Abstract
The encounter between invading microorganisms and dendritic cells (DC) triggers a series of events which include uptake and degradation of the microorganism, induction of a maturation process, and enhancement of DC migration to the draining lymph nodes. Various pathogens have developed strategies to counteract these events as a measure to evade the host defense. In the present study we found that interaction of the Yersinia pestis EV76 strain with DC has no effect on cell viability and is characterized by compliance with effective maturation, which is manifested by surface display of major histocompatibility complex class II, of costimulatory markers, and of the chemokine receptor CCR7. This is in contrast to maturation inhibition and cell death induction exerted by the related species Yersinia enterocolitica WA O:8. Y. pestis interactions with DC were found, however, to impair functions related to cytoskeleton rearrangement. DC pulsed with Y. pestis failed to adhere to solid surfaces and to migrate toward the chemokine CCL19 in an in vitro transmembrane assay. Both effects were dependent on the presence of the pCD1 virulence plasmid and on a bacterial growth shift to 37°C prior to infection. Moreover, while instillation of a pCD1-cured Y. pestis strain into mouse airways triggered effective transport of alveolar DC to the mediastinal lymph node, instillation of Y. pestis harboring the plasmid failed to do so. Taken together, these results suggest that virulence plasmid-dependent impairment of DC migration is the major mechanism utilized by Y. pestis to subvert DC function.
Dendritic cells (DC) play a key role at the interface of innate and adaptive immunity (1, 36, 49). Interactions between DC and bacterial pathogens are therefore important in determining the outcomes of the infectious processes.
DC reside in peripheral epithelial tissues in an immature state, where they serve as sentinels for invading microorganisms. Contact with the microorganism triggers a series of programmed events which begin by pathogen internalization and a concomitant stimulation of pattern recognition receptors such as Toll-like receptors, which subsequently leads to conversion of the immature DC into mature DC. Maturation is marked by a reduction in phagocytic ability, an increase in surface expression of major histocompatibility complex (MHC) class II and costimulatory molecules, and production of proinflammatory cytokines. This occurs together with degradation of the pathogen within the phagosome and presentation of microbial antigens on MHC molecules. Maturation is also characterized by changes in the repertoire of chemokine receptor expression, including the up-regulation of CCR7, which facilitates migration to the local lymph node (20, 59). This in turn allows DC to activate T cells in an MHC-specific manner and thus initiate an immune response, before undergoing apoptosis in the lymph node.
Many pathogens have developed a variety of mechanisms to modify DC functions as a means to disarm the host defense (43, 45). Bacteria such as Coxiella burnetii (57), and Legionella pneumophila (29) were found to impair DC maturation. Other bacteria promote premature apoptosis of DC (Streptococcus pneumoniae [10]) or inhibit cytokine secretion (Bacillus anthracis [7]). Moreover, certain intracellular bacteria, such as Brucella and Chlamydia, can use DC as their replication site and thus enhance invasiveness (4, 21). All this suggests that successful colonization of a host by a pathogen often entails interference with DC functions, as a way to subvert innate and adaptive immunity. Nevertheless, little is known about the interrelationship between DC and one of the most infectious bacterial agents, Yersinia pestis.
Y. pestis belongs to the genus Yersinia which includes two other human pathogens, Y. enterocolitica and Y. pseudotuberculosis. These three Yersinia species are closely related genetically yet differ significantly in their pathogenesis in humans. Infection by Y. pestis, the etiological agent of plague, leads to fatal bubonic or pneumonic plague. Y. enterocolitica and Y. pseudotuberculosis, on the other hand, cause mild and self-limiting gastrointestinal syndromes (8, 44).
In spite of the differences in pathogenesis, certain mechanisms for evading the host innate immunity are shared by all three Yersinia species. These mechanisms are governed by genes encoded on a ∼70-kb plasmid present in all three pathogenic Yersinia spp. (named pCD1 in Y. pestis and pYV in Y. enterocolitica and Y. pseudotuberculosis). The 70-kb plasmids, which are essential for virulence (2, 17), encode a type III secretion system (TTSS) comprised of a secretion apparatus, chaperones, and several secreted effectors (Yops) (for reviews see references 12, 39, 40, and 65). Yersinia TTSS expression is induced by temperature (shift to 37°C) and cell host contact, both of which lead to the transport of Yops from the bacterium into the host cell cytosol (50, 61).
Among the most pronounced effects of Yop translocation is the disruption of the signaling network and of cytoskeleton rearrangement, which are necessary for phagocytosis. These effects involve the action of several Yops, including YopE, YopH, YopO/YpkA, and YopT (11, 65). Another effector, designated YopP in Y. enterocolitica and YopJ in Y. pseudotuberculosis and Y. pestis, is involved in suppression of cytokine production and inhibition of mitogen-activated protein kinase and NF-κB pathways by preventing the activation of IKKβ and MKKs (41, 42, 51, 54, 67). In addition, YopP/YopJ of Y. enterocolitica and Y. pseudotuberculosis were implicated in induction of apoptosis in infected cells (37, 38, 52). In a recent study (67), however, we have demonstrated that Y. pestis is limited in its ability to confer programmed death in infected macrophages. This is attributed to shortcomings in the translocation of YopJ from Y. pestis to the target cell.
It should be noted that most studies on the virulence mechanisms of Yersinia are based on studies with infected macrophages, while research on the interaction of Yersinia and DC (6, 15, 30, 53, 55, 56, 64) is lagging behind. Interaction between murine bone marrow-derived DC (BMDC) and Y. enterocolitica was found recently to entail an array of adverse effects on DC functions (15, 64), some of which were not reported in a previous study conducted with human monocyte-derived DC (55). More recently, infection of murine alveolar DC with Y. pestis was not found to impair DC functions (6). However, a pCD1-deficient strain was used in that study, and adverse effects are not expected.
In this study we analyzed various effects of the Y. pestis EV76 strain, which carries pCD1, on DC functions, including effects on cell viability, effects on expression of costimulatory molecules and of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), and effects on the ability of DC to migrate. These analyses reveal a complex interrelationship between DC and Y. pestis, encompassing favorable as well as adverse effects of the plague bacterium on DC function. The most pronounced effect appears to be the paralysis of DC movement, which could be destructive to the function of the DC in presentation of Y. pestis antigens.
MATERIALS AND METHODS
Bacterial strains and cultivation.
Yersinia strains used in this study are listed in Table 1, and growth conditions are described elsewhere (19, 22). For cell infection experiments, bacteria were grown at 28°C on heart infusion broth overnight in a gyratory shaker. Bacteria were then diluted into heart infusion broth medium to an optical density at 660 nm of 0.1 and grown for additional 2.5 h at 28°C or 37°C at 100 rpm. Bacteria were washed and then resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The density was adjusted to the desired concentration for infection of eukaryotic cells.
TABLE 1.
Bacterial strains used in this study
| Species and strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Y. pestis | ||
| EV76 | Δpgm (Girard's vaccine strain) | 2 |
| EV76Δ70 | Spontaneously pCD1-cured EV76 | 19 |
| EV76GFP | EV76 carrying pGFPuv (Clontech) | 3 |
| EV76ΔyopJ +pyopP | yopJ-deleted EV76 expressing YopP of Y. enterocolitica WA 0:8 | 67 |
| Y. enterocolitica | ||
| ATCC 27729 | Virulent strain; WA 0:8 | ATCC |
| Δ70 | Spontaneously pYV-cured WA 0:8 | 34 |
BMDC preparation.
DC derived from bone marrow of BALB/c mice were grown in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1% (vol/vol) MEM-Eagle nonessential amino acid solution, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol (all components supplied by Biological Industries, Beit Haemek, Israel).
DC were prepared essentially according to the method developed by Lutz et al. (33). Briefly, bone marrow cells were flushed from femurs of 6- to 10-week-old male mice. Cells were passed through a cell restrainer, washed in phosphate-buffered saline (PBS), and resuspended in medium containing 50 ng/ml recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (R&D Systems) to a concentration of 1 × 106 to 1.5 × 106 cells/ml. Ten-milliliter aliquots of cell suspension were seeded into 100-mm petri dishes (Falcon 351029). After 3 days, an additional 10 ml of fresh medium containing 40 ng/ml rmGM-CSF was added. Three days later, half of the culture supernatant was collected by centrifugation, and the cell pellet was resuspended in 10 ml of fresh medium containing 20 ng/ml rmGM-CSF and added back to the culture. At day 8 nonadherent and slightly adherent cells were harvested by pooling cell supernatant and collecting cells by a gentle wash. Cells were centrifuged, resuspended in fresh medium containing 10 ng/ml rmGM-CSF but lacking antibiotics, and seeded onto 100-mm cell culture dishes (Falcon 353003). Nonadherent cells were collected on day 9 and concentrated by centrifugation and resuspension in growth medium containing 10 mM HEPES buffer to a suspension of 106 cells/ml to be used in the various experiments. More than 90% of the cells were CD11c positive as assessed by flow cytometry, indicating effective differentiation into DC. Light microscopy revealed the characteristic dendritic morphology as well as cell clustering.
Cell infection.
Cells (106 cells/well in 24-well plates) were infected by pulsing with bacteria at a multiplicity of infection (MOI) of 5 to 50. Infection was initiated by spinning (140 × g, 5 min) bacteria onto cells (this is referred to as the 0-h time point of infection). Pulsed cells were incubated at 37°C with 5%CO2 for 1 h before addition of gentamicin (Sigma) at the indicated concentration. Incubation was continued for an additional 1 to 23 h (depending on the specific protocols) before the cells were used for the various experiments. In an alternative protocol, bacteria were removed 1 h after gentamicin addition by spinning (140 × g, 10 min), followed by two PBS washes. Fresh medium containing gentamicin was then added, and incubation was continued. Parallel pulsing with 1 μg/ml of Escherichia coli 068:B6 lipopolysaccharide (LPS) (Sigma) served in many of the experiments as a positive control for DC activation.
Analysis of DC viability.
DC were stained with annexin V-fluorescein and propidium iodide (PI) by using the annexin V FLUOS staining kit from Roche (Penzberd, Germany) according to the protocol recommended by the manufacturers.
Surface marker staining.
For evaluation of surface marker expression, washed DC were incubated with fluorescein isothiocyanate-, phycoerythrin-, or allophycocyanin-conjugated antibodies against CD11c (clone N418), I-A/I-E MHC class II (clone M5/114.15.2), CD40 (clone 1C10) CD54 (clone YN1/1.7.4), CD80 (clone 16-10A1), CD83 (clone Michel-17), CD86 (clone GL1), and CCR7 (clone 4B12), as well as with the appropriate isotype-matched control antibodies. Reagents were purchased from eBioscience (San Diego CA). Staining was performed using standard incubation protocols (30 min at 4°C), except for CCR7 staining, which was conducted at 37°C. Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson), using the Cell Quest Pro software. Cell remains were excluded from the analysis by gating at the appropriate forward and side scatters.
TNF-α production.
The concentration of TNF-α secreted into the cell medium was determined by enzyme-linked immunosorbent assay, using the DuoSet mouse TNF-α immunoassay system (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Adhesion assay.
Adhesion of DC to plastic surfaces was assayed essentially as described by Waltzer et al. (66). DC were cultured (105 cells/well) in 96-well plates (TPP, Switzerland). Cells were pulsed either with LPS (1 μg/ml) or with bacteria at an MOI of 50. After the indicated period of time, medium was removed from wells. Nonadherent cells were gently washed away with PBS, and plates were frozen at −70°C. The plates were then thawed, and the amount of adhered cells was evaluated by measuring lactate dehydrogenase (LDH) released after cell lysis. For LDH determination we used the Cytotox 96 cytotoxicity assay (Promega, Madison, WI) according to the manufacturer's recommendations.
In vitro transmigration assay.
Infected cells were resuspended in RPMI containing 1% FCS to a concentration of 107 cells/ml and were placed (106 cells/well) in the upper compartments of Transwell migration chambers (Costar 3421). CCL19 (200 ng/ml; R&D Systems) diluted in RPMI plus 1% FCS was placed in the lower compartments to induce CCR7-dependent chemotaxis. Medium alone served to evaluate CCR7-independent migration. DC were allowed to migrate through polycarbonate (5-μm-pore-size mesh) at 37°C for 2 h, and cells migrating to the lower compartment were counted under a light microscope. The experiments were performed in triplicate, and migration was determined by calculating the percentage of migrating cells relative to input.
In vivo migration of respiratory DC.
Migration of alveolar DC to the peripheral lymph node was evaluated by adapting previously published protocols (31, 58). Briefly, the orange cell tracer CMTMR (Molecular Probes) was diluted in RPMI to a concentration of 8 mM, and 30 μl/mouse was administered intranasally to mice anesthetized with ketamine/xylazine. Five hours later mice were instilled with 5 × 106 bacteria per mouse (50 μl of bacterial suspension in PBS). At 18 h after addition of bacteria, mediastinal lymph nodes were isolated, shredded, and treated with 1 mg/ml collagenase D (Roche) for 30 min at 37°C. Cells were then passed through a 70-μm nylon mesh to generate a single-cell suspension. Cells were stained with allophycocyanin-conjugated CD11c (clone N418; eBioscience) and analyzed by flow cytometry analysis, and statistics were performed on 50,000 collected event.
RESULTS
Induction of DC death by Y. pestis is limited compared to that by Y. enterocolitica.
In a recent study (67), we have demonstrated that unlike Y. enterocolitica, which triggers programmed death in target cells (15, 37, 38, 52), Y. pestis is not effective in inducing apoptosis in infected macrophages. To examine the effects of the plague bacterium on DC viability, infection with the prototype Y. pestis vaccine strain EV76 was compared to infection with Y. enterocolitica. We used either strains which carry the corresponding virulence plasmids (pCD1 in EV76 and pYV in Y. enterocolitica WA 0:8) or their plasmid-cured derivatives (Table 1).
Infection conditions were designed to reflect early events of natural infection, where bacteria propagating in the flea gut at low temperature are exposed to mammalian body temperature. Accordingly, in most experiments we used bacteria pregrown at 28°C and shifted to 37°C for 2.5 h. Under these conditions, Yops are expressed and primed for translocation into phagocytic cells, yet bacteria do not carry the F1 capsule (67), which is known to inhibit Y. pestis phagocytosis (14).
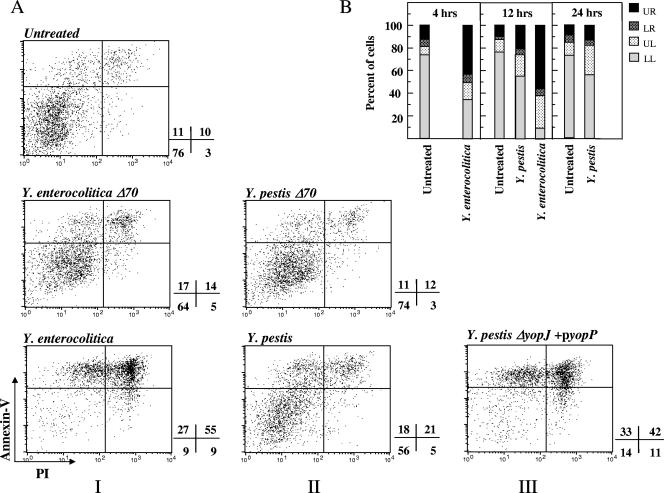
Murine bone marrow DC were pulsed with the various Yersinia strains and analyzed 12 h later for their ability to incorporate PI as a measure of membrane damage and to bind annexin V as a measure of accessibility to inner membrane phosphatidylserine. Both assays are markers for cell death, and the latter could also be correlated with apoptosis. Flow cytometry analysis of Y. enterocolitica-infected cells (Fig. 1A, column I) revealed that 55% of the Y. enterocolitica-infected cells were annexin+/PI+ and 27% were annexin+/PI−, while only 9% remained annexin−/PI−. In contrast, cells infected with Y. enterocolitica Δ70 exhibited a permeability profile (64% annexin−/PI− cells) similar to that of uninfected cells. Thus, Y. enterocolitica infection appears to trigger strong pYV-dependent cell death, as demonstrated previously (15).
FIG. 1.
Effect of Yersinia infection on DC viability. BMDC were pulsed at an MOI of 20 with the indicated bacterial strain by centrifugation. Gentamicin (20 μg/ml) was added 1 h later, and incubation was carried out for 4, 12, or 24 h. Cells were then stained with annexin V conjugated to Alexa 568 and propidium iodide and analyzed by flow cytometry. (A) Cell death at 12 h postinfection is expressed in the form of dot plots, and numbers adjacent to plots indicate the percentage of cells in each quadrant. (B) Summary of cell death data at 4, 12, and 24 h postinfection, presented as bar diagrams depicting the ratio between the various quadrants (UR, annexin+/PI+; LR, annexin−/PI+; UL, annexin+/PI−; LL, annexin−/PI−). Results are representative of three independent experiments.
Results obtained with Y. pestis-infected DC were markedly different (Fig. 1A, column II). Only 21% of the Y. pestis-infected cells stained annexin+/PI+ and 18% were annexin+/PI− (the corresponding background levels were 10 and 11%, respectively), while 56% were annexin−/PI−. The effects obtained with the plasmid-cured strain were again similar to background levels, as expected.
To characterize the kinetics of the cytotoxic process, membrane functions of infected DC were examined at 4 h and 24 h (Fig. 1B) in addition to 12 h postinfection. In the case of Y. enterocolitica, cell death was manifested already after 4 h, as reported previously (15). After pulsing with Y. pestis, close to 60% of cells appeared to be intact as late as 24 h postinfection, resembling values obtained after 12 h. Thus, a substantial fraction of DC remains viable for a rather prolonged period of time after infection with Y. pestis.
Taken together, these observations indicate that the limitation in Y. pestis-induced cell death, demonstrated previously in macrophages (67), is also valid in DC. In our previous study, we have shown that this limitation is due to an intrinsic handicap in the ability of the Y. pestis effector YopJ to translocate into the target cell. We have also shown that the cytotoxic potential of Y. pestis can be restored by replacing YopJ with the homologous effector YopP from Y. enterocolitica through the use of an engineered Y. pestis ΔyopJ+pyopP strain (67). We now show that this same bacterial strain can restore cell death induction in infected DC (Fig. 1A, column III), leading to generation of 42% annexin+/PI+ and 33% annexin+/PI− cells at 12 h postinfection (reintroduction of yopJ on a ΔyopJ background failed to do so [not shown]). Thus, 75% of cells interacting with Y. pestis ΔyopJ+pyopP exhibit positive staining with annexin V, compared to 39% and 83% of cells interacting with Y. pestis and Y. enterocolitica, respectively. This result suggests that the control of Y. pestis-induced cell death (67) in both macrophages and DC depends on common mechanisms.
Infection of DC by Y. pestis promotes up-regulation of costimulatory surface molecule expression.
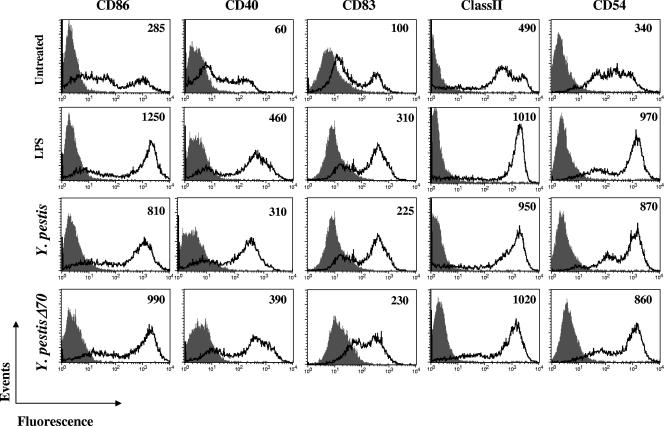
Maturation of DC following stimulus by pathogen-derived products usually triggers an increase in the expression of surface molecules which contribute to immunological synapse formation. To examine the effects of Y. pestis on this process, infected cells were examined by flow cytometry, at 24 h after pulsing, for expression of the costimulatory molecules CD86, CD40, CD83, and CD54 as well as MHC class II (Fig. 2). Pulsing of cells with Y. pestis resulted in a marked increase in surface expression of all markers examined. The levels of expression were similar to those achieved with the standard stimulant LPS. Notably, no marked difference was observed between cells pulsed with Y. pestis bearing the virulence plasmid pCD1 and cells pulsed with Y. pestis Δ70, which is cured of this plasmid. Moreover, a very similar profile of up-regulated expression was observed when cells were treated with killed bacteria (not shown).
FIG. 2.
Expression of costimulatory molecule and MHC class II on DC infected with Y. pestis. Cells were pulsed by centrifugation with LPS or with bacteria (shifted to grow at 37°C) at an MOI of 50. One hour later, 20 μg/ml gentamicin was added and cells were incubated for 23 h. Staining for the indicated surface markers and flow cytometry were performed as described in Materials and Methods. Gray areas denote the isotype-matched immunoglobulin control. Mean fluorescence intensities for surface marker staining are given at the right top corner of each diagram. Mean fluorescence intensities for isotype controls in all experiments presented in this figure ranged between 3 (class II) and 50 (CD83) and are therefore not presented. Three independent experiments exhibited similar results. Very similar results were obtained with an alternative infection protocol in which bacteria were washed away at 1 h after pulsing and cells were incubated in gentamicin-containing fresh medium for 23 h. In all these experiments optimal activation was observed at an MOI of 50, yet the same pattern of up-regulation was also obtained at an MOI of 20.
These results show that in Y. pestis, virulence plasmid-related functions do not impair DC maturation, and they thereby differ markedly from results obtained in a study with Y. enterocolitica WA 0:8-infected DC (15). In order to validate these differences, we conducted a side-by-side comparison of the two Yersinia species (Fig. 3). While 24 h is considered as the optimal time for analysis of surface markers, the rapid death of Y. enterocolitica-infected DC led us to perform the analysis at 4 h postinfection, a time period used previously in this type of analysis (15). The CD54 marker was used for the comparison, as its up-regulation upon stimulus occurs rather rapidly. The results presented in Fig. 3 underline the differences between the two Yersinia species. Y. pestis triggers effective up-regulation of CD54 expression even 4 h after pulsing, and this up-regulation can occur also in the presence of the pCD1 virulence plasmid. Y. enterocolitica, on the other hand, suppresses surface display of CD54 in a virulence plasmid-dependent manner.
FIG. 3.
Expression of CD54 on DC infected with Y. pestis or Y. enterocolitica strains. Cells were pulsed with the indicated bacterial strains as described in the legend to Fig. 2, except that incubation was terminated at 4 h postinfection. Mean fluorescence intensities for surface marker staining are given at the right top corner of each diagram. Mean fluorescence intensities for isotype controls ranged from 4.5 to 5.5. Three independent experiments exhibited similar results.
This difference can again be attributed to differences in YopJ/YopP functions of the two Yersinia spp. As in the case of cell death induction (Fig. 1A, column III), impairment of marker display can be bestowed on Y. pestis by expressing YopP of Y. enterocolitica in Y. pestis through the use of the engineered Y. pestis ΔyopJ(pyopP) strain (not shown).
The fact that two functions attributed to YopJ, impairment of surface marker display and induction of cell death, are not executed in Y. pestis-infected DC led us to examine TNF-α production by infected cells. Suppression of the induction of TNF-α expression is one of the hallmarks of the YopJ/YopP-dependent anti-inflammatory activities of Yersinia (5, 42). Infection of DC with Y. pestis Δ70 can effectively stimulate TNF-α secretion from DC, yet pulsing by Y. pestis which does carry the virulence plasmid results in a ∼7-fold reduction in the amount of secreted cytokine (Fig. 4). This variance between the two strains occurs only when bacteria are shifted to 37°C. In bacteria cultivated at 28°C, similar high induction levels can be observed.
FIG. 4.
Secretion of TNF-α by DC infected with Y. pestis. Cells were pulsed with the indicated bacterial strains at an MOI of 5 (bacteria were grown for 2.5 h at the indicated temperature prior to infection) or with LPS. One hour later, 20 μg/ml gentamicin was added and cells were incubated for 23 h. TNF-α was quantified in cell medium as described in Materials and Methods. Each experiment was performed in triplicate, and data represent averages and standard deviations. Two additional experiments performed under similar conditions and one experiment conducted at an MOI of 20 exhibited similar results.
Thus, in contrast to its failure to exert adverse effects on viability and marker display (Fig. 1 and 2), Y. pestis is capable of exerting an inhibitory effect on TNF-α production by DC in a pCD1- and temperature-dependent manner. This effect was demonstrated in a variety of cells infected by Yersinia, including DC infected with Y. enterocolitica (15) and macrophages infected with Y. pestis (67).
Transmembrane migration of DC is inhibited by Y. pestis carrying the virulence plasmid pCD1.
CCR7-dependent migration of mature DC from the periphery to the regional lymph node is essential for initiating immunity against newly encountered antigens (20, 24, 47). As a first step in evaluating the effect of Y. pestis on the potential for DC migration, we used the in vitro transmembrane chemotaxis assay.
DC were exposed to Y. pestis, at a low multiplicity of infection, for 0.5 to 1 h before addition of antibiotics to kill external bacteria. DC were examined, at 24 h after pulsing, for their ability to migrate through a nylon mesh towards the CCR7 ligand CCL19. This long time period is required for full manifestation of migratory properties and reflects the timetable for DC trafficking in vivo following infection. Nontreated cells and cells exposed to LPS served as controls.
Bacteria pregrown at 37°C and lacking the pCD1 virulence plasmid (Y. pestis Δ70) induced a marked increase in the ability of the cells to migrate towards the chemokine compared to nontreated or even to LPS-treated cells (Fig. 5A). In contrast, cells exposed to the pCD1-harboring bacteria (Y. pestis) failed to migrate through the membrane. Migration in this case was practically abolished, exhibiting levels that are 15-fold below those observed with Y. pestis Δ70 and 7-fold below those with untreated cells. It should be stressed that this strong paralyzing effect was observed only when bacteria were grown at 37°C prior to infection. When bacteria were grown at 28°C, the difference between migration efficiencies following treatment with the two Y. pestis strains was negligible (Fig. 5A). A temperature shift from 28°C to 37°C is a prerequisite for induction of the Yersinia TTSS, suggesting that the observed migratory paralysis could be related to translocation of bacterial Yops into the target DC. This conforms well with the documented adverse effects of some of the Yops on cytoskeleton rearrangement (reviewed in reference 65).
FIG. 5.
Effect of Y. pestis infection on DC transmembrane migration. Cells were pulsed without centrifugation with the indicated bacterial strains at an MOI of 5 (higher MOIs had adverse effects on DC migration independent of the Yersinia strain used). Gentamicin was added at 0.5 h after pulsing for infection with bacteria grown at 28°C and at 1 h for bacteria grown at 37°C. Twenty-four hours after pulsing, cells were examined for migration in Transwell chambers towards CCL19 (A) or control medium (B). Results are presented as percent cells migrating from the upper compartments to the lower compartment. Each experiment was performed in triplicate, and data represent averages and standard deviations. Two additional experiments performed under similar conditions exhibited similar results.
It is interesting to note that migration triggered by Y. pestis Δ70 pregrown at 37°C is much more effective than migration triggered by the same bacteria grown at 28°C. This could be due to the documented differences in the composition of the bacterial surface at these two temperatures, including changes in the LPS structure as well as some low level of TTSS induction (14, 28, 48).
Migration was less effective when examined in the absence of CCL19, yet in principle, the observations described above for effects of pCD1 and temperature remain valid (Fig. 5B). Pulsing with Y. pestis grown at 37°C leads to abolishment of cell migration. This effect could be relieved when bacteria were cured of the virulence plasmid or when they were cultured at 28°C. The similarity in effects observed in the presence or absence of CCL19 may suggest that impairment of migration is related not to chemoattraction per se but to the ability of the cells to undergo cytoskeleton remodeling necessary for movement. To better understand this, we have analyzed CCR7 surface expression as well as the effect of Y. pestis pulsing on DC adherence to solid surfaces, which is another DC feature involving cytoskeleton remodeling (62).
Expression of CCR7 is elevated in Y. pestis-infected DC.
Up-regulation of surface CCR7 expression by microbial stimuli was documented in maturing human DC (7, 25). The recently developed commercial reagents for immunostaining of mouse CCR7 allowed us to examine CCR7 expression in murine BMDC by flow cytometry (Fig. 6). As expected, exposure of cells to LPS resulted in an increase of surface CCR7. Pulsing with Y. pestis carrying or devoid of pCD1 up-regulated surface expression of the receptor as well (Fig. 6). As in the case of other surface markers (Fig. 2), we could not observe a plasmid-dependent deleterious effect on CCR7 surface expression. This suggests that the pCD1-carried genes are not involved in down-regulating the expression of the chemokine receptor examined and that the observed differences in migration (Fig. 5A) do not result from failure to display the CCR7 by Y. pestis.
FIG. 6.
Expression of CCR7 on DC infected with Y. pestis strains. Cells were infected and analyzed for CCR7 expression as described in legend to Fig. 3. Gray lines denote the isotype-matched immunoglobulin control. Mean fluorescence intensities for surface marker staining are given at the right top corner of each diagram. Mean fluorescence intensities for isotype controls are given at left top corner. Results are representatives of four independent experiments.
Adhesion to surfaces is impaired in Y. pestis-infected DC.
Adhesion of cells to solid surfaces is one of the many manifestations of their ability to remodel their cytoskeleton. Early stages of DC maturation are associated with changes in actin dynamics (62), which can also be manifested by increased adherence to plastic surfaces (66). To examine the effect of Y. pestis infection on cytoskeleton rearrangement of DC, we interacted DC with LPS, Y. pestis, and Y. pestis Δ70 and tested their ability to bind to the bottom of a microtiter plate at 1.5 h after pulsing (Fig. 7A). As expected, untreated cells were detached upon washing, while LPS-treated cells became adherent. DC treated with the Y. pestis strain devoid of the virulence plasmid acquired adherence, while those treated with the pCD1+ bacterial strain failed to bind to the bottom of the well (Fig. 7A). Microscopic examination revealed that upon interaction with Y. pestis, cells become rounded and lose their characteristic irregular morphology. In contrast, interaction with Y. pestis Δ70 resulted in stretching and spreading of the cells on the microplate bottom. To further examine the effects of Y. pestis on adherence, cell were pulsed with the two bacterial strains pregrown at either 28°C or 37°C, and the kinetics of binding was monitored (Fig. 7B). When bacteria were grown at 28°C, the two strains induced similar adhesion profiles: efficient adhesion occurred within 1.5 h, lasted about 2 h, and then was gradually lost (Fig. 7B, bottom panel). This transient binding profile resembles that reported for DC pulsed with LPS (66). Nevertheless, when cells were pulsed with plasmid-carrying bacteria grown at 37°C, substantial adherence failed to occur (Fig. 7B, top panel). Impairment of adhesion was not observed with plasmid-cured bacteria grown at 37°C. Taken together, these results indicate that acquisition of maturation-related adherence in DC is inhibited by Y. pestis through the pCD1-encoded temperature-regulated genes.
FIG. 7.
Effect of Y. pestis infection on DC adhesion. Pulsing was performed in 96-well microtiter plates at an MOI of 50 by centrifugation, and gentamicin (20 μg/ml) was added 1 h later. At the indicated times, medium and nonadherent cells were washed away, and the quantity of adhered cells was monitored by determining their LDH content (see Materials and Methods). Data are presented as the percentage of bound cells of those applied to the well (LDH content in bound cells of LDH content in input). (A) Comparison of adhesion induced at 1.5 h after pulsing with Y. pestis strains grown at 37°C to that induced by LPS and to spontaneous adhesion. (B) Kinetics of adhesion after pulsing with Y. pestis (black squares) and Y. pestis Δ70 (hatched squares) grown at 37°C (upper panel) and 28°C lower panel). Data are from a typical representative of three experiments, each performed in triplicates. Error bars indicate standard deviations.
Migration of respiratory DC to the draining lymph node is paralyzed by Y. pestis.
The in vitro analyses indicate that while bone marrow-derived DC pulsed with Y. pestis express the chemokine receptor CCR7 on their surface, they are unable to migrate towards the receptor ligand in an in vitro transmigration assay. To examine the effects of Y. pestis on DC trafficking in vivo, we used a recently developed procedure (31, 58) which allows assessment of migration of DC from the respiratory tract to the draining lymph node and actually provides an effective experimental system for monitoring DC migration in vivo. Cells within the respiratory tracts of BALB/c mice were first stained with the orange vital cell tracer CMTMR, followed by intranasal instillation of either Y. pestis (EV76) or the pCD1-cured strain Y. pestis Δ70, both grown at 37°C. Mice instilled with either PBS or LPS served as controls. Eighteen hours later, the mediastinal lymph nodes were examined for the presence of cells which are stained by the cell tracer and which carry the DC marker CD11c. Stimulation by LPS triggered the import of two major orange-stained cell populations from the respiratory tract to the draining lymph node (Fig. 8, compare panels A and B). One such population (R2) exhibits medium anti-CD11c staining, which could be nonspecific since it also interacts with the isotype control. The other population (R1) exhibits high and specific CD11c staining and thus reflects migration of DC from the respiratory tract to the draining lymph node upon stimulation. This is in agreement with previous reports on induction of DC migration in vivo upon stimulation with microbial components (16, 31). Analysis of mice treated with Y. pestis Δ70 bacteria revealed the presence of these two newly imported cell populations at much higher intensities (Fig. 8C). In this case the specific CD11chigh/CMTMR+ stained population constitute about 4% of the cells within the mediastinal lymph node. In contrast, DC migration was practically abolished in mice exposed to the pCD1-harboring Y. pestis (Fig. 8D). The amount of CD11chigh/CMTMR+ dropped to 0.4%, which is even lower then the basal level of CD11chigh cells migrating from the airways to the lymph node in nonstimulated mice (compare panels A and D in Fig. 8).
FIG. 8.
Migration of respiratory DC to draining lymph nodes after intranasal instillation of Y. pestis. Y. pestis and Y. pestis Δ70 suspensions were administered by intranasal instillation (two mice per group) in murine respiratory tracts prestained with CMTMR (see Materials and Methods). Instillation of 30 μg of LPS or PBS alone served as controls. After 18 h, mediastinal lymph nodes were examined by flow cytometry for evidence of DC immigrating from the airways. Two regions, R1 (CMTMR+/CD11c high) and R2 (CMTMR+/CD11cmedium), were gated. The tables adjacent to the dot plots provide gate statistics for staining with anti-CD11c and with the isotype-matched immunoglobulin control (isoC). Dot plots for isotype control staining are not shown. Two additional experiments performed under similar conditions exhibited similar results.
These observations suggest that Y. pestis carries bacterial constituents which are effective in triggering DC migration from the periphery to the draining lymph node, yet at the same time other factors harbored by the virulence plasmid pCD1 act to abolish this migration in vivo.
DISCUSSION
Interactions between bacteria and DC can have various consequences. They can range from full compliance of bacterial signals with DC functions, through impairment of one or several DC function, up to exploitation of the DC as a Trojan horse for bacterial invasion. Examination of the experimental data provided in this study indicates that the interaction between Y. pestis and DC is complex and multifaceted and bears characteristics of various interaction modes.
The interaction between DC and the virulence plasmid-cured strain Y. pestis Δ70 exemplifies full compliance with the expected functions of DC encountering invading pathogens. Upon pulsing by Y. pestis Δ70, induction of a maturation process is initiated, resulting in display of MHC class II, of costimulatory molecules, and of the chemokine receptor CCR7 on the DC surface (Fig. 2 and 6), as well as secretion of the prototype proinflammatory cytokine TNF-α (Fig. 4). DC maturation triggered by Y. pestis Δ70 also entails the characteristic programmed cytoskeleton rearrangement events. These include early events such as lamellipodium extension/retraction leading to increased adhesion (Fig. 7) and late events such as migration towards CCR7 ligands, which can be demonstrated by in vitro transmembrane experiments (Fig. 7A) as well as by an in vivo trafficking experiment (Fig. 8C). In most of these functions Y. pestis Δ70 resembles Y. pestis pregrown at 28°C, which simulates plague bacteria invading the host following a flea bite.
Interactions of DC with the virulence plasmid-harboring strains of Y. pestis are more complex. Some of the interactions comply with DC functions, while others lead to impairment of functions. Infection of DC by Y. pestis fails to trigger either an effective DC death or impairment of maturation (Fig. 1A [column II] and 2). This is in total contrast to the adverse effects exerted on DC by Y. enterocolitica (carrying the homologous virulence plasmid pYV), as observed previously (15) and also in this study (Fig. 1A [column I] and 3). Induction of cell death and inhibition of maturation, as manifested by failure to enhance surface marker display by Y. enterocolitica, are attributed to YopP (15, 37, 38, 52). In a recent study on Y. pestis-infected macrophages, we have demonstrated a functional limitation in the action of YopJ in macrophages compared to that of YopP (67). This could be also true in Y. pestis-infected DC and could explain the marked attenuation in cell death and the lack of inhibition of marker display observed in this study. Indeed, engineered expression of Y. enterocolitica WA 0:8-derived YopP in a Y. pestis genetic background restored effective cell death in infected DC (Fig. 1A, column III).
In contrast to the minor adverse effects on DC viability and marker display, effective pCD1-dependent impairment of TNF-α secretion, yet another YopJ/YopP-related function (15), was revealed in Y. pestis-infected DC (Fig. 4). It thus appears that certain YopJ-related functions are executed effectively, while others are not. Such discrimination was also observed in Y. pestis-infected macrophages (67) and was attributed to differences in intracellular effector levels needed for triggering the various effects.
The most notable adverse effects following DC infection by Y. pestis are impairments of functions related to actin modulation. The maturation of DC entails a cascade of various events requiring cytoskeleton rearrangement. These include: phagocytosis upon contact with the microorganism, entry into the lymphatic vessels which involves sliding and adhesion, chemotaxis-induced migration to the draining lymph node, and finally, cell-to-cell contact with T cells (47). All the cytoskeleton rearrangement functions examined by us were found to be severely impaired in DC infected by Y. pestis, in a pCD1-dependent and temperature-dependent manner. These include impairment of phagocytosis (not shown), of adhesion (Fig. 7), of migration through a porous membrane (Fig. 5), and of cell trafficking from the airways to the mediastinal lymph nodes (Fig. 8) in an in vivo migration model.
The roles of the various Yops in phagocytosis and reshaping of cellular morphology were dissected in numerous studies, and the involvement of YopE, YopT, YopO, and YopH in interfering with actin rearrangement was thoroughly examined (for reviews, see references 11, 39, and 65). It would be interesting to examine the individual contributions of these Yops to impairment of DC migration and to use the systems described here to address the effects of Yersinia on trafficking of immune cells in general.
It should be noted that results presented here were obtained with the attenuated EV76 strain. This is a conditionally lethal, Δpgm Y. pestis strain, and its virulence can be restored in mice by coinjection of iron (2, 8). EV76 does carry the virulence plasmid pCD1 and exhibits the characteristic wild-type phenotype related to TTSS and Yop functions. It is reasonable to assume that Yop-related abrogation of cytoskeleton modulation, which is conserved in all pathogenic Yersinia strains and which is most probably involved in impaired migration of DC, is not affected by the deletion of the pgm locus. In addition, the specific handicaps of Y. pestis in inducing DC death and impairment of maturation are both related to the limitations in YopJ function in Y. pestis. We have clearly demonstrated in our previous study that this limitation is shared by Y. pestis EV76 and by the fully virulent Kimberley 53 strain (67).
In summary, comparison of the interactions of Y. pestis and Y. enterocolitica with DC reveals differences in the strategies employed by the two Yersinia spp. to subvert antigen presentation by DC. Y. pestis relies mainly on paralyzing DC motility, to delay the migration of the infected cell to the lymph node and execute effective presentation of antigens to T cells. Other measures are used by Y. enterocolitica to counteract DC functions (15, 26, 64) (Fig. 1 and 3). These include abolishment of surface presentation of MHC class II and costimulatory molecules and premature killing of cells by induction of apoptosis. The effect of Y. enterocolitica on DC mobility is difficult to evaluate experimentally, since most infected DC are dead by the time migration can be evaluated in vitro or in vivo. Nevertheless, it is reasonable to assume that blocking of actin rearrangement in host cells by Y. enterocolitica will paralyze the few infected cells that have survived the apoptotic process and provide an additional arm for counteracting DC functions.
While the net effect of the strategies used by these related Yersinia species would be the same, namely, negation of effective onset of an innate immune response, the difference in the effort made by the two pathogens in achieving this goal is puzzling. One could speculate that in Y. enterocolitica infection, where disease is milder and self-limiting, development of an immune response is an effective measure for limiting infection and should be contested effectively by the pathogen. In Y. pestis, on the other hand, disease is fulminant and rapid, leading to host death within days. Fighting the onset of an adaptive immune response could therefore be less important.
Another aspect of the complex interrelationship between Yersinia and host cells involves mechanisms which promote the intracellular lifestyle of these bacteria. This is manifested by replication of Y. pestis in macrophages ex vivo (9, 46, 60) and by the presence of intracellular bacteria in macrophages of infected humans and animals (13, 18, 23, 32). The role of DC as a propagation site for Y. pestis is not clear. While no report on the presence of intracellular bacteria in DC of plague-infected mice is available, DC-bacterium interaction appears to occur in vivo, since Yop translocation into DC was recently demonstrated in vivo using a Δpgm KIM strain (35).
Preliminary experiments conducted by us with EV76 (which is also a Δpgm mutant) suggest that this strain does not replicate in DC but can be maintained in the cell in a viable form for at least 24 h. This information could suggest a role for DC in the intracellular life style of Y. pestis following infection and should be further examined.
Solving the complexity of the interactions between Y. pestis and DC is relevant not only for understanding the early events of infection and the onset of protection against the pathogen but also for the design of effective vaccines against Y. pestis, which is recognized as category A bioterror threat agent (27). A century of research effort has failed to provide a safe and effective plague vaccine (63). While killed and subunit vaccines are the main targets of ongoing research, replicating attenuated bacteria could be regarded as a future option (19) due to their obvious potential advantage. The study conducted here suggests that live vaccine candidates, such as the prevalent vaccine strain EV76, should be carefully analyzed for their compliance with antigen presentation by DC in order to identify factors that impair effective presentation. Deletion of such factors could result in the generation of improved live vaccine candidates for protection against plague.
Acknowledgments
We thank Hila Cohen for her excellent assistance with the experimental work, T. Waner for his help in establishing the in vivo migration system, and C. Kronman for critical reading of the manuscript.
Editor: D. L. Burns
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Gurion, R., and A. Shafferman. 1981. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid 5:183-187. [DOI] [PubMed] [Google Scholar]
- 3.Ber, R., E. Mamroud, M. Aftalion, A. Tidhar, D. Gur, Y. Flashner, and S. Cohen. 2003. Development of an improved selective agar medium for isolation of Yersinia pestis. Appl. Environ. Microbiol. 69:5787-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billard, E., C. Cazevieille, J. Dornand, and A. Gross. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 73:8418-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosio, C. M., A. W. Goodyear, and S. W. Dow. 2005. Early interaction of Yersinia pestis with APCs in the lung. J. Immunol. 175:6750-6756. [DOI] [PubMed] [Google Scholar]
- 7.Brittingham, K. C., G. Ruthel, R. G. Panchal, C. L. Fuller, W. J. Ribot, T. A. Hoover, H. A. Young, A. O. Anderson, and S. Bavari. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545-5552. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 83:348-363. [PubMed] [Google Scholar]
- 10.Colino, J., and C. M. Snapper. 2003. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 171:2354-2365. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 13.Davis, K. J., D. L. Fritz, M. L. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 14.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erfurth, S. E., S. Grobner, U. Kramer, D. S. Gunst, I. Soldanova, M. Schaller, I. B. Autenrieth, and S. Borgmann. 2004. Yersinia enterocolitica induces apoptosis and inhibits surface molecule expression and cytokine production in murine dendritic cells. Infect. Immun. 72:7045-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fainaru, O., D. Shseyov, S. Hantisteanu, and Y. Groner. 2005. Accelerated chemokine receptor 7-mediated dendritic cell migration in Runx3 knockout mice and the spontaneous development of asthma-like disease. Proc. Natl. Acad. Sci. USA 102:10598-10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferber, D. M., and R. R. Brubaker. 1981. Plasmids in Yersinia pestis. Infect. Immun. 31:839-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finegold, M. J. 1969. Pneumonic plague in monkeys. An electron microscopic study. Am. J. Pathol. 54:167-185. [PMC free article] [PubMed] [Google Scholar]
- 19.Flashner, Y., E. Mamroud, A. Tidhar, R. Ber, M. Aftalion, D. Gur, A. Zvi, N. Ariel, B. Velan, A. Shafferman, and S. Cohen. 2003. Identification of genes involved in Yersinia pestis virulence by signature-tagged mutagenesis. Adv. Exp. Med. Biol. 529:31-33. [DOI] [PubMed] [Google Scholar]
- 20.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23-33. [DOI] [PubMed] [Google Scholar]
- 21.Gervassi, A., M. R. Alderson, R. Suchland, J. F. Maisonneuve, K. H. Grabstein, and P. Probst. 2004. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 72:7231-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosfeld, H., S. Cohen, T. Bino, Y. Flashner, R. Ber, E. Mamroud, C. Kronman, A. Shafferman, and B. Velan. 2003. Effective protective immunity to Yersinia pestis infection conferred by DNA vaccine coding for derivatives of the F1 capsular antigen. Infect. Immun. 71:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarner, J., W. J. Shieh, P. W. Greer, J. M. Gabastou, M. Chu, E. Hayes, K. B. Nolte, and S. R. Zaki. 2002. Immunohistochemical detection of Yersinia pestis in formalin-fixed, paraffin-embedded tissue. Am. J. Clin. Pathol. 117:205-209. [DOI] [PubMed] [Google Scholar]
- 24.Gunn, M. D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L. T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson, M., A. Lundgren, K. Elgbratt, M. Quiding-Jarbrink, A. M. Svennerholm, and E. L. Johansson. 2006. Dendritic cells express CCR7 and migrate in response to CCL19 (MIP-3beta) after exposure to Helicobacter pylori. Microbes Infect. 8:841-850. [DOI] [PubMed] [Google Scholar]
- 26.Heesemann, J., A. Sing, and K. Trulzsch. 2006. Yersinia's stratagem: targeting innate and adaptive immune defense. Curr. Opin. Microbiol. 9:55-61. [DOI] [PubMed] [Google Scholar]
- 27.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, K. Tonat, et al. 2000. Plague as a biological weapon: medical and public health management. JAMA 283:2281-2290. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara, K., H. Tsukano, H. Watanabe, B. Lindner, and M. Matsuura. 2002. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 70:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi, T., T. Kobayashi, K. Gomi, T. Suzuki, Y. Tokue, A. Watanabe, and T. Nukiwa. 2004. Dendritic cells pulsed with live and dead Legionella pneumophila elicit distinct immune responses. J. Immunol. 172:1727-1734. [DOI] [PubMed] [Google Scholar]
- 30.Kramer, U., and C. A. Wiedig. 2005. Y. enterocolitica translocated Yops impair stimulation of T-cells by antigen presenting cells. Immunol. Lett. 100:130-138. [DOI] [PubMed] [Google Scholar]
- 31.Legge, K. L., and T. J. Braciale. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18:265-277. [DOI] [PubMed] [Google Scholar]
- 32.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 73:7142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 34.Mamroud, E., Y. Flashner, A. Tidhar, R. Ber, D. Gur, M. Aftalion, S. Lazar, B. Velan, A. Shafferman, and S. Cohen. 2003. Evaluation of protective immunity induced by Yersinia enterocolitica type-III secretion system mutants. Adv. Exp. Med. Biol. 529:425-430. [DOI] [PubMed] [Google Scholar]
- 35.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 37.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mota, L. J., and G. R. Cornelis. 2005. The bacterial injection kit: type III secretion systems. Ann. Med. 37:234-249. [DOI] [PubMed] [Google Scholar]
- 40.Navarro, L., N. M. Alto, and J. E. Dixon. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 8:21-27. [DOI] [PubMed] [Google Scholar]
- 41.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 42.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 43.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 44.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portnoy, D. A. 2005. Manipulation of innate immunity by bacterial pathogens. Curr. Opin. Immunol. 17:25-28. [DOI] [PubMed] [Google Scholar]
- 46.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randolph, G. J., V. Angeli, and M. A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617-628. [DOI] [PubMed] [Google Scholar]
- 48.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52:1363-1373. [DOI] [PubMed] [Google Scholar]
- 49.Rescigno, M. 2002. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 10:425-461. [DOI] [PubMed] [Google Scholar]
- 50.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saikh, K. U., T. L. Kissner, A. Sultana, G. Ruthel, and R. G. Ulrich. 2004. Human monocytes infected with Yersinia pestis express cell surface TLR9 and differentiate into dendritic cells. J. Immunol. 173:7426-7434. [DOI] [PubMed] [Google Scholar]
- 54.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyi, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 55.Schoppet, M., A. Bubert, and H. I. Huppertz. 2000. Dendritic cell function is perturbed by Yersinia enterocolitica infection in vitro. Clin. Exp. Immunol. 122:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoppet, M., and H. I. Huppertz. 2001. Differential stimulation of helper and cytotoxic T cells by dendritic cells after infection by Yersinia enterocolitica in vitro. Cell Immunol. 208:43-51. [DOI] [PubMed] [Google Scholar]
- 57.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Lack of dendritic cell maturation following infection by Coxiella burnetii synthesizing different lipopolysaccharide chemotypes. Ann. N. Y. Acad. Sci. 1063:154-160. [DOI] [PubMed] [Google Scholar]
- 58.Skinner, J. A., M. R. Pilione, H. Shen, E. T. Harvill, and M. H. Yuk. 2005. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J. Immunol. 175:4647-4652. [DOI] [PubMed] [Google Scholar]
- 59.Sozzani, S., P. Allavena, G. D'Amico, W. Luini, G. Bianchi, M. Kataura, T. Imai, O. Yoshie, R. Bonecchi, and A. Mantovani. 1998. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161:1083-1086. [PubMed] [Google Scholar]
- 60.Straley, S. C., and P. A. Harmon. 1984. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect. Immun. 45:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straley, S. C., and R. D. Perry. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310-317. [DOI] [PubMed] [Google Scholar]
- 62.Swetman, C. A., Y. Leverrier, R. Garg, C. H. Gan, A. J. Ridley, D. R. Katz, and B. M. Chain. 2002. Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur. J. Immunol. 32:2074-2083. [DOI] [PubMed] [Google Scholar]
- 63.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 64.Trulzsch, K., G. Geginat, T. Sporleder, K. Ruckdeschel, R. Hoffmann, J. Heesemann, and H. Russmann. 2005. Yersinia outer protein P inhibits CD8 T cell priming in the mouse infection model. J. Immunol. 174:4244-4251. [DOI] [PubMed] [Google Scholar]
- 65.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 66.Walzer, T., L. Galibert, M. R. Comeau, and T. De Smedt. 2005. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J. Immunol. 174:51-59. [DOI] [PubMed] [Google Scholar]
- 67.Zauberman, A., S. Cohen, E. Mamroud, Y. Flashner, A. Tidhar, R. Ber, E. Elhanany, A. Shafferman, and B. Velan. 2006. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect. Immun. 74:3239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]