Abstract
It is widely accepted that tight junctions are altered during infections by attaching and effacing (A/E) pathogens. These disruptions have been demonstrated both in vitro and more recently in vivo. For in vivo experiments, the murine model of A/E infection with Citrobacter rodentium is the animal model of choice. In addition to effects on tight junctions, these bacteria also colonize the colon at high levels, efface colonocyte microvilli, and cause hyperplasia and inflammation. Although we have recently demonstrated that tight junctions are disrupted by C. rodentium, the issue of direct effects of bacteria on epithelial cell junctions versus the indirect effects of inflammation still remains to be clarified. Here, we demonstrate that during the C. rodentium infections, inflammation plays no discernible role in the alteration of tight junctions. The distribution of the tight junction proteins, claudin-1, -3, and -5, are unaffected in inflamed colon, and junctions appear morphologically unaltered when viewed by electron microscopy. Additionally, tracer molecules are not capable of penetrating the inflamed colonic epithelium of infected mice that have cleared the bacteria. Finally, infected colonocytes from mice exposed to C. rodentium for 14 days, which have high levels of bacterial attachment to colonocytes as well as inflammation, have characteristic, altered claudin localization whereas cells adjacent to infected colonocytes retain their normal claudin distribution. We conclude that inflammation plays no discernible role in tight junction alteration during A/E pathogenesis and that tight junction disruption in vivo appears dependent only on the direct intimate attachment of the pathogenic bacteria to the cells.
Humans and animals infected with the attaching and effacing (A/E) pathogens enterohemorrhagic Escherichia coli, enteropathogenic E. coli (EPEC), and Citrobacter rodentium suffer from serious diarrhea, or diarrhea-like conditions, that can result in death. These pathogens attach to the surface epithelial cells of the intestine and inject effector proteins through a type III secretion system (TTSS) into host cells (18, 32). These pathogenic proteins commandeer various host cell functions and structures that contribute to the disease process. Some of the host structures targeted by A/E pathogens include the host cell's microvilli (7, 24), mitochondria (19, 27, 31), and tight junctions (3, 6, 23, 26).
In intestinal epithelial cells, tight junctions are the most luminal cell-cell junctions of the apical junction complex (10). These junctions have two functions. One is to act as permeability barriers, separating the luminal environment from the adluminal region of the epithelium; the other is to segregate apically located membrane proteins from those at the basolateral membrane. Tight junctions are often viewed as membrane fusions by transmission electron microscopy because the membranes of adjacent cells are held in extremely close opposition by the transmembrane protein occludin and members of the claudin family that form the junctions (11, 13, 45). Both of these protein groups contain four transmembrane-spanning regions but do not share any sequence homology (reviewed in reference 15). They attach intracellularly to the adaptor molecules zonula occludens 1 (ZO-1), ZO-2, and ZO-3. ZO-1 links the transmembrane proteins to the actin cytoskeleton (9, 45). Work on these two groups of proteins has yielded striking differences as to their importance in maintaining the barrier function of the tight junctions. Studies using occludin null mice have demonstrated that occludin is dispensable with regard to tight junction barrier function (36, 37). These mice, devoid of occludin, have tight junctions that function normally (36). Conversely, mice deficient in all claudins targeted thus far have catastrophic tight junction deficiencies (12, 16, 30). In the colon, claudin-1 (51), -2, -3, -4, and -5 (35) are present in colonocytes.
Until recently, most research on tight junction disruption induced by A/E pathogens has focused on EPEC infections in cell culture (in vitro) (6, 23, 43, 46, 48, 50). Generally it is accepted that in vitro, EPEC causes a redistribution of the tight junction proteins occludin, ZO-1, and claudin-1 as well as a decrease in transepithelial resistance (TER) (26). These alterations are thought to be due to the effector proteins EspF and Map (mitochondria-associated protein) (6, 23, 48). We previously have used a naturally occurring A/E murine infection model to test whether tight junctions are disrupted in vivo (17). This model uses the A/E pathogen C. rodentium to infect mice. This pathogen colonizes the colon, collapses microvilli, and produces a diarrhea-like phenotype in infected mice (22). We demonstrated that C. rodentium disrupts tight junctions in mice and that this disruption is dependent on the bacterial type III effector protein EspF but not Map (17).
Although the evidence is controversial, it has been hypothesized that inflammatory effects participate in tight junction alteration during infection (28). Studies have shown that the addition of tumor necrosis factor-α to cultured colonic cells (HT-29/B6) decreases tight junction strands (14) and that the addition of both tumor necrosis factor-α and gamma interferon reduces the expression of claudin-2 but not the expression of claudin-3 and -4 in T84 cells (34). However, the addition of interleukin-13 increases the expression of claudin-2, while the levels of claudin-3 and -4 are unchanged in T84 cells (34). This result occurs with an increase in dextran permeability and a decrease in TER (34). Western blots of claudin-1, -2, -3, and -5 from mice deficient in interleukin-2, which have colonic inflammation, show a dramatic increase in protein levels in membrane preparations from the colon (1). In humans with inflammatory bowel disease a report by Gassler and coworkers (13a) suggests that ZO-1, occludin, claudin-1, and claudin-2 are all reduced. These results are contrary to those in a recent report by Prasad and coworkers (34) in which they demonstrate that claudin-2 expression increases and claudin-3 and -4 expression decreases in patients with Crohn's disease and ulcerative colitis/inflammatory bowel disease.
There is some suggestion that EPEC causes the release of inflammatory mediators in vitro (in cell culture) (4, 38, 41). To date, only one study has attempted to link inflammatory effects with tight junction alteration caused by A/E pathogen infection (39). In that study EPEC-infected cells were pretreated with drugs that inhibit mitogen-activated protein (MAP) or extracellular signal-regulated kinase (ERK) kinase (39). Although these inhibitors have numerous effects on cells in general, they apparently had no effect on the decrease in TER during EPEC infection in vitro (39).
To determine what role A/E pathogen-induced inflammation has on tight junctions in vivo, we infected C57BL/6 mice with C. rodentium and then evaluated the status of colonic tight junctions at a time point (21 days) when bacteria have been cleared from the colon but inflammation persists. We confirmed that, at this time point, bacteria were not attached to colonocytes and that inflammatory cell infiltrate was evident in the lamina propria. Immunolocalization of claudin-1, -3, and -5 on infected tissue sections labeled the lateral boundaries of colonocytes and appeared unaltered compared to uninfected or ΔescN C. rodentium-infected controls. Electron microscopy validated these findings by demonstrating morphologically intact tight junctions. Functional tracer experiments showed that the tight junctions remained intact as the chemical tracers were retained in the lumen of the colon without any penetration into the mucosa. Additionally, we demonstrated that in tissue in which both bacteria are attached and inflammation persists, tight junctions are altered only where bacteria are intimately attached. These findings provide crucial in vivo evidence that the inflammatory response caused by A/E pathogens does not play a significant role in tight junction alteration; instead, it is direct bacterial contact that mediates junctional disruption.
MATERIALS AND METHODS
Chemicals and reagents.
Chemicals and reagents used in this study were obtained from Sigma-Aldrich Canada (Ontario, Canada). Paraformaldehyde was acquired from Canmeco (Quebec, Canada), and NaCl was from Fisher Scientific (British Columbia, Canada). Secondary antibodies conjugated to horseradish peroxidase and control immunoglobulins (immunoglobulin G [IgG]) were purchased from Jackson ImmunoResearch Laboratories, Inc. (Pennsylvania). All secondary antibodies conjugated to Alexa fluorochromes were purchased from Molecular Probes (Oregon), as was the Alexa 488-conjugated streptavidin.
Animals, bacterial strains, and mouse infections.
Four- to six-week-old female C57BL/6 mice were acquired from the Jackson Laboratory and Charles River Laboratories. Upon arrival at the University of British Columbia animal care center, animals were left undisturbed for at least 4 days prior to oral gavage infections with 4 × 108 to 5 × 108 ΔescN or wild-type C. rodentium (8). Infections persisted for 7, 14, or 21 days, at which point the mice were euthanized by cervical dislocation. All experiments were repeated at least three times.
Tissue preparation, immunolocalization, and neutrophil staining.
Tissue immunolocalization was performed as described previously (17). Briefly, tissue was fixed in 3% paraformaldehyde; 5-μm sections were cut by Wax-It Histology Services, Inc., and treated with 0.2% Triton X-100 in phosphate-buffered saline (PBS; 150 mM NaCl, 5 mM KCl, 0.8 mM KH2PO4, 3.2 mM Na2HPO4, pH 7.3), and samples were washed extensively in PBS and blocked with 5% normal goat serum in PBS containing 0.05% Tween-20 and 0.1% bovine serum albumin (TPBS-BSA). Primary antibodies consisted of rabbit anti-claudin-1, anti-claudin-3, and anti-claudin-5 antibodies used at a concentration of 0.005 mg/ml (Zymed Laboratories, Inc., California) as well as a previously characterized (47) rat serum anti-C. rodentium Tir antibody used at a 1:100 dilution and a rat anti-mouse F4/80 antibody directly conjugated to biotin and used at a concentration of 1.315 μg/ml to 3.85 μg/ml (Serotec) in TPBS-BSA with 1% normal goat serum. These antibodies were incubated on the tissue sections overnight at 4°C. The material was washed extensively with the TPBS-BSA and then incubated for 90 min at 37°C with a goat anti-rabbit secondary antibody conjugated to Alexa 568. The slides were again washed extensively and stained with DAPI (4′,6′-diamidino-2-phenylindole). Coverslips were mounted using Vectashield (Vector Labs, Ontario, Canada). The tissue was visualized using a Zeiss Axiophot microscope.
As controls for claudin-1, -3, and -5 immunolocalization, primary antibodies were replaced with normal rabbit IgG at identical concentrations to the primary antibodies. Specific staining was not detected on normal rabbit IgG-stained control sections (data not shown).
Neutrophils were labeled according to the manufacturer's instructions using a Naphthol AS-D Chloroacetate Esterase kit (Sigma). Tissue was counterstained using hematoxylin.
Electron microscopy and thick sections.
Two- to three-millimeter sections of colon were excised from euthanized mice and fixed at room temperature for 2 to 3 h by immersion in a solution consisting of 1.5% paraformaldehyde, 1.5% glutaraldehyde, and 0.1 M sodium cacodylate (pH 7.3). The sections of bowel were cut into smaller pieces (1 to 1.5 mm), washed three times (10 min each wash) in 0.1 M sodium cacodylate buffer (pH 7.3), and then postfixed on ice for 1 h in 1% OsO4 in 0.1 M sodium cacodylate (pH 7.3). The material was washed three times in distilled water and then stained en bloc in 1% aqueous uranyl acetate. After 1 h, the samples were washed with distilled water, dehydrated through an ascending series of ethyl alcohols, infiltrated through propylene oxide into resin (EPON 812), and then embedded in resin. Thick sections were cut at a 1-μm thickness, mounted on glass slides, stained with toluidine blue, and then photographed on a Zeiss Axiophot microscope. Thin sections were cut at about 700 Å, mounted on grids, stained with uranyl acetate and lead citrate, and photographed on a Philips 300 electron microscope operated at 60 kV.
Tracer experiments.
Tracer experiments have been described previously (17). Briefly, EZ-link Sulfo-NHS-Biotin (Pierce Chemical Co., Illinois) was diluted to 2 mg/ml in PBS-1 mM CaCl2 and slowly injected into the distal colon for 3.5 min. Following this, 1 cm of colon, just cranial to the area contacting the needle, was removed and fixed in 3% paraformaldehyde in PBS for 3 h. The tissue was then washed in PBS and sectioned by Wax-It Histology Services, Inc. Tissue sections were incubated with a 1:500 dilution of streptavidin conjugated to Alexa 488 for 30 min at room temperature and imaged using a Zeiss Axiophot microscope. As controls tissue sections that were not pretreated with biotin were incubated with streptavidin to investigate endogenous biotin activity. These controls were negative (data not shown).
RESULTS
Tight junctions remain morphologically and functionally intact in the presence of A/E bacterially induced inflammation in vivo.
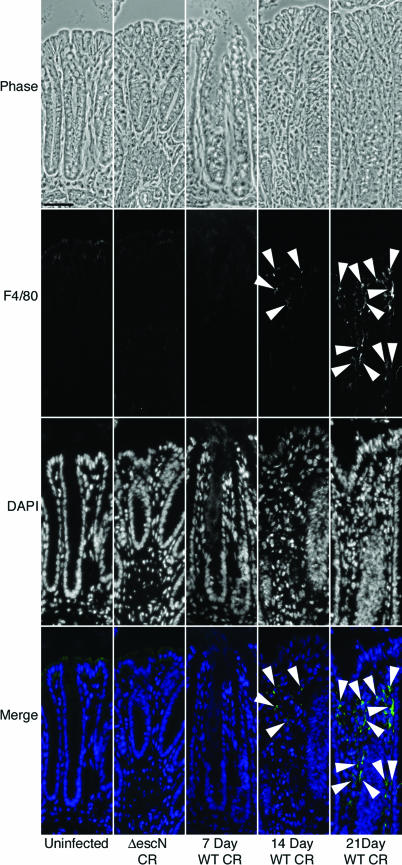
To assess what role the inflammatory response has on tight junction disruption caused by A/E pathogens, we infected mice with wild-type C. rodentium for 21 days and compared claudin-1, -3, and -5 localization on tissue sections with material from uninfected and 21-day ΔescN C. rodentium-infected mice as well as 7-day wild-type C. rodentium-infected tissue sections. The 21-day time point was studied because 21 days postinfection, C. rodentium is cleared by the animal (25), but significant inflammation persists. The TTSS mutant ΔescN C. rodentium is cleared by the animal by about day 7, does not attach to colonocytes, and, consequently, does not cause disease; an animal infected with ΔescN C. rodentium is indistinguishable from an uninfected animal (17). For these reasons, we use the ΔescN C. rodentium infection as a sham control. Following 21-day infection, localization of claudin-1, -3, and -5 in colonocytes appeared unaltered compared to uninfected or ΔescN C. rodentium-infected mice, whereas 7-day infections demonstrated extensive claudin redistribution away from the cell periphery (Fig. 1). The characteristic staining pattern remained along the lateral cell boundaries (Fig. 1, arrowheads). Wild-type C. rodentium-infected mice had inflamed colons, as demonstrated by increased cell infiltrate in the lamina propria (Fig. 1). The inflammatory cell infiltrate was confirmed by neutrophil and macrophage (F4/80) staining (Fig. 2 and 3).
FIG. 1.
Claudin-1, -3, and -5 and DAPI (DNA) localization on 21-day wild-type C. rodentium-infected mouse sections paired with 21-day ΔescN C. rodentium-infected tissue or uninfected tissue. Claudin staining of 7-day wild-type C. rodentium-infected tissue is also presented. Arrowheads indicate areas of staining at the lateral boundaries of colonocytes. The asterisks in the merged images indicate regions with high levels of cellular infiltrate in the lamina propria of 21-day wild-type C. rodentium-infected inflamed epithelium. WT, wild type; CR. C. rodentium. Scale bars, 50 μm (21-day micrographs) and 25 μm (7-day micrographs).
FIG. 2.
Neutrophil-labeled micrographs counterstained with hematoxylin. Neutrophil labeling on uninfected, 7-day ΔescN C. rodentium (ΔescN CR), or 7-day, 14-day or 21-day wild-type C. rodentium (WT CR) infections. Arrowheads indicate some of the neutrophils (pink staining). Increased numbers of neutrophils are present in the lamina propria of 14- and 21-day wild-type C. rodentium-infected colon sections compared to the uninfected, 7-day ΔescN C. rodentium-infected or 7-day wild-type C. rodentium-infected tissue. Scale bar, 50 μm.
FIG. 3.
Paired phase, F4/80 (macrophage marker), and DAPI micrographs of uninfected, ΔescN C. rodentium (ΔescN CR)-infected, and 7-, 14-, and 21-day wild-type C. rodentium (WT CR)-infected tissue. Positively labeled cells are not apparent in the lamina propria of uninfected, ΔescN C. rodentium-infected, or 7-day wild-type C. rodentium-infected colon sections. Positively labeled cells are present in the lamina propria of 14- and 21-day wild-type C. rodentium-infected tissue sections (arrowheads). Minimal nonspecific staining is present in the colonocytes and attached C. rodentium cells. Scale bar, 25 μm.
We then determined if the unaltered localization of the claudin proteins correlated with unaltered tight junction morphology. Careful comparison of colonic epithelial tight junction ultrastructure in 21-day wild-type C. rodentium-infected animals with that in mice infected with ΔescN C. rodentium (a sham infection; indistinguishable from uninfected tissue) indicated to us that there were no morphological differences with the tight junctions of 21-day wild-type-infected colonocytes, even though increased cellular infiltrate was observed in the lamina propria of wild-type-infected samples (Fig. 4).
FIG. 4.
Histologic and ultrastructure micrographs of infected tissue. The 21-day wild-type C. rodentium (WT CR)-infected tissue (B and B") is paired with 7-day ΔescN C. rodentium (ΔescN CR)-infected mouse tissue (A and A"). Toluidine blue (A and B) and electron micrographs (A′ and B′) of ΔescN and wild-type C. rodentium-infected tissue are shown. The A" and B" images are higher magnifications of the apical junction complexes in the A′ and B′ micrographs. The asterisk indicates a location of cell infiltration in the inflamed epithelium. TJ, tight junction; AJ, adherens junction; DS, desmosome. Scale bars, 50 μm (A), 1.0 μm (A′), and 0.2 μm (A").
Tight junctions maintain barrier function during A/E pathogen-induced inflammation.
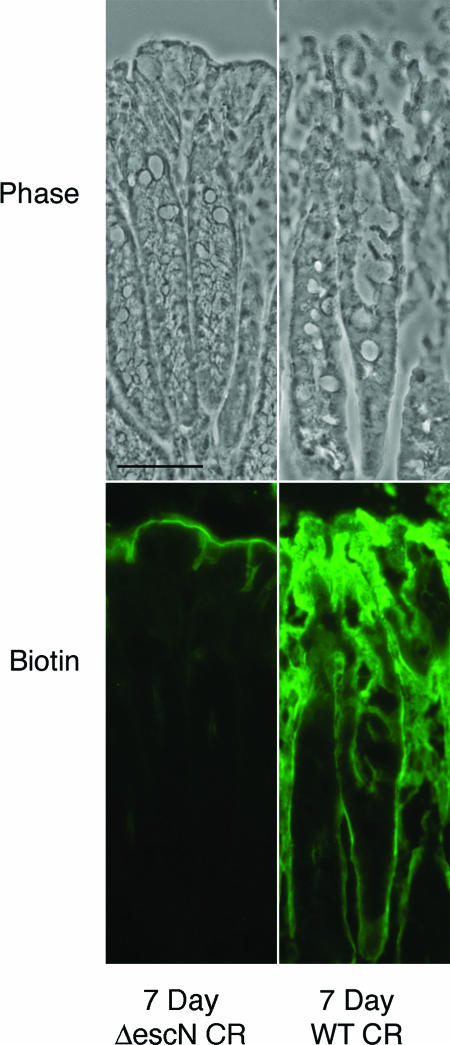
Tight junctions in the murine colon were assessed for functional integrity following 21-day infection with C. rodentium. The aim of this was to determine if the unaltered localization of claudin proteins corresponds to functionally intact tight junctions. We injected a molecular tracer (biotin) into the distal colon of 21-day wild-type C. rodentium-infected mice and compared these results to ΔescN C. rodentium-infected mice. We have used this technique previously to demonstrate that tight junctions are disrupted in the presence of wild-type C. rodentium at 7 days postinfection, when inflammatory cells are not evident in the lamina propria (17) (Fig. 5).
FIG. 5.
Barrier permeability micrographs of 7-day C. rodentium infections. Phase images of 7-day ΔescN C. rodentium (ΔescN CR)-infected and 7-day wild-type C. rodentium (WT CR)-infected tissue pretreated with a biotin tracer to assess barrier permeability. Biotin is held to the luminal border in ΔescN C. rodentium-infected tissue but permeates the epithelium into the lamina propria in 7-day wild-type C. rodentium-infected tissue. Scale bar, 50 μm.
Following biotin treatment of 21-day wild-type C. rodentium-infected animals, the biotin tracer lined the luminal boundary of the colonic epithelium and did not penetrate into the tissue (Fig. 6). This indicates that the tight junctions during the inflammatory events caused by A/E pathogen infection are intact and maintain the epithelial barrier (Fig. 6). No specific tissue labeling was present in controls (data not shown).
FIG. 6.
Barrier permeability micrographs. (A) Phase images of low magnification of 21-day ΔescN C. rodentium (ΔescN CR)-infected and wild-type C. rodentium (WT CR)-infected tissue pretreated with a biotin tracer to assess barrier permeability. Biotin is held to the luminal border in both ΔescN C. rodentium-infected and wild-type C. rodentium-infected tissue. (B) Higher magnification of biotin- and DAPI-treated murine tissue that was infected with ΔescN C. rodentium or wild-type C. rodentium for 21 days. Double-headed arrows demonstrate the difference in crypt depth between ΔescN C. rodentium-infected and wild-type C. rodentium-infected tissue to indicate that the disease phenotype has not resolved at this time point. The asterisks in the DAPI and merged images indicate some of the regions with high levels of cellular infiltrate in the 21-day wild-type C. rodentium-infected inflamed epithelium. Arrowheads point to regions with normal cellular load in the lamina propria. Scale bars, 100 μm (low magnification) and 50 μm (high magnification).
Tight junctions are altered by C. rodentium intimately attached to colonocytes in vivo.
To investigate whether or not the intimate attachment of C. rodentium to colonocytes is a prerequisite for tight junction disruption, murine colon sections from mice 14 days after C. rodentium infection were labeled with antibodies to claudin-3. Claudin-3 staining was pursued because of the superiority of the antibody over claudin-1 or -5 antibodies used in this study. We previously have shown that the appropriate localization of claudin-3 at the cell boundaries in colonocytes corresponds to intact tight junctions whereas the altered localization of claudin-3 corresponded to functional tight junction deficiencies in the colon (17).
At 14 days after infection, bacterial colonization and inflammation are at high levels. Claudin-3 localization was disrupted only in colonocytes that had intimately attached bacteria (Fig. 7). At areas of the same tissue devoid of bacteria, claudin-3 localization was unaffected (Fig. 7). These results further confirm our findings that tight junction disruption is unaffected by the inflammatory response during A/E bacterial infection and that any tight junction alteration is due to the direct contact of the pathogen to the host's cells (Fig. 7). Additionally, this evidence demonstrates that other host cell mediators likely do not alter tight junctions during in vivo A/E pathogen infections.
FIG. 7.
(A) Paired phase, C. rodentium Tir, and DAPI micrographs of infected tissue. Tir and DAPI colocalization demonstrates that bacteria labeled by DAPI in infected tissue sections is C. rodentium. Scale bar, 50 μm. (B) Paired phase, claudin-3, and DAPI micrographs of uninfected and 14-day wild-type C. rodentium (WT CR)-infected tissue. Arrows indicate regions of the epithelium without bacterial attachment that label the lateral boundaries of colonocytes. Arrowheads and white asterisks identify regions with bacterial colonization and direct attachment to colonocytes. All infected regions have altered localization of claudin-3. The black asterisks in the DAPI and merged images indicate areas with high levels of cellular infiltrate. Scale bar, 50 μm.
DISCUSSION
We have previously demonstrated that uninfected and ΔescN C. rodentium-infected mice display indistinguishable claudin phenotypes whereas wild-type C. rodentium infections cause overt tight junction disruption between infected colonocytes of mice that are exposed for 7 days. At this time point postinoculum, inflammatory cell infiltrate in the lamina propria of infected mice is not evident. However, 21 days after wild-type C. rodentium infection, inflammation persists in the absence of colonized bacteria. The temporal separation of inflammation from A/E bacterial colonization at 21 days provides a model to study the effects of bacterial colonization versus inflammation in vivo. We demonstrate here, morphologically and by the localization of claudin proteins, that tight junctions are unaltered in the presence of inflammatory cells during the inflammatory response caused by C. rodentium infection. These findings correspond to a functionally intact epithelial barrier.
Thus far, only one study has explored the role of A/E pathogen-induced inflammation on tight junctions (39). In that study, the MAP or ERK kinase inhibitor PD-98059 was used on T84 cells that were subsequently infected with EPEC. Although these kinases are capable of activating inflammatory cascades, they can also have direct effects on tight junctions (2, 21, 49) and can influence numerous other events such as cell proliferation, protection against apoptosis, and cell survival (5, 20, 29, 33, 44). Results from the Savkovic (39) study found equivalent decreases in TER caused by EPEC on cultured T84 monolayers in the presence or absence of PD-98059. This suggested to the authors that the relationship of cytokine release to tight junction alteration was not present because TER was still decreased in cells infected in the presence PD-98059. Although this study used an inhibitor that can directly act on tight junctions (2, 21, 49), the effects of both MAP and ERK in vivo are an area that should still be pursued in vivo.
Recently, a second mouse model has emerged for the study of A/E pathogen infections in vivo (40). In this model, the human A/E pathogen EPEC was used to infect mice. During infection, inflammation is minimal, and bacterial counts have been reported in various parts of the intestine at about 104 CFU (40). These counts correspond to those of C. rodentium lacking a functional type III secretion system (ΔescN), which does not colonize the colon, and signify extremely low levels of colonization of EPEC in mice. A recent study by this group using the EPEC murine model (42) suggested that tight junctions can be disrupted in vivo in a TTSS-dependent manner by EspF, but the presented photographic evidence indicated the absence of any bacteria intimately attached to the cells. Although this suggested that potential mediators might be released from infected host cells to influence tight junctions in noninfected cells, our evidence demonstrates that intimate contact of the bacteria to cells is essential for barrier alteration to occur.
Here, we present evidence that the inflammatory response caused by C. rodentium has no discernible effect on morphology or the barrier function of tight junctions. We also demonstrate that the alteration of claudin-3 localization in the infected murine colon is completely dependent on the intimate attachment of the pathogen to the colonic enterocytes. Our evidence further accentuates the importance of using relevant in vivo studies and infection models to assess the role of pathogenic infections on tissues.
Acknowledgments
The authors thank Wanyin Deng, Bruce Vallance, and Bryan Coburn for their critical review of the manuscript and Guntram Grassel for his technical assistance.
J.A.G. is a CAG/CIHR/AstraZeneca and MSFHR Postdoctoral Fellow. B.B.F. is a Howard Hughes International Research Scholar, a CIHR Distinguished Investigator, and the UBC Peter Wall Distinguished Professor. This study was funded through operating grants from the CIHR and the HHMI.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Barmeyer, C., M. Harren, H. Schmitz, U. Heinzel-Pleines, J. Mankertz, U. Seidler, I. Horak, B. Wiedenmann, M. Fromm, and J. D. Schulzke. 2004. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G244-G252. [DOI] [PubMed] [Google Scholar]
- 2.Basuroy, S., A. Seth, B. Elias, A. P. Naren, and R. Rao. 2006. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 393:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canil, C., I. Rosenshine, S. Ruschkowski, M. S. Donnenberg, J. B. Kaper, and B. B. Finlay. 1993. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect. Immun. 61:2755-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravortty, D., and K. S. Kumar. 1999. Interaction of lipopolysaccharide with human small intestinal lamina propria fibroblasts favors neutrophil migration and peripheral blood mononuclear cell adhesion by the production of proinflammatory mediators and adhesion molecules. Biochim. Biophys. Acta 1453:261-272. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 6.Dean, P., and B. Kenny. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 54:665-675. [DOI] [PubMed] [Google Scholar]
- 7.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanning, A. S., B. J. Jameson, L. A. Jesaitis, and J. M. Anderson. 1998. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273:29745-29753. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar, M. G., and G. E. Palade. 1963. Junctional complexes in various epithelia. J. Cell Biol. 17:375-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse, M., K. Fujimoto, N. Sato, T. Hirase, and S. Tsukita. 1996. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J. Cell Sci. 109:429-435. [DOI] [PubMed] [Google Scholar]
- 12.Furuse, M., M. Hata, K. Furuse, Y. Yoshida, A. Haratake, Y. Sugitani, T. Noda, A. Kubo, and S. Tsukita. 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156:1099-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuse, M., T. Hirase, M. Itoh, A. Nagafuchi, S. Yonemura, and S. Tsukita. 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Gassler, N., C. Rohr, A. Schneider, J. Kartenbeck, A. Bach, N. Obermuller, H. F. Otto, and F. Autschbach. 2001. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G216-228. [DOI] [PubMed] [Google Scholar]
- 14.Gitter, A. H., K. Bendfeldt, H. Schmitz, J. D. Schulzke, C. J. Bentzel, and M. Fromm. 2000. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann. N. Y. Acad. Sci. 915:193-203. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Mariscal, L., A. Betanzos, P. Nava, and B. E. Jaramillo. 2003. Tight junction proteins. Prog. Biophys. Mol. Biol. 81:1-44. [DOI] [PubMed] [Google Scholar]
- 16.Gow, A., C. M. Southwood, J. S. Li, M. Pariali, G. P. Riordan, S. E. Brodie, J. Danias, J. M. Bronstein, B. Kachar, and R. A. Lazzarini. 1999. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99:649-659. [DOI] [PubMed] [Google Scholar]
- 17.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell Microbiol. 8:634-645. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291:469-477. [DOI] [PubMed] [Google Scholar]
- 19.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 20.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 21.Lipschutz, J. H., S. Li, A. Arisco, and D. F. Balkovetz. 2005. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J. Biol. Chem. 280:3780-3788. [DOI] [PubMed] [Google Scholar]
- 22.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 23.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell Microbiol. 7:1697-1706. [DOI] [PubMed] [Google Scholar]
- 26.Muza-Moons, M. M., E. E. Schneeberger, and G. A. Hecht. 2004. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 6:783-793. [DOI] [PubMed] [Google Scholar]
- 27.Nagai, T., A. Abe, and C. Sasakawa. 2005. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280:2998-3011. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida, E., and Y. Gotoh. 1993. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18:128-131. [DOI] [PubMed] [Google Scholar]
- 30.Nitta, T., M. Hata, S. Gotoh, Y. Seo, H. Sasaki, N. Hashimoto, M. Furuse, and S. Tsukita. 2003. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nougayrede, J. P., and M. S. Donnenberg. 2004. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 6:1097-1111. [DOI] [PubMed] [Google Scholar]
- 32.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell Microbiol. 5:359-372. [DOI] [PubMed] [Google Scholar]
- 33.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 34.Prasad, S., R. Mingrino, K. Kaukinen, K. L. Hayes, R. M. Powell, T. T. MacDonald, and J. E. Collins. 2005. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 85:1139-1162. [DOI] [PubMed] [Google Scholar]
- 35.Rahner, C., L. L. Mitic, and J. M. Anderson. 2001. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411-422. [DOI] [PubMed] [Google Scholar]
- 36.Saitou, M., K. Fujimoto, Y. Doi, M. Itoh, T. Fujimoto, M. Furuse, H. Takano, T. Noda, and S. Tsukita. 1998. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141:397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou, M., M. Furuse, H. Sasaki, J. D. Schulzke, M. Fromm, H. Takano, T. Noda, and S. Tsukita. 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11:4131-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 39.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 40.Savkovic, S. D., J. Villanueva, J. R. Turner, K. A. Matkowskyj, and G. Hecht. 2005. Mouse model of enteropathogenic Escherichia coli infection. Infect. Immun. 73:1161-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma, R., S. Tesfay, F. L. Tomson, R. P. Kanteti, V. K. Viswanathan, and G. Hecht. 2006. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli (EPEC) on intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G685-G694. [DOI] [PubMed] [Google Scholar]
- 42.Shifflett, D. E., D. R. Clayburgh, A. Koutsouris, J. R. Turner, and G. A. Hecht. 2005. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab. Investig. 85:1308-1324. [DOI] [PubMed] [Google Scholar]
- 43.Simonovic, I., J. Rosenberg, A. Koutsouris, and G. Hecht. 2000. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2:305-315. [DOI] [PubMed] [Google Scholar]
- 44.Sturgill, T. W., and J. Wu. 1991. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim. Biophys. Acta 1092:350-357. [DOI] [PubMed] [Google Scholar]
- 45.Turksen, K., and T. C. Troy. 2004. Barriers built on claudins. J. Cell Sci. 117:2435-2447. [DOI] [PubMed] [Google Scholar]
- 46.Turner, J. R., J. M. Angle, E. D. Black, J. L. Joyal, D. B. Sacks, and J. L. Madara. 1999. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am. J. Physiol. 277:C554-C562. [DOI] [PubMed] [Google Scholar]
- 47.Vallance, B. A., W. Deng, M. De Grado, C. Chan, K. Jacobson, and B. B. Finlay. 2002. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen Citrobacter rodentium in infected mice. Infect. Immun. 70:6424-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viswanathan, V. K., A. Koutsouris, S. Lukic, M. Pilkinton, I. Simonovic, M. Simonovic, and G. Hecht. 2004. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect. Immun. 72:3218-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., J. Zhang, X. J. Yi, and F. S. Yu. 2004. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp. Eye Res. 78:125-136. [DOI] [PubMed] [Google Scholar]
- 50.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]
- 51.Zeissig, S., C. Bojarski, N. Buergel, J. Mankertz, M. Zeitz, M. Fromm, and J. D. Schulzke. 2004. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut 53:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]