Abstract
Acute Q fever is a zoonotic disease caused by the obligate intracellular bacterium Coxiella burnetii and can manifest as a flu-like illness, pneumonia, or hepatitis. A need exists in Q fever research for animal models mimicking both the typical route of infection (inhalation) and the clinical illness seen in human cases of Q fever. A guinea pig aerosol challenge model was developed using C. burnetii Nine Mile phase I (RSA 493), administered using a specialized chamber designed to deliver droplet nuclei directly to the alveolar spaces. Guinea pigs were given 101 to 106 organisms and evaluated for 28 days postinfection. Clinical signs included fever, weight loss, respiratory difficulty, and death, with the degree and duration of response corresponding to the dose of organism delivered. Histopathologic evaluation of the lungs of animals infected with a high dose showed coalescing panleukocytic bronchointerstitial pneumonia at 7 days postinfection that resolved to multifocal lymphohistiocytic interstitial pneumonia by 28 days. Guinea pigs receiving a killed whole-cell vaccine prior to challenge with the highest dose of C. burnetii were protected against lethal infection and did not develop fever. Clinical signs and pathological changes noted for these guinea pigs were comparable to those seen in human acute Q fever, making this an accurate and valuable animal model of human disease.
Q fever is a disease of worldwide importance for both humans and other animals and is caused by the obligately intracellular bacterium Coxiella burnetii. Sheep, goats, and cattle are the primary reservoirs of the organism and can shed it in milk, urine, feces, and birth products (2). Thus, occupational exposure of persons in contact with these animals, such as abattoir workers, farmers, and veterinarians, is associated with a higher risk of contracting Q fever. C. burnetii has also been found in numerous other mammals, birds (51, 55), reptiles (21, 52, 61), and arthropods (5, 9, 49). Humans are infected primarily through inhalation, and as few as 10 organisms are known to cause disease (4). Ingestion of contaminated dairy products (18, 24, 54) or bites from infected ticks (9, 10, 44) may also lead to infection.
C. burnetii has a high degree of resistance to physical and chemical agents (38, 46) and can withstand desiccation and remain infectious in contaminated soils for years (53). Due to the highly infectious nature of C. burnetii and its hardiness under adverse environmental conditions, the organism is considered a category B agent by the Centers for Disease Control and Prevention and has been included in the list of weapons of mass destruction likely to be used in bioterrorism and biological warfare (13, 23). Weaponization and mass production of this organism have already been accomplished (43, 48), reinforcing the need for a safe, efficacious vaccine that could be used to protect populations at risk following a deliberate or natural outbreak.
Human Q fever can present as either acute or chronic infection. Acute Q fever generally presents as a flu-like illness with severe periorbital headache, high fever, malaise, myalgia, rare nonproductive cough, and weight loss (29, 33). This illness can progress to Q fever pneumonia, characterized by gross lung consolidation and an interstitial pneumonia with bronchial and alveolar exudates and inflammatory infiltrates consisting primarily of lymphocytes and macrophages (29, 31, 37). Acute Q fever patients may also develop hepatitis (8), alone or in combination with the respiratory illness (30). Chronic Q fever often presents as endocarditis (14) and/or hepatitis and has been diagnosed occasionally in osteomyelitis cases (36, 41). An association has also been made between a chronic fatigue-like syndrome and Q fever (27, 40, 58, 60).
Animal models commonly used in the study of Q fever include mice, guinea pigs, nonhuman primates, and livestock. Mice are utilized most often due to the many relevant genetic and immunologic tools available, and differences in strain susceptibilities have been noted (47). Immunocompetent mice require a large number of organisms to develop clinical signs of illness, and splenomegaly is recognized as the primary indicator of disease (12), in contrast to fever as the primary indicator in guinea pigs (17), which can develop clinical illness after intraperitoneal (i.p.) infection with as few as 10 organisms (35). It has been shown that guinea pigs inoculated i.p. exhibit dose-dependent fever and have more pathologic changes associated with the liver, whereas those infected intranasally have greater involvement of the lungs (22). Mice are used essentially as a clearance model, relying on the mouse's ability to control infection after uptake of the organism. Guinea pigs can be used as a model of clinical disease and therefore would be more relevant for testing vaccines or antibiotic regimens for human use.
For this study, guinea pigs were infected with C. burnetii across a 5-log range of challenge doses through inhalation of small-particle aerosols. These exposures resulted in a dose-responsive relationship to clinical and pathologic changes. The disease produced in the guinea pig aerosol challenge model closely mimics human acute Q fever and Q fever pneumonia. The experiments presented here fill in gaps in the current knowledge concerning the consequences of aerosol infection with C. burnetii by using a physiologically relevant model, including the dose response to infection, kinetics of extrapulmonary dissemination, and pathologic and histopathologic changes resulting from aerosol exposure.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old female outbred Hartley guinea pigs obtained from Charles River Laboratories (Wilmington, MA) were housed in microisolator caging in a biosafety level 3 facility with a 12-h-12-h light-dark cycle and were given Harlan Teklad (Madison, WI) guinea pig diet and water ad libitum. Guinea pigs were acclimated to the facility and assessment procedures for 1 week prior to infection to reduce stress-related abnormalities. A modified Karnofsky performance status scoring system was used to determine if humane euthanasia was necessary after infection. All animal experimentation was reviewed and approved by the Texas A&M University Laboratory Animal Care Committee and was performed in an AAALAC-approved facility in accordance with university and federal regulations.
Purification of C. burnetii.
C. burnetii Nine Mile (RSA493), an isolate originating from a tick pool, was harvested from infected L929 mouse fibroblast cells by pooling infected cells and centrifuging them at 1,000 × g for 5 min. The supernatant was then centrifuged at 15,000 × g for 30 min to collect the naturally released bacteria, and the resulting pellet was resuspended in 0.25 M sucrose phosphate (SP) buffer (53.9 mM Na2HPO4, 12.8 mM KH2PO4, 72.6 mM NaCl, 250 mM sucrose). Double-distilled H2O was added to the original pellet. This suspension was passed through an 18-gauge canula, lysis was monitored by visual inspection, and an equal volume of 0.25 M SP was added when the majority of the cells were lysed. Benzonase, a nonspecific nuclease (Novagen, Madison, WI), was added to the lysed cell suspension for 5 min at room temperature. Cells were resuspended several times with a pipette before pelleting of host cells by centrifugation at 1,000 × g for 5 min. The supernatant was combined with the naturally released bacteria, and the combination was pelleted by centrifugation at 14,000 × g for 30 min. The supernatant was discarded, and the pellet was resuspended in SP. Host cell debris was pelleted by centrifugation at 1,000 × g for 5 min, and the pellet was discarded. Bacteria in the supernatant were pelleted by centrifugation at 14,000 × g for 30 min, the pellet was resuspended in SP, and the purity of the bacterial pellet was ascertained by examination of heat-fixed cells with Gimenez stain.
Quantification of C. burnetii inoculum.
C. burnetii Nine Mile (RSA493) was quantified by three methods. The optical density (OD) was used to determine the number of particles in the stock solution, as previously described (39). Particle counts were performed using a Live/Dead BacLight bacterial viability kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Primers amplifying the com-1 gene were used to enumerate C. burnetii genome copies by real-time PCR, as previously described (7). A dose of 2 × 106 organisms determined by OD measurement corresponded to 1.7 × 106 organisms by particle counting with 95% viability and to 1.1 × 106 genome equivalents by real-time PCR. Infectious doses described in this paper are those enumerated by OD measurement.
Infection.
Guinea pigs were exposed to phase I C. burnetii Nine Mile (RSA493) in phosphate-buffered saline (PBS) or to PBS alone (negative control), using a chamber specially designed to deliver droplet nuclei directly to the alveolar spaces (College of Engineering Shops, University of Wisconsin, Madison, WI) (34, 59). This chamber allows the infection of multiple guinea pigs simultaneously, ensuring uniform infection within each group.
Experimental design. (i) Experiment 1.
Fourteen guinea pigs were exposed to ∼2 × 106 C. burnetii organisms, and two negative control animals were sham infected with sterile PBS. Clinical assessments of disease progression were performed daily for 28 days, including assessments of behavior, weight, and rectal temperature, thoracic auscultation, and abdominal palpation. Temperatures of ≥39.5°C were defined as fever. Euthanasia by a ketamine-xylazine overdose followed by exsanguination was performed at 7, 14, or 28 days postinfection (p.i.) or as indicated by the Karnofsky score. Spleens and livers were weighed at necropsy, and hearts, lungs (perfused), livers, spleens, and kidneys were collected and fixed in formalin for evaluation by histopathology and immunohistochemistry.
(ii) Experiment 2.
Three guinea pigs per dose were exposed to ∼2 × 106, 2 × 105, 2 × 104, 2 × 103, 2 × 102, or 2 × 101 C. burnetii organisms, based on optical density-determined bacterial number correlation; three negative control animals were sham infected with PBS. Three vaccinated guinea pigs (see below) were infected with ∼2 × 106 C. burnetii organisms. Clinical and pathologic assessments were performed as described for experiment 1.
Guinea pigs infected with 2 × 106 C. burnetii organisms from experiment 2 were combined with those from experiment 1 to develop a survival curve.
Vaccination.
Guinea pigs were given subcutaneous injections of 100 μg formalin-killed phase I Nine Mile C. burnetii in incomplete Freund's adjuvant twice, with 2-week intervals between vaccinations and between the final vaccination and infection. Blood was collected from all animals prior to infection for serological confirmation of vaccination efficacy.
Histopathology and immunohistochemistry.
Tissues collected at necropsy were fixed in 10% buffered formalin for a minimum of 48 h. Tissues were sectioned and embedded in paraffin, cut to a thickness of 5 μm, allowed to adhere to slides, and stained routinely with hematoxylin and eosin. Unstained slides were prepared for immunohistochemical staining. A Vectastain ABC kit and a Vector NovaRED substrate kit (Vector Laboratories, Burlingame, CA) were used with rabbit anti-Nine Mile C. burnetii serum generated in our laboratory for immunostaining of C. burnetii in tissue sections (3); slides were counterstained with hematoxylin. All slides were evaluated in a blinded fashion.
Serology.
Serum samples collected at necropsy were tested by enzyme-linked immunosorbent assay in 96-well U-shaped polystyrene plates (Fisher Scientific, Houston, TX) coated with 50 μl of 0.5-μg/ml phase I Nine Mile C. burnetii antigen (formalin-killed whole cells) diluted in 0.1 M sodium carbonate buffer (pH 9.6) per well. After 24 h of incubation at 4°C, plates were emptied and blocked for 1 h at 37°C in 1% bovine serum albumin-PBS-Tween 20 (BSA-PBST). Serum was heat inactivated at 56°C for 30 min, dilutions of 1:400 to 1:102,400 were prepared in 1% BSA-PBST, and 50 μl of sample and 50 μl of 1% BSA-PBST were added to each well and incubated at room temperature for 2 h. Plates were washed four times with distilled H2O and then incubated with 100 μl of goat anti-guinea pig immunoglobulin G (Bethyl Laboratories, Montgomery, TX) diluted 1:1,000 for 2 h at room temperature before being washed again four times with distilled H2O. One hundred microliters of substrate (Sigma Fast o-phenylenediamine dihydrochloride; Sigma-Aldrich, St. Louis, MO) was incubated in each well for 5 to 10 min in the dark at room temperature, and the reaction was stopped with 100 μl 1 M H2SO4 per well. Plates were read at 490 nm in a Dynatech MR5000 microplate reader (Dynatech Laboratories, Chantilly, VA). Uninfected guinea pig serum was used as a negative control.
Statistical analysis.
Results were expressed as means ± standard errors and were compared using Student's t test. Differences were considered significant at P values of <0.05.
RESULTS
Clinical signs of infection with C. burnetii delivered via small-particle aerosol.
Guinea pigs infected with 2 × 106 C. burnetii organisms developed fever (≥39.5°C) by day 5 p.i. The temperature peaked at 40.8°C on day 7 p.i. and returned to normal by day 13 p.i. in surviving animals. Thereafter, the temperature remained within normal parameters for 28 days. Animals infected at all lower doses showed initial fever responses on later days. A dose-dependent relationship was noted for both the onset and degree of fever (Table 1).
TABLE 1.
Response to infection with C. burnetii
| Infectious dose (no. of organisms) | No. of animals with fever/total no. of animals | Earliest onset of fever (day)b | Avg duration of fever (days)b | Highest temp (°C) | No. of deaths/total no. of animals | No. of seroconversions/total no. of animals | Enzyme-linked immunosorbent assay titer (range) at 28 days p.i. |
|---|---|---|---|---|---|---|---|
| PBS control | 0/3 | NA | NA | 39.3 | 0/3 | 0/3 | 0 |
| 2 × 101 | 2/3 | 14 | 1 | 39.6 | 0/3 | 3/3 | 100 |
| 2 × 102 | 3/3 | 14 | 1.67 | 39.9 | 0/3 | 3/3 | 400-800 |
| 2 × 103 | 3/3 | 10 | 2.67 | 39.9 | 0/3 | 3/3 | 1,600-3,200 |
| 2 × 104 | 3/3 | 6 | 4 | 40.6 | 0/3 | 3/3 | 3,200-51,200 |
| 2 × 105 | 3/3 | 6 | 6 | 40.9 | 0/3 | 3/3 | 51,200-102,400 |
| 2 × 106 | 3/3 | 5 | 5a | 40.8 | 2/3 | 1/3a | 25,600 |
| 2 × 106 (given to vaccinated animals) | 0/3 | NA | NA | 39.4 | 0/3 | 3/3 | 25,600-102,400 |
Number may be skewed due to deaths.
NA, not available.
All guinea pigs infected with 2 × 104 to 2 × 106 C. burnetii organisms experienced inappetence and lethargy corresponding with the onset of fever. Weight loss began as early as day 4 p.i. in animals infected with 2 × 106 organisms, was statistically significant (P < 0.01) by day 5, and continued until death or until days 11 to 13 in surviving guinea pigs. Mild to moderate clinical dehydration was apparent by day 6 or 7 p.i. in these animals and only resolved with the alleviation of fever. Guinea pigs given 2 × 101 to 2 × 103 organisms had no significant weight loss or dehydration associated with disease.
Increased respiratory rates, sounds, and efforts were detected starting at days 5 and 6 p.i. for the infection group receiving 2 × 106 cells. Consolidation and crackles (rales) were noted on auscultation, with uniform distribution between the right and left sides. Upper respiratory obstruction was minimal. Paling and faint cyanosis of the muzzle were evident. Nasal discharge, though present, was rapidly cleaned away by the guinea pigs and therefore only noted sporadically throughout the main course of infection.
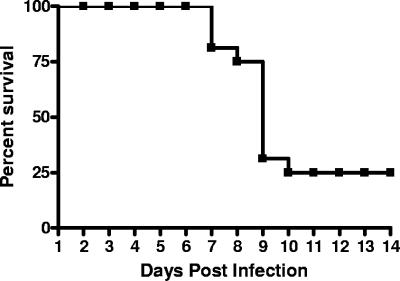
A 70.6% total mortality rate (12/17 animals from experiments 1 and 2) was noted for nonvaccinated guinea pigs infected with 2 × 106 C. burnetii organisms, with spontaneous death (75%) or humane euthanasia (25%) occurring from days 7 to 10 p.i. (Fig. 1); no animals died after 10 days postinfection. Groups infected at all lower doses had no fatalities, and the 50% lethal dose was calculated to be 2 × 105.7 by the method of Reed and Muench (42).
FIG. 1.
Percent survival after infection with 2 × 106 C. burnetii cells. Inoculation with 2 × 106 C. burnetii organisms resulted in mortality in 12/17 guinea pigs within the first 10 days of infection, with no deaths occurring thereafter.
Vaccination prior to infection conferred complete protection against the development of fever, respiratory abnormalities, and death. The lack of clinical signs of illness was comparable between the vaccinated, infected animals and those that were sham infected with PBS (Table 1).
Pathologic changes in guinea pigs infected with C. burnetii.
At 7 days p.i., guinea pigs exposed to 2 × 106 C. burnetii organisms displayed complete gross consolidation of cranial lung lobes and partial consolidation of caudal lobes at necropsy. Several multifocal, pinpoint, white foci were noted throughout the lungs in both the cranial and caudal lobes. The liver was mottled and pale, with a yellowish discoloration. These animals also had a noticeable lack of abdominal fat. By 14 days p.i., lung consolidation had largely resolved in surviving guinea pigs. The liver appeared as described for 7 days p.i., and there was still a lack of abdominal fat. Moderate, transient splenomegaly (P < 0.01) was also noted (data not shown). A calculation of relative spleen-to-body-weight ratios also showed statistical significance (P < 0.05) at 7 days p.i., although this was likely an artifact due to the rapid, severe weight loss of the animals at that time. Multifocal, pinpoint-to-4-mm, white foci were present in the lungs at 28 days p.i. The liver continued to appear somewhat mottled, but coloration returned to normal, and pinpoint-to-2-mm, white-to-tan foci were present in some animals.
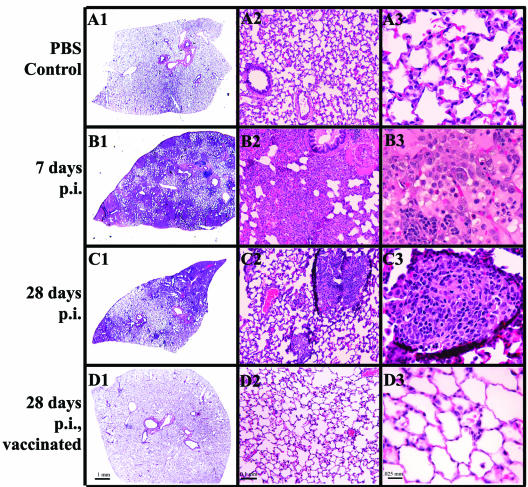
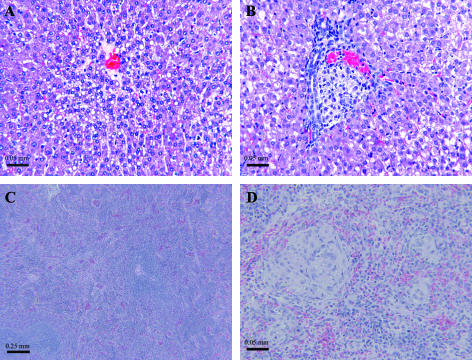
Histopathologic evaluation (Fig. 2) at 7 days p.i. revealed a coalescing, panleukocytic, bronchointerstitial pneumonia (Fig. 2B, panels 1 to 3). Lungs were hyperemic, and alveolar walls were thickened due to cellular infiltration consisting primarily of neutrophils and lymphocytes, although all inflammatory cell types were present to some extent. The amount of bronchus-associated lymphoid tissue was increased (Fig. 2B, panel 1, arrows). Extensive bronchial and alveolar exudates were noted throughout the sections. Purulent bronchial exudates (Fig. 2B, panel 2) were prominent, with degeneration and sloughing of the bronchial epithelial lining. Alveolar exudates (Fig. 2B, panel 3) were a deep pink, indicating a high protein content. By 28 days p.i., lung changes resolved to multifocal, lymphohistiocytic, interstitial pneumonia with granuloma formation (Fig. 2C, panels 1 to 3). Granulomas (Fig. 2C, panel 2, arrows, and panel 3) varied in size and distribution and consisted primarily of aggregates of macrophages surrounded by lymphocytes.
FIG. 2.
Q fever pneumonia. Hematoxylin- and eosin-stained lung tissues from guinea pigs infected with 2 × 106 C. burnetii organisms at 7 and 28 days p.i. were compared to those from PBS-treated controls (A1 to A3). Panleukocytic bronchointerstitial pneumonia with bronchial and alveolar exudates was seen at 7 days p.i. (B1 to B3) (arrows indicate bronchus-associated lymphoid tissue). Granulomatous pneumonia was present at 28 days p.i. (C1 to C3) (arrows indicate pulmonary granulomas). No significant pathological change was noted in guinea pigs vaccinated prior to infection (D1 to D3).
Severe, diffuse hepatic lipid accumulation (Fig. 3A) was present at 7 days p.i. Centrilobular hepatocellular degeneration and vacuolization were noted in the liver, along with periportal lymphocyte infiltration and multiple small granulomas at 14 and 28 days p.i. (Fig. 3B). One guinea pig evaluated at 14 days p.i. was noted to have extensive focal necrosis and mineralization in a section of the liver. Disruption of normal splenic architecture (Fig. 3C) was most apparent at 14 days p.i., corresponding to the gross splenomegaly observed at necropsy, and granulomas were present in the spleen at both 14 and 28 days p.i. (Fig. 3D). Vascular thrombi were noted in several tissues at 28 days p.i. Mineralization of kidney tubules was occasionally noted. Although some guinea pigs exhibited a mild lymphocytic myocarditis or pericarditis, there was no evidence of valvular endocarditis at any time postinfection.
FIG. 3.
Q fever hepatopathies and splenic changes. Hematoxylin- and eosin-stained liver and spleen sections show (A) hepatic lipidosis at 7 days p.i., (B) a hepatic granuloma at 14 days p.i., (C) disruption of normal splenic architecture at 14 days p.i., and (D) splenic granulomas at 14 days p.i. in guinea pigs infected with 2 × 106 C. burnetii organisms.
Animals infected at lower doses and necropsied at 28 days p.i. displayed progressively less severe signs of pneumonia and hepatic degeneration (Table 2). No significant pathologic changes were noted in negative control and vaccinated guinea pigs (Fig. 2A, panels 1 to 3, and D, panels 1 to 3).
TABLE 2.
| Infectious dose (no. of organisms) | Score
|
|||||
|---|---|---|---|---|---|---|
| Lungs
|
Livers
|
Spleens
|
||||
| H | I | H | I | H | I | |
| PBS control | 0 | − | 0 | − | 0 | − |
| 2 × 101 | 1 | − | 0 | − | 0 | + |
| 2 × 102 | 1 | + | 1 | − | 0 | + |
| 2 × 103 | 2 | + | 1 | + | 1 | ++ |
| 2 × 104 | 3 | + | 2 | ++ | 1 | ++ |
| 2 × 105 | 3 | ++ | 2 | ++ | 1 | +++ |
| 2 × 106 | 3 | ++ | 3 | ++ | 2 | +++ |
| 2 × 106 (given to vaccinated animals) | 0 | − | 0 | − | 0 | + |
The severity of histopathology (H) is based on the amount of architectural change, cellular infiltration, and the presence or absence of necrosis, as follows: 0, none; 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; 5, severe.
For immunohistochemistry (I), the following score system was used: −, no organisms present in the tissue section; +, the presence of the organism is rare and focal; ++, few organisms are multifocally present; +++, many organisms are diffusely present; ++++, an abundance of organisms are diffusely present. Scores are the averages for sections evaluated for three guinea pigs per group at 28 days p.i. The evaluation of tissue sections was performed in a blinded fashion.
Immunohistochemistry confirmed the presence of C. burnetii organisms in infected animals in the lungs, liver, and spleen (Table 2). Bacteria were most prominent in macrophages but were also noted in other cell types, primarily pneumocytes in the lungs.
Serologic response to infection.
Infected guinea pigs in all dose groups seroconverted by the time of euthanasia, with the exception of animals necropsied at 7 days p.i. The extent of seroconversion was dose dependent (Table 1). No PBS control animals seroconverted.
DISCUSSION
A need exists in Coxiella research for an animal model that simulates both the natural route of infection and common clinical presentations associated with human acute Q fever infection. Such a physiologically relevant model would be valuable not only for testing vaccines and therapeutic agents but also for evaluating the comparative virulence of different C. burnetii isolates. Moos and Hackstadt showed a difference in virulence between two isolates, Nine Mile and Priscilla, in an i.p. challenge guinea pig model (35), and Kazar et al. also demonstrated a lower virulence of Priscilla than of the Nine Mile and S isolates (20). No histologic changes were described by Stein et al. for Q212-infected mice compared to multiple pathological changes in Nine Mile-infected animals (50). The guinea pig aerosol challenge model described here is able to discern differences in disease manifestation within and between the six C. burnetii genomic groups (unpublished data). This model would also be useful for examining the kinetics of extrapulmonary distribution of C. burnetii, especially in whole blood and serum, in the very early stages of infection for the purpose of improving diagnostic tests for use in humans following a suspected exposure or outbreak.
The dose-responsive nature of infection in the guinea pig aerosol challenge model was expected and consistent within each group. This clinical dose-response effect has been noted previously for humans (4, 32) and cynomolgus macaques (15). Guinea pigs receiving small numbers of organisms took longer to develop a low-grade fever for a brief period of time, in some cases as little as 1 day. Conversely, guinea pigs receiving larger numbers of organisms developed a high fever which persisted longer than the case for the former group. Fatalities noted for animals exposed to 2 × 106 C. burnetii organisms were likely due to severe respiratory insufficiency. Guinea pigs receiving 2 × 103 to 2 × 105 organisms, though also experiencing respiratory difficulty and showing histologic evidence of pneumonia, were able to overcome the illness. A 50% lethal dose of 2 × 105.7 C. burnetii organisms was determined for this model. The aerosol challenge mouse model described by Stein et al. required 108 C. burnetii organisms before any pathological changes were noted at early times postinfection, suggesting that a large amount of the organism must be present in aerosols for the development of Q fever (50), whereas the guinea pig model displays clinical and pathological evidence of Q fever over a wider and lower range of doses at more times postinfection, and the consistent development of fever, easily measurable antemortem, makes the guinea pig a much more sensitive model. As opposed to reports using high doses of C. burnetii for infection via inhalation in guinea pigs (20), this study showed that guinea pigs exposed to as few as 2 × 101 organisms are able to develop acute Q fever, as is the case for humans.
The histologic characteristics of the pneumonia which developed in the guinea pigs in this study corresponded to those seen for human Q fever pneumonia (29, 31, 37). The change from the panleukocytic bronchointerstitial pneumonia seen at 7 days p.i. in animals infected with 2 × 106 organisms to a lymphohistiocytic or granulomatous pneumonia by 28 days p.i. showed resolution of the pneumonia and recovery, but it is unknown at this time how long the pulmonary (and hepatic) granulomas may persist postinfection. Should these granulomas remain and contain viable organisms, it is possible that they could be sites of persistent or latent infection, like the placenta (41), bone marrow (28), and liver (16) in humans.
For many animals, hepatic lipidosis has been associated with periods of anorexia, similar to that seen for guinea pigs infected at higher doses in this study, leading to an excess of triglycerides in the liver, intrahepatic cholestasis, and liver failure. Guinea pigs are known to develop fatty livers as a result of reduced carbohydrate intake and mobilization of fat as an energy source, most often associated with fasting metabolic pregnancy toxemia (45). The hepatic steatosis seen at 7 days p.i. in our guinea pigs infected via aerosol with 2 × 106 C. burnetii organisms has been reported previously for i.p. inoculated guinea pigs at 3 to 4 days p.i. Increases in triglycerides, unesterified fatty acids, and cholesterol have been noted during early Q fever infection in these animals, and it is thought that the development of a fatty liver is due to a failure of the liver to export these lipids after their mobilization to the liver (6). An association has also been suggested between fatty liver development and changes in the plasma membrane peptide composition which alter lipid transport (25). This steatohepatitis resolved after the main course of infection when the animals resumed eating and was no longer apparent at 14 and 28 days p.i., when centrilobular hepatocellular degeneration and vacuolization were noted. Centrilobular hepatic degeneration is a zonal change centered about the central vein (hepatic venule) and is generally caused by low oxygen tension or a high concentration of enzymes associated with bioactivation of toxic compounds. Low oxygen tension, in this case, could be caused by the severe respiratory insufficiency and resulting hypoxia displayed by guinea pigs infected with high doses of C. burnetii.
There is currently no licensed vaccine against Q fever in the United States, although a killed whole-cell vaccine (QVax; CLS Ltd.) available in Australia has been shown to be highly successful in the prevention of clinical disease in humans (19) as well as in rodent (56) and nonhuman primate (57) models. However, this vaccine can cause severe necrotic lesions or granuloma development at the injection site in humans previously exposed to C. burnetii (11, 26) and thus requires skin testing prior to vaccination. A new, efficacious vaccine without such deleterious side effects is needed, and appropriate animal models of human Q fever will be required to evaluate the safety and efficacy of new vaccine candidates. Guinea pigs in this study that were vaccinated with whole killed cells were completely protected from fever development and death when given a high-dose challenge, again correlating with the human response to vaccination and challenge (1). The guinea pig aerosol challenge model presented here mimics both the clinical and pathologic changes seen in human acute Q fever and Q fever pneumonia cases and will provide an accurate and valuable tool for the study of the general pathogenesis of C. burnetii infection, for vaccine assessment, and for evaluations of host immune responses.
Acknowledgments
This work was supported by funding from NIH NIAID grants KO8 AI055664 and U54 AI057156.
We thank Andres de la Concha and Ian Tizard for assistance in reviewing this paper.
Editor: R. P. Morrison
REFERENCES
- 1.Ackland, J. R., D. A. Worswick, and B. P. Marmion. 1994. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985-1990. Med. J. Aust. 160:704-708. [PubMed] [Google Scholar]
- 2.Arricau Bouvery, N., A. Souriau, P. Lechopier, and A. Rodolakis. 2003. Experimental Coxiella burnetii infection in pregnant goats: excretion routes. Vet. Res. 34:423-433. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner, W., H. Dettinger, N. Schmeer, and E. Hoffmeister. 1988. Evaluation of different fixatives and treatments for immunohistochemical demonstration of Coxiella burnetii in paraffin-embedded tissues. J. Clin. Microbiol. 26:2044-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benenson, A. S., and W. D. Tigertt. 1956. Studies on Q fever in man. Trans. Assoc. Am. Physicians 69:98-104. [PubMed] [Google Scholar]
- 5.Bernasconi, M. V., S. Casati, O. Peter, and J. C. Piffaretti. 2002. Rhipicephalus ticks infected with Rickettsia and Coxiella in Southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2:111-120. [DOI] [PubMed] [Google Scholar]
- 6.Bernier, R. D., T. Haney, and D. Paretsky. 1974. Changes in lipids of liver and plasma during Q fever. Acta Virol. 18:75-80. [Google Scholar]
- 7.Brennan, R. E., and J. E. Samuel. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J. Clin. Microbiol. 41:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, K., J. J. Yan, H. C. Lee, K. H. Liu, N. Y. Lee, and W. C. Ko. 2004. Acute hepatitis with or without jaundice: a predominant presentation of acute Q fever in southern Taiwan. J. Microbiol. Immunol. Infect. 37:103-108. [PubMed] [Google Scholar]
- 9.Davis, G. E., and H. R. Cox. 1938. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersonii, reactions in animals, and filtration. Public Health Rep. 53:2259. [Google Scholar]
- 10.Dyer, R. E. 1938. A filter-passing infectious agent isolated from ticks. Public Health Rep. 53:2277-2282. [Google Scholar]
- 11.Fairweather, P., T. O'Rourke, and G. Strutton. 2005. Rare complication of Q fever vaccination. Australas. J. Dermatol. 46:124-125. [DOI] [PubMed] [Google Scholar]
- 12.Franti, C. E., D. E. Behymer, J. E. Goggin, and M. E. Wright. 1974. Splenomegaly, sex, and other characteristics of laboratory animals used for primary isolations of Coxiella burnetii. Lab. Anim. Sci. 24:656-665. [PubMed] [Google Scholar]
- 13.Franz, D. R., P. B. Jahrling, A. M. Friedlander, D. J. McClain, D. L. Hoover, W. R. Bryne, J. A. Pavlin, G. W. Christopher, and E. M. Eitzen, Jr. 1997. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 278:399-411. [DOI] [PubMed] [Google Scholar]
- 14.Gami, A. S., V. S. Antonios, R. L. Thompson, H. P. Chaliki, and N. M. Ammash. 2004. Q fever endocarditis in the United States. Mayo Clin. Proc. 79:253-257. [DOI] [PubMed] [Google Scholar]
- 15.Gonder, J. C., R. A. Kishimoto, M. D. Kastello, C. E. Pedersen, Jr., and E. W. Larson. 1979. Cynomolgus monkey model for experimental Q fever infection. J. Infect. Dis. 139:191-196. [DOI] [PubMed] [Google Scholar]
- 16.Harris, R. J., P. A. Storm, A. Lloyd, M. Arens, and B. P. Marmion. 2000. Long-term persistence of Coxiella burnetii in the host after primary Q fever. Epidemiol. Infect. 124:543-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heggers, J. P., L. H. Billups, D. J. Hinrichs, and L. P. Mallavia. 1975. Pathophysiologic features of Q fever-infected guinea pigs. Am. J. Vet. Res. 36:1047-1052. [PubMed] [Google Scholar]
- 18.Huebner, R. J., W. L. Jellison, and M. D. Beck. 1949. Q fever studies in southern California. III. Effects of pasteurization on survival of Coxiella burnetii in naturally infected milk. Public Health Rep. 64:499-511. [PMC free article] [PubMed] [Google Scholar]
- 19.Izzo, A. A., B. P. Marmion, and D. A. Worswick. 1988. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J. Infect. Dis. 157:781-789. [DOI] [PubMed] [Google Scholar]
- 20.Kazar, J., M. Lesny, P. Propper, D. Valkova, and R. Brezina. 1993. Comparison of virulence for guinea pigs and mice of different Coxiella burnetii phase I strains. Acta Virol. 37:437-448. [PubMed] [Google Scholar]
- 21.Lang, G. H. 1990. Coxiellosis* (Q fever) in animals, p. 23-49. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla. [Google Scholar]
- 22.La Scola, B., H. Lepidi, and D. Raoult. 1997. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect. Immun. 65:2443-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leggiadro, R. J. 2000. The threat of biological terrorism: a public health and infection control reality. Infect. Control Hosp. Epidemiol. 1:53-56. [DOI] [PubMed] [Google Scholar]
- 24.Lennette, E. H., W. H. Clark, M. M. Abinanti, O. Brunetti, and J. M. Covert. 1952. Q fever studies. XIII. The effect of pasteurization on Coxiella burnetii in naturally infected milk. Am. J. Hyg. 55:246-253. [PubMed] [Google Scholar]
- 25.Marecki, N., F. Becker, O. G. Baca, and D. Paretsky. 1978. Changes in liver and L-cell plasma membranes during infection with Coxiella burnetii. Infect. Immun. 19:272-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marmion, B. P., R. A. Ormsbee, M. Kyrkou, J. Wright, D. Worswick, S. Cameron, A. Esterman, B. Feery, and W. Collins. 1984. Vaccine prophylaxis of abattoir-associated Q fever. Lancet ii:1411-1414. [DOI] [PubMed] [Google Scholar]
- 27.Marmion, B. P., M. Shannon, I. Maddocks, P. Storm, and I. Penttila. 1996. Protracted debility and fatigue after acute Q fever. Lancet 347:977-978. (Letter.) [DOI] [PubMed] [Google Scholar]
- 28.Marmion, B. P., P. A. Storm, J. G. Ayres, L. Semendric, L. Mathews, W. Winslow, M. Turra, and R. J. Harris. 2005. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 98:7-20. [DOI] [PubMed] [Google Scholar]
- 29.Marrie, T. J. 1990. Acute Q fever, p. 125-160. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla. [Google Scholar]
- 30.Marrie, T. J. 1990. Q fever hepatitis, p. 171-178. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla. [Google Scholar]
- 31.Marrie, T. J. 2004. Q fever pneumonia. Curr. Opin. Infect. Dis. 17:137-142. [DOI] [PubMed] [Google Scholar]
- 32.Marrie, T. J., H. Durant, J. C. Williams, E. Mintz, and D. M. Waag. 1988. Exposure to parturient cats: a risk factor for acquisition of Q fever in maritime Canada. J. Infect. Dis. 158:101-108. [DOI] [PubMed] [Google Scholar]
- 33.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMurray, D. N. 1994. Guinea pig model of tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 35.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 55:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nourse, C., A. Allworth, A. Jones, R. Horvath, J. McCormack, J. Bartlett, D. Hayes, and J. M. Robson. 2004. Three cases of Q fever osteomyelitis in children and a review of the literature. Clin. Infect. Dis. 39:e61-e66. [DOI] [PubMed] [Google Scholar]
- 37.Okimoto, N., N. Asaoka, K. Osaki, T. Kurihara, K. Yamato, T. Sunagawa, K. Fujita, H. Ohba, J. Nakamura, and K. Nakada. 2004. Clinical features of Q fever pneumonia. Respirology 9:278-282. [DOI] [PubMed] [Google Scholar]
- 38.Ormsbee, R. A. 1965. Q fever rickettsia, p. 1144-1160. In F. L. Horsfall and I. Tamm (ed.), Viral and rickettsial diseases of man. J. B. Lippincott, Philadelphia, Pa.
- 39.Ormsbee, R. A., and M. G. Peacock. 1976. Rickettsial plaque assay and cloning procedure. Tissue Culture Assoc. 2:475-478. [Google Scholar]
- 40.Penttila, I. A., R. J. Harris, P. Storm, D. Haynes, D. A. Worswick, and B. P. Marmion. 1998. Cytokine dysregulation in the post-Q-fever fatigue syndrome. QJM 91:549-560. [DOI] [PubMed] [Google Scholar]
- 41.Raoult, D., A. Raza, and T. J. Marrie. 1990. Q fever endocarditis and other forms of chronic Q fever, p. 179-200. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla. [Google Scholar]
- 42.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 43.Regis, E. 1999. The biology of doom: the history of America's secret germ warfare project. Henry Holt and Associates, New York, N.Y.
- 44.Rolain, J. M., F. Gouriet, P. Brouqui, D. Larrey, F. Janbon, S. Vene, V. Jarnestrom, and D. Raoult. 2005. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin. Infect. Dis. 40:82-88. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffer, D. O., and T. M. Donnelly. 1997. Disease problems of guinea pigs and chinchillas, p. 260-281. In E. V. Hillyer and K. E. Quesenberry (ed.), Ferrets, rabbits, and rodents: clinical medicine and surgery. W. B. Saunders Co., Philadelphia, Pa.
- 46.Scott, G. H., and J. C. Williams. 1990. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann. N. Y. Acad. Sci. 590:291-296. [DOI] [PubMed] [Google Scholar]
- 47.Scott, G. H., J. C. Williams, and E. H. Stephenson. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691-700. [DOI] [PubMed] [Google Scholar]
- 48.Spicer, A. J. 1978. Military significance of Q fever: a review. J. R. Soc. Med. 71:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spitalska, E., and E. Kocianova. 2003. Detection of Coxiella burnetii in ticks collected in Slovakia and Hungary. Eur. J. Epidemiol. 18:263-266. [DOI] [PubMed] [Google Scholar]
- 50.Stein, A., C. Louveau, H. Lepidi, F. Ricci, P. Baylac, B. Davoust, and D. Raoult. 2005. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73:2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein, A., and D. Raoult. 1999. Pigeon pneumonia in Provence: a bird-borne Q fever outbreak. Clin. Infect. Dis. 29:617-620. [DOI] [PubMed] [Google Scholar]
- 52.Stephen, S., and K. N. Rao. 1979. Coxiellosis in reptiles of South Kanara district, Karnataka. Indian J. Med. Res. 70:937-941. [PubMed] [Google Scholar]
- 53.Tigertt, W. D., A. S. Benenson, and W. S. Gochenour. 1961. Airborne Q fever. Bacteriol. Rev. 25:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tissot Dupont, H., D. Raoult, P. Brouqui, F. Janbon, D. Peyramond, P. J. Weiller, C. Chicheportiche, M. Nezri, and R. Poirier. 1992. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am. J. Med. 93:427-434. [DOI] [PubMed] [Google Scholar]
- 55.To, H., R. Sakai, K. Shirota, C. Kano, S. Abe, T. Sugimoto, K. Takehara, C. Morita, I. Takashima, T. Maruyama, T. Yamaguchi, H. Fukushi, and K. Hirai. 1998. Coxiellosis in domestic and wild birds from Japan. J. Wildl. Dis. 34:310-316. [DOI] [PubMed] [Google Scholar]
- 56.Waag, D. M., M. J. England, and M. L. M. Pitt. 1997. Comparative efficacy of a Coxiella burnetii chloroform:methanol residue (CMR) vaccine and a licensed cellular vaccine (Q-Vax) in rodents challenged by aerosol. Vaccine 15:1779-1783. [DOI] [PubMed] [Google Scholar]
- 57.Waag, D. M., M. J. England, R. F. Tammariello, W. R. Byrne, P. Gibbs, C. M. Banfield, and M. L. Pitt. 2002. Comparative efficacy and immunogenicity of Q fever chloroform:methanol residue (CMR) and phase I cellular (Q-Vax) vaccines in cynomolgus monkeys challenged by aerosol. Vaccine 20:2623-2634. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe, A. 2004. Various clinical types of Q-fever disease. Intern. Med. 43:1-2. [DOI] [PubMed] [Google Scholar]
- 59.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith. 1970. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422-429. [DOI] [PubMed] [Google Scholar]
- 60.Wildman, M. J., E. G. Smith, J. Groves, J. M. Beattie, E. O. Caul, and J. G. Ayres. 2002. Chronic fatigue following infection by Coxiella burnetii (Q fever): ten-year follow-up of the 1989 UK outbreak cohort. QJM 95:527-538. [DOI] [PubMed] [Google Scholar]
- 61.Yadav, M. P., and M. S. Sethi. 1980. A study on the reservoir status of Q-fever in avifauna, wild mammals and poikilotherms in Uttar Pradesh (India). Int. J. Zoonoses 7:85-89. [PubMed] [Google Scholar]