Abstract
There is a relationship between schistosomiasis and anemia, although the magnitude and exact mechanisms involved are unclear. In a cohort of 580 Schistosoma japonicum-infected 7- to 30-year-old patients from Leyte, The Philippines, we evaluated the impact of reinfection with S. japonicum after treatment with praziquantel on the mean hemoglobin level, iron-deficiency (IDA) and non-iron-deficiency anemia (NIDA), and inflammatory markers. All participants were treated at baseline and followed up every 3 months for a total of 18 months. At each follow-up, participants provided stools to quantify reinfection and venous blood samples for hemograms and measures of iron status and inflammation. After 18 months, reinfection with S. japonicum was associated with a lower mean hemoglobin level (−0.39 g/dl; 95% confidence interval [95% CI], −0.63 to −0.16) and 1.70 (95% CI, 1.10 to 2.61) times higher odds of all-cause anemia than those without reinfection. Reinfection was associated with IDA for high reinfection intensities only. Conversely, reinfection was associated with NIDA for all infection intensities. Reinfection was associated with serum interleukin-6 responses (P < 0.01), and these responses were associated with NIDA (P = 0.019) but not with IDA (P = 0.29). Our results provide strong evidence for the causal relationship between S. japonicum infection and anemia. Rapidly reinfected individuals did not have the positive treatment effect on hemoglobin seen in nonreinfected individuals. The principle mechanism involved in S. japonicum-associated anemia is that of proinflammatory cytokine-mediated anemia, with iron deficiency playing a role in high-intensity infections. Based on the proposed mechanism, anemia is unlikely to be ameliorated by iron therapy alone.
Schistosomiasis remains a global public health problem, with an estimated 600 million people residing in regions where the disease is endemic and approximately 200 million individuals being infected at any given time (21, 28). Schistosoma japonicum infects approximately 2.4 million individuals, and 70 million people are at risk of infection, mainly in China and Southeast Asia (21). Evidence from cross-sectional studies and randomized controlled trials supports a relationship between schistosomiasis and anemia (7, 28), although design-related issues and conflicting results make the magnitude of the relationship unclear (4). Only one randomized, controlled trial treating patients solely for schistosomiasis has demonstrated a beneficial effect of praziquantel on hemoglobin (Hgb) concentration (17).
The exact mechanisms involved in schistosomiasis-associated anemia are unclear (4). Schistosomiasis may cause intestinal blood loss and subsequent iron deficiency as eggs pass through the intestinal or bladder wall into the lumen or through the induction of inflammatory lesions of intestinal or bladder mucosa. However, there is little evidence that the quantity of blood lost is sufficient to produce iron deficiency and anemia in the context of S. japonicum infection, except possibly at high infection intensities (6, 33). Two studies have reported an association between schistosome egg counts and decreased iron stores (30). However, the traditional markers of iron status used in these studies (ferritin and erythrocyte protoporphyrin) are likely influenced by inflammation, complicating their interpretation. Moreover, because poverty increases the risk of both schistosomiasis and dietary deficiency of iron, studies that do not adjust for socioeconomic status (SES) may be confounded by poor iron intake.
Alternatively, schistosomiasis may cause anemia by inducing proinflammatory cytokine-mediated dyserythropoiesis, as seen in anemia associated with inflammation (10, 19). During S. japonicum infection, only a proportion of the 500 to 3,500 eggs shed daily by each female worm reach the intestine (29). The remainder of these heavily immunogenic eggs are trapped in the intestinal wall or the liver, causing an inflammatory reaction and granuloma formation. Anemia in the setting of acute/chronic inflammation is mediated by (i) decreased erythropoietin production and/or responsiveness of erythrocyte precursors in the bone marrow, (ii) a decreased erythrocyte life span, and (iii) shunting of bioavailable iron to storage forms and, possibly, a reduced uptake of dietary iron in the gut (19). In addition to anemia associated with inflammation, schistosomiasis may result in anemia secondary to increased sequestration of erythrocytes and/or increased hemolysis in the spleens of individuals with schistosomiasis-associated splenomegaly (5, 14, 36).
In a cohort of 580 7- to 30-year-old S. japonicum-infected individuals in a region of The Philippines where S. japonicum is endemic, we evaluated (i) the impact of reinfection with S. japonicum after treatment with praziquantel on the mean hemoglobin level and (ii) the contributions of potential mechanisms involved in S. japonicum-associated anemia, particularly iron deficiency and inflammation.
MATERIALS AND METHODS
Study area and population.
This prospective treatment-reinfection study was originally designed to investigate immune correlates of resistance to reinfection. The study was conducted in three rice-farming villages where S. japonicum is endemic in Leyte, The Philippines. Malaria is not endemic in this study area. In total, 74.3% (1,262/1,699) of individuals between the ages of 7 and 30 years residing in these villages were screened for the presence of S. japonicum infection by duplicate Kato-Katz examination of three stool samples prior to enrollment. The prevalence of infection with S. japonicum for this age range was 60.0%. Subjects were eligible for participation if they were infected with S. japonicum, lived primarily in a study village, were 7 to 30 years old, were not pregnant or lactating, and provided both child assent and parental consent or adult consent. Subjects with severe hepatomegaly or fibrosis on ultrasound examination, as well as subjects with severe anemia or severe wasting, were excluded from participation and referred for medical treatment.
In total, 616 participants living in 331 households were enrolled in two separate cohorts, in October 2002 and April 2003. Table 1 shows the baseline characteristics of the participants. After blood collection and physical examination, all participants were treated with a split dose of 60 mg of praziquantel/kg of body weight. Subsequently, participants were followed up at approximately 1, 3, 6, 9, 12, 15, and 18 months posttreatment. At each time point, stool and blood samples were collected and a physical examination was performed. All participants were transported to the study clinic by study staff for enrollment and follow-up visits. Brown University and The Philippines Research Institute of Tropical Medicine institutional review boards approved this study. All S. japonicum-reinfected subjects as well as subjects infected with geohelminths were treated at the end of the study.
TABLE 1.
Baseline characteristics of patients in this study
| Characteristic | Value |
|---|---|
| Age (yr) (mean [SD]) | 15.5 (6.1) |
| Sex (no. of males/total no. of patients [%]) | 364/580 (62.8) |
| Schistosoma japonicum egg count (epg) (geometric mean [SD]) | 42 (32) |
| No. of patients infected with hookworm/total no. tested (%) | 345/570 (60.5) |
| Hookworm egg count (epg) (geometric mean [SD]) | 212 (161) |
| Hemoglobin level (g/dl) (mean [SD]) | 12.49 (1.79) |
| No. of patients with anemiaa/total no. tested (%) | |
| Iron-deficiency anemia | 116/570 (20.4) |
| Non-iron-deficiency anemia | 73/570 (12.8) |
| Moderate all-cause anemia | 18/573 (3.1) |
Iron-deficiency anemia is defined as a hemoglobin level below the WHO cutoff and an SF level of ≤30 ng/ml. Non-iron-deficient anemia is defined as a hemoglobin level below the WHO cutoff and an SF level of >30 ng/ml. Moderate all-cause anemia is defined as a hemoglobin level of ≥7 and <9 g/dl.
Stool examination.
At 4 weeks posttreatment, a single stool sample was collected from each individual to assess the treatment response. In the week prior to each 3-month follow-up, three consecutive stool samples were collected from each participant at their home. Each of the stool specimens was examined in duplicate for Schistosoma japonicum, Ascaris lumbricoides, Trichuris trichiura, and hookworm eggs within 24 h of collection by the Kato-Katz method. For each of the stool specimens, the average number of eggs per gram (epg) for the duplicate exam was determined. For each time point, the overall mean epg was derived by averaging the egg counts for the three individual specimens.
SES.
A summary SES score based on questionnaire data addressing parental and child educational status, occupation, ownership of home/land, and assets was calculated for each participant as described previously (12).
Blood collection.
At each trimonthly follow-up visit, venipuncture was performed, and blood was collected into Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing EDTA as an anticoagulant (for hemograms) or serum separator gel (for serum assays). A complete hemogram was obtained on a Serono Baker 9000 hematology analyzer (Serono Baker Diagnostics, Allentown, PA). The hematology analyzer was maintained in compliance with the College of American Pathologists guidelines, with daily controls and necessary calibration. Because 248/2,958 (8.4%) hemoglobin measurements were performed on a non-quality-controlled hematology analyzer outside of our main study lab, they were coded as missing and were excluded from analysis.
Serum samples were aliquoted and stored at −80°C. Serum ferritin (SF), serum transferrin receptor (sTfR), C-reactive protein (CRP), and serum cytokines (interleukin-1β [IL-1β], IL-6, gamma interferon, tumor necrosis factor alpha [TNF-α], TNF receptor I, and TNF receptor II) were analyzed using a multiplex bead-based platform (Bio-Rad, Hercules, CA) with custom sandwich- or competitive-style bead kits and commercial controls as described previously (2). SF is a measure of stored iron, and sTfR is a circulating form of the transferrin protein receptor, derived mostly from red blood cell precursors, that measures functional iron deficiency or increased erythropoiesis. Total and direct bilirubin levels were assayed using commercial kits (ThermoDMA, Louisville, CO), and indirect bilirubin was calculated and used as a measure of hemolysis.
Ultrasound.
Study subjects were evaluated by ultrasound, using a Hitachi EUB-200 instrument with a 3.5-MHz probe (Hitachi Medical Corp., Tokyo, Japan), at baseline and at the 12-month follow-up. Spleen size was measured in the left intercostal oblique view and reported in centimeters.
Definitions.
Reinfection was defined as having S. japonicum eggs in the stool after treatment. In total, 355/1,955 (18.2%) posttreatment stool results were intermittently negative following reinfection at a previous follow-up. These results were considered to be false-negative results (falling under the limit of detection for stool examination), and egg counts were imputed using the “last value carried over” method (31). The duration of reinfection was defined as the time since the first positive stool sample plus 1.5 months, the midpoint between observations. Individuals without a negative stool sample at 4 weeks posttreatment were considered reinfected shortly after treatment. Subjects who were not reinfected following successful treatment were regarded as the reference group in the analyses of reinfection.
All-cause anemia was defined based on the following age- and gender-specific Hgb cutoffs recommended by the WHO (35): Hgb level of <11.5 g/dl for children under 12 years old, Hgb level of <12 g/dl for children of 12 to 14 years and females of ≥15 years, and Hgb level of <13 g/dl for males of ≥15 years. Mild, moderate, and severe anemia were defined as hemoglobin levels below the WHO cutoff but ≥9 g/dl, ≥7 and <9 g/dl, and <7 g/dl, respectively.
Recent studies evaluating the usefulness of SF for determining iron deficiency in anemic individuals from different populations concluded that a single cutoff SF level of ≤30 ng/ml has a high sensitivity and specificity for detecting iron-deficiency anemia (IDA), even when there is concurrent inflammation (16, 32). IDA was defined as the presence of anemia with an SF level of ≤30 ng/ml. Non-iron-deficiency anemia (NIDA) was defined as the presence of anemia with an SF level of >30 ng/ml. Notwithstanding the good performance of the single SF measure, several studies have shown that the addition of an sTfR measure has a higher sensitivity than does using SF alone (20, 27). However, due to the lack of an international standard for sTfR and a lack of commercial control for our assay, we were unable to utilize sTfR for our definition of IDA. Nevertheless, to assess the effect of potential misclassification that occurs when using a single SF cutoff, we used an alternative SF cutoff of 70 ng/ml to define iron deficiency in individuals with concurrent inflammation (defined as a CRP level of >8.2 μg/ml).
IL-6 responders were defined as individuals with detectable levels of IL-6 in serum (>1.45 pg/ml). Hyperbilirubinemia was defined as an indirect (unconjugated) bilirubin level of >1 mg/dl. Splenomegaly was defined as a spleen size of >2 standard deviations (SD) above the reference mean for a healthy Chinese population (13).
Statistical analyses.
Data forms collected in the field were bar coded and entered using Filemaker 5.5 software (Filemaker Inc., Santa Clara, CA). Variables that were not normally distributed (all egg counts and SF, sTfR, CRP, and all cytokine levels) were loge transformed [ln(n + 1)]. Analyses were implemented in SAS, version 9.1.3 (SAS Institute, Cary, NC). Cross-sectional comparisons of observations at baseline and at the final follow-up were made using linear and logistic regression. For longitudinal analyses, repeated-measure linear regression models and marginal logistic regression models with generalized estimating equations (GEE) were used to account for within-person correlations for continuous and dichotomous outcomes, respectively (3). β-Estimates presented for linear regression models represent mean changes of the outcome per unit change of the exposure. β-Estimates presented for logistic regression represent changes in log odds of the outcome per unit change of the exposure. All cross-sectional analyses and longitudinal linear regression analyses took clustering of observations within households into account, using hierarchical linear models (3). Adjustment for both within-household and within-person correlations was not technically possible for marginal logistic regression analyses and may have led to slightly smaller standard errors. Robust standard errors and P values are reported. P values of <0.05 were considered statistically significant.
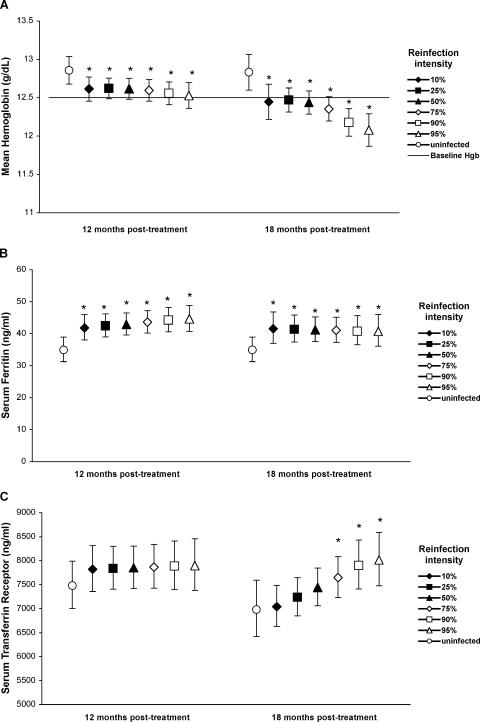
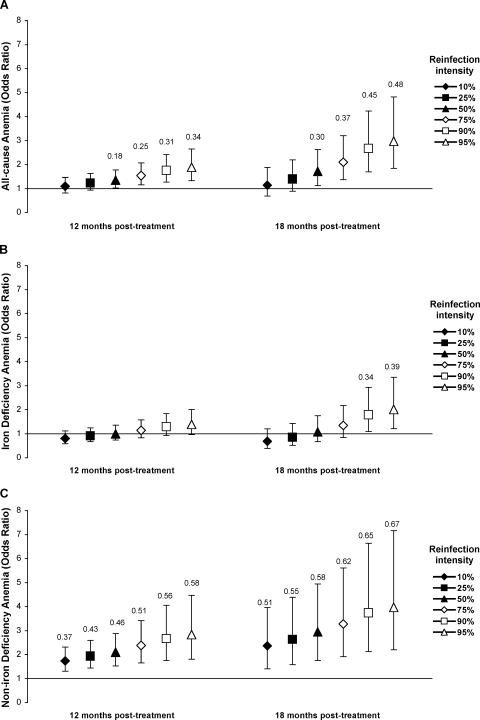
Our analytic approach was to simultaneously model (i) the temporal pattern of effects of reinfection (duration of reinfection), (ii) the intensity-associated differences in effects (intensity of reinfection), and (iii) any effect modification between the two (interactions). By allowing nonlinear (quadratic) associations between the outcomes and durations of reinfection, we were able to differentiate between acute and delayed effects of reinfection. Model selection was based on the best fit of observed data. An a priori decision was made to adjust all models for potential confounding by age, sex, SES, concurrent hookworm infection, and S. japonicum egg count at baseline. The baseline egg count reflects the exposure to S. japonicum prior to treatment and was included as a proximate marker of susceptibility to infection and cumulative exposure, which may influence immunologic reactions to reinfection and thus susceptibility to anemia. Figure 1 presents estimated mean Hgb, SF, and sTfR levels for an “average” individual (individual with a median duration of reinfection and a mean value for each covariate) with different intensities of reinfection at the 12- and 18-month follow-ups, calculated using the regression model parameters presented in Table 2. Similarly, Fig. 2 presents estimated odds ratios for all-cause anemia, IDA, and NIDA for an average individual with different intensities of reinfection at the 12- and 18-month follow-ups, calculated using the regression model parameters presented in Table 3. The attributable fraction in the exposed population (AFE) was estimated for anemia outcomes, using adjusted prevalence estimates obtained from the GEE models described above. The AFE is interpreted as the proportion of anemia in the S. japonicum-infected group that would not have occurred if S. japonicum reinfection would have been prevented.
FIG. 1.
Effects of reinfection with S. japonicum on mean hemoglobin (A), geometric mean serum ferritin (B), and serum transferrin receptor (C) levels at 12 and 18 months posttreatment. Means (symbols) and 95% confidence limits (error bars) were estimated using multilevel repeated-measure linear regression and were adjusted for potential confounding influences of age, sex, socioeconomic status, baseline S. japonicum egg count, and concurrent hookworm infection and for within-person correlation and clustering within households. Means were estimated by assuming the median reinfection duration at each follow-up, i.e., 7.5 and 13.5 months at the 12- and 18-month follow-ups, respectively, for different representative infection intensities based on the distribution of egg counts at each follow-up (3, 10, 22, 73, 212, and 383 epg at the 12-month follow-up and 3, 10, 30, 83, 287, and 483 epg at the 18-month follow-up). Asterisks represent significant differences compared to the group not (yet) reinfected. In panel a, the horizontal line represents the overall mean hemoglobin level at baseline.
TABLE 2.
Multilevel repeated-measure linear regression models of the effects of reinfection with Schistosoma japonicum on mean hematological and iron status measures in 580 7- to 30-year-old patientsa
| Variable | Hemoglobin
|
Serum ferritinb
|
Serum transferrin receptorb
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| Interceptc | 12.187 | 0.071 | <0.0001 | 3.266 | 0.05 | <0.0001 | 8.706 | 0.026 | <0.0001 |
| T3 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| T6 | 0.403 | 0.053 | <0.0001 | 0.240 | 0.042 | <0.0001 | −0.126 | 0.018 | <0.0001 |
| T9 | 0.429 | 0.085 | <0.0001 | 0.101 | 0.042 | 0.015 | −0.075 | 0.024 | 0.002 |
| T12 | 0.664 | 0.071 | <0.0001 | 0.311 | 0.048 | <0.0001 | 0.213 | 0.030 | <0.0001 |
| T15 | 0.796 | 0.093 | <0.0001 | 0.314 | 0.059 | <0.0001 | 0.169 | 0.035 | <0.0001 |
| T18 | 0.639 | 0.102 | <0.0001 | 0.205 | 0.067 | 0.002 | 0.144 | 0.042 | <0.001 |
| Aged | 0.145 | 0.010 | <0.0001 | 0.058 | 0.005 | <0.0001 | −0.022 | 0.003 | <0.0001 |
| Sexd | −0.487 | 0.105 | <0.0001 | −0.196 | 0.066 | 0.003 | 0.066 | 0.044 | 0.13 |
| Socioeconomic statusd | 0.279 | 0.065 | <0.0001 | 0.137 | 0.037 | <0.001 | −0.086 | 0.023 | <0.001 |
| Hookworm egg counte | 0.003 | 0.007 | 0.66 | −0.008 | 0.005 | 0.14 | 0.001 | 0.003 | 0.62 |
| Baseline S. japonicum egg countd | −0.086 | 0.039 | 0.027 | −0.029 | 0.025 | 0.25 | 0.031 | 0.015 | 0.037 |
| Durationf | −0.038 | 0.019 | 0.042 | 0.021 | 0.007 | 0.002 | 0.015 | 0.008 | 0.066 |
| S. japonicum egg countg | −0.072 | 0.041 | 0.081 | 0.037 | 0.011 | <0.001 | 0.003 | 0.006 | 0.65 |
| Duration × S. japonicum egg counth | 0.015 | 0.009 | 0.11 | −0.003 | 0.001 | 0.024 | −0.002 | 0.002 | 0.25 |
| S. japonicum egg count × S. japonicum egg counti | 0.016 | 0.008 | 0.052 | ||||||
| Duration × S. japonicum egg count × S. japonicum egg counth | −0.003 | 0.001 | 0.009 | ||||||
| Duration × durationi | −0.001 | 0.001 | 0.017 | ||||||
| Duration × duration × S. japonicum egg counth | 0.0003 | 0.0001 | 0.028 | ||||||
β-Estimates (excluding the intercept) represent mean changes of the outcome per unit change of the exposure. β-Estimates and SE were adjusted for within-person correlation, for clustering within households, and for confounding by covariates. T3 to T18 represent the trimonthly posttreatment follow-ups, and the 3-month follow-up is the reference category.
Serum ferritin and serum transferrin receptor were ln transformed.
The intercept represents the outcome mean at the 3-month follow-up for an average individual (i.e., an individual with a mean value for each of the covariates). The P value indicates whether the intercept is significantly different from zero.
The confounders age, sex, SES, and baseline ln-transformed S. japonicum egg count were centered on their baseline means.
ln-transformed hookworm egg counts were centered on the mean for all posttreatment observations.
Defined as the time since reinfection with S. japonicum.
ln-transformed S. japonicum egg count.
Interaction term.
Quadratic function (indicates a nonlinear relationship between the exposure and the outcome).
FIG. 2.
Effects of reinfection with S. japonicum on odds of all-cause anemia (A), iron-deficiency anemia (B), and non-iron-deficiency anemia (C) at 12 and 18 months posttreatment. Odds ratios (symbols) and 95% confidence limits (error bars) were estimated using marginal logistic (GEE) regression and were adjusted for potential confounding influences of age, sex, socioeconomic status, baseline S. japonicum egg count, and concurrent hookworm infection and for within-person correlation. Odds ratios were estimated by assuming the median reinfection duration at each follow-up, i.e., 7.5 and 13.5 months at the 12- and 18-month follow-ups, respectively, for different representative infection intensities based on the distribution of egg counts at each follow-up (3, 10, 22, 73, 212, and 383 epg at the 12-month follow-up and 3, 10, 30, 83, 287, and 483 epg at the 18-month follow-up). Numbers above the error bars represent the attributable fractions in the exposed population, given for significant comparisons only.
TABLE 3.
Marginal logistic regression models of the effects of reinfection with Schistosoma japonicum on the odds of anemia in 580 7- to 30-year-old patientsa
| Variable | All-cause anemiab
|
Iron-deficiency anemiab
|
Non-iron-deficiency anemiab
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| Interceptc | −0.287 | 0.095 | 0.0024 | −0.635 | 0.102 | <0.0001 | −1.431 | 0.135 | <0.0001 |
| T3 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| T6 | −0.430 | 0.094 | <0.0001 | −0.385 | 0.092 | <0.0001 | −0.429 | 0.146 | 0.003 |
| T9 | −0.201 | 0.127 | 0.11 | −0.219 | 0.142 | 0.12 | −0.184 | 0.17 | 0.28 |
| T12 | −0.699 | 0.144 | <0.0001 | −0.618 | 0.154 | <0.0001 | −0.685 | 0.194 | <0.001 |
| T15 | −0.915 | 0.18 | <0.0001 | −0.934 | 0.193 | <0.0001 | −0.851 | 0.245 | <0.001 |
| T18 | −0.670 | 0.200 | <0.001 | −0.591 | 0.223 | 0.008 | −0.668 | 0.253 | 0.008 |
| Aged | −0.091 | 0.014 | <0.0001 | −0.103 | 0.017 | <0.0001 | −0.077 | 0.017 | <0.0001 |
| Sexd | 0.063 | 0.156 | 0.68 | −0.048 | 0.187 | 0.80 | 0.125 | 0.181 | 0.49 |
| Socioeconomic statusd | −0.278 | 0.083 | <0.001 | −0.368 | 0.102 | <0.001 | −0.168 | 0.099 | 0.091 |
| Hookworm egg counte | 0.001 | 0.016 | 0.97 | 0.008 | 0.019 | 0.68 | −0.024 | 0.021 | 0.26 |
| Baseline S. japonicum egg countd | 0.082 | 0.051 | 0.11 | 0.099 | 0.059 | 0.094 | 0.067 | 0.058 | 0.25 |
| Durationf | −0.012 | 0.023 | 0.59 | −0.054 | 0.025 | 0.032 | 0.052 | 0.021 | 0.012 |
| S. japonicum egg countg | 0.017 | 0.033 | 0.61 | −0.014 | 0.034 | 0.69 | 0.109 | 0.039 | 0.005 |
| Duration × S. japonicum egg counth | 0.014 | 0.005 | 0.003 | 0.018 | 0.005 | <0.0001 | |||
β-estimates (excluding the intercept) represent changes in log odds of the outcome per unit change of the exposure. β-Estimates and SE were adjusted for within-person associations and for confounding by covariates. T3 to T18 represent the trimonthly posttreatment follow-ups, and the 3-month follow-up is the reference category.
All-cause anemia is defined as a hemoglobin level below the WHO cutoff. Iron-deficiency anemia is defined as a hemoglobin level below the WHO cutoff and an SF level of ≤30 ng/ml. Non-iron-deficiency anemia is defined as a hemoglobin level below the WHO cutoff and an SF level of >30 ng/ml.
The intercept represents the log odds of the outcome at the 3-month follow-up for an average individual (i.e., an individual with a mean value for each of the covariates). The P value indicates whether the intercept is significantly different from zero.
The confounders age, sex, socioeconomic status, and baseline ln-transformed S. japonicum egg count were centered on the baseline means.
ln-transformed hookworm egg counts were centered on the mean of all posttreatment observations.
Defined as the time since reinfection with S. japonicum.
ln-transformed S. japonicum egg count.
Interaction term.
RESULTS
Study sample.
We screened 1,262 individuals between the ages of 7 and 30 years, 60% of whom were infected with S. japonicum. In total, 616 individuals were eligible to participate and were treated with praziquantel at baseline. Of these, 20 (3.2%) could not be included in the analyses because missing data did not allow assessments of reinfection status, and 16 (2.6%) did not participate in any of the follow-up surveys, which functionally excluded them from analyses as well. For the remaining 580 individuals, participation was good: 62.4% were present at all six posttreatment follow-ups, and 89.8% were present at three or more posttreatment follow-ups. Hemoglobin measurements were available for 2,710/2,958 (91.6%) observations. Stored serum samples were available for 2,948/2,958 (99.7%) observations. Stool results were available for 2,846/2,958 (95.2%) observations. Treatment with praziquantel was effective: 4 weeks after treatment, 512/550 (93.2%) individuals present had negative stool samples. Table 1 presents baseline characteristics for the cohort.
Hemoglobin concentrations following treatment.
Relative to baseline Hgb levels and after adjusting for the effect of reinfection with S. japonicum and other confounders, we saw a significant drop in the mean hemoglobin level at 3 months posttreatment (mean, −0.30 g/dl; 95% confidence interval [95% CI], −0.39 to −0.21) and a subsequent rise to a peak at 15 months posttreatment (mean, 0.52 g/dl; 95% CI, 0.33 to 0.70). Further details of the posttreatment nutritional responses in this cohort have been published elsewhere (1).
Reinfection with S. japonicum.
After 12 months of follow-up, 410/496 individuals present (82.7%) were reinfected, and the median duration of reinfection after praziquantel treatment was 7.5 months. After 18 months, 434/486 individuals present (89.3%) were reinfected, and the median duration of reinfection was 13.5 months. Median egg counts for infected individuals were 22 epg (interquartile range, 10 to 73 epg) and 30 epg (interquartile range, 10 to 83 epg) at 12 and 18 months posttreatment, respectively.
Hemoglobin concentration and anemia at 18 months posttreatment.
Individuals who were reinfected had a decreased mean Hgb level (−0.49 g/dl; 95% CI, −0.82 to −0.16 [P = 0.004]) and increased odds of all-cause anemia (prevalence odds ratio [95% CI], 4.20 [1.65 to 10.65] [P = 0.003]) compared to individuals who were not reinfected at the end of the study. In total, 164/390 S. japonicum-reinfected individuals with a Hgb measurement at the final follow-up were anemic (42.1%); of these anemic, reinfected individuals, 50.6% were classified as having IDA and 49.4% were classified as having NIDA. Only 9/390 (2.3%) and 3/390 (0.8%) reinfected individuals had moderate and severe anemia, respectively.
Impact of reinfection on Hgb.
Using a repeated-measure linear regression model, we assessed the time-dependent relationships between the mean Hgb level and the duration and intensity of reinfection. There was a significant interaction between the duration and intensity of reinfection (egg count squared) (Table 2), indicating that the magnitude of the per-month drop in Hgb level after reinfection increased as a quadratic function of the reinfection intensity. Figure 1A compares pretreatment mean Hgb levels to the estimated mean Hgb levels at the 12- and 18-month follow-ups for uninfected individuals and individuals reinfected at various intensities (i.e., percentiles of the egg count distribution at that follow-up) for the median duration of reinfection at that follow-up. The mean hemoglobin level was lower for all intensities of infection in reinfected individuals than that in uninfected individuals. Importantly, the mean hemoglobin level was lower than or equal to the mean pretreatment hemoglobin level (12.49 g/dl) in the most heavily reinfected individuals (≥95th percentile) at 12 months and in all reinfected individuals at 18 months, indicating a complete reversal or absence of treatment effect after 6 months of high-intensity reinfection or 12 months of reinfection at any intensity.
The decreased mean Hgb level in reinfected compared to uninfected individuals coincided with an increased risk of all-cause anemia in all but the lowest-intensity infections (≤25th percentile) (Fig. 2A). On average, 30% of all-cause anemia cases in S. japonicum-reinfected individuals at the 18-month follow-up were attributable to reinfection (AFE = 0.30) (Fig. 2A).
Impact of reinfection on iron status.
Compared to that in uninfected individuals, the mean SF level was raised significantly after reinfection, regardless of infection intensity (Fig. 1B), likely as part of the acute-phase response (see below). There was a significant interaction between the duration (duration squared) and intensity of reinfection in the model of mean sTfR levels (Table 2). The mean sTfR level in the uninfected group was higher at 12 months than at 18 months (Fig. 1C). Although this difference was not significant, it may reflect increased erythropoiesis following treatment or a natural fluctuation in iron status. More importantly, after 13.5 months of reinfection, high-intensity infections (≥75th percentile) were associated with higher mean sTfR levels than those in uninfected individuals (Fig. 1C). Because the increase in mean sTfR level in heavily infected individuals coincided with the decrease in mean Hgb level for this group (Fig. 1A), elevated sTfR in these individuals likely reflects iron deficiency, although compensatory erythropoiesis cannot be excluded.
Impact of reinfection on type of anemia.
To further evaluate the potential mechanisms involved in S. japonicum-associated anemia, we assessed the associations between different types of anemia and the duration and intensity of reinfection. There was a significant interaction between the duration and intensity of reinfection in the model assessing the probability of IDA but not in the model for NIDA (Table 3). The probability of IDA was not significantly different for reinfected than for uninfected individuals after 7.5 months of reinfection (Fig. 2B). However, the probability of IDA was significantly increased for the heaviest-intensity infections (≥90th percentile) after 13.5 months of reinfection (Fig. 2B). This delayed effect suggests an S. japonicum-associated decrease in iron stores that is slowly progressive and leads to IDA only after prolonged exposure. The use of an alternative definition of IDA (see Materials and Methods) led to similar conclusions, with the only difference being that the increased probability of IDA for the heaviest-intensity infections was already apparent after 7.5 months of reinfection and that the odds ratios at 12 and 18 months were approximately 10% and 17% higher, respectively (data not shown).
The odds of NIDA were significantly raised at all reinfection intensities, but the odds and the AFE of NIDA increased with the duration and intensity of reinfection (Fig. 2C). The use of the alternative definition of NIDA resulted in similar conclusions, although the odds ratios at 12 and 18 months were approximately 4.5% and 9% lower, respectively (data not shown).
Impact of reinfection on CRP and serum cytokines.
There was a significant interaction between the duration and intensity of reinfection in our repeated-measure model of serum CRP (for duration, β = 0.013, standard error [SE] = 0.007, and P = 0.073; for S. japonicum egg count, β = 0.038, SE = 0.011, and P < 0.001; and for duration × S. japonicum egg count, β = 0.005, SE = 0.002, and P = 0.001). As expected, the mean CRP level for reinfected individuals was significantly raised relative to that for uninfected individuals, regardless of the duration or intensity of reinfection.
There was no interaction between the duration and intensity of reinfection in our model of the IL-6 response (P = 0.71). The intensity and duration of reinfection were both associated with the probability of the IL-6 response (P < 0.0001 and P = 0.002 for intensity and duration, respectively); the log odds of an IL-6 response increased 0.316 (95% CI, 0.212 to 0.420) for every log increase in S. japonicum egg count and decreased −0.081 (95% CI, −0.134 to −0.029) for every month of reinfection. Together, these findings indicate that the probability of an IL-6 response, for any infection intensity, is highest shortly after reinfection but decreases thereafter.
None of the other serum cytokines involved in anemia associated with inflammation (gamma interferon, TNF-α, TNF receptor I, TNF receptor II, and IL-1β) were associated with reinfection with S. japonicum (data not shown).
Inflammation and anemia.
To evaluate the potential mediating role of S. japonicum-associated inflammation in the etiology of anemia, we assessed whether CRP and IL-6 responses predicted mean Hgb and iron status measures and the probability of anemia (Table 4). CRP was significantly associated with all outcomes. Importantly, the positive association between CRP and the mean sTfR level was not confounded by S. japonicum intensity (data not shown), suggesting a direct effect of inflammation on the mean sTfR level. Serum IL-6 responses were associated with increased SF and increased odds of NIDA (Table 4).
TABLE 4.
Impact of S. japonicum-associated cytokines on hematological and iron status measures and prevalence of anemia
| Variable | β-Estimate (95% CI), P valuec
|
||||
|---|---|---|---|---|---|
| Hemoglobina | Serum ferritina | Serum transferrin receptora | Iron-deficiency anemiab | Non-iron-deficiency anemiab | |
| C-reactive protein | −0.152 (−0.214 to −0.089), <0.0001 | 0.243 (0.209 to 0.277), <0.0001 | 0.037 (0.017 to 0.057), <0.001 | 0.126 (0.024 to 0.227), 0.016 | 0.577 (0.414 to 0.740), <0.0001 |
| IL-6 response | −0.035 (−0.181 to 0.110), 0.63 | 0.358 (0.268 to 0.449), <0.0001 | −0.001 (−0.046 to 0.044), 0.98 | −0.141 (−0.399 to 0.117), 0.285 | 0.484 (0.079 to 0.888), 0.019 |
β-Estimates and P values were obtained from multilevel repeated-measure linear regression models; β represents the change in the mean of the outcome per (log) unit increase of the predictor. Estimates were adjusted for potential confounding influences of age, sex, socioeconomic status, baseline S. japonicum egg count, and concurrent hookworm infection and for within-person correlation and clustering within households.
β-Estimates and P values were obtained from marginal logistic regression (GEE) models; β represents the change in log odds of the outcome per (log) unit increase of the predictor. Estimates were adjusted for potential confounding influences of age, sex, socioeconomic status, baseline S. japonicum egg count, and concurrent hookworm infection and for within-person association.
SF, sTfR, and C-reactive protein levels were ln transformed. An IL-6 response was defined as having a detectible concentration (>1.45 pg/ml) of serum IL-6. Iron-deficiency anemia was defined as an Hgb level below the WHO cutoff and an SF level of ≤30 ng/ml. Non-iron-deficiency anemia was defined as an Hgb level below the WHO cutoff and an SF level of >30 ng/ml.
Macrocytosis, hyperbilirubinemia, and splenomegaly.
Finally, we assessed other mechanisms that may be responsible for anemia in this population. In only 7/2,674 (0.3%) complete blood counts, a mean cell volume of >100 μm3 was detected, and on no two occasions did the same person have an increased mean cell volume. Spleen size was determined by ultrasound at 12 months posttreatment for 437 subjects; only 2 individuals (0.5%) had splenomegaly. Hyperbilirubinemia was detected in 265/2,948 (9.0%) measurements, half of which were detected on only one occasion in a single person. Importantly, hyperbilirubinemia was not associated with the S. japonicum reinfection duration or intensity, nor was it associated with decreased Hgb or an increased probability of anemia (data not shown).
DISCUSSION
In a large cohort of 7- to 30-year-old S. japonicum-infected inhabitants of Leyte, The Philippines, we found that reinfection with S. japonicum after treatment with praziquantel had a major impact on hemoglobin levels and the risk of anemia. There was a clear dose-response relationship between reinfection intensity, mean Hgb levels, and the odds of anemia that progressed in magnitude with increasing durations of infection. We estimated that 30% of all-cause anemia cases in the group reinfected with S. japonicum were attributable to reinfection. Extrapolating these findings to the population level for this setting of high (60%) S. japonicum prevalence among children, adolescents, and young adults, approximately one-fifth of all-cause anemia cases are attributable to S. japonicum infection, which is a large proportion for a single infectious etiology.
By simultaneously measuring the impact of S. japonicum on hemoglobin, iron status, and inflammatory measures, we were able to assess the potential mechanisms involved with S. japonicum-associated anemia. It is generally held that iron deficiency due to extracorporeal blood loss is the main cause of schistosomiasis-associated anemia (29). A previously published cross-sectional study conducted with this cohort reported increased odds of detectable occult blood in the stools of individuals with high-intensity infection at baseline (≥400 epg), suggesting that extracorporeal blood loss due to translocation of eggs through the bowel wall or through inflammatory lesions of intestinal mucosa occurs during high-intensity infections (6). In our current analyses, we found evidence of iron deficiency, increased odds of IDA, and increased mean sTfR levels, even for individuals with significantly lower intensities of reinfection (≥80 epg). A lack of evidence of occult blood loss for individuals with <400 epg at baseline may be related to the poor sensitivity of the Hemoccult test. Alternatively, inflammation may be responsible for a reduced intake or absorption of iron, which is supported by the current finding of a positive association between CRP and mean sTfR levels, independent of the S. japonicum reinfection intensity.
Inflammation may mediate iron deficiency in two ways. First, proinflammatory cytokines, in particular TNF-α, IL-1, and IL-6, cause anorexia (11, 26) and thus lead to a decreased intake of dietary iron. Second, several recent studies have found that production of the novel iron regulatory protein hepcidin is up-regulated during inflammation through mediation of IL-6 (22), which in turn inhibits iron efflux from intestinal epithelial cells through its influence on ferroportin (23), thus reducing iron absorption through the gut (24, 34). Whether this mechanism leads to iron depletion in humans with chronic inflammatory disease remains to be investigated. Additional research is needed to assess whether infection with schistosomiasis negatively affects hematological responses to iron supplementation.
Although the overall prevalence of IDA was high (∼20%) and we did find an association between S. japonicum and IDA, this type of anemia was only attributable to S. japonicum infection in a minority of individuals with higher-than-average intensities of reinfection, implying that iron deficiency was not a central mechanism in S. japonicum-associated anemia within this 18-month follow-up period. Conversely, approximately 50% of the non-iron-deficiency anemia cases in S. japonicum-reinfected individuals were attributable to S. japonicum. Note that increased odds of NIDA were apparent for all reinfected individuals, regardless of reinfection intensity or duration. We found no evidence that S. japonicum-associated anemia was due to hemolysis or sequestration of erythrocytes due to splenomegaly, nor was macrocytic anemia prevalent. Clinically relevant hemoglobinopathies are likely uncommon in this study area (8, 9). While there is no direct method to assess the presence of anemia associated with inflammation, the exclusion of iron deficiency and other causes of anemia, together with the associations we found between NIDA and inflammatory markers (CRP and IL-6), justifies the interpretation of NIDA as anemia associated with inflammation. In light of these results, the overall decline in hemoglobin 3 months after treatment may also be attributed to proinflammatory immune responses to worm antigens released as worms disintegrate after treatment with praziquantel.
The reliable assessment of iron status is difficult in situations where inflammation is prevalent. This is particularly problematic when attempting to assess whether iron deficiency plays a role in the etiology of an infectious disease. Although several recent clinical studies have provided compelling evidence supporting the use of the sTfR-to-SF ratio for the diagnosis of IDA when there is concurrent inflammation (15, 27), no population-based studies have validated its use in the tropics. The usefulness of different iron status measures in a tropical setting has been evaluated in several recent studies, but most of these are difficult to interpret due to the lack of a gold standard definition of iron deficiency. Although the precise magnitude of the effects of S. japonicum on different types of anemia is likely influenced by misclassification based on the definitions used, conclusions from our definition-based approach (type of anemia) and from our continuous-outcome approach (hemoglobin and sTfR levels) are consistent. In addition, analyses using alternative definitions of IDA and NIDA led to the same conclusions. Nevertheless, the lack of sensitivity and specificity in the definition of outcomes reduces the precision of our estimates and makes it difficult to detect subtle differences between groups.
While our study sample is likely representative of many S. japonicum-infected populations, generalizations of our findings to other populations should be made with care. In this study area, reinfection rates are very high, in part due to the abundance of nonhuman hosts for S. japonicum (18), and therefore the time-dependent associations presented are likely specific to areas with similar transmission ecology. The inflammation-mediated mechanisms discussed above may also apply to other human-specific Schistosoma species. Nevertheless, because differences in the numbers of eggs excreted and, possibly, differences in adaptation of the human host to different species may influence these mechanisms (25), more research is required to assess the proportions of these causes in other Schistosoma species. Another limitation lies in the definition of reinfection, as some patients classified as reinfected may represent individuals who did not experience a full cure. This should not affect the relationships between infection status and anemia type, unless one proposes a very different immune response for new infections versus continued old infections which may be down-modulated. Finally, given the 22% attrition rate from baseline to the final visit, potential bias in the loss of patients to follow up should be discussed. Overall, this might be due to poor subjects not returning, but this was minimized by the provision of transportation and compensation with a day's worth of lost wages. It may also be due to healthy subjects feeling well and not returning. Although either scenario can influence the prevalence of anemia, it is unlikely to affect relationships between the type of anemia and schistosomiasis reinfection, as these were based only on those who attended.
In general, national schistosomiasis control programs targeting communities and schools use annual treatment rounds. Although the mean hemoglobin level in reinfected individuals at 1 year posttreatment was higher than that at baseline, it was significantly lower than that for individuals who were not reinfected at 1 year. This implies that reinfection led to an attenuation of any potential treatment effect within the 1-year treatment interval. Importantly, for individuals reinfected shortly after treatment or at a higher intensity, no net treatment effect was observed after 1 year. Our findings suggest that individuals with high levels of exposure will need repeated chemotherapy, at least once per year, to treat and prevent anemia. However, in areas of intense transmission, schistosomiasis control based on treatment alone is unlikely to be sustainable. Measures to prevent reinfection by reducing exposure should be included, such as improved sanitation, vector (snail) control, and treatment of nonhuman hosts.
In conclusion, our results, together with those of others (17), provide strong evidence for the causal relationship between both chronic and acute S. japonicum infections and anemia. Schistosomiasis japonica, a readily treatable disease, may be responsible for approximately one-fifth of all-cause anemia cases in settings with a high prevalence of this infection. The principle mechanism involved in S. japonicum-associated anemia is that of proinflammatory cytokine-mediated anemia, with iron deficiency playing a role in high-intensity infections. Our findings highlight the complex nature of anemia in tropical settings and underscore the need for combined approaches to treatment, as iron therapy in the context of anemia associated with inflammation will be much less efficacious due to the alteration of iron metabolism.
Acknowledgments
This work was funded by National Institutes of Health grants R01AI48123 and K23AI52125.
We thank our field staff for their diligence and energy. We thank the study participants from Macanip, Buri, and Pitogo in Leyte, The Philippines.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Coutinho, H. M., L. P. Acosta, S. T. McGarvey, B. Jarilla, M. Jiz, A. Pablo, L. Su, D. L. Manalo, R. M. Olveda, J. D. Kurtis, and J. F. Friedman. 2006. Nutritional status improves after treatment of Schistosoma japonicum-infected children and adolescents. J. Nutr. 136:183-188. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho, H. M., S. T. McGarvey, L. P. Acosta, D. L. Manalo, G. C. Langdon, T. Leenstra, H. K. Kanzaria, J. Solomon, H. Wu, R. M. Olveda, J. D. Kurtis, and J. F. Friedman. 2005. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192:528-536. [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice, G. M., N. M. Laird, and J. H. Ware. 2004. Applied longitudinal analysis. John Wiley & Sons, Inc., New York, N.Y.
- 4.Friedman, J. F., H. K. Kanzaria, and S. T. McGarvey. 2005. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 21:386-392. [DOI] [PubMed] [Google Scholar]
- 5.Friis, H., D. Mwaniki, B. Omondi, E. Muniu, F. Thiong'o, J. Ouma, P. Magnussen, P. W. Geissler, and K. F. Michaelsen. 2003. Effects on haemoglobin of multi-micronutrient supplementation and multi-helminth chemotherapy: a randomized, controlled trial in Kenyan school children. Eur. J. Clin. Nutr. 57:573-579. [DOI] [PubMed] [Google Scholar]
- 6.Kanzaria, H. K., L. P. Acosta, G. C. Langdon, D. L. Manalo, R. M. Olveda, S. T. McGarvey, J. D. Kurtis, and J. F. Friedman. 2005. Schistosoma japonicum and occult blood loss in endemic villages in Leyte, the Philippines. Am. J. Trop. Med. Hyg. 72:115-118. [PubMed] [Google Scholar]
- 7.King, C. H., K. Dickman, and D. J. Tisch. 2005. Reassessment of the cost of chronic helminthic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365:1561-1569. [DOI] [PubMed] [Google Scholar]
- 8.Ko, T. M., A. P. Caviles, Jr., H. L. Hwa, C. W. Liu, P. M. Hsu, and Y. P. Chung. 1998. Prevalence and molecular characterization of beta-thalassemia in Filipinos. Ann. Hematol. 77:257-260. [DOI] [PubMed] [Google Scholar]
- 9.Ko, T. M., H. L. Hwa, C. W. Liu, S. F. Li, J. Y. Chu, and Y. P. Cheung. 1999. Prevalence study and molecular characterization of alpha-thalassemia in Filipinos. Ann. Hematol. 78:355-357. [DOI] [PubMed] [Google Scholar]
- 10.Konijn, A. M. 1994. Iron metabolism in inflammation. Baillieres Clin. Haematol. 7:829-849. [DOI] [PubMed] [Google Scholar]
- 11.Kotler, D. P. 2000. Cachexia. Ann. Intern. Med. 133:622-634. [DOI] [PubMed] [Google Scholar]
- 12.Leenstra, T., L. P. Acosta, G. C. Langdon, D. L. Manalo, L. Su, R. M. Olveda, S. T. McGarvey, J. D. Kurtis, and J. F. Friedman. 2006. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am. J. Clin. Nutr. 83:371-379. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y. S., R. Kardorff, J. Richter, K. Y. Sun, H. Zhou, D. P. McManus, and C. Hatz. 2004. Ultrasound organometry: the importance of body height adjusted normal ranges in assessing liver and spleen parameters among Chinese subjects with Schistosoma japonicum infection. Acta Trop. 92:133-138. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud, A. A., and A. W. Woodruff. 1972. Mechanisms involved in the anaemia of schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 66:75-84. [DOI] [PubMed] [Google Scholar]
- 15.Malope, B. I., A. P. MacPhail, M. Alberts, and D. C. Hiss. 2001. The ratio of serum transferrin receptor and serum ferritin in the diagnosis of iron status. Br. J. Haematol. 115:84-89. [DOI] [PubMed] [Google Scholar]
- 16.Mast, A. E., M. A. Blinder, A. M. Gronowski, C. Chumley, and M. G. Scott. 1998. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin. Chem. 44:45-51. [PubMed] [Google Scholar]
- 17.McGarvey, S. T., G. Aligui, K. K. Graham, P. Peters, G. R. Olds, and R. Olveda. 1996. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. Am. J. Trop. Med. Hyg. 54:498-502. [DOI] [PubMed] [Google Scholar]
- 18.McGarvey, S. T., H. Carabin, E. Balolong, Jr., P. Bélisle, T. Fernandez, L. Joseph, V. Tallo, R. Gonzales, M. Tarafder, P. Alday, A. L. Willingham, and R. Olveda. 2006. Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar Province, Philippines. Bull. W. H. O. 84:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Means, R. T., Jr. 2000. The anaemia of infection. Baillieres Best Pract. Res. Clin. Haematol. 13:151-162. [DOI] [PubMed] [Google Scholar]
- 20.Means, R. T., Jr., J. Allen, D. A. Sears, and S. J. Schuster. 1999. Serum soluble transferrin receptor and the prediction of marrow aspirate iron results in a heterogeneous group of patients. Clin. Lab. Haematol. 21:161-167. [DOI] [PubMed] [Google Scholar]
- 21.Murray, C. J. L., and A. D. Lopez. 1996. Global health statistics: a compendium of incidence, prevalence and mortality estimates for over 200 conditions. Harvard University Press, Boston, Mass.
- 22.Nemeth, E., S. Rivera, V. Gabayan, C. Keller, S. Taudorf, B. K. Pedersen, and T. Ganz. 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 113:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth, E., M. S. Tuttle, J. Powelson, M. B. Vaughn, A. Donovan, D. M. Ward, T. Ganz, and J. Kaplan. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090-2093. [DOI] [PubMed] [Google Scholar]
- 24.Nicolas, G., M. Bennoun, A. Porteu, S. Mativet, C. Beaumont, B. Grandchamp, M. Sirito, M. Sawadogo, A. Kahn, and S. Vaulont. 2002. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 99:4596-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olds, G. R., R. Olveda, G. Wu, P. Wiest, S. McGarvey, G. Aligui, S. Zhang, B. Ramirez, B. Daniel, P. Peters, R. Romulo, P. Fevidal, W. Tiu, J. Yuan, E. Domingo, and B. Blas. 1996. Immunity and morbidity in schistosomiasis japonicum infection. Am. J. Trop. Med. Hyg. 55:121-126. [DOI] [PubMed] [Google Scholar]
- 26.Plata-Salaman, C. R. 1998. Cytokine-induced anorexia. Behavioral, cellular, and molecular mechanisms. Ann. N. Y. Acad. Sci. 856:160-170. [DOI] [PubMed] [Google Scholar]
- 27.Punnonen, K., K. Irjala, and A. Rajamaki. 1997. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89:1052-1057. [PubMed] [Google Scholar]
- 28.Stephenson, L. S. 1993. The impact of schistosomiasis on human nutrition. Parasitology 107(Suppl.):S107-S123. [DOI] [PubMed] [Google Scholar]
- 29.Strickland, G. T., and B. L. Ramirez. 2000. Schistosomiasis, p. 804-832. In G. T. Strickland (ed.), Hunter's tropical medicine and emerging infectious diseases, 8th ed. W. B. Saunders Company, Philadelphia, Pa.
- 30.Tatala, S., U. Svanberg, and B. Mduma. 1998. Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi district of Tanzania. Am. J. Clin. Nutr. 68:171-178. [DOI] [PubMed] [Google Scholar]
- 31.Twisk, J., and W. de Vente. 2002. Attrition in longitudinal studies. How to deal with missing data. J. Clin. Epidemiol. 55:329-337. [DOI] [PubMed] [Google Scholar]
- 32.van den Broek, N. R., E. A. Letsky, S. A. White, and A. Shenkin. 1998. Iron status in pregnant women: which measurements are valid? Br. J. Haematol. 103:817-824. [DOI] [PubMed] [Google Scholar]
- 33.Warren, K. S., D. L. Su, Z. Y. Xu, H. C. Yuan, P. A. Peters, J. A. Cook, K. E. Mott, and H. B. Houser. 1983. Morbidity in schistosomiasis japonica in relation to intensity of infection. A study of two rural brigades in Anhui Province, China. N. Engl. J. Med. 309:1533-1539. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein, D. A., C. N. Roy, M. D. Fleming, M. F. Loda, J. I. Wolfsdorf, and N. C. Andrews. 2002. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 100:3776-3781. [DOI] [PubMed] [Google Scholar]
- 35.WHO. 2001. Iron deficiency anaemia: assessment, prevention and control. World Health Organization, Geneva, Switzerland.
- 36.Woodruff, A. W., A. Z. Shafei, H. K. Awwad, L. E. Pettitt, and H. H. Abaza. 1966. Anaemia in patients with schistosomiasis and gross splenomegaly. Trans. R. Soc. Trop. Med. Hyg. 60:343-351. [DOI] [PubMed] [Google Scholar]