 30 min at 4°C, and the column was eluted first with 300 ml 0.05 M Tris-HCl buffer, pH 7.0, containing 0.15 M NaCl, followed by a second elution with 300 ml 0.05 M Tris-HCl buffer, pH 7.0, containing 0.5 M NaCl. Protein in the collected fractions was monitored by absorbance at 280 nm and elution of hemoglobin at 420 nm. Unbound or loosely bound proteins were eluted from the hemoglobin-Sepharose by these elution steps, which were continued until the absorbance at 280 nm returned to baseline. Then, haptoglobin was eluted with freshly prepared 8 M urea (Ultra Pure; ICN Biomedicals, Inc. Aurora, Ohio) dissolved in 0.05 M Tris-HCl buffer, pH 7.0. Fractions were collected at 7-min intervals at an initial flow rate of 0.5 ml/min. The fractions containing the haptoglobin peak eluted by 8 M urea were immediately pooled and then dialyzed in a Slide-A-Lyzer (Pierce, Rockford, Ill.) against 8 liters of PBS at 4°C for 24 h with two changes of the dialyzing solution. Subsequently the purity of the haptoglobin, its molecular weight, and its ability to bind rat hemoglobin (Sigma-Aldrich) were established by gel exclusion chromatography on Sephacryl S-200, by denaturing and nondenaturing polyacrylamide gel electrophoresis, and by immunoblotting.
Statistics.
Bacteremic titers are expressed as geometric means, typically from groups of 10 animals. Statistical comparisons of mean bacteremic titers between two groups of animals infected with different strains were made with the Kruskal-Wallis test. The fraction of animals that exhibited a particular finding, e.g., bacteremia at a specific time after infection with a given strain, is expressed as a percentage based on a typical group size of at least 10 animals. Statistical comparison of the significance of percentage differences between two groups was made by the Fisher exact test. Analyses were performed with SigmaStat (SPSS Inc., Chicago, Ill.) and Analyze-It (Analyze-It Software, Ltd., Leeds, England) software. A P value of <0.05 was taken as statistically significant.
RESULTS
Virulence of wild-type strain HI689 in invasive disease production in infant rats.
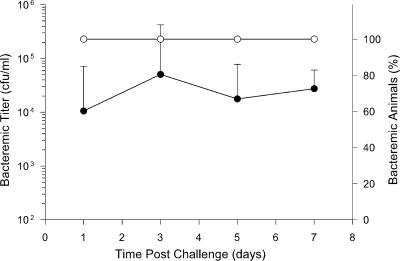
The wild-type strain HI689 was chosen for the current study for several reasons. This strain had been isolated from a patient with invasive disease and was a serotype b strain likely to produce invasive disease in this animal model, and the three nonidentical hgp genes present in its genome had been described. Because this strain's ability to produce invasive disease in 5-day-old infant rats was uncharacterized, we first established its virulence properties and the reliability of infection after intraperitoneal challenge with this animal model. An inoculum of 200 CFU produced bacteremia in 90 to 100% of pups in each of three different experimental cohorts (n = 10/cohort) by 24 h postchallenge. A representative experiment is shown in Fig. 1. Mean bacterial titers in blood specimens from infant animals challenged with strain HI689 were typically in the range of 1 × 104 to 5 × 104 CFU/ml 1 day after challenge. All pups that were bacteremic at 1 day postchallenge remained so for at least 1 week. Over the weeklong postchallenge period, mean bacterial titers in blood reached a maximum of ∼105 to 106 CFU/ml. This maximum typically occurred 3 to 5 days after challenge. Two control groups of infant rats, one receiving no injections and the other injected with the sterile vehicle (n = 10 each), failed to develop bacteremia (data not shown).
FIG. 1.
Demonstration of the ability of H. influenzae strain HI689 to produce bacteremia in infant rats challenged intraperitoneally with 200 CFU at 5 days of age. Values are expressed as geometric mean CFU per ml blood (filled circles) or the percentage of infant animals (n = 10) that were bacteremic at a specified time after challenge (open circles).
Following challenge, the infant rats were examined daily for additional abnormal neurological signs, including tremor, loss of righting ability, coma, and rigidity, as well as for nonspecific systemic signs of severe infection, including hypothermia, immobility/lethargy, anorexia, and in older pups, poor grooming/ruffled fur. Marked tremor associated with histopathologically documented meningitis occurred in 100% of challenged pups at 2 to 3 days after infection. These tremors were not observed in vehicle-injected or noninjected control animals. Severity and frequency of tremors declined with increasing age and were undetectable at 7 to 10 days after infection. Despite the high frequency of documented bacteremia and significant bacteremic titers for these pups, nonspecific clinical signs of severe responses to infection, other than meningitis, were an uncommon occurrence (≤5% of animals). In this minority of pups, bacteremic titers continued to increase to values of >5 × 106 CFU/ml. Such pups were severely debilitated and were euthanized.
H. influenzae is an uncommon but sometimes devastating cause of human endophthalmitis that frequently results in blindness (2). In infant rats, H. influenzae serotype b strains of independent origin are reported to cause endogenous endophthalmitis (46, 50, 61) that appears to stem from hematogenous seeding (50). Endophthalmitis was observed as a late clinical finding associated with systemic infection of infant rats by strain HI689. Upon opening their eyes (day 15 to 16 after birth) or shortly thereafter, 20% (6/30) of the bacteremic infant rats scored for this invasive infection exhibited unilateral or bilateral proptosis, an index of acute disease due to secondary glaucoma, and/or various degrees of corneal opacity. No clinically observable eye disease was noted in any of 30 control animals (n = 20 injected with vehicle; n = 10 noninjected). The fraction of animals with endophthalmitis after infection with strain HI689 differed significantly from that observed in uninfected controls (P = 0.01) but was similar to the 30% incidence reported by Myerowitz et al. for bacteremic pups infected intraperitoneally with an unrelated serotype b clinical isolate of H. influenzae (50).
Taken together, these observations demonstrate that the wild-type strain HI689 has retained the ability of the original clinical isolate to produce invasive disease. It reliably produced bacteremia, meningitis, and endophthalmitis in the infant rat.
Comparison of invasive disease production in the infant rat by the hgp null mutant strain HI1717 and its wild-type progenitor.
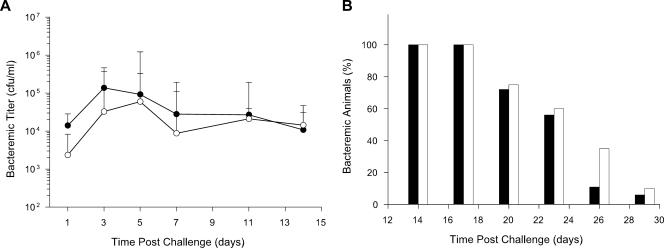
The complement of three hgp genes—hgpA, hgpB, and hgpC—present in strain HI689 was deleted to produce the isogenic hgp null mutant, strain HI1717. This mutant strain is unable to utilize hemoglobin-haptoglobin as a heme source for growth in vitro and has an impaired ability to utilize hemoglobin at low concentrations (43). Three different indices of bacteremia were used to compare the virulence of strains HI689 and HI1717 in the infant rat model. The ability to initiate bacteremia was established by the fraction of infant rats becoming bacteremic 24 h after challenge with 200 CFU of a single strain. The percentage of infant rats with demonstrable bacteremia after 24 h was very high (97%) for both strains (respectively, 57/59 and 56/58 pups for strain HI1717 and strain HI689 from 6 different experimental groups of pups for each strain). Bacterial titers at various times after challenge and clearance of bacteremia also were evaluated (Fig. 2). Mean bacterial titers in blood specimens from infant animals challenged with either strain HI689 or strain HI1717 did not differ from one another in a statistically significant manner. The outcome of a representative experiment is shown in Fig. 2A. Persistence of bacteremia brought about by these strains was noteworthy; nearly all infant rats that developed bacteremia remained bacteremic for at least 2 weeks. To determine if the clearance of the mutant strain was more rapid than that of the wild-type strain, a group of pups with verified bacteremia at 14 days after challenge was followed for an additional 15 days (Fig. 2B). The fraction of pups remaining bacteremic began to decline at 20 days postchallenge. H. influenzae was undetectable in ≥90% of their blood specimens 9 days later. No statistically significant reduction in the clearance rate of the strain HI1717 relative to that of strain HI689 was observed.
FIG. 2.
Comparison of the abilities of the H. influenzae wild-type strain HI689 and the isogenic hgp mutant strain HI1717 to initiate and sustain bacteremia in infant rats challenged at 5 days of age. (A) Bacteremic titers. In this cohort, infant animals (n = 10 for each strain) were challenged with either strain HI689 (filled circles) or strain HI1717 (open circles). Values are geometric mean CFU per ml blood. (B) Clearance of bacteremia. Blood samples were obtained at 3-day intervals from a group of pups all of which exhibited bacteremia 14 days after challenge with either strain HI689 (filled bars; n = 18) or strain HI1717 (open bars; n = 20). Values are the percentages of pups exhibiting bacteremia.
The occurrence of meningitis and endophthalmitis also was evaluated in infant rats after challenge by each of these two H. influenzae strains. A marked tremor was observed in infant rats 2 to 3 days following challenge with strain HI689 or strain HI1717. This clinical manifestation of meningitis was confirmed by histopathology. In a cohort of pups, 10/10 pups with marked tremor after challenge with strain HI689 had histopathological findings indicative of meningitis. Similarly, 9/10 pups challenged with strain HI1717 exhibited clinical and histopathological findings indicative of meningitis. The observed frequencies of occurrence of confirmed meningitis in pups infected with the two H. influenzae strains did not differ statistically. Additionally, we found no statistically significant difference in the frequency or severity of endophthalmitis among bacteremic rat pups infected with either of the two strains. The fraction of bacteremic pups developing endophthalmitis was 17% (5/30) after infection with strain HI1717 and 20% (6/30) after infection with the wild-type strain, HI689.
These observations indicated that deletion of the complement of hgp genes from H. influenzae did not attenuate virulence in invasive disease production in the infant rat challenged at 5 days of age. To clarify the cause(s) of this effect, we investigated several mechanisms that could contribute to the lack of effect of the hgp null mutant on virulence.
Hypohaptoglobinemia in the infant rat.
An assumption of the experiments described above was that the plasma haptoglobin concentration in infant rats is sufficiently high to complex all of the extracellular hemoglobin. If haptoglobin levels were low compared to hemoglobin concentrations in infant rat plasma, then free hemoglobin could potentially be available as a heme source. While hemoglobin-haptoglobin complexes cannot be utilized as a heme source for aerobic growth by the Δhgp mutant strain HI1717, this mutant and the wild-type strain can utilize free hemoglobin for growth in vitro, since this hemoprotein is utilized by both hgp-dependent and hgp-independent mechanisms (6, 39, 40, 43). Thus, a haptoglobin concentration insufficient to bind all or most of the extracellular hemoglobin might permit the Hgp-deficient mutant to proliferate in vivo. To determine if infant rats were hypohaptoglobinemic relative to adult animals, we used immunoblotting to quantify the relative amounts of haptoglobin present in plasma of infant rats at different ages.
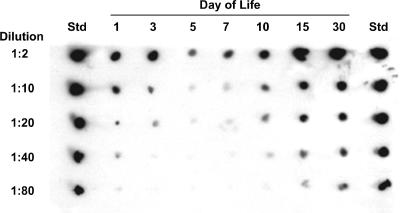
Rat haptoglobin, an α2β2 tetramer with a mass of ∼90 kDa, has the same quaternary structure as and high homology to the simplest polymorphic type of human haptoglobin, type 1-1 (20, 35). Because a commercial ELISA assay kit was not available for the quantification of rat haptoglobin at the time these experiments were undertaken, we first established that a commercially available rabbit anti-human haptoglobin (type 1-1) antibody cross-reacted with both free rat haptoglobin that we purified by affinity chromatography and rat haptoglobin bound to rat hemoglobin (data not shown). This antibody preparation was used to examine the relative concentrations of haptoglobin in plasma obtained from rats of different ages (n = 5/age). By immunoblotting and direct comparison to standards containing various concentrations of human haptoglobin type 1-1, we demonstrated that the haptoglobin concentration of plasma obtained from uninfected infant rats changed significantly as a function of postnatal age. A representative immunoblot is shown in Fig. 3. Haptoglobin was detectable in infant rats 1 day after birth. On subsequent days, plasma haptoglobin concentrations declined until a nadir was reached at 5 days of age, the same age at which animals typically are challenged in the infant rat model of invasive disease produced by H. influenzae. At the nadir, the plasma haptoglobin concentration for infant rats was at least 40-fold lower than we observed in adult rats. As postnatal age increased, plasma haptoglobin levels increased until adult levels were reached by about 14 days of age. The observed changes in the haptoglobin concentration suggested that hypohaptoglobinemia occurring coincidentally with the age at challenge might underlie the initial lack of effect of the hgp mutations on establishment of invasive disease.
FIG. 3.
Demonstration of hypohaptoglobinemia in plasma of 5-day-old infant rats by immunoblotting. Pooled plasma specimens from uninfected rat pups of the specified postnatal ages (n = 5 from different litters per pool) were diluted in PBS, and their relative haptoglobin concentrations (assessed as combined free and hemoglobin-bound forms) were determined by comparison to a similarly diluted human type 1-1 haptoglobin standard (1 mg/ml) using a commercial antihaptoglobin antibody. The immunoblot shown is representative of those observed in five separate experiments. The plasma haptoglobin concentration changed markedly with age and reached its lowest level at 5 days of age, returning to adult-like concentration by 2 weeks of age.
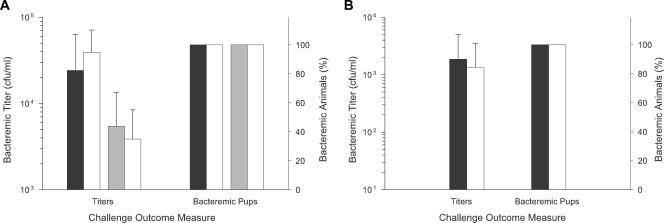
However, several additional findings negate the likelihood that low plasma haptoglobin concentrations in 5-day-old infant rats account for the ability of the Hgp-deficient strain to produce bacteremia (Fig. 4). If hypohaptoglobinemia leads to the increased availability of free hemoglobin at concentrations sufficient to support wild-type-like growth of strain HI1717, then administration of exogenous haptoglobin to infant rats should decrease hemoglobin availability by increasing the formation of hemoglobin-haptoglobin complexes. The resulting starvation of strain HI1717 for heme should reduce its growth in vivo. This was not observed. Neither the administration of human nor rat haptoglobin at the highest practical dosages (human type 1-1 haptoglobin [0.1 mg/pup i.p.] or rat haptoglobin [1 mg/pup i.p.]) to infant rats 2 h prior to challenge with strain HI1717 significantly reduced this strain's ability to produce subsequent bacteremia compared to vehicle-injected animals (Fig. 4A). Both bacterial titers in blood and the frequency of infant rats developing bacteremia were unaffected by prior administration of haptoglobin. Our observation that 14-day-old rat pups have significantly high, adult-like levels of plasma haptoglobin compared to 5-day-old infant animals provided another approach to testing this hypothesis that did not require exogenous haptoglobin injection. Upon challenge of pups this age with either strain HI689 or strain HI1717, both strains again exhibited comparable abilities to produce bacteremia (Fig. 4B).
FIG. 4.
Effect of exogenous administration of haptoglobin to infant rats and of the age-dependent increase in the plasma haptoglobin concentration on the ability of the H. influenzae Δhgp mutant strain HI1717 to produce bacteremia. (A) Exogenous haptoglobin administration. Human haptoglobin (0.1 mg/kg i.p.; black shaded bars), rat haptoglobin (1 mg/kg i.p.; gray shaded bars), or vehicle (PBS; open bars) was injected i.p. into 5-day-old infant rats (n = 9 to 10/treatment group) 2 h prior to challenge with strain HI1717. (B) Age dependence. Production of bacteremia by either strain HI689 (shaded bars) or strain HI1717 (open bars) in rats (n = 9 to 10) challenged at 2 weeks of age when plasma haptoglobin is adult-like in concentration. Titers are expressed as geometric mean CFU/ml.
An alternate source of heme in infant rat blood.
A plausible explanation for the absence of an effect of deleting the hgp genes on virulence in this animal model is that infant rat plasma contains one or more heme sources in addition to hemoglobin-haptoglobin. Release of heme from methemoglobin could result in the presence of free heme if plasma levels of hemopexin and albumin in 5-day-old infant rats are low or formation of heme-hemopexin and heme-albumin complexes if their concentrations are high. Aerobic growth of H. influenzae in vitro is supported by each of these heme sources by mechanisms other than those involving the Hgps (38, 40, 63). The hypothesis that plasma from infant rats contains heme sources capable of supporting the growth of strain HI1717 was evaluated first in plate bioassays on hdBHI agar (Table 2). As expected, the two strains failed to grow in vitro in the absence of a heme source but did so when supplied with heme or hemoglobin. Hemoglobin-haptoglobin supported the growth of the wild-type strain but not of strain HI1717. Nonhemolyzed plasma specimens from multiple rat pups also supported the growth of both strains. This finding indicated plasma specimens from infant rats contained one or more heme sources that could be utilized by the Hgp-deficient strain.
TABLE 2.
Abilities of various purified heme sources and plasma from infant rats 5 days of age to support the growth of H. influenzae strains in plate bioassaysa
| Strain | Genotype | Growth of strain with:
|
|||
|---|---|---|---|---|---|
| Heme | Hb | Hb-Hp | Plasma | ||
| HI689 | hgpA+hgpB+hgpC+hxuC+ | + | + | + | + |
| HI1717 | ΔhgpA ΔhgpB ΔhgpC hxuC+ | + | + | − | + |
| HI1787 | hgpA+hgpB+hgpC+ ΔhxuC | + | + | + | + |
| HI1810 | ΔhgpA ΔhgpB ΔhgpC ΔhxuC | + | − | − | − |
Concentrations were as follows: heme, 100 μg/ml; Hb (human hemoglobin), 500 μg/ml; Hb-Hp (human hemoglobin-haptoglobin), 500 μg/ml (hemoglobin equivalent); plasma (nonhemolyzed), undiluted. Rat Hb and Hb-Hp supported growth of the strains in a manner similar to that of their respective human forms. Symbols: +, growth; −, no growth after 24 to 48 h of incubation at 37°C.
The hxuCBA gene cluster has been implicated in binding and utilization of low concentrations of free heme, heme-hemopexin complexes, and hemoglobin but does not mediate utilization of hemoglobin-haptoglobin as a heme source (6, 7, 9, 40). To further verify the presence of plasma heme sources in addition to hemoglobin-haptoglobin, we constructed two additional isogenic mutants of strain HI689, an hxuC mutant (strain HI1787) and a quadruple mutant in which the hgp genes and the hxuC gene were deleted (strain HI1810). Both mutants grew well in vitro in the presence of high concentrations of heme or hemoglobin (Table 2). This observation showed that the quadruple mutant was not generally growth impaired. Infant rat plasma also supported the growth in vitro of strain HI1787 but did not support the growth of strain HI1810 (Table 2). These findings indicated that infant rat plasma contains heme sources whose utilization proceeds via mechanisms involving both the Hgp- and HxuC-mediated pathways of heme acquisition. Involvement of an additional (alternative) pathway for heme acquisition was unlikely, because infant rat plasma failed to support the growth of the quadruple mutant.
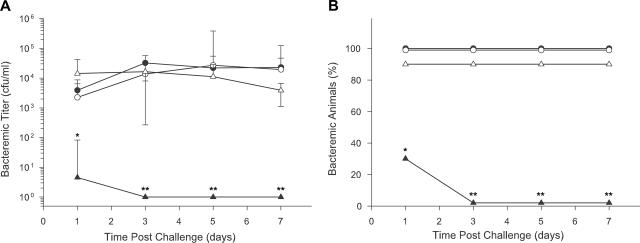
To determine if these observations made in vitro were relevant to heme source availability in vivo, we next compared the virulence of strains HI689, HI1717, HI1787, and HI1810 in infant rats challenged at 5 days of age (Fig. 5). A cohort of 40 infant rats was divided into four groups (n = 10/group), and each group was infected with 200 CFU of a single strain. Bacteremic titers (Fig. 5A), the percentages of pups exhibiting bacteremia (Fig. 5B), and the duration of bacteremia were compared across infecting strains. Three strains—the wild-type strain HI689, the Δhgp strain HI1717, and the ΔhxuC strain HI1787—produced bacteremia in infant rats within 24 h after infection (Fig. 5A) and maintained titers of ∼104 to 105 CFU/ml for 7 days postchallenge. As shown in Fig. 5B, 90 to 100% of infant rats challenged with these strains remained bacteremic for at least a week. In contrast, strain HI1810 carrying null mutations in both its hgp and hxuC genes exhibited a significant reduction in virulence compared to each of these strains. After challenge with the latter strain, the titer of bacteria in blood of infant animals was significantly lower (P < 0.01) 24 h after challenge (Fig. 5A) and the fraction of pups developing bacteremia was significantly reduced (30%; P < 0.003) compared to results with the wild-type, Δhgp, and ΔhxuC strains (Fig. 5B). By the third postchallenge day, strain HI1810 had been cleared from the blood of all pups; this difference in the persistence of bacteremia between strain HI1810 and the other strains was highly statistically significant (P < 0.0001). As expected from their decreased bacteremia, infant rats infected with strain HI1810 did not exhibit the tremors associated with meningitis, in contrast to rats infected with each of the other strains. These in vivo findings support the previous in vitro evidence suggesting the availability of more than one heme source to H. influenzae in the infant rat and indicate that sufficient heme to support growth of H. influenzae in vivo can be acquired through either the Hgp- or Hxu-mediated pathway.
FIG. 5.
Effect of an hxuC null mutation in combination with an hgp+ or Δhgp genetic background on the ability of H. influenzae to initiate and sustain bacteremia in the infant rat. The outcome of a representative experiment employing four isogenic strains (HI689 [hgp+ hxuC+], HI1717 [Δhgp hxuC+], HI1787 [hgp+ ΔhxuC], and HI1810 [Δhgp ΔhxuC]) to individually challenge infant rats 5 days of age (n = 9 to 10/strain) is shown. (A) Bacteremic titers. Values are expressed as geometric mean CFU per ml of blood. Zero values were arbitrarily assigned a value of 1 in order to calculate the geometric means for strain HI1810. The same symbols are used to identify these strains in both panels: HI689, filled circles; HI1717, open circles; HI1787, open triangles; HI1810, filled triangles. Statistical significance of the decrease in titers of infant rats challenged with strain HI1810 versus each of the other strains: *, P < 0.01; **, P < 0.0001. (B) Frequency of infant rats developing bacteremia. Statistical significance of the decrease in bacteremic pups challenged with strain HI1810 versus results with each of the other strains: *, P < 0.003; **, P < 0.0001.
Decreased ability of plasma from rat pups 30 days of age to support growth of hgp and hxuC mutants.
The observation that the plasma haptoglobin concentration changes as a function of age alerted us to the possibility that heme source availability might change with age as well. Susceptibility of infant rats to invasive infection by H. influenzae decreases with increasing age but is unrelated to serum bactericidal antibodies (61). If maturation of components of innate immunity limits availability of nutrients, such as iron and heme, to invading bacteria, older rats, such as weanlings, might provide a more stringent environment for heme acquisition and be a more sensitive indicator of the effects of hgp and hxuC mutations on virulence of H. influenzae. When the abilities of plasma samples obtained from infant and weanling rats (5 and 30 days old, respectively) to support growth of strains HI689, HI1717, HI1787, and HI1810 were compared in plate bioassays, plasma from weanling rats, unlike infant specimens, failed to support growth of any of the strains (Table 3). Mixtures (1:1 [volume/volume]) of plasma specimens from infant and weanling rats supported the growth of strain HI689, HI1717, or HI1787. These findings suggest that plasma from weanling animals is deficient in one or more heme sources rather than containing a growth inhibitor. While it is currently unclear whether this pathogen experiences a similar variation in heme source availability in the human host (since the identities and concentrations of heme sources in both humans and rats are so incompletely characterized), this seems possible, because plasma haptoglobin and hemopexin concentrations vary with developmental age in humans (32).
TABLE 3.
Evidence from plate bioassays that heme sources in infant rat plasma supporting growth of H. influenzae become restricted with increasing agea
| Strain | Genotype | Growth of strain with:
|
|||
|---|---|---|---|---|---|
| Heme | Hb | Plasma
|
|||
| 5-day pups | 30-day pups | ||||
| HI689 | hgpA+hgpB+hgpC+hxuC+ | + | + | + | − |
| HI1717 | ΔhgpA ΔhgpB ΔhgpC hxuC+ | + | + | + | − |
| HI1787 | hgpA+hgpB+hgpC+ ΔhxuC | + | + | + | − |
| HI1810 | ΔhgpA ΔhgpB ΔhgpC ΔhxuC | + | − | − | − |
Concentrations of the test substances were as follows: heme, 100 μg/ml; hemoglobin (Hb), 500 μg/ml; plasma, undiluted. Symbols: +, growth; −, no growth after 24 to 48 h of incubation at 37°C.
Bacteremia in weanling rats challenged at 30 days of age with strain HI689 or strain HI1717.
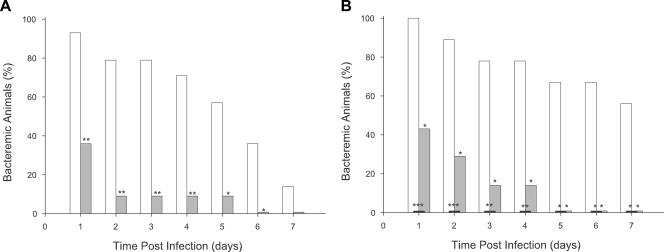
As anticipated from the in vitro growth studies, maximal bacteremic titers were significantly decreased and the clearance of bacteremia was markedly increased for both strains in weanling rats challenged at 30 days of age compared to results with infant rats challenged at 5 days of age. Low and quite variable titers of H. influenzae ranging from 0 to 103 CFU/ml were observed in blood specimens from weanlings 24 h after challenge with strain HI689 or HI1717. Because of this large interanimal variation in blood titers, a better indicator of the relative virulences of the two strains in these older animals was the percentage of pups becoming and remaining bacteremic after challenge. Strains HI689 and HI1717 differed significantly in their abilities to establish and sustain bacteremia in weanling rat pups. In a representative experiment (Fig. 6A), 93% of the weanlings challenged with strain HI689 became bacteremic 24 h after infection, whereas only 36% of the weanlings challenged with strain HI1717 were bacteremic (P < 0.01). The percentage of weanling animals exhibiting bacteremia after challenge with the wild-type strain remained significantly higher than that occurring after challenge with strain HI1717 for 6 days postinfection. In three independent experiments, at least 50% of pups challenged with strain HI689 remained bacteremic for 5 to 7 days. However, after challenge with strain HI1717, the percentage of bacteremic weanlings was ≤50% at 24 h and decreased to 0 to 10% thereafter. When weanling rats were coinfected with the HI689 and HI1717 strains, none of 50 H. influenzae colonies recovered from bacteremic rats had the antibiotic resistance profile expected for strain HI1717 (data not shown). This coinfection experiment independently confirmed the reduced ability of strain HI1717 to establish and sustain bacteremia relative to its wild-type progenitor. This reduced ability of strain HI1717 to cause bacteremia in weanling rats reflects the loss of function of the total complement of hgp genes rather than the loss of a single, selective Hgp, since isogenic double-mutant strains HI1714, HI1715, and HI1716, each expressing a single nonidentical hgp gene, were as effective as the wild-type strain in establishing and sustaining bacteremia in weanling rats (data not shown).
FIG. 6.
Evaluation of the relative abilities of the H. influenzae wild-type strain HI689 and its isogenic Δhgp, ΔhxuC, and Δhgp ΔhxuC derivatives to initiate and sustain bacteremia in weanling rats challenged at 30 days of age. (A) Comparison of the occurrence of bacteremia in 30-day-old pups challenged with either strain HI689 (open bars; n = 14) or strain HI1717 (shaded bars; n = 11). (B) Comparison of the occurrence of bacteremia in 30-day-old pups challenged with strain HI689 (open bars; n = 9), the ΔhxuC mutant strain HI1787 (gray shaded bars; n = 7), or the Δhgp ΔhxuC mutant strain HI1810 (black shaded bars; n = 7). Statistical significance of the decreased frequency of bacteremic pups between the wild-type and mutant strains: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Challenge of weanling rats with strain HI689, HI1787, or HI1810.
The effect of the ΔhxuC and Δhgp ΔhxuC mutations on the ability of H. influenzae to produce bacteremia in weanling rats was investigated with another cohort of animals (Fig. 6B). None of the rat pups infected with the quadruple-mutant strain HI1810 became bacteremic, while all pups infected with the wild-type strain, HI689, were bacteremic 1 day after infection. This difference in the percentage of bacteremic pups following challenge was highly statistically significant (P < 0.001). The rapid clearance of strain HI1810 from weanling rats compared to that of strain HI689 resembled that observed in infant rats challenged at 5 days of age. Virulence of strain HI1787 also was attenuated in weanling rats. One day after challenge with this hxuC null mutant, fewer than half of the weanling pups exhibited bacteremia (P < 0.05 compared to results with the wild-type strain). Subsequent clearance of bacteremia caused by strain HI1787 was more rapid than that of bacteremia caused by the wild-type strain. The observed detrimental effect of the ΔhxuC mutation carried by strain HI1787 in weanling rats contrasted with the lack of its effect on bacteremia production in infant rats. Together these findings suggest that heme sources present in infant rats are utilized via the Hgp- and/or the HxuC-mediated acquisition pathway of H. influenzae and that these sources subsequently decrease in availability as the rat pups mature.
Effect of an exogenous heme source on clearance of strain HI1717 from weanling rats.
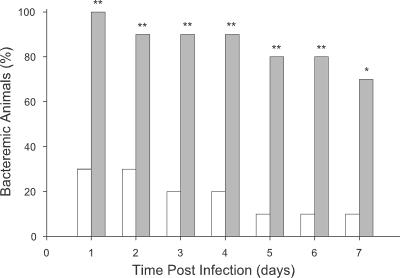
If limitation of heme source availability contributes to the increased clearance of the Δhgp strain from weanling rats, then administration of an exogenous heme source whose utilization is not dependent on Hgp function should enhance the ability of this strain to produce bacteremia. Hemoglobin administration had been shown by others to increase the virulence of pathogenic bacteria in the rat (14). Using the procedure of Eaton et al. (14), we first established that intraperitoneal injection of a single hemoglobin dose (20 mg/rat) had no overt adverse clinical effects on the animals as judged by the indices of weight gain, water and food consumption, maintenance of grooming, or activity level (data not shown). Administration of human hemoglobin prior to challenge with strain HI1717 led to a statistically significant increase in the percentage of animals developing bacteremia compared to results for vehicle-treated controls (from 30% to 100%; P < 0.01) and extension of the duration of bacteremia (from a t1/2 value of <1 day to >7 days; P < 0.05) (Fig. 7). This result is consistent with the apparent decrease in plasma heme source availability shown by the in vitro studies described earlier and provides further support for the view that in vivo availability of heme sources utilized by non-Hgp-mediated pathways becomes growth limiting with increased age.
FIG. 7.
Effect of human hemoglobin administration on the reduced ability of strain HI1717 to produce and sustain bacteremia in weanling rats. Two hours prior to challenge, rats 30 days of age (n = 10 for each treatment group) were injected i.p. with either 1 ml of sterile vehicle (open bars) or filter sterilized human hemoglobin solution (20 mg/ml; shaded bars). Subsequently the same inoculum of strain HI1717 was used to challenge rats of both treatment groups. Statistical significance of the increased frequency of bacteremic pups between treatment groups: *, P < 0.05; **, P < 0.01.
DISCUSSION
The present study was undertaken to determine whether heme acquisition via the hemoglobin and hemoglobin-haptoglobin binding proteins of an H. influenzae serotype b strain contributes to virulence in the infant rat model of invasive disease. The infant rat model of hematogenous meningitis has been widely used to investigate the influence of bacterial virulence factors, host protective factors, and vaccine candidates on invasive disease caused by H. influenzae (21, 26, 39, 61, 66). Few investigations have examined the roles of hemoglobin and hemoglobin-haptoglobin binding proteins encoded by H. influenzae or by other bacterial species in proliferation in vivo and in pathogenesis (10, 28, 38, 62).
In a prior study we characterized the contribution of hgp genes to bacterial proliferation and the severity of pathological changes in middle ear infections caused by a nontypeable strain (86-028 NP) of H. influenzae in the chinchilla model of otitis media (38). This is a widely accepted model of noninvasive disease caused by H. influenzae (1, 24, 56). An isogenic mutant strain from which the set of three hgp genes had been deleted exhibited a significant reduction in virulence compared to its wild-type progenitor. Onset of bacterial proliferation in the middle ear was delayed, maximal bacterial load in ear fluid was reduced, and the duration of middle ear infection was shortened in animals infected with the mutant strain compared to results with those infected with the wild-type strain. The reduction in growth of the mutant strain in vivo was associated with a reduced severity of ear pathology associated with otitis media. While these observations established a role for the hgp genes in heme acquisition in vivo and in virulence of H. influenzae in otitis media, it was clear that this nontypeable strain could acquire heme from sources other than hemoglobin-haptoglobin in the middle ear of the chinchilla. These alternative heme sources supported submaximal proliferation of H. influenzae in the middle ear. Thus, the complexity and redundancy of heme acquisition mechanisms possessed by H. influenzae are likely to reflect the availability of particular heme sources or combinations of sources in the various niches occupied by this opportunistic pathogen when it enters the human body.
Here we evaluated the contribution of the hgp genes to the production of invasive disease in infant rats challenged with the H. influenzae strain HI689, a clinical isolate from a patient with bacteremia. Like strain 86-028 NP, this strain contains three nonidentical hgp genes (41, 43). The virulence of this wild-type clinical isolate was established by its ability to produce bacteremia of long duration (up to 30 days), endophthalmitis, and histopathologically confirmed meningitis in infant rats infected at 5 days of age. An isogenic mutant derivative (strain HI1717) from which the complement of hgp genes had been deleted was unimpaired in its ability to produce invasive disease in this animal model. The presumption that deletion of the hgp genes would decrease virulence was based upon the expectation that heme sources other than hemoglobin-haptoglobin would be insufficient to meet the heme/iron requirements for proliferation of H. influenzae in infant rats.
Acquisition of alternative heme sources in the extracellular milieu of the infant rat via Hgp-independent pathways would explain the lack of a detrimental effect of hgp gene deletions on production of invasive disease in this model. Several lines of evidence support this explanation. (i) At least one alternative heme source must be available to H. influenzae systemically infecting the infant rat, because the ability to grow aerobically is critical for the establishment of invasive disease by H. influenzae in this model (26) and H. influenzae requires heme for aerobic growth (15, 53). Hemoglobin-haptoglobin, while serving as a heme source for the wild-type strain, does not support the aerobic growth of the Δhgp mutant strain in vitro. (ii) Because the hxu operon of H. influenzae plays an important role in heme acquisition from hemoglobin and heme-hemopexin but not from hemoglobin-haptoglobin (6-9, 40), we also investigated the impact on invasive disease of an isogenic hxuC deletion mutant (strain HI1787) and its combination with deletion of the hgp gene complement (strain HI1810). The hxuC mutant exhibited a phenotype in vitro (reduced growth in medium supplemented with low concentrations of heme or hemoglobin) but was unimpaired in its ability to produce invasive disease in infant rats. However, virulence of the Δhgp ΔhxuC quadruple mutant was markedly impaired. These findings indicate that heme acquisition by H. influenzae in the infant rat proceeds by both pathways, each of which alone is sufficient to support a wild-type level of virulence. It is unclear whether the in vivo effect of the hxuC mutation arose solely from the inactivation of this gene or from polar effects on expression of the downstream hxuBA genes, since complementation of the hxuC mutation was not evaluated. (iii) Nonhemolyzed plasma specimens obtained from infant rats contained heme sources capable of supporting growth in vitro of the wild-type strain and of mutant strains lacking either a functional hgp or hxuC gene but failed to support the growth of the strain lacking both heme acquisition pathways. It is unclear whether infant rat plasma contains a single heme source (e.g., hemoglobin) that can be acquired by both the Hgp- and Hxu-mediated pathways or multiple heme sources whose acquisition is mediated separately by independent pathways. Identification of the number and types of the heme sources present in plasma and other body fluids of the infant rat was beyond the scope of the present study.
Changes in hematological values, iron absorption, and regulation of genes involved in iron/heme homeostasis are known to occur with age during infancy in both humans and rats (12, 13, 32, 36, 51). Among proteins involved in iron/heme scavenging, plasma haptoglobin levels are very low in newborn human infants and do not reach adult-like levels until approximately 6 months of age (32). Significant hemopexin levels occur even in newborns (32). Such developmental and individual changes have the potential to markedly influence the concentration and spectrum of heme sources available to infecting H. influenzae. Hemoglobin released by the spontaneous intravascular lysis of red blood cells in normal individuals is bound with high affinity by haptoglobin present in plasma at concentrations (0.5 to 2 mg/ml) far in excess of free hemoglobin (∼1 to 10 μg/ml) (27, 33, 35). Hemoglobin-haptoglobin complexes are bound by the CD163 receptor on monocytes and macrophages and removed from blood (33). Severe hypohaptoglobinemia could result in the accumulation of elevated plasma levels of hemoglobin and/or heme-hemopexin if heme is released from methemoglobin, and this could contribute to the presence of multiple alternative heme sources in blood of infant rats. We did observe significant changes in the plasma haptoglobin concentration with developmental age in the infant rat. In the 2 days following birth, haptoglobin concentrations in plasma were high, consistent with the reported overexpression of the liver haptoglobin gene during the transition from intrauterine to extrauterine existence (19). The haptoglobin concentration subsequently decreased and reached a nadir (a >40-fold decrement compared to plasma levels observed for neonatal rat pups or adult rats) at 5 days of age, precisely the age at which infant rats are usually challenged with H. influenzae in this model. By 2 weeks of age, the plasma haptoglobin concentration increased to adult-like levels. A prior publication in the German literature utilizing a different method described a similar age-dependent change in the plasma haptoglobin concentration for infant rats (37). However, the transient hypohaptoglobinemia in 5-day-old infant rats that we observed did not in itself account for the failure of the multiple Δhgp mutations in strain HI1717 to reduce virulence. Nevertheless, the identification of large quantitative, age-dependent changes in haptoglobin alerted us to the possibility that the heme sources present in infant rats might also change with age.
Another focus of the present study was to determine whether functional hgp and hxuC genes were necessary for the production of invasive disease in older animals. An age-dependent decline in the incidence and severity of invasive disease produced by H. influenzae is known to occur with the infant rat model (34, 61). Older animals often develop transient bacteremia when challenged with H. influenzae, but bacterial titers in blood are lower and meningitis does not occur. We reasoned that an age-dependent increase in the stringency of heme source limitation might contribute to the reduction in susceptibility to invasive infection by H. influenzae in older animals and speculated that mutants deficient in specific heme acquisition pathways should be at a considerable disadvantage compared to a wild-type strain under these conditions. To test this hypothesis, we challenged a cohort of 30-day-old weanling rats with either the wild-type strain HI689, the hgp null mutant strain HI1717, the hxuC null mutant strain HI1787, or the hgp hxuC quadruple mutant strain HI1810. Nearly all weanling pups developed bacteremia after challenge with the wild-type strain or mutants expressing a single functional hgp gene. In contrast, the virulence of the mutants lacking one or both of the heme acquisition pathways was severely impaired. Reduced virulence of the Δhgp strain HI1717 was confirmed by its failure to compete with the wild-type strain in weanling rats coinfected with the two strains. Administration of hemoglobin (a heme source whose acquisition proceeds by multiple pathways [6, 39, 40, 43]) to pups prior to challenge significantly enhanced the ability of strain HI1717 to produce bacteremia. Taken together, these observations indicate that heme source availability to H. influenzae decreases with age in the infant rat, and they establish that both the hgp genes and the gene(s) of the hxu operon play a vital role in heme acquisition to support the production of invasive disease in weanling rats.
In summary, the H. influenzae outer membrane proteins binding hemoglobin and hemoglobin-haptoglobin (the Hgps) and hemoglobin and heme-hemopexin (HxuC) each contribute importantly to bacterial proliferation during invasive infection of infant rats. Evidence from both in vitro and in vivo experiments provides support for the presence of multiple heme sources in infant rat plasma that decline in concentration with age. This is the first report demonstrating that either of two different heme acquisition pathways suffices for the establishment and/or persistence of H. influenzae invasive infections in infant animals. Our findings also indicate that mutations disrupting either pathway significantly decrease the limited ability of H. influenzae to initiate and sustain invasive disease in weanling rats. Thus, both the hgp genes and the hxu gene(s) are virulence determinants in the rat model of human invasive disease. The demonstration of important functions of the Hgp-mediated pathway for two animal models of human disease, the infant rat model of hematogenous meningitis and the chinchilla model of otitis media, and the documented occurrence of hgp genes in all clinical isolates of H. influenzae evaluated to date further support the potential of the Hgps as vaccine candidates for prevention of infections caused by both typeable and nontypeable strains of H. influenzae.
Acknowledgments
We thank Larissa Madore for skillful assistance in the construction of certain mutant strains used in this study and Scotty Shriner for technical assistance with in vitro growth studies, haptoglobin immunoblotting, and the isolation and purification of rat hemoglobin and haptoglobin.
This work was supported by Public Health Service grant AI29611 to T.L.S. from the National Institute of Allergy and Infectious Disease. Support of the Children's Medical Research Institute of Oklahoma also is gratefully acknowledged.
Editor: F. C. Fang
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Alper, C. M., A. Andalibi, L. O. Bakaletz, C. Buchman, P. Caye-Thomasen, S. O. Hellstrom, P. Herman, A. Hermansson, B. Hussl, Y. Iino, H. Kawauchi, M. M. Paparella, I. Sando, J. D. Swarts, and T. Takasaka. 2002. Recent advances in otitis media. 4. Anatomy, cell biology, pathology, and animal models. Ann. Otol. Rhinol. Laryngol. 111(Suppl. 188):36-51. [DOI] [PubMed] [Google Scholar]
- 2.Alrawi, A. M., K. C. Chern, V. Cevallos, T. Lietman, J. P. Whitcher, T. P. Margolis, and E. T. Cunningham. 2002. Biotypes and serotypes of Haemophilus influenzae ocular isolates. Br. J. Opthalmol. 86:276-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajanca, P., M. Canica, and The Multicenter Study Group. 2004. Emergence of nonencapsulated and encapsulated non-b-type invasive Haemophilus influenzae isolates in Portugal (1989-2001). J. Clin. Microbiol. 42:807-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, J. R., and M. E. Pichichero. 2004. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr. Infect. Dis. J. 23:824-828. [DOI] [PubMed] [Google Scholar]
- 5.Cope, L. D., Z. Hrkal, and E. J. Hansen. 2000. Detection of phase variation in expression of proteins involved in hemoglobin and hemoglobin-haptoglobin binding by nontypeable Haemophilus influenzae. Infect. Immun. 68:4092-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope, L. D., R. P. Love, S. E. Guinn, A. Gilep, S. Usanov, R. W. Estabrook, Z. Hrkal, and E. J. Hansen. 2001. Involvement of HxuC outer membrane protein in utilization of hemoglobin by Haemophilus influenzae. Infect. Immun. 69:2353-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope, L. D., S. E. Thomas, Z. Hrkal, and E. J. Hansen. 1998. Binding of heme-hemopexin complexes by soluble HxuA protein allows utilization of this complexed heme by Haemophilus influenzae. Infect. Immun. 66:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope, L. D., S. E. Thomas, J. L. Latimer, C. A. Slaughter, U. Muller-Eberhard, and E. J. Hansen. 1994. The 100 kDa haem:haemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol. Microbiol. 13:863-873. [DOI] [PubMed] [Google Scholar]
- 9.Cope, L. D., R. Yogev, U. Muller-Eberhard, and E. J. Hansen. 1995. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J. Bacteriol. 177:2644-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox, A. J., M. L. Hunt, J. D. Boyce, and B. Adler. 2003. Functional characterization of HgbB, a new hemoglobin binding protein of Pasteurella multocida. Microb. Pathog. 34:287-296. [DOI] [PubMed] [Google Scholar]
- 11.Delers, F., C. Lombart, M. Domingo, and S. Musquera. 1981. A novel and specific method for the purification of hemoglobin-binding proteins. Anal. Biochem. 118:353-357. [DOI] [PubMed] [Google Scholar]
- 12.Dewhirst, F. E., B. J. Paster, I. Olsen, and G. J. Fraser. 1992. Phylogeny of 54 representative strains of species in the family Pasteurellaceae as determined by comparison of 16S rRNA sequences. J. Bacteriol. 174:2002-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubuque, S. H., B. Dvorak, S. S. Woodward, R. S. McCuskey, and P. J. Kling. 2002. Iron-deficient erythropoiesis in neonatal rats. Biol. Neonate 81:51-57. [DOI] [PubMed] [Google Scholar]
- 14.Eaton, J. W., P. Brandt, J. R. Mahoney, and J. T. Lee Jr. 1982. Haptoglobin: a natural bacteriostat. Science 215:691-693. [DOI] [PubMed] [Google Scholar]
- 15.Evans, N. M., D. D. Smith, and A. J. Wicken. 1974. Haemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae and Haemophilus parainfluenzae. J. Med. Microbiol. 7:359-365. [DOI] [PubMed] [Google Scholar]
- 16.Evans, R. W., J. B. Crawley, C. L. Joannou, and N. D. Sharma. 1999. Iron proteins, p. 27-86. In J. J. Bullen and E. Griffiths (ed.), Iron and infection: molecular, physiological and clinical aspects. John Wiley & Sons, Inc., New York, N.Y.
- 17.Garcia-Rodriguez, J. A., and M. J. Fresnadillo Martinez. 2002. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J. Antimicrob. Chemother. 50(Suppl. S2):59-73. [DOI] [PubMed] [Google Scholar]
- 18.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial haem capture. Mol. Microbiol. 39:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Glibetic, M., D. Bogojevic, S. Matic, and L. Sevaljevic. 1992. The expression of liver acute-phase protein genes during rat development and in response to inflammation of the dam. Differentiation 50:35-40. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein, L. A., and E. C. Heath. 1984. Nucleotide sequence of rat haptoglobin cDNA. Characterization of the αβ-subunit junction region of prohaptoglobin. J. Biol. Chem. 259:9212-9217. [PubMed] [Google Scholar]
- 21.Granert, C., H. Abdalla, L. Lindquist, A. Diab, M. Bahkiet, K. J. Tracey, and J. Andersson. 2000. Suppression of macrophage activation with CNI-1493 increases survival in infant rats with systemic Haemophilus influenzae infection. Infect. Immun. 68:5329-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gromkova, R. C., P. B. Rowji, and H. J. Koornhof. 1989. Induction of competence in nonencapsulated and encapsulated strains of Haemophilus influenzae. Curr. Microbiol. 19:241-245. [Google Scholar]
- 23.Harlow, E., and D. Lane. 1999. Immunoblotting, p. 267-309. In Using antibodies; a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Harris, R. H., D. Wilk, C. L. Bevins, R. S. Munson, Jr., and L. O. Bakaletz. 2004. Identification and characterization of a mucosal antimicrobial peptide expressed by the chinchilla (Chinchilla lanigera) airway. J. Biol. Chem. 279:20250-20256. [DOI] [PubMed] [Google Scholar]
- 25.Harrison, A., D. W. Dyer, A. Gillaspy, W. C. Ray, R. Mungur, M. B. Carson, H. Zhong, J. Gipson, M. Gipson, L. S. Johnson, L. Lewis, L. O. Bakaletz, and R. S. Munson, Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187:4627-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbert, M., A. Kraiss, A. K. Hilpert, S. Schlor, and J. Reidl. 2003. Aerobic growth deficient Haemophilus influenzae mutants are non-virulent: implications on metabolism. Int. J. Med. Microbiol. 293:145-152. [DOI] [PubMed] [Google Scholar]
- 27.Huang, G., J. Ouyang, J. R. Delanghe, W. R. Baeyens, and Z. Dai. 2004. Chemiluminescent image detection of haptoglobin phenotyping after polyacrylamide gel electrophoresis. Anal. Chem. 76:2997-3004. [DOI] [PubMed] [Google Scholar]
- 28.Jerse, A. E., E. T. Crow, A. N. Bordner, I. Rahman, C. N. Cornelissen, T. R. Moench, and K. Mehrazar. 2002. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect. Immun. 70:2549-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 30.Jin, H., Z. Ren, J. M. Pozsgay, C. Elkins, P. W. Whitby, D. J. Morton, and T. L. Stull. 1996. Cloning of a DNA fragment encoding a heme-repressible hemoglobin-binding outer membrane protein from Haemophilus influenzae. Infect. Immun. 64:3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin, H., Z. Ren, P. W. Whitby, D. J. Morton, and T. L. Stull. 1999. Characterization of hgpA, a gene encoding a hemoglobin/hemoglobin-haptoglobin binding protein of Haemophilus influenzae. Microbiology 145:905-914. [DOI] [PubMed] [Google Scholar]
- 32.Kanakoudi, F., V. Drossou, V. Tzimouli, E. Diamanti, T. Konstantinidis, A. Germenis, and G. Kremenopoulos. 1995. Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin. Chem. 41:605-608. [PubMed] [Google Scholar]
- 33.Knutson, M., and M. Wessling-Resnick. 2003. Iron metabolism in the reticuloendothelial system. Crit. Rev. Biochem. Mol. Biol. 38:61-88. [DOI] [PubMed] [Google Scholar]
- 34.Koedel, U., and H. W. Pfister. 1999. Models of experimental bacterial meningitis. Role and limitations. Infect. Dis. Clin. N. Am. 13:549-577, vi. [DOI] [PubMed] [Google Scholar]
- 35.Langlois, M. R., and J. R. Delanghe. 1996. Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 42:1589-1600. [PubMed] [Google Scholar]
- 36.Leong, W. I., C. L. Bowlus, J. Tallkvist, and B. Lonnerdal. 2003. Iron supplementation during infancy—effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am. J. Clin. Nutr. 78:1203-1211. [DOI] [PubMed] [Google Scholar]
- 37.Moldenhauer, H., and H. Rose. 1970. Entwicklung der serumeiweisse von ratten im ersten monat nach der geburt. Acta Biol. Med. Ger. 25:469-472. [PubMed] [Google Scholar]
- 38.Morton, D. J., L. O. Bakaletz, J. A. Jurcisek, T. M. VanWagoner, T. W. Seale, P. W. Whitby, and T. L. Stull. 2004. Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin-haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microb. Pathog. 36:25-33. [DOI] [PubMed] [Google Scholar]
- 39.Morton, D. J., A. Smith, L. L. Madore, T. M. VanWagoner, T. W. Seale, P. W. Whitby, and T. L. Stull. 2004. Identification of a haem utilization protein (Hup) in Haemophilus influenzae. Microbiology 150:3923-3933. [DOI] [PubMed] [Google Scholar]
- 40.Morton, D. J., and T. L. Stull. 2004. Haemophilus, p. 273-292. In J. H. Crosa, A. R. Mey, and S. M. Payne (ed.), Iron transport in bacteria. American Society for Microbiology, Washington, D.C.
- 41.Morton, D. J., T. M. VanWagoner, T. W. Seale, P. W. Whitby, and T. L. Stull. 2006. Differential utilization by Haemophilus influenzae of hemoglobin complexed to the three human haptoglobin phenotypes. FEMS Immunol. Med. Microbiol. 46:426-432. [DOI] [PubMed] [Google Scholar]
- 42.Morton, D. J., T. M. VanWagoner, T. W. Seale, P. W. Whitby, and T. L. Stull. 2006. Utilization of myoglobin as a heme source by Haemophilus influenzae requires binding of myoglobin to haptoglobin. FEMS Microbiol. Lett. 258:235-240. [DOI] [PubMed] [Google Scholar]
- 43.Morton, D. J., P. W. Whitby, H. Jin, Z. Ren, and T. L. Stull. 1999. Effect of multiple mutations in the hemoglobin- and hemoglobin-haptoglobin-binding proteins, HgpA, HgpB, and HgpC of Haemophilus influenzae type b. Infect. Immun. 67:2729-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton, D. J., and P. Williams. 1989. Utilization of transferrin-bound iron by Haemophilus species of human and porcine origins. FEMS Microbiol. Lett. 53:123-127. [DOI] [PubMed] [Google Scholar]
- 45.Motley, S. T., B. J. Morrow, X. Liu, I. L. Dodge, A. Vitiello, C. K. Ward, and K. J. Shaw. 2004. Simultaneous analysis of host and pathogen interactions during an in vivo infection reveals local induction of host acute phase response proteins, a novel bacterial stress response, and evidence of a host-imposed metal ion limited environment. Cell Microbiol. 6:849-865. [DOI] [PubMed] [Google Scholar]
- 46.Moxon, E. R., A. L. Smith, D. R. Averill, and D. H. Smith. 1974. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J. Infect. Dis. 129:154-162. [DOI] [PubMed] [Google Scholar]
- 47.Murphy, T. F., L. O. Bakaletz, J. M. Kyd, B. Watson, and D. L. Klein. 2005. Vaccines for otitis media: proposals for overcoming obstacles to progress. Vaccine 23:2696-2702. [DOI] [PubMed] [Google Scholar]
- 48.Murphy, T. F., A. L. Brauer, A. T. Schiffmacher, and S. Sethi. 2004. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170:266-272. [DOI] [PubMed] [Google Scholar]
- 49.Musser, J. M., S. J. Barenkamp, D. M. Granoff, and R. K. Selander. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect. Immun. 52:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myerowitz, R. L., R. Klaw, and B. L. Johnson. 1976. Experimental endogenous endophthalmitis caused by Haemophilus influenzae type b. Infect. Immun. 14:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikkila, H., J. D. Gitlin, and U. Muller-Eberhard. 1991. Rat hemopexin. Molecular cloning, primary structural characterization, and analysis of gene expression. Biochemistry 30:823-829. [DOI] [PubMed] [Google Scholar]
- 52.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panek, H., and M. R. O'Brian. 2002. A whole genome view of prokaryotic haem biosynthesis. Microbiology 148:2273-2282. [DOI] [PubMed] [Google Scholar]
- 54.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pidcock, K. A., J. A. Wooten, B. A. Daley, and T. L. Stull. 1988. Iron acquisition by Haemophilus influenzae. Infect. Immun. 56:721-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poolman, J. T., L. Bakaletz, A. Cripps, P. A. Denoel, A. Forsgren, J. Kyd, and Y. Lobet. 2000. Developing a nontypeable Haemophilus influenzae (NTHi) vaccine. Vaccine 19 (Suppl. 1):S108-S115. [DOI] [PubMed] [Google Scholar]
- 57.Ren, Z., H. Jin, D. J. Morton, and T. L. Stull. 1998. hgpB, a gene encoding a second Haemophilus influenzae hemoglobin- and hemoglobin-haptoglobin-binding protein. Infect. Immun. 66:4733-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribeiro, G. S., J. N. Reis, S. M. Cordeiro, J. B. Lima, E. L. Gouveia, M. Petersen, K. Salgado, H. R. Silva, R. C. Zanella, S. C. Almeida, M. C. Brandileone, M. G. Reis, and A. I. Ko. 2003. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J. Infect. Dis. 187:109-116. [DOI] [PubMed] [Google Scholar]
- 59.Schaible, U. E., and S. H. Kaufmann. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2:946-953. [DOI] [PubMed] [Google Scholar]
- 60.Schryvers, A. B. 1989. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J. Med. Microbiol. 29:121-130. [DOI] [PubMed] [Google Scholar]
- 61.Smith, A. L., D. H. Smith, D. R. Averill, J. Marino, and E. R. Moxon. 1973. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect. Immun. 8:278-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens, M. K., S. Porcella, J. Klesney-Tait, S. Lumbley, S. E. Thomas, M. V. Norgard, J. D. Radolf, and E. J. Hansen. 1996. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect. Immun. 64:1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stull, T. L. 1987. Protein sources of heme for Haemophilus influenzae. Infect. Immun. 55:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turk, D. C. 1984. The pathogenicity of Haemophilus influenzae. J. Med. Microbiol. 18:1-16. [DOI] [PubMed] [Google Scholar]
- 65.Vogel, L., F. Geluk, H. Jansen, J. Dankert, and L. van Alphen. 1997. Human lactoferrin receptor activity in non-encapsulated Haemophilus influenzae. FEMS Microbiol. Lett. 156:165-170. [DOI] [PubMed] [Google Scholar]
- 66.Watson, M. E., Jr., J. Jarisch, and A. L. Smith. 2004. Inactivation of deoxyadenosine methyltransferase (dam) attenuates Haemophilus influenzae virulence. Mol. Microbiol. 53:651-664. [DOI] [PubMed] [Google Scholar]
- 67.Watt, J. P., O. S. Levine, and M. Santosham. 2003. Global reduction of Hib disease: what are the next steps? Proceedings of the meeting Scottsdale, Arizona, September 22-25, 2002. J. Pediatr. 143:S163-S187. [DOI] [PubMed] [Google Scholar]
- 68.Whitby, P. W., K. E. Sim, D. J. Morton, J. A. Patel, and T. L. Stull. 1997. Transcription of genes encoding iron and heme acquisition proteins of Haemophilus influenzae during acute otitis media. Infect. Immun. 65:4696-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams, P., D. J. Morton, K. J. Towner, P. Stevenson, and E. Griffiths. 1990. Utilization of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae and H. paraphrophilus. J. Gen. Microbiol. 136:2343-2350. [DOI] [PubMed] [Google Scholar]
- 70.Wong, J. C. Y., J. Holland, T. Parsons, A. Smith, and P. Williams. 1994. Identification and characterization of an iron-regulated hemopexin receptor in Haemophilus influenzae type b. Infect. Immun. 62:48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]