Abstract
Moraxella catarrhalis immunoglobulin D-binding protein (MID) is a complex antigen with unique immunoglobulin D (IgD)-binding, adhesion, and hemagglutination properties. Previous studies have shown that antibodies raised against MID764-913 in rabbits inhibited M. catarrhalis adhesion to human alveolar epithelial cells, and immunization with MID764-913 resulted in an increased pulmonary clearance in a murine model. Strong immune responses against MID have also consistently been shown in humans. Here, the MID-specified IgG responses were compared to those of ubiquitous surface proteins A1 and A2 (UspA1/A2) using a series of recombinant fragments that spanned all three proteins. Sera were obtained from young children, aged 6 months to 1 year (n = 8) and 2 to 3 years (n = 15), and healthy adults (n = 16). Acute- and convalescent-phase sera from chronic obstructive pulmonary disease (COPD) patients with M. catarrhalis infective exacerbations (n = 23) were also analyzed. Young children, who are at risk of M. catarrhalis infection, had low levels of anti-MID and anti-UspA1/A2 antibodies. Healthy adults and the majority of COPD patients (16/23) had high levels of antibodies directed against, among others, the adhesive domain of MID and the fibronectin- and C3-binding domains of UspA1/A2. Among eight COPD patients in whom a rise in antibody levels could be detected, these functional domains were also the main regions targeted by the antibodies. In addition, human IgG directed against MID was bactericidal and anti-MID antibodies were additive to antibodies targeting UspA1/A2. Hence, the functional domains in these three antigens may have significant potential in a future vaccine against M. catarrhalis.
The nonencapsulated gram-negative bacterium Moraxella catarrhalis is the third most common cause of acute otitis media after Streptococcus pneumoniae and Haemophilus influenzae (24, 25). It is also, after nontypeable H. influenzae, the second leading bacterial cause of exacerbations in adults with chronic obstructive pulmonary disease (COPD) (41). In addition, sinusitis and other respiratory infections are often caused by M. catarrhalis (4, 44). The percentage of clinical isolates producing beta-lactamase has increased significantly over the last two decades, with more than 90% of isolates being resistant to the aminopenicillin antibiotics (13, 14). The economic burden of infection due to this pathogen is significant. In the young, antibiotic treatment may be required. Occasionally, surgery, such as an adenoidectomy and/or the insertion of tympanostomy tubes, may be necessary for the management of recurrent otitis media. In patients with COPD, M. catarrhalis infections result in 2 to 4 million cases of exacerbations per year in the United States (5, 32). The search for a suitable vaccine candidate against M. catarrhalis has thus increased lately. However, unlike S. pneumoniae and H. influenzae, M. catarrhalis has no protective capsule against which a vaccine can be developed. Instead, the various outer membrane proteins (OMPs) have been the focus of research. These include the ubiquitous surface proteins A1 and A2 (UspA1/A2), transferrin-binding protein A, transferrin-binding protein B, CopB, CD, E, McaP, LbpA, LbpB, and M. catarrhalis immunoglobulin D (IgD)-binding protein (MID) (also called Hag by other authors [39]) among others (for a review, see reference 31). The main beneficiaries for a future vaccine are expected to be the very young and the COPD patients since these two groups are the ones with the highest risks of M. catarrhalis infection.
During exacerbations in COPD patients, significant antibody responses (IgG, IgM, and IgA) are raised against some major OMPs of M. catarrhalis (10). Among the majority who cleared M. catarrhalis from the respiratory tract, the serum immunoglobulins were targeted against mainly UspA1, UspA2, MID/Hag, TbpB, and OMP CD (33). In the young, little is known of the relative importance of each of these M. catarrhalis antigens, although only natively acquired UspA1 and -A2 antibodies have been shown to be bactericidal (8). In fact, while antibodies against a few of the Moraxella OMPs were bactericidal in animals, only UspA1 and -A2 antibodies have been shown to be bactericidal in humans to date (27). The UspA family consists of UspA1, UspA2, and the hybrid protein UspA2H (26, 28). UspA1 and -A2 share homology to a significant extent in the central regions where there are amino acid motifs and repeats found in both (11). They exist as oligomeric structures, forming a lollipop-like head at the tip, and cover M. catarrhalis as a dense layer (20, 39). A wide range of functions have been attributed to UspA1/A2, including adhesion to epithelial cell-associated fibronectin, carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) on epithelial cells, and laminin on the basement membrane (19, 42, 43). UspA2 also interacts with C4-binding protein (C4BP), C3, and vitronectin, and is one of the main virulence factors involved in the complement resistance of M. catarrhalis (3, 21, 35, 36). It is thus not surprising that antibodies against UspA1/A2 are beneficial, as the complement-dependent bactericidal activity is not impeded and adhesion is blocked.
An increased number of IgD molecules have been observed in human bronchus-associated lymphoid tissue, and bacterial IgD-binding proteins are believed to play important roles in the pathogenesis of infection (6). To date, only M. catarrhalis and H. influenzae have been found to strongly bind IgD (17). MID is a highly conserved OMP with hemagglutination properties (also designated Hag) and functions as an adhesin that interacts with epithelial cells (7, 15, 39). The adhesive domain is located in the sequence MID764-913 of M. catarrhalis Bc5. The identity and similarity in this area between strains are 60 to 96 and 69 to 97%, respectively (15). The IgD-binding domain (MID962-1200) forms a complex multimeric structure likely located near the tip of the protein (37). Interestingly, the immunization with the adhesive domain was protective, with improved pulmonary clearance in a mouse model (16).
The importance of MID/Hag is underlined by the consistently strong immune response observed in humans. For example, Meier et al. showed that a salivary immune response could be detected in 70% of young children of less than 24 months of age (29). In that study, other OMPs appeared to be less important, although this interpretation is limited by the possibility of nonrepresentative antigenicity of using a single strain. MID also stimulates a prominent mucosal immune response in COPD patients. Most patients develop mucosal IgA against MID, UspA1, and -A2, whereas fewer patients develop IgA responses against other OMPs like TbpB and CopB (32). Besides stimulating a significant mucosal response, a strong serum IgG response to MID can also be found in the majority of COPD patients who cleared the organism (33).
In the present study, we characterized this human serological response against MID and compared it with those against UspA1 and -A2, which are the only known bactericidal antibodies elicited in native infection to date (27).
MATERIALS AND METHODS
Bacteria.
M. catarrhalis RH4 and BBH18 and their corresponding MID, UspA1, and -A2 mutants were as described elsewhere (35, 38). They were grown on brain heart infusion (BHI) agar plates overnight before being harvested for bactericidal assays or absorption with human serum containing anti-Moraxella IgG. BHI agar plates supplemented with antibiotics were used for the growth of the mutants (35, 38).
Antibodies.
Horseradish peroxidase-conjugated rabbit anti-human IgG and fluorescein isothiocyanate-conjugated rabbit anti-human IgG (CH2) were from Dakopatts (Glostrup, Denmark). Human anti-MID and anti-UspA1/A2 IgG were prepared by absorbing M. catarrhalis RH4Δmid mutant or RH4ΔuspA1/A2 double mutants to remove all other M. catarrhalis IgG. Nonspecific human IgG was prepared by absorbing with M. catarrhalis RH4 wild type. The source of the serum was a healthy adult donor. The IgG fraction was isolated using the Melon Gel IgG purification kit (Pierce, Rockford, Ill.). This was subsequently heat inactivated to remove any residual complement activity.
Human serum samples.
Paired serum samples (n = 23) from COPD patients with lower respiratory tract infections with M. catarrhalis were used (9). Their sputum, endotracheal aspirates, or bronchial suctions had revealed the presence of granulocytes with few or no squamous epithelial cells. The patient characteristics were reported elsewhere (9). Convalescent-phase sera were taken 1 to 2 weeks after the acute samples. In addition, 39 serum samples were collected from a group of 8 children aged 6 to 12 months, 15 children aged 2 to 3 years, and 16 healthy adults. All sera from healthy donors were collected at the HS Blood Bank of Copenhagen University Hospital (Denmark) with the ethics committee's approval. The presence of M. catarrhalis colonization or infection of these subjects was unknown.
Recombinant protein construction and expression.
Recombinant proteins corresponding to multiple regions spanning UspA150-770, UspA230-539, and MID69-2139 were used (37, 43). Two additional truncated proteins, UspA1360-680 (primers 5′ GAGGTGGATCCATTAGGCGAAGAGATTAACTCAC and 5′CTTGAAGCTTGGCTTTATTTTGCTCAACCAATGC) and UspA2200-480 (primers 5′CAAAAGGATCCTCTTAAAGGCTTGATAACAAAC and 5′GTTTGCAAGCTTTAATTTGTCATGCTCTTTATC) were manufactured as previously described (43).
Enzyme-linked immunosorbent assay (ELISA).
Human IgG in sera reactive to UspA1, -A2, and MID recombinant fragments was analyzed according to a previously published protocol (2). The recombinant fragments spanned the entire coding regions except for the signal peptides and the C-terminal ends. The fragments were suspended in 100 mM NaHCO3 buffer, pH 9.6 (coating buffer), and plated at a concentration of 40 nM in wells of flat-bottomed MaxiSorp microtiter plates (Nunc, Roskilde, Denmark). Following an overnight incubation at 4°C and blocking with 2% bovine serum albumin in phosphate-buffered saline (PBS) for 1 h, human serum samples were diluted 1:100 and, in some, 1:1,000 in PBS with 0.05% Tween 20 and 2% bovine serum albumin and then incubated for 2 h at room temperature. Plates were washed four times with 0.05% Tween 20 in PBS, followed by the addition of horseradish peroxidase-labeled rabbit anti-human IgG (Dakopatts). After 1 h of incubation and subsequent washing steps, the plates were developed. Each sample was tested in duplicate.
Flow cytometry.
The specificity of human anti-MID, anti-UspA1/A2 IgG was checked by flow cytometry against M. catarrhalis (RH4 and BBH18) wild-type strains and their corresponding MID- and UspA1/A2-deficient mutants. These bacteria were grown overnight and washed twice in PBS containing 3% fish gelatin (PBS-gelatin). The bacteria (108 CFU) were then incubated with either human anti-UspA1/A2 or anti-MID antiserum (1/20) at 4°C for 1 h. After washes, bacteria were incubated for 30 min at room temperature with anti-human IgG (CH2) fluorescein isothiocyanate-conjugated polyclonal antibody (diluted according to the manufacturer's instructions) for 30 min. After three additional washes, bacteria were analyzed by flow cytometry (Epics XL-MCL; Coulter). All incubations were kept in a final volume of 100 μl of PBS-gelatin, and the washings were performed with the same buffer. MID- and UspA1/A2-deficient mutants were used as negative controls for each strain analyzed. Wild-type M. catarrhalis RH4 and BBH18 were used as positive controls.
Complement-dependent bactericidal assay.
The complement source was a pool of immunoglobulin-depleted human sera from five healthy individuals. The immunoglobulin was removed by passing 5 ml of pooled human serum over a protein G column (Amersham Biosciences) twice at 4°C. The immunoglobulin-depleted sera were stored in small aliquots at 20°C. The bactericidal assay was performed by mixing 60 μl of a bacterial suspension (approximately 1,000 CFU in PBS containing 1 mM CaCl2 and 0.2 mM MgCl2) with human anti-MID and anti-UspA1/A2 IgG or both and then incubated at 4°C for half an hour. The complement fraction (30%) was then added, and the mixture was incubated at 37°C. Surviving bacteria (CFU) were documented by aliquoting 10 μl at 10-min intervals and plated on BHI agar. The plates were incubated for 12 h at 35°C with 5% carbon dioxide. The bactericidal activity of nonspecific human IgG (i.e., human IgG that was absorbed with the M. catarrhalis RH4 wild type) against wild-type bacteria and bactericidal activity of anti-MID IgG against MID-deficient mutants were used as negative controls. In addition, antibodies against UspA1/A2 (human IgG that was absorbed with M. catarrhalis RH4ΔuspA1/A2 double mutants) were used as positive controls.
Statistical methods.
Comparisons between different groups of donor sera were assessed by the Mann-Whitney U test using GraphPad InStat 3.06 for Windows from GraphPad Software, San Diego, CA.
RESULTS
Comparison of IgG levels directed against truncated MID and UspA1/A2 in healthy children and adults.
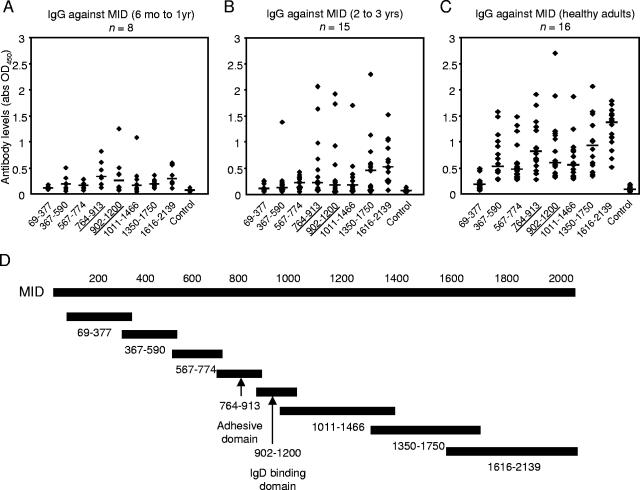
To examine the immunodominant regions of MID, a series of recombinant proteins that spanned the entire coding region, except for the signal peptides and the C-terminal ends, were recombinantly produced. The proteins were used as antigens to detect and quantify the amount of antibodies against each particular region of the MID protein. Human sera were diluted 1:100, and the amount of IgG bound to the antigens was detected by rabbit anti-human IgG. The IgD-binding MID902-1200 and adhesion domain MID764-913 were found to be relatively immunodominant. There were, however, other stretches that also induced strong immune responses. Young children (6 months to 1 year old) did not have high titers of antibodies against MID (Fig. 1A). With age, some children developed antibodies against the adhesive and IgD-binding domains. There was also a rise in antibody levels against the relatively conserved regions from amino acids 1350 to 2139 (Fig. 1B). A more heterogeneous rise in IgG was seen in healthy adults (Fig. 1C). Antibodies against all MID fragments, except those against MID69-377, were significantly higher (P < 0.05 to 0.0001) in adults. The general rise in IgG is in keeping with published literature about the rise of M. catarrhalis antibodies with age (12). Moreover, this is usually associated with decreased rates of colonization (12).
FIG. 1.
The rise in IgG against MID was directed against several parts of the molecule, including the IgD-binding MID902-1200 and the adhesive MID764-913 domains. Human sera were diluted 1:100. The horizontal bars represent the median values. All values are the averages of two separate ELISAs performed independently. (A) Low levels were found in children aged 6 months to 1 year. (B) Several young children of 2 to 3 years old had high levels of antibodies against the IgD-binding MID902-1200 and the adhesive MID764-913 domains in addition to the relatively conserved regions from amino acids 1350 to 2139. (C) A more heterogeneous antibody pattern was seen in healthy adults. (D) Recombinant truncated fragments spanning the entire M. catarrhalis Bc5 MID were used as antigens. Abbreviations: abs, antibodies; OD450, optical density at 450 nm.
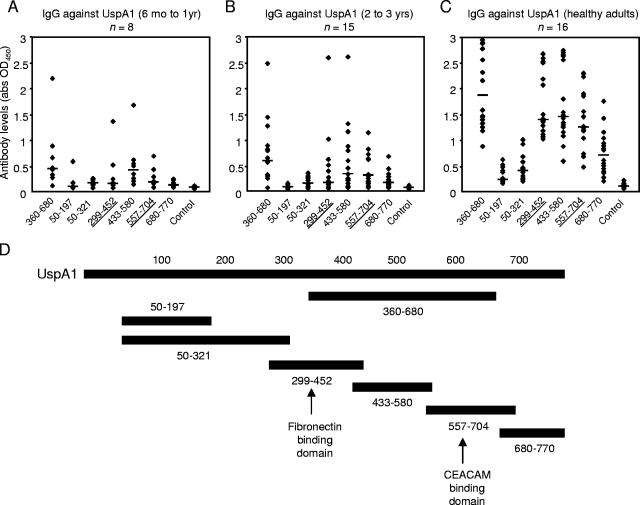
To investigate the immunodominant regions of UspA1, recombinant protein fragments that contained the coding region, except for the signal peptides and the C-terminal ends, were used. Higher levels of anti-UspA1 IgG were found in healthy adults, and they were directed against a central portion (amino acids 360 to 680). Truncated UspA1 proteins (UspA1299-452, UspA1433-580, and UspA1557-704) spanning this region were also immunogenic. It is notable that, within the central region, UspA1 shares much sequence similarity with UspA2 (11). Only two to three out of eight children's (6 months to 1 year old) sera had significant reactivity against the major UspA1 fragments (Fig. 2A). With increasing age, the rise in IgG against the relatively conserved domains spanning amino acids 360 to 680 was apparent (Fig. 2B). In adults, higher levels of antibodies against these regions were evident in the healthy population (P was <0.0001 for antibodies against UspA1360-680 relative to that in children of 2 to 3 years of age) (Fig. 2C).
FIG. 2.
The concentration of antibodies directed against UspA1360-680 containing the fibronectin-binding (UspA1299-452) and CEACAM-binding (UspA1557-704) domains increased with age. Human sera were diluted in 1:100. The horizontal bars represent the median values. Data shown are the averages of two separate ELISAs performed independently. (A) Low levels of antibodies directed against UspA1 were found in children aged 6 months to 1 year. (B) Elevated levels of antibodies found with increasing age were directed mainly against relatively conserved domains in UspA1360-680. (C) High levels of antibodies directed against UspA1360-680 were found in adults. (D) Recombinant truncated UspA1 fragments spanning the entire protein were used as bait in ELISAs. Abbreviations: abs, antibodies; OD450, optical density at 450 nm.
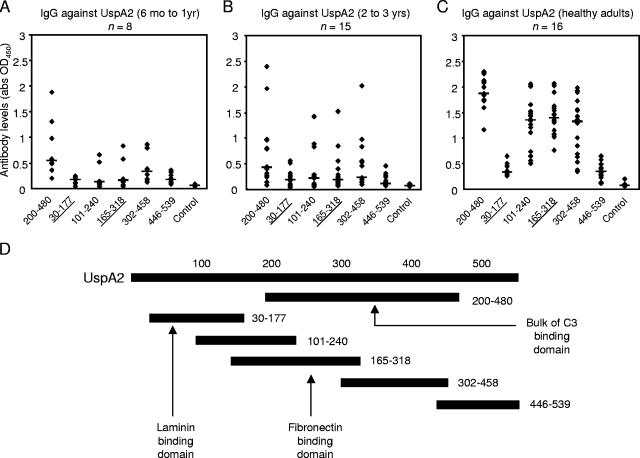
To study the immunodominant regions of UspA2, truncated proteins that spanned the entire coding region, except for the signal peptides and the C-terminal ends, were recombinantly produced and used in ELISAs. Like anti-UspA1 IgG, higher levels of anti-UspA2 antibodies were found with increasing age. The antibodies were mainly targeted at the biologically active domains in the central regions that are homologous with UspA1. These include the fibronectin-binding UspA2165-318 and the C3-binding region found in amino acids 200 to 458. Most sera from children 6 months to 1 year old had low reactivity towards UspA2 (Fig. 3A). With increasing age, there were considerably more children with higher serum reactivity towards UspA2 (Fig. 3B). A clear pattern of reactivity directed against the conserved and shared regions of UspA1 and UspA2 was seen in the adult population. The median levels for the representative UspA2200-480 were significantly higher compared to those for both groups of young children (P < 0.0001). These data are in keeping with those from Chen et al., who found that the IgG levels were age dependent (8). In addition, we were able to document that the rise in UspA1 and -A2 antibodies targeted central regions of UspA1 and -A2. In these regions, there is a significant conservation of different amino acid motifs among different strains (11).
FIG. 3.
The rise in UspA2 antibodies was directed against UspA2200-480 containing conserved areas that share identity with UspA1. Human sera were diluted in 1:100. The horizontal bars represent median values. All values are the averages of two separate ELISAs performed independently. (A) Most children in the younger group (6 months to 1 year old) had low IgG reactivity towards UspA2. (B) In the older group (2 to 3 years old), there were more children with higher reactivity towards some regions of UspA2, although median values remain low. (C) A clear pattern of reactivity against the conserved and shared regions of UspA2 was seen in the healthy adult population. (D) Recombinant truncated UspA2 fragments spanning the entire protein were used as antigens in ELISAs. Abbreviations: abs, antibodies; OD450, optical density at 450 nm.
Comparison of IgG directed against MID and UspA1/A2 in COPD patients.
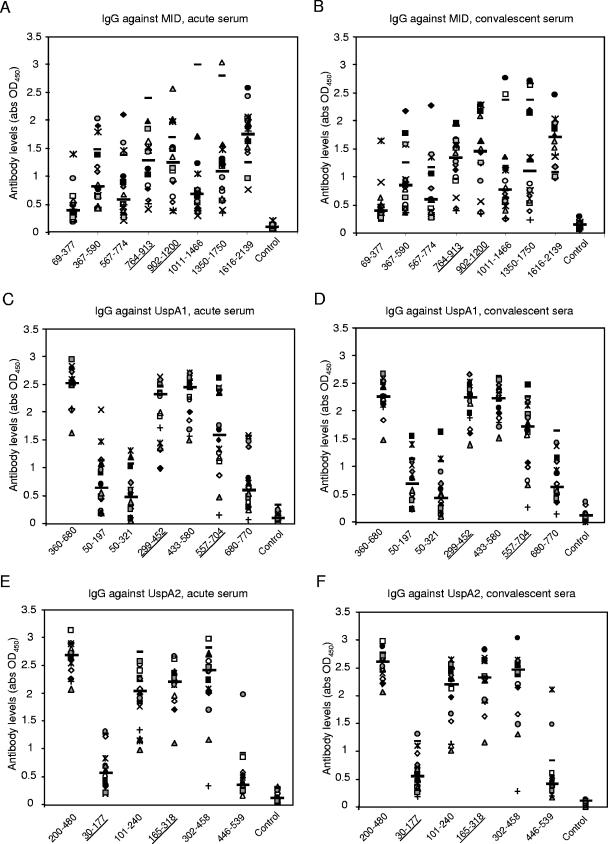
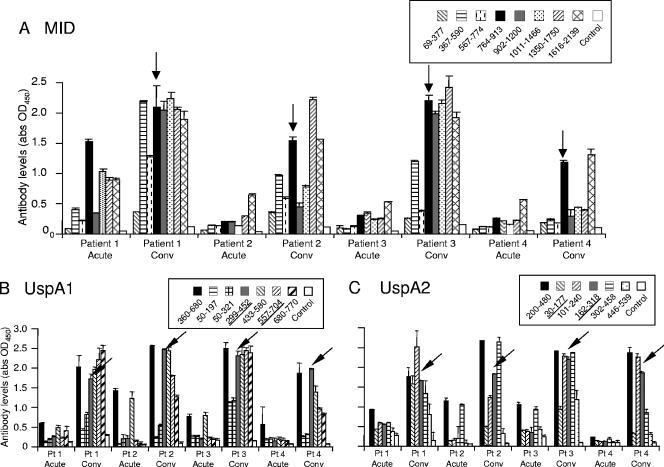
To further validate whether the immunodominant regions of MID, UspA1, and -A2 would be important in the natural immune response of M. catarrhalis infection, 23 pairs of acute- and convalescent-phase sera were tested in ELISAs. These sera were collected from COPD patients with lower respiratory tract infections caused by M. catarrhalis (9). Most (n = 16) had high levels of antibodies against MID, UspA1, and UspA2 fragments that were similar to the distribution of the healthy adults. They showed little changes in the convalescent-phase sera (at 1:100 dilution) (Fig. 4A to F). A minority (n = 3) had minimal reactivity to all of the antigens tested in both acute- and convalescent-phase sera (data not shown). Four sera showed a significant rise in IgG levels during convalescent phase (at a dilution of 1:100) (Fig. 5A). After infection, there was a rise in antibodies directed against MID764-913 and MID1616-2139 in these four sera. Interestingly, MID764-913 contains adhesive epitopes for which antibodies are protective in a mouse model (16). The convalescent-phase sera in these four patients also had a variable rise in titers against epitopes found in amino acids 1350 to 2139, which are relatively conserved (30). With regards to UspA1 and -A2, the rise in antibodies corresponded closely to the pattern seen in the natural rise of antibodies with age. These convalescent-phase sera targeted regions found in UspA1360-680 and UspA2200-480 (Fig. 5B and C). Notably, it is also within these two regions where the adhesive epitopes (Fig. 5B and C) for fibronectin binding are located (43). Among the 16 patients for whom high levels of antibodies were found in both acute- and convalescent-phase samples, ELISAs with further dilutions were also performed. Four more patients had rises in IgG against both UspA1 and UspA2 in a pattern similar to those found at 1:100 dilution. Two of these patients had rises in IgG against MID1616-2139 (data not shown).
FIG. 4.
High IgG reactivity in the majority (16 of 23 patients) of COPD patients to various regions of MID, UspA1, and UspA2 is similar in distribution to that of the healthy adult population. The acute (A, C, and E)- and convalescent (B, D, and F)-phase sera that were directed against different regions of the outer membrane proteins MID (A and B), UspA1 (C and D), and UspA2 (E and F) showed little changes. Each symbol represents the average of two separate IgG ELISAs performed independently. The longer horizontal bars represent median values.
FIG. 5.
Analysis of the IgG reactivity against MID, UspA1, and UspA2 in acute- and convalescent-phase sera from four patients. (A) Significant rises in IgG antibodies directed against the adhesive domain MID764-913 were found in the convalescent (conv) sera (arrow). (B) Significant rises in IgG antibodies directed against UspA1360-680 were found in the convalescent-phase sera. Smaller constituent fragments, such as those of the fibronectin-binding domain UspA1299-452 (arrow), were immunodominant. (C) Significant rises in IgG antibodies directed against UspA2200-480 were found in the convalescent-phase sera. Smaller constituent fragments spanning this region, including the fibronectin-binding domain UspA2162-318 (arrow), were also reactive. These ELISAs were performed as described for Fig. 3. Mean values of two separate ELISAs are shown. Error bars indicate standard deviations.
Biological activity of human anti-MID antibodies.
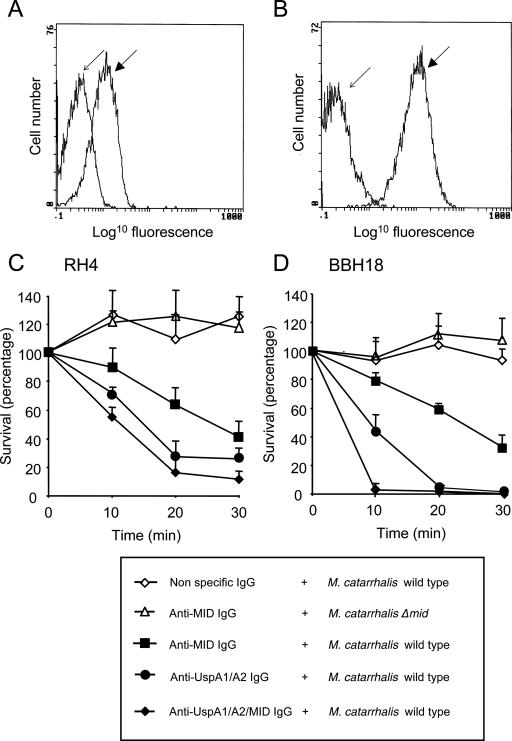
Our data show that antibodies are mounted against epitopes other than the defined adhesive and IgD-binding regions. Since healthy adults rarely get M. catarrhalis infection and a strong MID reactivity is found in both healthy adults and in postinfectious sera, we tested the anti-MID IgG for bactericidal activity. The anti-MID IgG (i.e., human IgG that was preabsorbed with the M. catarrhalis RH4Δmid mutant) was first confirmed to be specific for MID in flow cytometry (Fig. 6A). Similar experiments were performed with anti-UspA1/A2 IgG (human IgG that was preabsorbed with M. catarrhalis RH4ΔuspA1/A2 double mutant) (Fig. 6B). Complement-dependent bactericidal assays were then performed. At 30 min, only 40.3 and 31.3% of M. catarrhalis RH4 and BBH18, respectively, were found to survive when incubated with human anti-MID antibodies, together with IgG-depleted serum as a complement source (Fig. 6C and D). The bactericidal activity of nonspecific human IgG (i.e., human IgG that was preabsorbed with the M. catarrhalis RH4 wild type) on the M. catarrhalis wild type and those of anti-MID IgG against MID-deficient mutants were used as negative controls. In addition, antibodies against UspA1/A2 (human IgG that was preabsorbed with M. catarrhalis RH4ΔuspA1/A2 double mutant) were used as positive controls. Interestingly, the effect of combining both sets of antibodies resulted in more efficient bactericidal activity (Fig. 6C and D).
FIG. 6.
Specific human anti-MID IgG antibodies are bactericidal. (A) Flow cytometry profile of human anti-MID IgG against the M. catarrhalis RH4 wild type (dark arrow) and its corresponding MID-deficient mutant (light arrow). (B) Profile of anti-UspA1/A2 IgG against M. catarrhalis RH4 wild type (dark arrow) and its UspA1/A2-deficient mutant (light arrow). M. catarrhalis RH4 (C) and BBH18 (D) wild-types or MID-deficient mutants were incubated with nonspecific IgG (1/20), human anti-MID IgG (1/20), anti-UspA1/A2 IgG (1/50), or a combination of anti-MID/anti-UspA1/A2 IgG in PBS supplemented with 1 mM CaCl2 and 0.2 mM MgCl2 at 4°C for 30 min. Complement (30%) was added, and the suspensions were incubated at 37°C. Bacteria were collected at the indicated time points. After overnight incubation, CFU were counted. CFU at the initiation of the experiments was defined as 100%. Mean values of three to five separate experiments are shown. Error bars indicate standard errors of the means.
DISCUSSION
Our data show that levels of serum IgG antibodies directed against MID/Hag of M. catarrhalis naturally increase with age. This corresponds to a decreased risk of colonization in adulthood (23). The importance of natural immunity against MID/Hag is likely to be significant. This is evidenced by the fact that it is one of the antigens that COPD patients raised antibodies against upon the clearance of M. catarrhalis infection (33). In addition to UspA1 and -A2, MID/Hag is the only major antigen that mucosal IgA is directed against in the mucosal response during COPD exacerbations (32). Moreover, in young children, anti-MID antibodies are part of the salivary immune repertoire, detectable in up to 70% of children younger than 24 months of age (29).
In our results with COPD patients, a strong rise in anti-MID 6/23 (26.1%) and anti-UspA1/A2 IgG 8/23 (34.8%) was detectable. Excluding those whose infections resulted in rises in antibodies against these antigens, there were still 14 and 12 patients with high levels of UspA1/A2 and MID IgG, respectively. These patients were seemingly infected by M. catarrhalis. This is not surprising for the following reasons. (i) Respiratory virus infections are associated with 30% of COPD exacerbations (40). It is conceivable that some of these M. catarrhalis isolates were actually colonizers and the patients had viral infections. (ii) The short interval (1 to 2 weeks) between the acute- and convalescent-phase serum samples would have missed identifying some subjects who had even greater rises in titers. (iii) Lastly, the duration of carriage of M. catarrhalis is long in both exacerbations and asymptomatic colonization, with median durations of 31 days and 40 days, respectively (34). A recent exacerbation might thus be responsible for the high titers in the acute-phase serum.
A strong immune response against MID may well be protective. In an animal model, immunization with a peptide corresponding to the putative adhesive epitope results in significantly more efficient pulmonary clearance (16). IgG against the adhesive domain MID764-913 was evident in most adult patients; 13 of 16 healthy adults (Fig. 1C) and 15 of 16 patients with COPD had values above 0.5 (Fig. 4A), while only a few young children had levels above this arbitrary cutoff. However, it is clear that there are other epitopes that also stimulate strong immune responses. These regions are found in the C-terminal half of the protein and are relatively conserved (30).
When MID antibody levels were compared with those of UspA1/A2, there was an evident rise both with age and with infection. The rise in UspA1/A2 IgG was represented predominantly against a middle stretch where the conservation of motifs is found in different M. catarrhalis strains (11). Interestingly, these regions also encompassed domains that are responsible for most of the virulence functions of these proteins. The adhesion to epithelial cell-associated fibronectin and C3 binding is mediated in this region (UspA1299-452, UspA2165-318, and conserved amino acid stretches in UspA2200-480) (36, 43). Antibodies against these domains might improve clearance in humans. In a murine model, a monoclonal antibody (17C7) enhanced pulmonary clearance of both homologous and heterologous isolates (18). This monoclonal antibody reacts to a motif found in both UspA1 and -A2 within the central regions and that is found across most strains (1).
We have recently shown that both UspA1 and -A2 can bind to laminin (42). Interestingly, healthy adults and COPD patients do not have high levels of IgG against UspA230-177 (Fig. 3C and 4C). This might explain the higher rates of M. catarrhalis infection in COPD patients. However, whether antibodies directed against the laminin-binding domain of UspA2 are protective remains to be seen.
The most immunogenic antigens in M. catarrhalis have consistently been shown to include at least UspA1/A2 and MID (29, 32, 33). Of these, anti-UspA1 and -A2 antibodies have been shown to be the major source of bactericidal activity in serum (8). Since MID is able to stimulate a robust immune response in patients who can clear M. catarrhalis, the question of a possible bactericidal effect arose. We found that anti-MID IgG was bactericidal and comparable with anti-UspA1/A2 IgG. Moreover anti-MID and anti-UspA1/A2 IgG acted in an additive manner. A reason for the relatively modest bactericidal activity of anti-MID IgG (or any other antibody against other antigens) compared to that of anti-UspA1/A2 IgG is the fact that UspA2 is one of the key proteins conveying serum resistance in M. catarrhalis. These proteins counteract the complement pathway by binding and inactivating the complement factor C3 (36). Moreover, both UspA1 and -A2 interact with C4BP, which inhibits the formation and accelerates the decay of the C3 convertase (C4bC2a). C4BP also serves as a cofactor to factor I in the proteolytic degradation of C4b, and C4BP bound to Moraxella retains its cofactor activity (35). Finally, UspA2 also interferes with the proper formation of the membrane attack complex in bacterial outer membranes (3). The complement cascade initiated by antibodies binding to various antigens could thus be interrupted by these interactions.
M. catarrhalis is one of the most important bacterial respiratory tract pathogens after S. pneumoniae and H. influenzae. With the wider usage of pneumococcal vaccines, an ecologic niche is available that may be filled by pathogens such as M. catarrhalis. Such a phenomenon appears likely given that a shift towards an increased carriage of nonvaccine pneumococcal serotypes has already been documented (22). Hence, a further understanding of MID and its serological response might pave the way for its consideration as a vaccine candidate.
In conclusion, the serological IgG responses to MID/Hag are at least targeted at the adhesive epitopes on MID and the relatively conserved C terminus. Natural human IgG against M. catarrhalis MID can be bactericidal and act in an additive manner with the other known bactericidal antibodies (anti-UspA1 and -A2 antibodies) in humans. Our data suggest that UspA1/A2 and MID/Hag could be important components in a potential vaccine against M. catarrhalis. Further studies, however, are warranted.
Acknowledgments
This work was supported by grants from the Alfred Österlund, the Anna and Edwin Berger, the Crafford, and the Greta and Johan Kock Foundations; the Swedish Medical Research Council; the Swedish Society of Medicine; and the Cancer Foundation at the University Hospital in Malmö, Sweden.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., H. Janson, M. Ruan, and A. Forsgren. 1996. Biological activity of serum antibodies to a nonacylated form of lipoprotein D of Haemophilus influenzae. Infect. Immun. 64:4586-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attia, A. S., S. Ram, P. A. Rice, and E. J. Hansen. 2006. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect. Immun. 74:1597-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamberger, D. M. 1991. Antimicrobial treatment of sinusitis. Semin. Respir. Infect. 6:77-84. [PubMed] [Google Scholar]
- 5.Bluestone, C. D. 1989. Modern management of otitis media. Pediatr. Clin. N. Am. 36:1371-1387. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., S. T. Gjeruldsen, F. Korsrud, K. Baklien, P. Berdal, and J. Ek. 1979. The human secretory immune system shows striking heterogeneity with regard to involvement of J chain-positive IgD immunocytes. J. Immunol. 122:503-510. [PubMed] [Google Scholar]
- 7.Bullard, B., S. L. Lipski, and E. R. Lafontaine. 2005. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect. Immun. 73:5127-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, D., V. Barniak, K. R. VanDerMeid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen, J. J., J. Renneberg, B. Bruun, and A. Forsgren. 1995. Serum antibody response to proteins of Moraxella (Branhamella) catarrhalis in patients with lower respiratory tract infection. Clin. Diagn. Lab. Immunol. 2:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, J. J., N. Q. Hansen, and B. Bruun. 1996. Serum antibody response to outer membrane proteins of Moraxella (Branhamella) catarrhalis in patients with bronchopulmonary infection. Clin. Diagn. Lab. Immunol. 3:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejlertsen, T., E. Thisted, P. A. Ostergaard, and J. Renneberg. 1994. Maternal antibodies and acquired serological response to Moraxella catarrhalis in children determined by an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 1:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felmingham, D., and J. Washington. 1999. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens—findings of the Alexander Project 1992-1996. J. Chemother. 11(Suppl. 1):5-21. [DOI] [PubMed] [Google Scholar]
- 14.Felmingham, D., A. R. White, M. R. Jacobs, P. C. Appelbaum, J. Poupard, L. A. Miller, and R. N. Gruneberg. 2005. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 56(Suppl. 2):ii3-ii21. [DOI] [PubMed] [Google Scholar]
- 15.Forsgren, A., M. Brant, M. Karamehmedovic, and K. Riesbeck. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 71:3302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsgren, A., M. Brant, and K. Riesbeck. 2004. Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J. Infect. Dis. 190:352-355. [DOI] [PubMed] [Google Scholar]
- 17.Forsgren, A., and A. O. Grubb. 1979. Many bacterial species bind human IgD. J. Immunol. 122:1468-1472. [PubMed] [Google Scholar]
- 18.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867-872. [DOI] [PubMed] [Google Scholar]
- 19.Hill, D. J., and M. Virji. 2003. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol. Microbiol. 48:117-129. [DOI] [PubMed] [Google Scholar]
- 20.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hol, C., C. M. Verduin, E. E. Van Dijke, J. Verhoef, A. Fleer, and H. van Dijk. 1995. Complement resistance is a virulence factor of Branhamella (Moraxella) catarrhalis. FEMS Immunol. Med. Microbiol. 11:207-211. [DOI] [PubMed] [Google Scholar]
- 22.Huang, S. S., R. Platt, S. L. Rifas-Shiman, S. I. Pelton, D. Goldmann, and J. A. Finkelstein. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408-e413. [DOI] [PubMed] [Google Scholar]
- 23.Jousimies-Somer, H. R., S. Savolainen, and J. S. Ylikoski. 1989. Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J. Clin. Microbiol. 27:2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 25.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 26.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19(Suppl. 1):S101-S107. [DOI] [PubMed] [Google Scholar]
- 28.McMichael, J. C., M. J. Fiske, R. A. Fredenburg, D. N. Chakravarti, K. R. VanDerMeid, V. Barniak, J. Caplan, E. Bortell, S. Baker, R. Arumugham, and D. Chen. 1998. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect. Immun. 66:4374-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier, P. S., S. Freiburghaus, A. Martin, N. Heiniger, R. Troller, and C. Aebi. 2003. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr. Infect. Dis. J. 22:256-262. [DOI] [PubMed] [Google Scholar]
- 30.Mollenkvist, A., T. Nordstrom, C. Hallden, J. J. Christensen, A. Forsgren, and K. Riesbeck. 2003. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 185:2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, T. F. 2005. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev. Vaccines 4:843-853. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect. Immun. 73:8161-8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect. Immun. 73:3471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordstrom, T., A. M. Blom, A. Forsgren, and K. Riesbeck. 2004. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J. Immunol. 173:4598-4606. [DOI] [PubMed] [Google Scholar]
- 36.Nordstrom, T., A. M. Blom, T. T. Tan, A. Forsgren, and K. Riesbeck. 2005. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J. Immunol. 175:3628-3636. [DOI] [PubMed] [Google Scholar]
- 37.Nordstrom, T., A. Forsgren, and K. Riesbeck. 2002. The immunoglobulin D-binding part of the outer membrane protein MID from Moraxella catarrhalis comprises 238 amino acids and a tetrameric structure. J. Biol. Chem. 277:34692-34699. [DOI] [PubMed] [Google Scholar]
- 38.Nordstrom, T., J. Jendholm, M. Samuelsson, A. Forsgren, and K. Riesbeck. 2006. The IgD-binding domain of the Moraxella IgD-binding protein MID (MID962-1200) activates human B cells in the presence of T cell cytokines. J. Leukoc. Biol. 79:319-329. [DOI] [PubMed] [Google Scholar]
- 39.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St. Geme 3rd, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sethi, S. 2000. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 117:380S-385S. [DOI] [PubMed] [Google Scholar]
- 41.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan, T. T., A. Forsgren, and K. Riesbeck. 2006. The respiratory pathogen Moraxella catarrhalis binds laminin through ubiquitous surface proteins A1 and A2. J. Infect. Dis. 194:493-497. [DOI] [PubMed] [Google Scholar]
- 43.Tan, T. T., T. Nordstrom, A. Forsgren, and K. Riesbeck. 2005. The respiratory pathogen Moraxella catarrhalis adheres to epithelial cells by interacting with fibronectin through ubiquitous surface proteins A1 and A2. J. Infect. Dis. 192:1029-1038. [DOI] [PubMed] [Google Scholar]
- 44.Wald, E. R., G. J. Milmoe, A. Bowen, J. Ledesma-Medina, N. Salamon, and C. D. Bluestone. 1981. Acute maxillary sinusitis in children. N. Engl. J. Med. 304:749-754. [DOI] [PubMed] [Google Scholar]