Abstract
Several lines of evidence from different model systems suggest that gamma interferon (IFN-γ) is an important regulator of T-cell contraction after antigen (Ag)-driven expansion. To specifically investigate the role of IFN-γ in regulating the contraction of Ag-specific CD4 T cells, we infected IFN-γ−/− and IFN-γR1−/− mice with attenuated Listeria monocytogenes and monitored the numbers of Ag-specific CD4 T cells during the expansion, contraction, and memory phases of the immune response to infection. In the absence of IFN-γ or the ligand-binding portion of its receptor, Ag-specific CD4 T cells exhibited normal expansion in numbers, but in both strains of deficient mice there was very little decrease in the number of Ag-specific CD4 T cells even at time points later than day 90 after infection. This significant delay in contraction was not due to prolonged infection, since mice treated with antibiotics to conclusively eliminate infection exhibited the same defect in contraction. In addition to altering the number of Ag-specific CD4 T cells, the absence of IFN-γ signaling also changed the phenotype of cells generated after infection. IFN-γR1−/− Ag-specific CD4 T cells reacquired expression of CD127 more quickly than wild-type cells, and more IFN-γR1−/− CD4 T cells were capable of producing both IFN-γ and interleukin 2 following Ag stimulation. From these data we conclude that IFN-γ regulates the contraction, phenotype, and function of Ag-specific CD4 T cells generated after infection.
Two of the hallmarks of adaptive immunity are specificity and establishment of long-term memory. Over the past decade, with the advent of new technologies to detect antigen (Ag)-specific T cells, we are beginning to gain significant insight into how Ag-specific T-cell responses evolve (1, 12, 28). Following infection, naive T-cell precursors specific for peptide antigens derived from the infectious agent become activated via interaction between their T-cell receptors and peptide-major histocompatibility complex (MHC) complexes on mature dendritic cells. Following this initial antigen recognition and requisite costimulatory signals, Ag-specific T cells undergo vigorous proliferation (42).
Ag-specific CD4 and CD8 T cells require only a brief exposure to antigen in order to begin the expansion phase of their response and will continue to divide even after the infection has been terminated and antigen is absent (7, 14, 25, 27, 41, 43). Interestingly, populations of T cells specific for different antigens expand nearly synchronously but can reach drastically different peak numbers of cells due to differences in Ag presentation, numbers of naive precursors, and efficiency of precursor recruitment (9, 11, 24). Ag-specific CD4 T cells expand with kinetics similar to those of Ag-specific CD8 T cells but typically do not reach the same magnitude, in terms of numbers, as Ag-specific CD8 T cells (14, 20, 24, 38).
Following the peak of the expansion phase, Ag-specific T cells begin to contract in numbers. This is a necessary process for maintaining lymphocyte homeostasis. Contraction of Ag-specific T cells prevents the accumulation of lymphocytes following multiple infections and maintains the immunologic space required to generate new responses to recently encountered antigens. The onset of contraction of both CD4 and CD8 T cells is programmed early in the response and is independent of the continued presence of infection or antigen (8, 14). During the contraction phase of the Ag-specific T-cell response, approximately 90% of the T cells present at the peak will die. The cells that survive form the initial memory pool that will evolve into a long-lived population of Ag-specific memory cells equipped to respond and provide protection against secondary infection (23).
The mechanisms involved in regulating the contraction phase remain largely unknown. The cells that are eliminated following expansion die via apoptosis, but CD95 and TNFR1, death receptors required for activation-induced cell death (AICD), are not required for contraction (29). Several lines of evidence suggest that gamma interferon (IFN-γ) is a key regulator of CD8 T-cell contraction. Following infection of BALB/c IFN-γ−/− mice with either Listeria monocytogenes or lymphocytic choriomeningitis virus (LCMV), Ag-specific CD8 T cells undergo severely delayed contraction, resulting in increased numbers of T cells at >60 days postinfection (p.i.) compared to numbers of T cells in wild-type (wt) mice (9). Although the exact mechanism(s) by which IFN-γ regulates contraction is unknown, recent evidence suggests that it may act directly on Ag-specific CD8 T cells versus an indirect mechanism on another cell type (19).
Much of what is known about the effects of IFN-γ on CD4 T cells has been gathered from in vitro studies (3, 30). Th1 CD4+ T-cell lines polarized in vitro maintain expression of IFN-γR1 but become unresponsive to IFN-γ due to downregulation of IFN-γR2 (3, 30). In the absence of IFN-γ or STAT1, in vitro-activated CD4 T cells fail to undergo AICD due to a lack of caspase upregulation (32). These data indicate that CD4 T cells activated in vitro evade signals delivered by IFN-γ, perhaps to prevent the induction of apoptosis. Although in vivo studies with Ag-specific CD4 T cells have not been described, there are published data consistent with a role for IFN-γ in the regulation of CD4 T-cell contraction. Activated-phenotype (CD44hi) CD4 T cells accumulate in IFN-γ−/− mice after infection with Mycobacterium bovis bacillus Calmette-Guérin or induction of experimental autoimmune encephalomyelitis (13, 15). These model systems represent chronic infection and/or persistent exposure of CD4 T cells to antigen. However, no single Ag-specific CD4 T-cell population was tracked in vivo in these studies. Thus, the influence IFN-γ has on contraction of Ag-specific CD4 T cells in an acute infectious model system where antigen is demonstrably cleared has not been determined.
In the current study, we sought to determine if IFN-γ is involved in the regulation of Ag-specific CD4 T-cell contraction in vivo after an acute infection. We chose to use L. monocytogenes infection as our model, because there is a well-defined MHC class II epitope derived from the L. monocytogenes protein listerolysin O (LLO) and presented in C57BL/6 mice (17), which would allow us to carefully track the kinetics of expansion and contraction of these Ag-specific CD4 T cells in the presence or absence of IFN-γ or its receptor. We recently demonstrated that Th1-polarized Ag-specific CD4 T cells can be generated in IFN-γR1−/− and IFN-γR2−/− mice after L. monocytogenes or LCMV infections, indicating that IFN-γ signaling in naive CD4 T cells is not required for the generation of a Th1 response in vivo (18). In addition, clearance of L. monocytogenes can be easily monitored, and infection can be truncated with the use of ampicillin treatment (8, 14, 27). Our results show that IFN-γ is a major regulator of Ag-specific CD4 T-cell contraction in vivo in the absence of persistent infection. In the absence of this critical cytokine, Ag-specific CD4 T cells exhibit normal expansion but minimal contraction after expansion. In addition, a greater portion of Ag-specific CD4 T cells generated in the absence of IFN-γ signaling gain the ability to produce both IFN-γ and interleukin 2 (IL-2) than is the case in wt mice. Thus, IFN-γ signaling influences the function of Ag-specific CD4 T cells as they progress through a primary immune response.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). Breeding pairs of C57BL/6 IFN-γ−/− and C57BL/6 IFN-γR1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred and maintained at the University of Iowa. L. monocytogenes-infected mice were housed in accordance with biosafety regulations. All animal experiments followed approved Institutional Animal Care and Use Committee protocols.
Bacteria and infection of mice.
L. monocytogenes that had been engineered to express ovalbumin (OVA) was a gift from Leo Lefrancois (University of Connecticut) and Hao Shen (University of Pennsylvania) (31). An attenuated version of this strain was created by introducing an in-frame deletion in the actA gene as previously described (37) (referred to as actA-deficient L. monocytogenes OVA) (19). This was the only strain of L. monocytogenes used in these experiments. Bacteria were grown and quantified as previously described (21, 22). All infections were via intravenous injection. The number of bacteria injected was determined by plate count.
Antibodies and peptides.
Synthetic OVA257-264 (SIINFEKL) and LLO190-201 (NEKYAQAYPNVS) peptides were obtained from Biosynthesis (Lewisville, TX). Antibodies with the following specificities were used: PerCP-conjugated anti-mouse CD4 (RM4-5), PerCP-conjugated anti-mouse CD8 (53-6.7), fluorescein isothiocyanate- or allophycocyanin-conjugated anti-mouse Thy1.2 (53-2.1), phycoerythrin (PE)-conjugated anti-mouse CD127 (A7R34; eBioscience, San Diego, CA), PE- or fluorescein isothiocyanate-conjugated anti-mouse IFN-γ (XMG1.2), PE-conjugated anti-mouse IL-2 (JES6-5H4), PE-conjugated anti-mouse CD44 (IM7; eBioscience), and PE-conjugated anti-mouse CD62L (MEL-14). All antibodies are from BD PharMingen (San Jose, CA) unless otherwise indicated.
Detection and calculation of the number of Ag-specific T cells.
Ag-specific T cells were detected by surface staining for CD4 or CD8 and Thy1.2 together with intracellular staining for IFN-γ as previously described (4) after a 5.5-h stimulation with 200 nM OVA257-264 peptide or 5 μM LLO190-201 peptide in the presence of GolgiPlug (BD PharMingen, San Jose, CA) diluted 1:1,000. The total number of Ag-specific T cells per spleen was calculated by multiplying the frequency of CD4 or CD8+ Thy1.2+ IFN-γ+ cells after stimulation with specific peptide by the total number of splenocytes. The number of cells producing cytokine in unstimulated samples was subtracted.
CFU assay and antibiotic treatment.
Numbers of CFU per spleen or per gram liver were determined on days 2 and 5 after infection as previously described (22). In some experiments, mice were treated with water containing 2 mg/ml ampicillin to terminate L. monocytogenes infection as previously described (7). Mice were given ampicillin-containing drinking water for a total of 7 days.
BrdU labeling and detection.
In some experiments, mice were given an initial intraperitoneal injection of 1 mg bromodeoxyuridine (BrdU) diluted in phosphate-buffered saline and then given drinking water containing 0.8 mg/ml BrdU for the next 7 or 10 days. BrdU-containing drinking water was prepared fresh and replaced daily. In other experiments, mice were given a single 2-mg injection of BrdU 24 h before spleens were harvested for staining and analysis of T-cell proliferation. BrdU incorporation in Ag-specific T cells as a measure of in vivo proliferation was detected using the BrdU Flow kit as per the manufacturer's instructions (BD Pharmingen).
RESULTS
Significantly delayed contraction of Ag-specific CD4 T cells in IFN-γ−/− mice.
To determine the role of IFN-γ in the contraction of Ag-specific CD4 T cells in vivo after acute infection, we infected IFN-γ−/− and wt mice with attenuated actA-deficient L. monocytogenes OVA. It was necessary to use attenuated bacteria, because IFN-γ is required for innate resistance to L. monocytogenes and IFN-γ−/− mice succumb to extremely low doses of virulent L. monocytogenes (22). However, IFN-γ−/− mice not only tolerate infection with attenuated L. monocytogenes as well as wt mice but also generate protective Ag-specific CD8 T-cell responses (22). Mice on a B6 background were used in these experiments, since there are no strong L. monocytogenes-derived MHC class II epitopes presented in BALB/c mice. With B6 mice, a vigorous CD4 T-cell response is generated to residues 190 to 201 of the LLO protein (LLO190) presented on I-Ab (14, 17). By using an attenuated strain of L. monocytogenes that expresses ovalbumin, we could track an endogenous CD8 T-cell response to OVA257-264 for comparison.
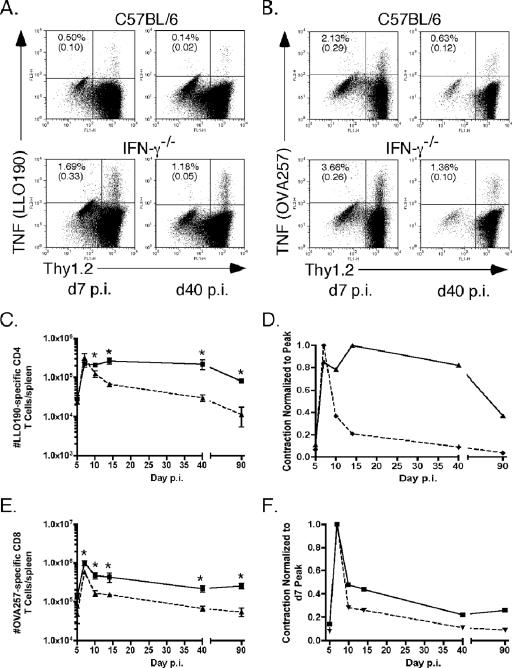
IFN-γ−/− and wt B6 mice were infected with L. monocytogenes, and at several times p.i. starting at day 5, Ag-specific CD4 and CD8 T cells were detected in the spleen using intracellular cytokine staining (ICS) for tumor necrosis factor (TNF) following a brief in vitro peptide stimulation. Staining for Thy1.2 was included to decrease detection of background TNF production by non-T cells in the spleen. The Ag-specific CD4 T cells in IFN-γ−/− mice expanded to almost the same number as wt cells; however, in the absence of IFN-γ, Ag-specific CD4 T cells exhibited virtually no contraction through day 40 p.i. (Fig. 1A, C, and D). In contrast, Ag-specific CD4 T cells contracted by >85% by day 14 p.i. in wt mice (Fig. 1A, C, and D). There was a decline in the number of memory Ag-specific CD4 T cells in both IFN-γ−/− and wt mice between day 40 and day 90 p.i. It has been shown previously that CD4 T-cell memory begins to significantly wane during this time frame after a primary infection (24, 33). However, there were still increased numbers of LLO190-201-specific CD4 T cells in IFN-γ−/− mice compared to numbers of the cells in wt mice at day 90 p.i. (Fig. 1C). These data suggest that IFN-γ regulates contraction of Ag-specific CD4 T cells.
FIG. 1.
IFN-γ regulates Ag-specific CD4 T-cell responses. The numbers of Ag-specific CD4 and CD8 T cells in IFN-γ−/− and wt B6 mice were monitored at various times following infection using intracellular cytokine staining for TNF after peptide stimulation. (A) ICS of CD4 T cells from wt (top) or IFN-γ−/− (bottom) mice on day 7 (d7) (left) or day 40 (d40) (right) p.i. after stimulation with LLO190-201. Plots were first gated on CD4+ cells. Top numbers in each plot are the percentage of CD4+ Thy1.2+ cells TNF positive after peptide stimulation. Numbers in parentheses are the percent TNF+ cells with no peptide stimulation. (B) ICS of CD8 T cells from wt (top) or IFN-γ−/− (bottom) mice day 7 (left) or day 40 (right) p.i. after stimulation with OVA257-264. Plots were first gated on CD8+ cells. Top numbers are the percentage of CD8+ Thy1.2+ cells TNF positive after peptide stimulation. Numbers in parentheses are the percent TNF+ cells with no peptide stimulation. (C) Total numbers of LLO190-201-specific CD4 T cells per spleen at the days p.i. indicated on the x axis calculated as described in Materials and Methods. Squares are the numbers of cells for IFN-γ−/− mice. Triangles are the numbers of cells for wt B6 mice. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test. (D) Kinetics of the LLO190-201-specific CD4 T-cell response normalized to the peak of T-cell numbers, which was on day 7 p.i. for wt B6 mice and on day 14 p.i. for IFN-γ−/− mice. (E) Total numbers of OVA257-264-specific CD8 T cells per spleen at the days p.i. indicated on the x axis. Squares are the numbers of cells for IFN-γ−/− mice. Triangles are the numbers of cells for wt B6 mice. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test. (F) Kinetics of the OVA257-264-specific CD8 T-cell response normalized to the peak of T-cell numbers, which was on day 7 p.i. for both types of mice.
Ag-specific CD8 T cells in IFN-γ−/− mice expanded slightly more after L. monocytogenes infection and exhibited an initial abrupt contraction phase that was less extensive than what was seen with OVA257-264-specific CD8 T cells in wt mice (Fig. 1B, E, and F). Because of the increased expansion and decreased contraction, there were increased numbers of memory Ag-specific CD8 T cells in IFN-γ−/− mice (Fig. 1E), and when normalized to peak numbers of cells, IFN-γ−/− Ag-specific CD8 cells still exhibit less contraction than wt cells at day 90 p.i. (Fig. 1F). Thus, in contrast to the lack of contraction in Ag-specific CD4 T cells, the absence of IFN-γ in B6 mice had less effect on contraction of Ag-specific CD8 T cells.
Aberrant contraction phenotype is preserved in Ag-specific CD4 T cells generated in IFN-γR1−/− mice.
IFN-γR1 is the ligand-binding portion of the IFN-γ receptor (2); thus, IFN-γR1−/− Ag-specific CD4 T cells should exhibit the same delayed contraction after infection that was detected in IFN-γ−/− mice. In addition, we wanted to further characterize the effector functions of Ag-specific CD4 T cells generated in the absence of signals delivered by IFN-γ. By using the IFN-γR1−/− mice, we could investigate the ability of these cells to make IFN-γ following antigen stimulation.
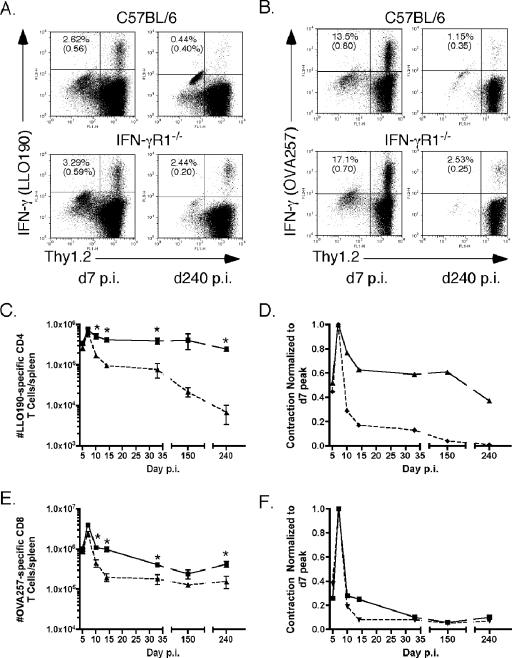
Using ICS for IFN-γ, we tracked Ag-specific CD4 and CD8 T-cell responses in wt and IFN-γR1−/− mice following infection with actA-deficient L. monocytogenes OVA. As predicted, IFN-γR1−/− Ag-specific CD4 T cells expanded to the same level as wt cells but exhibited extremely protracted contraction out to day 240 p.i. (Fig. 2A, C, and D). wt Ag-specific CD4 T cells began to contract after day 7 p.i., maintained relatively stable cell numbers out to day 32 p.i., and then began to decline at later times after primary infection (Fig. 2C). The numbers of Ag-specific CD4 T cells in IFN-γR1−/− mice declined minimally between day 7 and day 14 after infection and remained stable until day 150 before beginning to wane (Fig. 2C). Even at day 240 p.i., when Ag-specific CD4 T cells in wt mice have declined almost below the limit of detection, the numbers of IFN-γR1−/− Ag-specific CD4 T cells remained elevated at 40% of the number present at the peak of the response (Fig. 2D). These data demonstrate that in the absence of IFN-γ or IFN-γR1, Ag-specific CD4 T cells exhibit decreased contraction after Ag-driven expansion.
FIG. 2.
Absence of IFN-γ signaling results in aberrant contraction of Ag-specific CD4 T cells. The numbers of Ag-specific T cells in IFN-γR1−/− and wt B6 mice were monitored at various times after infection using intracellular cytokine staining for IFN-γ. (A) ICS of cells from wt (top) or IFN-γR1−/− (bottom) mice on day 7 (d7) (left) or day 240 (d240) (right) p.i. after stimulation with LLO190-201. Plots were first gated on CD4+ cells. Top numbers are the percentage of CD4+ Thy1.2+ cells that are IFN-γ positive after peptide stimulation. Numbers in parentheses are the percent IFN-γ+ cells with no peptide stimulation. (B) ICS of cells from wt (top) or IFN-γR1−/− (bottom) mice on day 7 (left) and day 240 (right) p.i. after stimulation with OVA257-264. Plots were first gated on CD8+ cells. Top numbers are the percentage of CD8+ Thy1.2+ cells that are IFN-γ positive after peptide stimulation. Numbers in parentheses are the percent IFN-γ+ cells with no peptide stimulation. (C) Total numbers of LLO190-201-specific CD4 T cells per spleen at the days p.i. indicated on the x axis, calculated as described in Materials and Methods. Squares are the numbers of cells for IFN-γR1−/− mice. Triangles are the numbers of cells for wt B6 mice. The number of mice per group per time point was three to nine. *, P value of <0.05 as determined using the Student t test. (D) Kinetics of the LLO190-201-specific CD4 T-cell response normalized to the peak of T-cell numbers, which was on day 7 p.i. for both types of mice. (E) Total numbers of OVA257-264-specific CD8 T cells per spleen at the days p.i. indicated on the x axis. Squares are the numbers of cells for IFN-γR1−/− mice. Triangles are the numbers of cells for wt B6 mice. The number of mice per group per time point was three to nine. *, P value of <0.05 as determined using the Student t test. (F) Kinetics of the OVA257-264-specific CD8 T-cell response normalized to the peak of T-cell numbers, which was on day 7 p.i. for both types of mice.
IFN-γR1−/− Ag-specific CD8 T cells expanded to slightly higher numbers than wt cells and, as was observed with IFN-γ−/− mice, exhibited a modest delay in contraction compared to wt cells (Fig. 2B, E, and F). We observed a precipitous decline in the frequency and total numbers of wt IFN-γ-producing Ag-specific CD8 T cells from day 7 to day 240 for wt mice, with most of the decline occurring between day 7 and day 14 p.i. (Fig. 2B and E). There was also a large drop in numbers of OVA257-264-specific CD8 T cells in IFN-γR1−/− mice between days 7 and 10 p.i., and then cell numbers waned more slowly between days 10 and 32 p.i. than was the case with cells of wt mice. However, similar to the data generated with IFN-γ−/− mice, by an early memory time point (day 32 p.i.), IFN-γR1−/− cells had contracted to the same degree as wt Ag-specific CD8 T cells when normalized to the number of cells present at the peak (Fig. 2F).
Contraction phenotype is not due to prolonged infection.
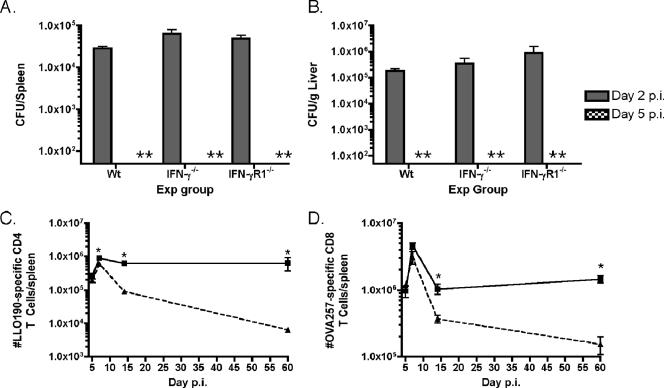
To rule out prolonged infection as a possible explanation for delayed contraction of Ag-specific CD4 T cells in IFN-γ−/− and IFN-γR1−/− mice, we monitored clearance of actA-deficient L. monocytogenes OVA from the spleens and livers of infected mice on days 2 and 5 p.i. IFN-γ−/− and IFN-γR1−/− mice had slightly higher levels of infection on day 2 p.i., both in their spleens and in their livers, than wt B6 mice (Fig. 3A and B). However, all mice had cleared bacteria below the level of detection (50 CFU/spleen; 50 CFU/g liver) by day 5 p.i.
FIG. 3.
Infection in IFN-γ and IFN-γR1 mice is not significantly prolonged compared to infection in wt B6 mice. (A and B) Numbers of bacteria in the spleens (A) or livers (B) of wt B6 (Wt), IFN-γ−/−, or IFN-γR1−/− mice on day 2 (shaded columns) or 5 (hatched columns) p.i. A double asterisk indicates none detected. The limit of detection of the assay was 50 CFU/organ. Exp group, experimental group. (C) Numbers of LLO190-201-specific CD4 T cells in ampicillin-treated IFN-γR1−/− mice (squares) or wt B6 mice (triangles) were determined using ICS for IFN-γ on the days after infection indicated on the x axis. The number of mice per group per time point was three. *, P value of <0.05 as determined using the Student t test. (D) Numbers of OVA257-264-specific CD8 T cells in ampicillin-treated mice. The number of mice per group per time point was three. *, P value of <0.05 as determined using the Student t test.
To further confirm that the prolonged presence of bacteria (below the level of detection in the CFU assay) and Ag exposure was not preventing contraction of Ag-specific CD4 T cells in IFN-γR1−/− mice, we infected wt and IFN-γR1−/− mice and then gave them ampicillin-containing drinking water on day 4 p.i. to eliminate any possibility of remaining bacteria. The numbers of Ag-specific CD4 and CD8 T cells were quantitated in the spleens of antibiotic-treated mice at various times p.i., using ICS for IFN-γ. Treatment with ampicillin to conclusively end infection on day 4 p.i. did not alter the kinetics of the CD4 or CD8 T-cell response compared with results for nontreated mice (Fig. 3C and D). The LLO190-201-specific CD4 T cells from ampicillin-treated IFN-γR1−/− mice contracted very little in contrast to the wt cells, which dramatically decreased in numbers by day 60 p.i. (Fig. 3C). The OVA257-264-specific CD8 T cells from ampicillin-treated IFN-γR1−/− mice still expanded to a similar degree and exhibited slightly delayed contraction compared to Ag-specific CD8 T cells generated in wt mice treated with ampicillin (Fig. 3D). These data rule out prolonged infection in the absence of IFN-γR1 as the explanation for aberrant contraction of Ag-specific CD4 T cells. Therefore, from these data we conclude that IFN-γ regulates the kinetics of the Ag-specific CD4 T-cell response after L. monocytogenes infection.
Increased proliferation of Ag-specific CD4 T cells in the absence of IFN-γ signaling is not the mechanism responsible for prolonged contraction.
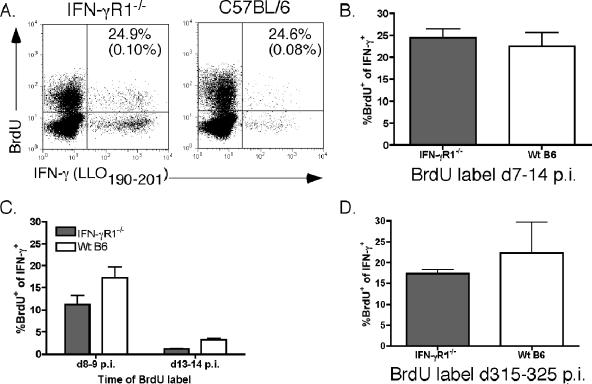
The observed decrease in contraction of Ag-specific CD4 T cells in IFN-γR1−/− mice could be the result of a longer period of proliferation by these cells than was seen for wt cells. To investigate this potential mechanism, we treated mice with BrdU from days 7 to 14 p.i. to label proliferating cells. Using codetection of intracellular IFN-γ and incorporated BrdU, we determined that the frequency of proliferating LLO190-201-specific CD4 T cells during this time frame, which corresponded to the normal contraction phase in wt mice, was not different between IFN-γR1−/− and wt mice (Fig. 4A and B). To more precisely monitor proliferation during shorter intervals, mice were labeled with BrdU for two separate 24-h periods during the contraction phase (days 8 to 9 p.i. and days 13 to 14 p.i.). Similar to what was observed with the 1-week BrdU label, the rates of proliferation of LLO190-201-specific CD4 T cells were not higher in the absence of IFN-γ signaling during these short-term BrdU labeling periods (Fig. 4C), further supporting the conclusion that increased proliferation during the normal contraction phase does not account for the lack of contraction in Ag-specific CD4 T-cells observed in the absence of IFN-γ signaling.
FIG. 4.
Ag-specific CD4 T cells do not exhibit increased proliferation or decreased apoptosis compared to wt T cells. (A) Intracellular staining of cells from IFN-γR1−/− (left) or wt B6 (right) mice for intracellular IFN-γ and incorporated BrdU or control immunoglobulin. Dot plots are of splenocytes gated on CD4+ Thy1.2+ cells. Numbers are the frequency of IFN-γ+ cells that were also BrdU+. Numbers in parentheses are frequencies of IFN-γ+ cells that stained positive with an isotype control for the BrdU antibody. (B) Average frequency of IFN-γ+ BrdU+ CD4 T cells found in each group of mice after labeling from day 7 to day 14 (d7-14) p.i. There were three mice in each group. (C) Average frequency of IFN-γ+ BrdU+ CD4 T cells detected after short-term (24 h) BrdU labeling from day 8 to day 9 (d8-9) p.i. or day 13 to day 14 (d13-14) p.i. There were three mice per group. (D) Average frequency of IFN-γ+ BrdU+ memory CD4 T cells detected after labeling with BrdU from day 315 to day 325 (d315-325) p.i. There were three mice per group.
There are increased numbers of memory Ag-specific CD4 T cells in IFN-γR1−/− mice, even at times after infection when numbers of wt memory Ag-specific CD4 T cells have begun to wane significantly (Fig. 2C). To test if the maintenance of elevated numbers of memory Ag-specific CD4 T cells in IFN-γR1−/− mice was due to increased proliferation, we labeled mice from days 315 to 325 p.i. with BrdU. We did not detect increased proliferation by Ag-specific CD4 T cells in IFN-γR1−/− mice during this portion of the memory phase (Fig. 4D), suggesting that this was not the mechanism supporting increased numbers of memory cells in these mice.
An alternative mechanism that could explain the increased numbers of IFN-γR1−/− Ag-specific CD4 T cells is decreased apoptosis compared to that of wt cells. Unfortunately, current methodologies used to detect apoptosis directly ex vivo, such as annexin V staining or labeling with fluorogenic substrates for activated caspases, are incompatible with identification of Ag-specific CD4 T cells with ICS due to the fixation requirement in the ICS protocol. Therefore, although we were unable to directly analyze apoptosis with Ag-specific CD4 T cells, this remains an attractive explanation for the lack of contraction observed in the absence of IFN-γ signaling, since IFN-γ has been shown to affect apoptosis of CD4 T cells activated in vitro (32).
IFN-γ signaling regulates the phenotype of Ag-specific CD4 T cells in addition to their total numbers.
IFN-γ is a pleiotropic cytokine and has the potential to influence the expression of hundreds of genes (10). We further characterized the Ag-specific CD4 T cells generated in the absence of IFN-γ signaling to see if/how this affected the phenotype of the cells in addition to regulating the total numbers of Ag-specific CD4 T cells.
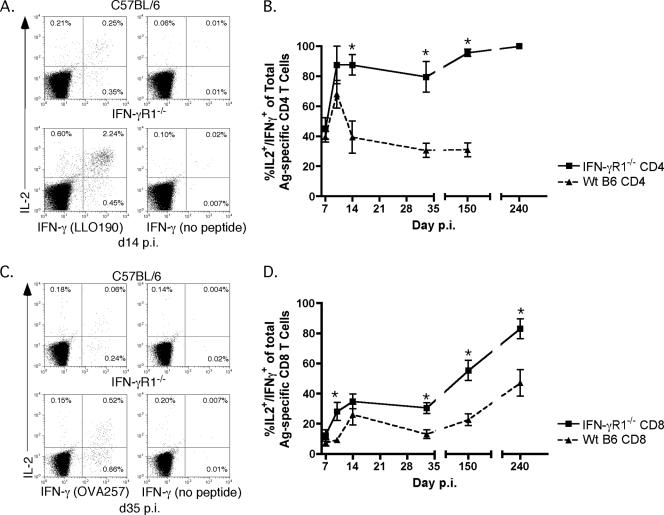
The ability of Ag-specific CD8 T cells to produce IL-2 in addition to IFN-γ after peptide stimulation is often used as a marker of a memory-like phenotype (6). Much less is known about the ability of Ag-specific CD4 T cells to be dual cytokine producers and what this may mean functionally. A recent study documented the predominance of IFN-γ+ TNF+ IL-2+ CD4 T cells in the peritoneal cavity of mice infected intraperitoneally with L. monocytogenes beginning at day 10 p.i. (16). We compared the frequencies of Ag-specific CD4 (Fig. 5A and B) and CD8 (Fig. 5C and D) T cells that produce both IFN-γ and IL-2 in wt and IFN-γR1−/− mice following L. monocytogenes infection. In the absence of IFN-γ signaling, a much higher frequency of Ag-specific CD4 T cells produced both IFN-γ and IL-2 than was observed in wt mice (Fig. 5A and B). By day 14 p.i., more than 80% of IFN-γR1−/− Ag-specific CD4 T cells were able to make both cytokines after peptide stimulation (Fig. 5A), and this frequency increased to virtually 100% by day 150 p.i. (Fig. 5B). In contrast, the frequency of dual IFN-γ/IL-2-producing wt CD4 T cells leveled off at approximately 40% at day 14 p.i. (Fig. 5A) and did not increase with further time (Fig. 5B).
FIG. 5.
Capacity of Ag-specific T cells to make both IL-2 and IFN-γ is dependent on cell type and is influenced by IFN-γ signaling. (A) Example of dual IL-2 and IFN-γ ICS of splenocytes from wt B6 (top row) or IFN-γR1−/− (bottom row) mice on day 14 p.i. Dot plots were first gated on CD4+ Thy1.2+ cells. Frequencies are of the gated CD4+ Thy1.2+ population. (B) Frequencies of IL-2+ IFN-γ+ cells of total IFN-γ+ LLO190-201-specific CD4 T cells from wt B6 (triangles) or IFN-γR1−/− (squares) mice. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test. (C) Example of dual ICS of splenocytes from wt B6 (top row) or IFN-γR1−/− (bottom row) mice for IFN-γ and IL-2 on day 35 p.i. Dot plots were first gated on CD8+ Thy1.2+ cells. Frequencies are of the gated CD8+ Thy1.2+ population. (D) Frequencies of IL-2+ IFN-γ+ cells of total IFN-γ+ OVA257-264-specific CD8 T cells from wt B6 (triangles) or IFN-γR1−/− (squares) mice. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test.
The absence of IFN-γ signaling also affected the phenotype of Ag-specific CD8 T cells generated after L. monocytogenes infection. A larger number of memory Ag-specific CD8 T cells were dual IFN-γ/IL-2-producing cells in IFN-γR1−/− mice than in wt mice (Fig. 5C and D). Early during the response (day 7 p.i.) the frequencies of IFN-γ+ IL-2+ Ag-specific CD8 T cells were very similar. However, by day 35 p.i., approximately 38% of IFN-γR1−/− cells made both IFN-γ and IL-2, whereas only 20% of Ag-specific CD8 T cells from wt mice could make both cytokines (Fig. 5C). At later memory time points (day 150 and day 240 p.i.), IFN-γR1−/− mice contained almost twice the frequency of dual IFN-γ/IL-2-producing Ag-specific CD8 T cells as that of wt mice (Fig. 5D). Even though the absence of IFN-γ signaling in Ag-specific CD8 T cells only modestly affected their contraction after Ag-driven expansion, it did influence the ability of the memory Ag-specific CD8 T cells to make both IFN-γ and IL-2.
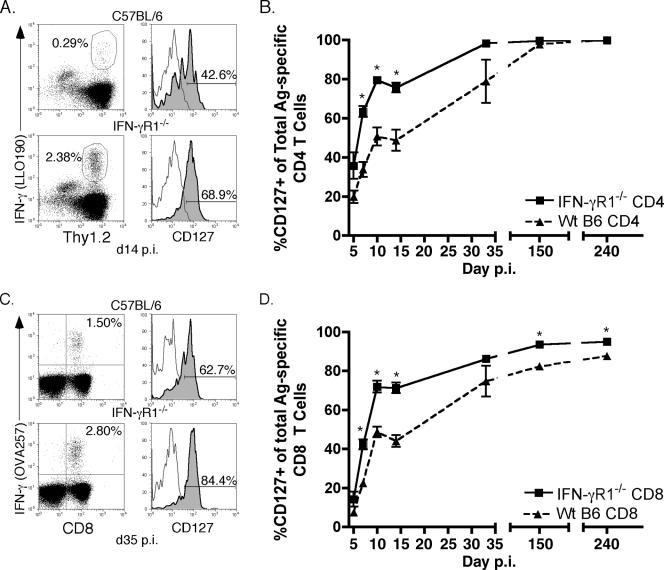
In addition to the ability to produce effector cytokines, such as IFN-γ and IL-2, after peptide stimulation, the expression of specific surface markers is often used to characterize the phenotype of Ag-specific T cells. One of these markers is the α-chain of the IL-7 receptor (CD127), which is downregulated by effector T cells but is expressed by naive and memory cells (26, 34, 35). We were able to determine expression of CD127 on Ag-specific CD4 (Fig. 6A) and CD8 (Fig. 6C) T cells using surface staining for CD127 in combination with intracellular staining for IFN-γ. After initial downregulation, IFN-γR1−/− Ag-specific CD4 (Fig. 6B) and CD8 (Fig. 6D) T cells reacquired expression of CD127 with accelerated kinetics compared to that of wt Ag-specific T cells. By day 10 p.i., 80% of IFN-γR1−/− Ag-specific CD4 T cells and 70% of IFN-γR1−/− Ag-specific CD8 T cells expressed CD127, compared to approximately 50% of wt Ag-specific CD4 and CD8 T cells. Even at an early memory time point (day 32 p.i.), a higher number of Ag-specific T cells from IFN-γR1−/− mice expressed CD127 than wt cells. At much later memory time points (day 150 and day 240 p.i.), the expression of CD127 on IFN-γR1−/− and wt Ag-specific T cells had become the same (Fig. 6B, CD4s) or more similar (Fig. 6D, CD8s). These data further highlight a role for IFN-γ in shaping the phenotypes of both CD4 and CD8 Ag-specific T cells after L. monocytogenes infection, in addition to its function as a key regulator of the contraction of Ag-specific CD4 T cells.
FIG. 6.
Expression of CD127 by Ag-specific CD4 and CD8 T cells is higher in the absence of IFN-γR1. (A) Example of combined surface staining for CD127 and ICS for IFN-γ on splenocytes from wt B6 (top row) or IFN-γR1−/− (bottom row) mice on day 14 p.i. Dot plots were first gated on CD4+ cells. Histograms are gated on CD4+ Thy1.2+ IFN-γ+ cells. Frequency IFN-γ positive is of the gated CD4+ population. Frequency CD127 positive is of the gated CD4+ Thy1.2+ IFN-γ+ population. (B) Frequency of LLO190-201-specific CD4 T cells that were CD127 positive in wt B6 (triangles) or IFN-γR1−/− (squares) mice. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test. (C) Example of combined surface staining for CD127 and ICS for IFN-γ on splenocytes from wt B6 (top row) or IFN-γR1−/− (bottom row) mice on day 35 p.i. Dot plots were first gated on Thy1.2+ cells. Histograms are gated on CD8+ Thy1.2+ IFN-γ+ cells. Frequency IFN-γ positive is of the gated CD8+ Thy1.2+ population. Frequency CD127 positive is of the gated CD8+ Thy1.2+ IFN-γ+ population. (D) Frequency of OVA257-264-specific CD8 T cells that were CD127 positive in wt B6 (triangles) or IFN-γR1−/− (squares) mice at the times p.i. indicated on the x axis. The number of mice per group per time point was three to six. *, P value of <0.05 as determined using the Student t test.
Ag-specific T cells in IFN-γR1−/− mice respond to secondary infection.
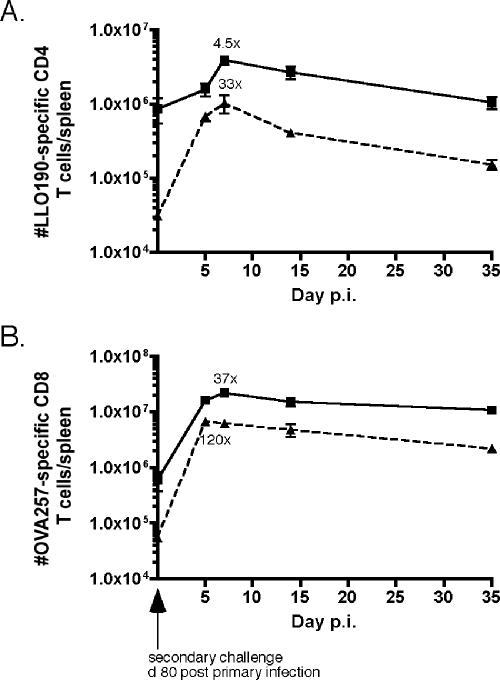
Even though memory Ag-specific CD4 and CD8 T cells persisted long term in IFN-γR1−/− mice (Fig. 2), it was unknown how T cells generated in the absence of IFN-γ signaling would respond to secondary antigen exposure. After the starting number of memory Ag-specific T cells was determined, IFN-γR1−/− and wt mice on day 80 post-primary infection were given a secondary infection, and the expansion in numbers of memory cells was monitored. Both Ag-specific CD4 (Fig. 7A) and CD8 (Fig. 7B) T cells in IFN-γR1−/− mice responded by proliferating, reaching a peak number of cells on day 7 postchallenge. The degree of increase in IFN-γR1−/− Ag-specific T cells was less than the degree of increase observed for wt mice, which is most likely the result of greater starting numbers of both CD4 and CD8 memory T cells in IFN-γR1−/− mice. This phenomenon of higher starting memory cell numbers resulting in less expansion by memory cells has been documented previously for Ag-specific CD8 T cells (5). These data demonstrate that IFN-γ signaling is not required for the generation of memory CD4 and CD8 T cells capable of responding to secondary antigen exposure.
FIG. 7.
IFN-γR1−/− Ag-specific T cells proliferate in response to secondary infection. IFN-γR1−/− and wt mice on day 80 post-primary infection were challenged with actA-deficient L. monocytogenes OVA. Numbers of Ag-specific T cells were determined by ICS for IFN-γ. (A) Kinetics of LLO190-201-specific CD4 T cells responding to secondary infection for IFN-γR1−/− (squares) or wt (triangles) B6 mice. There were three mice per group per time point. (B) Kinetics of OVA257-264-specific CD8 T cells responding to secondary infection for IFN-γR1−/− (squares) or wt B6 (triangles) mice. There were three mice per group per time point.
DISCUSSION
Ag-specific T-cell responses follow a remarkably coordinate pattern of expansion, contraction, and establishment of memory regardless of their different peptide specificities. The regulatory mechanisms controlling T-cell kinetics following infection must be elucidated before strategies can be designed to manipulate the immune system in specific ways, such as eliciting more effective T-cell responses via vaccination, curbing overexuberant immune responses, as is the case with autoimmune diseases, and developing treatments that assist in the elimination of cancerous T cells.
The contraction of T-cell populations following Ag-driven expansion is critical for the maintenance of homeostasis within the total lymphocyte population. Sometimes overshadowed by the focus on T-cell death is an equally critical phenomenon, which is the preservation of a small population of T cells that will survive the death phase to become the initial memory T-cell population. What mechanisms ultimately regulate one or both of these processes and when during the immune response these mechanisms exert their effect are subjects of intense investigation.
There are multiple studies that highlight IFN-γ as playing a key role in regulating contraction of Ag-specific T cells (7, 9, 13, 15). In this report, we have shown that in the absence of IFN-γ or the ligand-binding portion of its receptor, contraction of Ag-specific CD4 T cells is significantly delayed, resulting in the accumulation of Ag-specific CD4 T cells well beyond the time point when the numbers of wt memory Ag-specific CD4 T cells have begun to decline. Aberrant contraction of Ag-specific CD4 T cells is not due to delayed clearance of actA-deficient L. monocytogenes OVA from IFN-γ−/− or IFN-γR1−/− mice and therefore is specifically attributed to the absence of IFN-γ or signaling of IFN-γ through its receptor.
Several experiments using BrdU labeling did not support increased proliferation of Ag-specific CD4 T cells during the contraction phase or later during the memory phase as the mechanism supporting larger numbers of T cells in the absence of IFN-γ signaling (Fig. 4). However, decreased rates of apoptosis compared to those for wt T cells could not be ruled out. This mechanism would be consistent with findings of other studies highlighting a role for IFN-γ in activation-induced cell death of in vitro-activated CD4 T cells (32).
In our experiments, we detected equal or greater expansion of Ag-specific CD4 and CD8 T cells in IFN-γ−/− and IFN-γR1−/− mice than that in wt mice following infection with actA-deficient L. monocytogenes OVA. In a previous study, we also demonstrated equivalent expansion of Ag-specific CD8 T cells in BALB/c IFN-γ−/− mice following L. monocytogenes infection but decreased expansion following LCMV infection (9). In addition, other studies have demonstrated that after LCMV infection (39, 40) or immunization of mice with peptide plus lipopolysaccharide (LPS) (36), IFN-γ was required for optimal expansion of Ag-specific CD4 and CD8 T cells. After adoptive transfer into wt mice, CD8 T cells lacking IFN-γR1 expanded five- to sixfold less than wt T cells did after LCMV infection, suggesting that IFN-γ acted directly on the Ag-specific CD8 T cells to promote their expansion (40). Similarly, IFN-γR1−/− Ag-specific CD4 T cells failed to expand normally after LCMV infection (39). In a different study, wt CD8 T cells transferred into IFN-γ−/− mice expanded fivefold less than cells transferred into wt mice after immunization with peptide plus LPS (36). Interestingly, in this study, wt CD8 T cells transferred into IFN-γR1−/− mice expanded twofold more than cells transferred into wt mice (36). Although the exact mechanism(s) by which IFN-γ promotes the expansion of Ag-specific CD4 and CD8 T cells is not known, it is clear that the nature of the infection/T-cell stimulus is an important variable. Perhaps due to a unique characteristic of the innate immune response directed against L. monocytogenes or by virtue of pathogen tropism, IFN-γ plays a lesser role in regulating Ag-specific CD4 and CD8 T-cell expansion after infection with L. monocytogenes than after infection with LCMV or peptide immunization.
In the experiments reported here, we used Ag-induced production of IFN-γ and IL-2 to identify and count Ag-specific CD4 T cells because it is a dependable, widely used assay for detecting Ag-specific T cells. It is possible that using ICS for IFN-γ and IL-2 may hinder our ability to distinguish between alterations in T-cell differentiation (i.e., the ability to produce specific cytokines) and kinetics of Ag-specific CD4 T cells in IFN-γR1−/− and wt mice. However, we recently demonstrated that there is no increase in Th2 Ag-specific CD4 T cells for IFN-γ−/−, IFN-γR1−/−, or IFN-γR2−/− mice compared to the number of T cells for wt mice after L. monocytogenes or LCMV infections, indicating that IFN-γ signaling is not required for the generation of IFN-γ-producing Th1 CD4 T-cell responses in vivo (18).
Through the results of other studies, we strongly favor the hypothesis that IFN-γ exerts its regulatory mechanism(s) directly on T cells very early during the immune response (7, 19). T cells have the most exposure to IFN-γ during the first 24 h after L. monocytogenes infection. In fact, there is no detectable IFN-γ mRNA or protein in mice at time points corresponding to the contraction phase (19). We have demonstrated that Ag-specific CD8 T cells receive IFN-γ signals within 12 h p.i. and then become unresponsive to further IFN-γ signals for the remainder of the expansion phase (19). In addition, when mice were pretreated with antibiotics prior to actA-deficient L. monocytogenes OVA infection to significantly decrease inflammation and IFN-γ production at the time of T-cell priming, Ag-specific CD8 T cells expanded but did not contract (7). Regulation of IFN-γ receptor expression by Ag-specific CD4 T cells responding to infection in vivo has not been determined, and when during the immune response IFN-γ regulates Ag-specific CD4 T-cell contraction remains to be determined.
The absence of IFN-γ signaling affected the ability of Ag-specific T cells to make both IFN-γ and IL-2 after peptide stimulation. In IFN-γR1−/− mice, a greater number of Ag-specific CD4 T cells were capable of making both effector cytokines than in wt mice (Fig. 4A). Perhaps the increased number of IL-2-producing cells assists in the maintenance of elevated Ag-specific CD4 T-cell numbers. Although the pattern of gene regulation that permits the generation of Ag-specific T cells with the ability to make both IFN-γ and IL-2 is not known, our data suggest that the lack of IFN-γ signals permits expression/repression of the genes required to produce cells of this phenotype. At early times p.i., a larger number of Ag-specific CD4 T cells, both in wt and in IFN-γR1−/− mice, were capable of making IFN-γ and IL-2 after antigen stimulation than Ag-specific CD8 T cells. This most likely reflects the importance to each of these T-cell populations of IL-2 secretion as an effector function.
The findings generated with C57BL/6 IFN-γ−/− mice presented in this report differ somewhat from our previously published data using BALB/c IFN-γ−/− mice in which Ag-specific CD8 T cells exhibit dramatically delayed contraction following L. monocytogenes infection (9). These new data suggest that other background genes may influence the degree to which IFN-γ regulates contraction of Ag-specific CD8 T cells. The C57BL/6 IFN-γ−/− model seems to be unique in one aspect. To our knowledge this is the first example of a model system in which Ag-specific CD4 and CD8 T-cell responses exhibit significantly disparate kinetics, suggesting that, at least for B6 background mice, IFN-γ may regulate CD4 and CD8 T-cell response kinetics via different mechanisms.
The downstream changes in gene expression after IFN-γ signaling that result in contraction of T cells are not precisely known. IFN-γ has the capacity to regulate hundreds of genes, mostly but not entirely through the Jak/STAT signaling pathway. Cellular responses to IFN-γ by both immune and nonimmune cells vary widely and include, but are not restricted to, increased susceptibility to AICD/apoptosis and acquisition of antiproliferative properties (10). Microarray analyses of wt and IFN-γR1−/− T cells after infection may identify candidate genes regulated by IFN-γ signaling that are involved in T-cell contraction.
Acknowledgments
This work was supported by National Institutes of Health grants AI42767, AI46653, and AI50073 (to J.T.H.) and American Cancer Society grant no. PF-05-142-01-LIB (to J.S.H.).
We thank Rebecca Podyminogin for excellent laboratory assistance.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Bach, E. A., M. Aguet, and R. D. Schreiber. 1997. The IFN γ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563-591. [DOI] [PubMed] [Google Scholar]
- 3.Bach, E. A., S. J. Szabo, A. S. Dighe, A. Ashkenazi, M. Aguet, K. M. Murphy, and R. D. Schreiber. 1995. Ligand-induced autoregulation of IFN-γ receptor β chain expression in T helper cell subsets. Science 270:1215-1218. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac, V. P., and J. T. Harty. 2000. Intracellular staining for TNF and IFN-γ detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Methods 238:107-117. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., K. A. Messingham, S. E. Hamilton, and J. T. Harty. 2003. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J. Immunol. 170:4933-4942. [DOI] [PubMed] [Google Scholar]
- 6.Badovinac, V. P., K. A. Messingham, A. Jabbari, J. S. Haring, and J. T. Harty. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11:748-756. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5:809-817. [DOI] [PubMed] [Google Scholar]
- 8.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3:619-626. [DOI] [PubMed] [Google Scholar]
- 9.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 10.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 11.Busch, D. H., K. Kerksiek, and E. G. Pamer. 1999. Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection. Immunol. Rev. 172:163-169. [DOI] [PubMed] [Google Scholar]
- 12.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, C. Q., S. Wittmer, and D. K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbin, G. A., and J. T. Harty. 2004. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J. Immunol. 173:5679-5687. [DOI] [PubMed] [Google Scholar]
- 15.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman, M. M., and H. K. Ziegler. 2005. Simultaneous Th1-type cytokine expression is a signature of peritoneal CD4+ lymphocytes responding to infection with Listeria monocytogenes. J. Immunol. 175:394-403. [DOI] [PubMed] [Google Scholar]
- 17.Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877-1884. [DOI] [PubMed] [Google Scholar]
- 18.Haring, J. S., V. P. Badovinac, M. R. Olson, S. M. Varga, and J. T. Harty. 2005. In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-γ receptor. J. Immunol. 175:3117-3122. [DOI] [PubMed] [Google Scholar]
- 19.Haring, J. S., G. A. Corbin, and J. T. Harty. 2005. Dynamic regulation of IFN-γ signaling in antigen-specific CD8+ T cells responding to infection. J. Immunol. 174:6791-6802. [DOI] [PubMed] [Google Scholar]
- 20.Haring, J. S., L. L. Pewe, and S. Perlman. 2001. High-magnitude, virus-specific CD4 T-cell response in the central nervous system of coronavirus-infected mice. J. Virol. 75:3043-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harty, J. T., and M. J. Bevan. 1992. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J. Exp. Med. 175:1531-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 23.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 24.Homann, D., L. Teyton, and M. B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 25.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 27.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 28.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, L. T., K. McKall-Faienza, A. Zakarian, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 2000. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur. J. Immunol. 30:683-688. [DOI] [PubMed] [Google Scholar]
- 30.Pernis, A., S. Gupta, K. J. Gollob, E. Garfein, R. L. Coffman, C. Schindler, and P. Rothman. 1995. Lack of interferon γ receptor β chain and the prevention of interferon γ signaling in Th1 cells. Science 269:245-247. [DOI] [PubMed] [Google Scholar]
- 31.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. (Erratum, 166:5840.) [DOI] [PubMed] [Google Scholar]
- 32.Refaeli, Y., L. Van Parijs, S. I. Alexander, and A. K. Abbas. 2002. Interferon γ is required for activation-induced death of T lymphocytes. J. Exp. Med. 196:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiemann, M., V. Busch, K. Linkemann, K. M. Huster, and D. H. Busch. 2003. Differences in maintenance of CD8+ and CD4+ bacteria-specific effector-memory T cell populations. Eur. J. Immunol. 33:2875-2885. [DOI] [PubMed] [Google Scholar]
- 34.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 35.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269-279. [DOI] [PubMed] [Google Scholar]
- 36.Sercan, O., G. J. Hammerling, B. Arnold, and T. Schuler. 2006. Cutting edge: innate immune cells contribute to the IFN-γ-dependent regulation of antigen-specific CD8+ T cell homeostasis. J. Immunol. 176:735-739. [DOI] [PubMed] [Google Scholar]
- 37.Tvinnereim, A. R., S. E. Hamilton, and J. T. Harty. 2002. CD8+-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect. Immun. 70:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga, S. M., and R. M. Welsh. 2000. High frequency of virus-specific interleukin-2-producing CD4+ T cells and Th1 dominance during lymphocytic choriomeningitis virus infection. J. Virol. 74:4429-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmire, J. K., N. Benning, and J. L. Whitton. 2005. Cutting edge: early IFN-γ signaling directly enhances primary antiviral CD4+ T cell responses. J. Immunol. 175:5624-5628. [DOI] [PubMed] [Google Scholar]
- 40.Whitmire, J. K., J. T. Tan, and J. L. Whitton. 2005. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 201:1053-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams, M. A., and M. J. Bevan. 2004. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 173:6694-6702. [DOI] [PubMed] [Google Scholar]
- 42.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29-70. [DOI] [PubMed] [Google Scholar]
- 43.Wong, P., and E. G. Pamer. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166:5864-5868. [DOI] [PubMed] [Google Scholar]