Abstract
The rationale for the present study was to determine how different species of bacteria interact with cells of the human meninges in order to gain information that would have broad relevance to understanding aspects of the innate immune response in the brain. Neisseria lactamica is an occasional cause of meningitis in humans, and in this study we investigated the in vitro interactions between N. lactamica and cells derived from the leptomeninges in comparison with the closely related organism Neisseria meningitidis, a major cause of meningitis worldwide. N. lactamica adhered specifically to meningioma cells, but the levels of adherence were generally lower than those with N. meningitidis. Meningioma cells challenged with N. lactamica and N. meningitidis secreted significant amounts of the proinflammatory cytokine interleukin-6 (IL-6), the C-X-C chemokine IL-8, and the C-C chemokines monocyte chemoattractant protein 1 (MCP-1) and RANTES, but it secreted very low levels of the cytokine growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF). Thus, meningeal cells are involved in the innate host response to Neisseria species that are capable of entering the cerebrospinal fluid. The levels of IL-8 and MCP-1 secretion induced by both bacteria were essentially similar. By contrast, N. lactamica induced significantly lower levels of IL-6 than N. meningitidis. Challenge with the highest concentration of N. lactamica (108 CFU) induced a small but significant down-regulation of RANTES secretion, which was not observed with lower concentrations of bacteria. N. meningitidis (106 to 108 CFU) also down-regulated RANTES secretion, but this effect was significantly greater than that observed with N. lactamica. Although both bacteria were unable to invade meningeal cells directly, host cells remained viable on prolonged challenge with N. lactamica, whereas N. meningitidis induced death; the mechanism was overwhelming necrosis with no significant apoptosis. It is likely that differential expression of modulins between N. lactamica and N. meningitidis contributes to these observed differences in pathogenic potential.
Meningitis, inflammation of the meninges that surround the brain and the spinal cord (63), is the most common serious infection of the central nervous system. In humans, the meninges comprise the pachymenix (dura mater) and the leptomeninges, which consists of the arachnoid and pia mater together with the trabeculae that traverse the cerebrospinal fluid (CSF)-filled subarachnoid space (SAS) (4, 25). The bacterial etiology of meningitis is broad, and susceptibility to the causative organisms varies with age, with different groups of organisms affecting neonates, children, and adults (28). Neonatal bacterial meningitis is due mainly to transmission of bacteria from the maternal genital tract to the newborn infant, and the main organisms that cause disease are Streptococcus agalactiae (group B hemolytic streptococcus) and Escherichia coli K1 (28, 47). After the neonatal period, the most common bacterial agents causing classical meningitis are Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis (11, 43, 56).
Bacteremia and penetration of the blood-CSF barrier is necessary for development of the overwhelming majority of cases of meningitis. However, in addition to meningitis caused by spread of bacteria by the hematogenous route, meningitis has also been reported following infection with other bacteria as a complication of traumas, surgical procedures, and developmental malformations or as secondary infections to chronic ear infections. Regardless of the route of entry of bacteria, whether by hematogenous spread or by nonhematogenous routes, the host defenses within the SAS are inadequate to control infection. Classical bacterial meningitis is predominantly a leptomeningitis, with both the inflammatory response and bacteria largely contained within the SAS and with little or no involvement of the dura mater or the underlying brain.
Neisseria meningitidis is not the only species within the genus Neisseria capable of inducing intracranial infection. Leptomeningitis caused by the closely related pathogen Neisseria gonorrhoeae, the causative agent of gonorrhoeae, has been reported as an extremely rare event during disseminated gonococcal infection (17). Neisseria lactamica also shares a close relationship with Neisseria meningitidis, and frequent interspecies genetic exchange has been reported to occur (6, 24, 38). Although N. lactamica has a predilection for associating primarily with epithelial cells of the nasopharynx, it is an occasional cause of meningitis (18, 29, 31, 36) and septicemia (10, 42, 51, 66).
Although the mechanisms are not entirely understood, the interactions of bacteria with host cells within the SAS are likely to contribute to the inflammatory response. Recent studies have shown that cells derived from the leptomeninges are active participants in the host response to infection with meningococci (13, 26, 35, 64). The rationale for the present study was therefore to determine how different species of bacteria interact with cells of the leptomeninges. The information gained would have broad relevance to understanding the innate immune response in the SAS. Thus, we investigated the in vitro interactions of different species of Neisseria (N. lactamica and N. meningitidis) with cells derived from the human leptomeninges and compared the nature of the inflammatory response induced.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Neisseria meningitidis strain MC58 (B:15:P1.7,16b; Cap+ Opa+ Opc+ PorA+ PorB+ Pil+ [type IV; class I] Rmp+ LPS+) was isolated from an outbreak of meningococcal infections that occurred in Stroud, Gloucestershire, United Kingdom, in the mid-1980s (41). Neisseria lactamica NCTC10617 (34) (Cap− Opa− Opc− PorA− PorB+ Pil+ [type IV, class II] Rmp+ LPS+) was obtained from the National Collection of Type Cultures (Colindale, United Kingdom). Both bacteria were grown on proteose-peptone agar and incubated at 37°C in an atmosphere containing 5% (vol/vol) CO2. The presence of capsule was detected using both a slide agglutination assay (Wellcome meningococcal serogrouping antisera) and a negative staining protocol with a solution of 1% (wt/vol) Congo red stain. The presence of type IV pili was confirmed by transmission electron microscopy following a negative staining protocol adapted from the method of Lin and colleagues (37). The presence of lipopolysaccharide (LPS) was confirmed by low-Mr sodium dodecyl sulfate-polyacrylamide gel electrophoresis using the method of Schagger and von Jagow (50) with silver staining (33). The phenotypes of strain MC58 and N. lactamica were defined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with specific antibodies to Opa, Opc, PorA, PorB, Rmp, and pilus proteins as described previously (32, 62).
Outer membranes (OM) were prepared from both meningococci and N. lactamica by extraction of whole cells with lithium acetate as described previously (54).
Culture of human cells.
Culture of meningioma cells of the meningothelial histological subtype was carried out as described previously (32). Briefly, fresh meningioma tissue was obtained from surgically removed tumors and digested with 0.125% (wt/vol) trypsin-0.02% (wt/vol) ETDA in Hanks balanced salt solution. The cells were then cultured on collagen (type I from rat tail; 5 μg cm−2; Sigma)-coated flasks in Dulbecco's modified Eagle's medium (DMEM) with glutamax-1 and sodium pyruvate (Invitrogen) supplemented with 10% (vol/vol) decomplemented fetal calf serum (dFCS), 100 IU ml−1 penicillin, 10 μg ml−1 streptomycin, and 10 μg ml−1 gentamicin. The meningioma cells were characterized by immunohistochemical staining with antibodies against the specific cellular markers of desmosomal desmoplakin, epithelial membrane antigen, vimentin, and cytokeratin as described previously (32). In addition, the absence of staining in the monolayers after incubation with antibody to CD68 (Dako) confirmed that macrophages were not present.
Interactions of N. lactamica and N. meningitidis with human meningioma cells. (i) Determination of bacterial adherence and invasion.
Human meningioma cells from passages 6 to 9 were grown to confluence (∼90%; 5 × 104 cells) on collagen-coated 24-well tissue culture plates in culture medium without antibiotics. The medium was removed, and the cell monolayers were washed in phosphate-buffered saline (PBS) containing 0.1% dFCS (wash buffer) and then were challenged with concentrations of bacteria (1 ml per monolayer) ranging from 1 × 102 (multiplicity of infection [MOI] of bacteria to cells, 0.002), 1 × 104 (MOI, 0.2), and 1 × 106 (MOI, 20) to 1 × 108 (MOI, 2,000) CFU ml−1. Each bacterial inoculum was prepared in experimental medium that had been optimized in preliminary experiments so that the relative growth rates of both meningococci and N. lactamica in the absence of cell monolayers were similar (data not shown). Thus, inocula of both bacteria were prepared in DMEM-glutamax containing 1.0% (vol/vol) dFCS.
During the experiments, sampling was carried out from triplicate wells at intervals of up to 24 h. To quantify total bacterial CFU associated with the cells, washed monolayers were lysed by incubation with 1% (wt/vol) saponin in wash buffer for 15 min, and organisms were quantified by viable counting (32). Bacterial internalization was investigated after 24 h by first incubating the washed monolayers with 200 microliters of a gentamicin solution (200 μg ml−1) for 30 min to eliminate all extracellular bacteria. Monolayers were subsequently washed and lysed, and internalized bacteria were quantified by viable counting (60). The involvement of actin polymerization was investigated by preincubating the cell monolayers with DMEM containing 0.1% (vol/vol) dFCS and 2 μg ml−1 cytochalasin D. To validate the invasion assay, meningioma cells were infected for 9 h with E. coli strain IH3080, which we have previously shown is able to invade meningioma cells, in contrast to other meningeal pathogens, including Neisseria meningitidis, which are unable to invade this cell type (26). The relative association and internalization of N. meningitidis and N. lactamica was evaluated using one-way analysis of variance to compare the level of significance between mean values, with P < 0.05 being significant.
(ii) Antibodies to N. lactamica.
A group of 15 mice was housed under standard conditions of heating and lighting with access to food ad libitum. Each animal was immunized subcutaneously on days 1, 14, and 28 with 10 μg of OM, which had been adsorbed to aluminum hydroxide (2% [wt/vol] Superfos; Biosector a/s, Vedbaek, Denmark) as previously described (14). Animals were bled 10 days later, and antisera were stored at −20°C until used. All antisera recognized whole N. lactamica cells and did not cross-react significantly with either N. meningitidis or cultured cells, as judged by immunofluorescense. Subsequently, a pool of murine antisera was prepared for confocal labeling experiments.
(iii) Visualization of bacterial association with human cells by laser-scanning confocal microscopy (LSCM).
Human meningioma cells were grown to confluence in culture medium on 0.4 μm-pore-size cell culture inserts (12-well format with a surface area equivalent to 24-well culture plates; Falcon, Becton Dickinson Biosciences, Bedford, Mass.), and each monolayer was challenged with various concentrations of each bacterium (1-ml volume in the upper chamber). At intervals of up to 24 h, the challenge medium was removed and the monolayers were washed three times in PBS before being fixed in ice-cold methanol (100%) for 10 min at 25°C. The fixative was removed, the monolayers were washed a further three times in PBS, and then they were blocked for 1 h at 25°C with PBS containing 10% (vol/vol) normal rabbit serum (Gibco). Adherent bacteria were immunostained by interaction at 4°C for 16 to 18 h with primary polyclonal antibodies (each used at a dilution of 1/100) specific for each bacterial pathogen. Meningococci were detected with mouse polyclonal antibodies raised against OM of Neisseria meningitidis MC58 (32) and N. lactamica detected with murine antibodies described above. After washing in PBS, the monolayers were incubated with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse antibody (Zymed, Calif.) and used at a concentration of 1/100 for 1 h at 37°C. The cells were washed and counterstained with a solution of 0.0025% (wt/vol) Evans Blue in PBS for 20 min at 25°C. Cell monolayers were washed and examined immediately on a Leica model TCS 4D confocal microscope (Leitz), and images were obtained by simultaneous two-channel scanning at wavelengths of 488 and 568 nm to excite FITC and counterstain, respectively. Optical sections (25 to 30 per sample) were usually taken at intervals of approximately 1.0 μm and used to reconstruct three-dimensional confocal images.
Measurement of cytokine and chemokine production.
The levels of cytokine and chemokine proteins produced on bacterial challenge of human meningioma cells, cultured in 24-well plates, were quantified by sandwich immunoassay using matched pairs of specific antibodies, as described previously (13). The antibodies used were directed against the proinflammatory cytokine mediators interleukin-1α (IL-1α), IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) (all from R&D systems); the anti-inflammatory cytokines IL-10 and transforming growth factor beta (TGF-β) (R&D); the growth factor granulocyte-macrophage-colony stimulating factor (GM-CSF) (R&D); the C-C chemokine family members monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES (R&D); and the C-X-C family chemokine IL-8 (R&D). The concentration of each cytokine or chemokine was determined by comparison with standard solutions of the corresponding purified recombinant protein (Peprotech, London, United Kingdom) similarly treated. A two-sample t-test was used to compare the mean levels of cytokine secretion following particular treatments, with P < 0.05 being significant.
Investigating cell death induced by bacteria. (i) Viability/cytotoxicity assay.
Human meningioma cells were grown to confluence (∼90%) on 8-chamber Falcon tissue culture-treated slides (Becton Dickinson Biosciences). The viability of human cells following challenge with approximately 106 and 108 CFU/monolayer of bacteria was assessed at intervals up to 96 h with the LIVE/DEAD reduced biohazard viability/cytotoxicity assay from Molecular Probes, as described previously (26). All cell monolayers were examined immediately with LSCM as described above.
(ii) Quantification of cell viability.
Human meningioma cells were grown to confluence on collagen-coated 96-well plates, and cultures were challenged with concentrations of bacteria (0.2 ml per monolayer) ranging from 1 × 102, 1 × 104, and 1 × 106 to 1 × 108 CFU ml−1. Cell viability was determined from triplicate wells at 24, 48, and 96 h after challenge using the WST-1 Cell Proliferation Assay kit (Chemicon) according to the manufacturer's instructions. Briefly, the monolayers were gently washed three times with PBS and DMEM-glutamax containing 0.1% (vol/vol) dFCS, and WST-1 reagent (10 μl/well) was then added to each well (100 μl/well). The cultures were incubated for 2 h at 37°C, and absorbance was then measured at 450 nm (Anthos microplate spectrophotometer). A two-sample t-test was used to compare the mean levels of cell viability following particular treatments, with P < 0.05 being significant.
(iii) Detection of apoptosis with annexin V.
Human meningioma cells were grown to confluence (∼90%) on 8-chamber Falcon tissue culture-treated slides (Becton Dickinson Biosciences) and challenged with approximately 106 and 108 CFU/monolayer of bacteria. The combination of annexin V-FITC conjugate (R&D) and propidium iodide was used for detecting the presence of apoptotic and necrotic cells at intervals of up to 96 h after challenge, according to the manufacturer's instructions (R&D). This differentiation was also examined in additional cell monolayers by dual staining with acridine orange and ethidium bromide as described previously (9, 46). Uninfected cells were used as negative controls at each time point, and treatment with staurosporine (Sigma; 1 μM for 6 h) was used as a positive control for apoptosis (40). Cells were viewed immediately by LSCM as described above, and the number of apoptotic cells was quantified as the mean percentage of cells (from a minimum of 6 fields) of the total number of cells (from a minimum of 6 fields) present in normal monolayers.
(iv) Assay for DNA fragmentation.
Human meningioma cells were grown to confluence on collagen-coated 96-well plates and challenged with approximately 106 to 108 CFU ml−1 of bacteria (0.2 ml per monolayer). At 24, 48, and 96 h, cells were removed by treatment with trypsin, and genomic DNA was isolated from 9 wells for each challenge and from control, uninfected cells, using the Wizard Genomic DNA purification kit (Promega UK, Southampton, England) according to the manufacturer's instructions. As a positive control for DNA fragmentation, cells were exposed to UV radiation. Samples of genomic DNA were analyzed by electrophoresis in 1.5% (wt/vol) agarose gels, run in 1× Tris-acetate-EDTA (TAE) buffer for 1 h at 90 V, and visualized by staining for 20 min in 1× TAE buffer containing ethidium bromide (0.5 μg ml−1).
(v) Determination of caspase-3 and caspase-8 activity.
Human meningioma cells were grown to confluence on collagen-coated 24-well plates, and quadruplicate wells were challenged with 106 to 108 CFU/monolayer. Caspase activity was determined at 6, 24, 48, and 96 h using commercially available colorimetric assay kits according to the manufacturer's instructions (Sigma). Uninfected cells were used as negative controls at each time point, and treatment with staurosporine (Sigma; 1 μM for 6 h) was used as a positive control for apoptosis (40).
RESULTS
The interactions of Neisseria lactamica with meningioma cells.
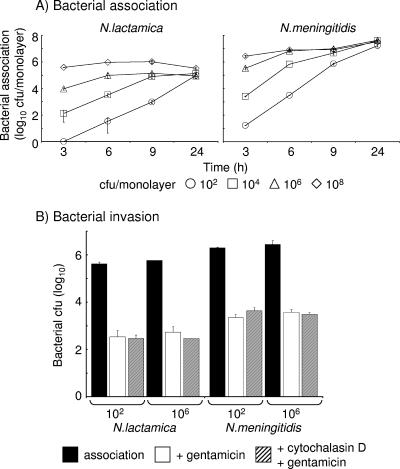
Meningioma cells were challenged with various concentrations of N. lactamica, and association was determined over time. Challenge with 102 to 104 CFU/monolayer of N. lactamica demonstrated concentration-dependent increases in association with cell monolayers over time (Fig. 1A). However, rapid saturation of the cell monolayers was observed as early as 3 to 6 h following challenge with concentrations of 106 to 108 CFU/monolayer of N. lactamica (Fig. 1A). Nevertheless, by 24 h, the levels of association of N. lactamica were similar (P > 0.05) regardless of the initial infecting concentrations tested (Fig. 1A). Significant differences (P < 0.05) were observed between the levels of association of N. lactamica and N. meningitidis: in general, at every time point sampled and for each concentration of bacterium tested, N. meningitidis associated with meningioma cells in approximately 10-fold greater numbers (P < 0.05) than N. lactamica, and this disparity was still evident at the saturating levels achieved at 24 h after challenge (Fig. 1A).
FIG. 1.
(A) Association of Neisseria lactamica and Neisseria meningitidis with human meningioma cells. Confluent monolayers of meningothelial meningioma cells were challenged over time with various doses of bacteria, and total association to cells was quantified by viable counting. Infection experiments were carried out in triplicate with two different meningioma cell lines. Data are shown from typical experiments with meningioma cells from one patient, with individual points representing the mean bacterial CFU counts per 5 × 104 cells in monolayer. The error bars represent the standard deviations from triplicate-infected wells. (B) Neisseria lactamica and Neisseria meningitidis do not invade meningioma cells. Confluent monolayers of meningothelial meningioma cells were challenged for 24 h with various doses of bacteria, and total association to cells was quantified by viable counting. Treatment with gentamicin was used to remove all extracellular bacteria. Cells were also pretreated with cytochalasin D prior to bacterial infection and then incubated with gentamicin after 24 h. Experiments were carried out in duplicate with meningioma cells from two different patients, and typical data are from one experiment with one patient cell line, with bars representing the mean bacterial CFU counts and the error bars representing the standard deviations from triplicate-infected wells.
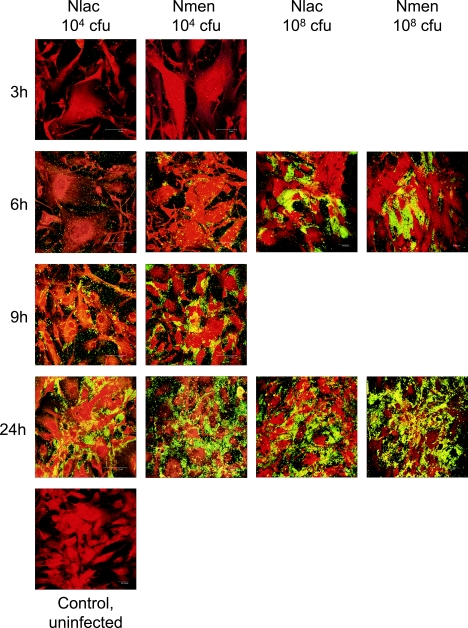
The differential association of N. lactamica and N. meningitidis with meningioma cells was also studied with confocal microscopy (Fig. 2). Infection of monolayers with a nonsaturating concentration (104 CFU/monolayer) of each bacterium confirmed the viable counting data (Fig. 1A) and clearly demonstrated that N. meningitidis adhered more rapidly to the cells by 9 h than N. lactamica. Notably, N. lactamica association was concentrated at the curvilinear edges of the cells rather than over the cytoplasm, whereas N. meningitidis adherence was observed over the whole cell surface. As expected, when saturating concentrations (108 CFU/monolayer) of bacteria were used, higher levels of adherence of both bacteria were observed by 6 h compared with the lower concentration (Fig. 2). By 24 h, regardless of initial bacterial concentration, N. meningitidis showed greater association than N. lactamica with meningioma cells, which was in agreement with the viable count data (Fig. 1A).
FIG. 2.
Laser-scanning confocal microscopy of human meningioma cells infected with Neisseria lactamica (Nlac) and Neisseria meningitidis (Nmen). Confluent monolayers were infected with various doses of bacteria, and at intervals the monolayers were processed for confocal microscopy. The images shown are for adherence of bacteria determined at 3, 6, 9, and 24 h after infection with a concentration of 104 CFU/monolayer (nonsaturating). For comparison, images are shown of rapid bacterial saturation of monolayers at 6 and 24 h after infection with a concentration of 108 CFU/monolayer (saturating). Images are representative of experiments carried out in triplicate with two different meningioma cell lines.
We next investigated whether N. lactamica and N. meningitidis invaded meningioma cells by infecting monolayers with a concentration of each bacterium that did not saturate the monolayers (102 CFU) and comparing them with a concentration that led to rapid saturation (106 CFU). After 24 h of infection with bacteria, gentamicin treatment was then used to remove all extracellular bacteria. At each concentration tested, and compared to the total number of associated bacteria, <0.1% of either bacterium was recovered from the monolayers after gentamicin treatment (Fig. 1B). These numbers were not significantly different (P > 0.05) from the numbers of either species recovered from monolayers that had been pretreated with cytochalasin D (Fig. 1B), demonstrating the absence of internalization by a microfilament-dependent process, which is necessary for invasion of epithelial and endothelial cells by N. meningitidis (59, 60). These data demonstrate that both N. lactamica and N. meningitidis were unable to invade meningioma cells, an observation that is similar to our previous studies showing that meningioma cells provide an effective barrier to Neisseria species (32).
Cytokine and chemokine production by meningioma cells infected with Neisseria lactamica.
In order to investigate whether N. lactamica induced the production of inflammatory mediators, meningioma cell monolayers were challenged with various concentrations of bacteria, and the dose-dependent kinetics of cytokine and chemokine secretion were measured over time and compared to those of N. meningitidis. Supernatants were collected for 48 h, since previous studies have shown that bacterial infection induced the late secretion of some chemokines (13).
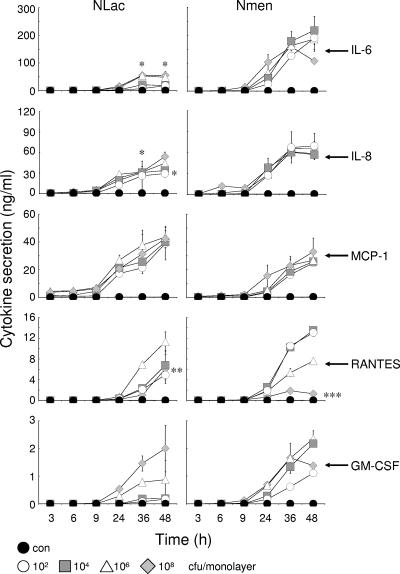
Differences were observed in the levels and patterns of cytokine and chemokine secretion by meningioma cells challenged with N. lactamica compared with N. meningitidis (Fig. 3). Challenge with various concentrations of N. lactamica induced the secretion of between 18 to 55 ng/ml of the proinflammatory cytokine IL-6 by 48 h, but these levels were significantly lower (P < 0.05) than the levels of IL-6 secretion induced by challenge with N. meningitidis (107 to 218 ng/ml).
FIG. 3.
Cytokine and chemokine secretion by human meningioma cells infected with Neisseria lactamica (Nlac) and Neisseria meningitidis (Nmen). Confluent monolayers of meningioma cells were infected with various doses of bacteria, and culture supernatants were assayed over time for cytokine and chemokine protein secretion. Infection experiments were carried out in triplicate with two different meningioma cell lines. Data are shown from a typical experiment with meningioma cells from one patient, with individual points representing the mean levels of cytokine secretion and the error bars representing the standard deviations from triplicate-infected wells. An asterisk denotes significantly lower (P < 0.05) levels of cytokine/chemokine induced by N. lactamica compared to levels induced by N. meningitidis. Two asterisks denotes significantly lower (P < 0.05) levels of RANTES secretion following challenge with 108 CFU of N. lactamica compared with levels induced by 106 CFU of N. lactamica. Three asterisks denotes significantly lower (P < 0.05) levels of RANTES secretion following challenge with 108 CFU of N. meningitidis compared with secretion induced by all other concentrations of N. meningitidis.
N. lactamica also induced secretion of the C-X-C chemokine IL-8 by meningioma cells, but after 36 h the levels were approximately twofold lower (P < 0.05) than those observed following challenge with N. meningitidis (Fig. 3). This twofold difference was still significant (P < 0.05) by 48 h at lower concentrations (102 to 104 CFU/ml) of N. lactamica (29 to 33 ng/ml) and N. meningitidis (57 to 69 ng/ml). By contrast, at the higher concentrations tested (106 to 108 CFU/ml), there were no significant differences (P > 0.05) in the induction of IL-8 secretion by N. lactamica (44 to 54 ng/ml) and N. meningitidis (58 to 66 ng/ml) (Fig. 3).
N. lactamica also induced secretion of the C-C chemokines MCP-1 and RANTES by meningioma cells. In contrast to the different patterns of secretion of IL-6 and IL-8 induced by the two bacteria, the levels of MCP-1 secretion after 48 h following challenge with N. lactamica (39 to 43 ng/ml) were not significantly different (P > 0.05) from the levels induced by N. meningitidis (25 to 33 ng/ml). However, a more pronounced difference was observed for RANTES. Challenge with various concentrations of N. lactamica resulted in the accumulation of between 5 and 10 ng/ml of RANTES by 48 h (Fig. 3). However, challenge with the highest concentration of N. lactamica tested (108 CFU) resulted in a small but significant down-regulation (P < 0.05) of RANTES secretion relative to the levels that were induced by 106 CFU of bacteria (Fig. 3). In contrast, the ability of N. meningitidis to down-regulate RANTES production was more pronounced. Significantly lower (P < 0.05) concentrations of RANTES (1 ng/ml) were detected by 48 h in samples challenged with the highest concentration of N. meningitidis (108 CFU) compared to the levels (8 to 13 ng/ml) secreted following challenge with smaller numbers of bacteria (102 to 106 CFU) (Fig. 3). Thus, increased concentrations of N. meningitidis down-regulated secretion of RANTES, confirming the observations from our previous study (13), whereas minor down-regulation by N. lactamica was only observed with the highest concentration tested.
Both bacteria induced the secretion of the cytokine growth factor GM-CSF, but they did so at very low levels compared with the other inflammatory molecules (Fig. 3). Nevertheless, minor differences were seen in the patterns of GM-CSF secretion induced by both bacteria. Challenge with 102 to 106 CFU/monolayer of N. lactamica induced the accumulation of between 0.2 to 0.9 ng/ml GM-CSF by 48 h, and these levels were approximately twofold lower (P < 0.05) than the levels of secretion induced by similar concentrations of N. meningitidis (1 to 2 ng/ml, respectively). By contrast, at the highest concentration tested (108 CFU/monolayer), no significant difference (P > 0.05) was observed (Fig. 3). Nevertheless, it should be noted that the biological significance of differences in GM-CSF induction may not be critical given the low levels of cytokine observed. Neither bacterium induced the secretion of IL-1α, IL-1β, TNF-α, MIP-1α, MIP-1β, TGF-β, IL-10, or IL-12 cytokines.
Stimulation of cytokine and chemokine production by outer membranes (OM) of Neisseria lactamica.
It is clear that OM vesicles released by pathogenic Neisseria species are important during infection due to their ability to stimulate host cell inflammatory responses (58), and recent studies have shown that OM from meningococci can induce cytokine production by meningeal cells (13). Since N. lactamica could induce the production of inflammatory mediators by meningeal cells, we investigated whether OM isolated from the organism also played a significant role in cytokine and chemokine secretion. OM from N. lactamica induced the secretion of IL-6, IL-8, MCP-1, and RANTES but not GM-CSF from meningeal cells, and these results were similarly observed with OM from N. meningitidis (Table 1). In addition, for each cytokine examined, there was no significant difference (P > 0.05) in the levels of secretion induced by either OM preparation.
TABLE 1.
Effects of OM from N. lactamica and N. meningitidis on cytokine and chemokine secretion by human meningioma cellsa
| Challenge | Dose (μg/monolayer) | Cytokine level (ng/ml)
|
||||
|---|---|---|---|---|---|---|
| IL-8 | IL-6 | MCP-1 | RANTES | GM-CSF | ||
| N. lactamica-OM | 1.0 | 5.8 ± 0.8 | 4.8 ± 1.1 | 35.9 ± 1.0 | 9.2 ± 1.2 | 0.2 |
| N. meningitidis-OM | 1.0 | 7.8 ± 2.0 | 6.1 ± 2.7 | 30.2 ± 4.0 | 6.2 ± 2.0 | 0.2 ± 0.1 |
| Control | Medium only | 0.3 ± 0.1 | 0.5 ± 0.7 | 8.3 ± 1.0 | 0.9 ± 0.2 | 0.1 |
Human meningioma cells were treated with a range of concentrations (1, 0.1, 0.01, and 0.001 μg/monolayer) of OM from Neisseria lactamica and Neisseria meningitidis for up to 48 h. Data are shown for the highest concentration tested at the 48-h time point; dose-dependent decreases in secretion of all cytokines were observed with the other concentrations tested (data not shown).
However, the levels of secretion by 48 h of IL-6 and IL-8 induced by N. lactamica-OM preparation (used at a concentration of 1 μg LPS/monolayer) were approximately 10-fold lower (P < 0.05) than the levels observed following challenge with viable bacteria (106 CFU bacteria, containing a similar amount of LPS) (13). By contrast, there was no significant difference in the levels of either MCP-1 or RANTES secreted following treatment with either viable bacteria or N. lactamica OM (Table 1).
Influence of bacterial challenge on viability of meningioma cells.
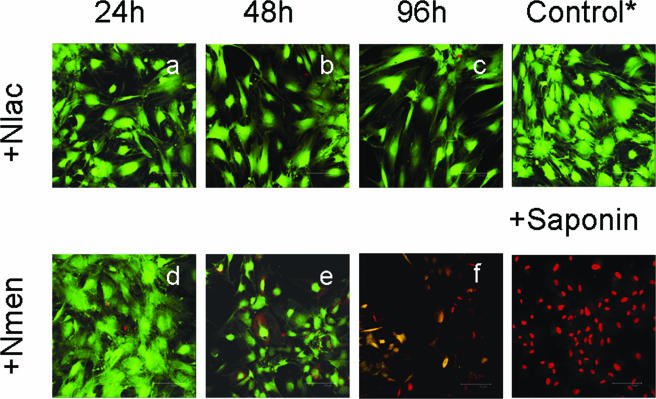
During the course of challenge experiments, it was apparent by visual inspection that death and detachment of meningioma cells within the monolayers was occurring by 48 to 96 h in response to high concentrations of N. meningitidis but not to N. lactamica. In order to investigate this observation in more detail, meningioma cell cultures were challenged with 106 to 108 CFU of bacteria and viability was assessed using a fluorescence-based method, with simultaneous determination of live and dead cells using two probes that measure intracellular esterase activity and plasma membrane integrity (Fig. 4). N. lactamica did not induce cell death during contact with monolayers for 48 h (Fig. 4a and b). By contrast, while N. meningitidis similarly did not affect the viability of meningioma cells by 24 h (Fig. 4d), significant thinning of monolayers and some cell detachment was demonstrated in cultures by 48 h (Fig. 4e). Furthermore, prolonged contact with N. meningitidis resulted in gross destruction of the monolayers by 96 h (Fig. 4f), and cells sloughed into the culture medium were dead. By contrast, the viability of monolayers challenged with N. lactamica for the same period of time was unaffected (Fig. 4c).
FIG. 4.
Viability of human meningioma cells following infection with Neisseria lactamica (Nlac) and Neisseria meningitidis (Nmen). Confluent monolayers of human meningioma cells were challenged with bacteria, and host cell viability was determined over time with a live/dead cytotoxicity assay and compared with control, uninfected cells. Confocal images are representative of experiments carried out in triplicate with two different meningioma cell lines infected with 108 CFU/monolayer of bacteria. The green fluorescence denotes viable cells, whereas uptake of red fluorescence denotes necrotic cell death. Saponin treatment of an uninfected monolayer was used as a positive control for cell death.
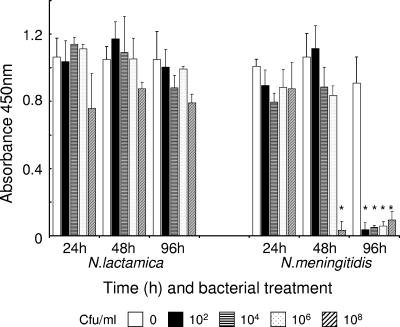
A spectrophotometric assay was next used to quantify total host cell viability following bacterial challenge over time by measuring the cleavage of the tetrazolium salt WST-1 to formazan by host cell mitochondrial dehydrogenases (Fig. 5). N. lactamica did not affect the viability of meningioma cells at any time up to 96 h and at any of the concentrations tested (Fig. 5), which confirmed the results of the fluorescence-based assays (Fig. 4c). Meningioma cell viability was also unaffected by challenge with concentrations of N. meningitidis from 102 to 106 CFU, but bacteria at 108 CFU induced >95% reduction in cell viability by 48 h (Fig. 5). However, prolonged contact of monolayers with N. meningitidis for 96 h resulted in total reduction of cell viability (>95%) regardless of the initial concentrations of bacteria used (Fig. 5), and this correlated with the absence of viable cells (Fig. 4f).
FIG. 5.
Viability of human meningioma cells determined by measuring the cleavage of the tetrazolium salt WST-1 to formazan by host cell mitochondrial dehydrogenases. Confluent monolayers of human meningioma cells were challenged over time with various doses of Neisseria lactamica and Neisseria meningitidis, and formazan dye was quantified with a spectrophotometric assay as a measure of total host cell viability. Control cells were maintained in normal culture medium. Infection experiments were carried out in triplicate with two different meningioma cell lines. Data are shown from a typical experiment with meningioma cells from one patient, with the columns representing mean absorbance (A450) and the error bars representing the standard deviations from triplicate-infected wells. An asterisk indicates statistical significance (P < 0.05) compared with control, uninfected cell monolayers. Incubation of bacteria alone (approximately 108 CFU/ml) with WST-1 did not result in any production of formazan dye.
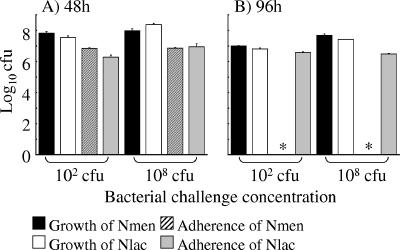
Taken together, these data demonstrate that after prolonged contact, N. meningitidis but not N. lactamica is able to induce death in meningioma cells. However, it is possible that differences in the growth rates of N. lactamica and N. meningitidis could account for the observed differences in the ability to induce cell death. In order to exclude this possibility, growth of bacteria in culture medium and attachment to cell monolayers was quantified at 48 and 96 h, when cell death was apparent. No significant differences (P > 0.05) were observed in the numbers of viable N. lactamica and N. meningitidis cells grown from culture medium at 48 h, when either a low (102) or high (108 CFU) concentration of each bacterium was used to infect the monolayers (Fig. 6). This was similarly the case by 96 h. Therefore, the differences between the two bacteria in their abilities to induce cell death were unrelated to differences in bacterial growth during the infection experiments. However, differences were observed in bacterial association: by 96 h, no significant numbers of N. meningitidis bacteria were recovered, and this observation correlated with the absence of viable cell monolayers (Fig. 4f and 5), whereas the recovery of adherent N. lactamica correlated with the presence of intact monolayers (Fig. 4c and 5).
FIG. 6.
Comparison of the growth of Neisseria lactamica (Nlac) and Neisseria meningitidis (Nmen) in culture and association to meningioma cells at time points when cell death is apparent. Monolayers of meningioma cells were infected with various concentrations of N. lactamica and N. meningitidis, and growth in culture and association to cell monolayers were quantified at 48 and 96 h. Data are shown for infection with the low, nonsaturating (102 CFU) concentration or high, saturating (108 CFU) concentration of each bacterium. Infection experiments were carried out in triplicate with two different meningioma cell lines, and data are shown from a typical experiment with meningioma cells from one patient. The columns represent mean log10 bacterial CFU, and the error bars represent the standard deviations from triplicate-infected wells. An asterisk denotes no recovery of N. meningitidis bacteria from monolayers.
We next investigated whether this cell death induced by N. meningitidis was solely a consequence of necrosis or whether apoptosis was involved. In a previous study of meningococcal interactions with meningeal cells, microarray technology revealed a general trend in down-regulation of proapoptotic genes, with the exception of the interferon regulatory factor 1 gene and an up-regulation of antiapoptotic genes (64). These data suggested that the bacteria inhibited potential apoptotic mechanisms at least at the transcriptional level, but the phenotype of this differential gene expression was not investigated. In the current study, the apoptotic phenotype was investigated at early (6 to 24 h) and late time points (48 to 96 h) by determining annexin V binding as a marker for the externalization of phosphatidylserine, a hallmark change in the cell surface during apoptosis (48); by dual staining with acridine orange and ethidium bromide to differentiate between live, necrotic, and apoptotic cells (9, 46); by identifying the appearance of DNA fragments (65); and by determining the upregulation of caspase-3 and -8 enzymes (15).
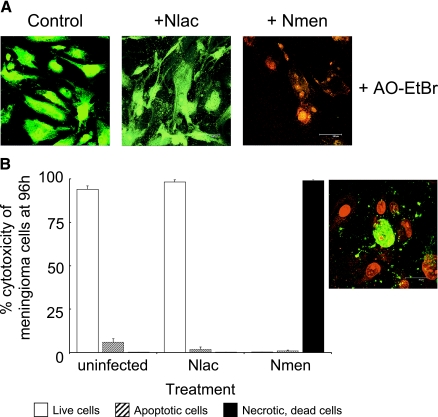
Dual staining with acridine orange and ethidium bromide showed the presence of necrotic cells and the absence of apoptotic cells in cultures following 96 h of infection with N. meningitidis, whereas cells infected with N. lactamica remained viable (Fig. 7A). Labeling with annexin V demonstrated that control, uninfected meningioma cell monolayers were viable (>95%) and contained small numbers of apoptotic cells (<5% of the total cell population) by 96 h (Fig. 7B). Infection with N. lactamica did not induce any significant change in the percentage of cells labeled with annexin V (P > 0.05) over time, and viability remained >95% by 96 h (Fig. 7B). This was similarly the case in monolayers that were still intact during meningococcal infection at early time points (data not shown). However, with gross destruction of the monolayers by 96 h, all meningioma cells detached from the substratum (>95% of the monolayer population) were necrotic (Fig. 7B), as were the few remaining attached cells, with only an occasional cell showing signs of late apoptosis (Fig. 7B, inset).
FIG. 7.
Prolonged infection with Neisseria meningitidis (Nmen) but not Neisseria lactamica (Nlac) induces necrotic cell death in human meningioma cells. Confluent monolayers of meningioma cells from two patients were infected in triplicate for up to 96 h with 108 CFU of bacteria. (A) Confocal images are from one of the experiments with cells from one patient, and they are typically representative of monolayers after 96 h of challenge with bacteria. Dual staining with acridine orange and ethidium bromide (AO-EtBr) was also done; in each panel, green fluorescence denotes viable cells and red fluorescence denotes necrotic cells. (B) Percentages of live, apoptotic, and necrotic cells in meningioma cell monolayers after 96 h of challenge with bacteria. Typically representative data are from one of the experiments with cells from one patient. Apoptotic and necrotic cells were visualized by annexin V and propidium iodide staining, respectively, and they were quantified as the mean percentage of cells (from a minimum of six confocal microscopy fields) of the total number of cells (from a minimum of six fields, each containing approximately 200 cells) present in normal monolayers. The inset figure shows a representative confocal image of a monolayer after 96 h of infection with meningococci, in which gross destruction is evident and the remaining cells are necrotic (counterstained red with propidium iodide) with only an occasional cell showing signs of late apoptosis (green fluorescence surrounding a red nucleus). Control cells and cells infected with N. lactamica show little or no annexin V staining (images not shown).
In addition, challenge with either bacterium did not induce DNA laddering characteristic of apoptosis or increases in caspase enzyme activity at any time point (data not shown). Thus, prolonged bacterial challenge did not induce any apoptotic phenotype in meningioma cell cultures above the low levels associated with normal cellular turnover.
DISCUSSION
In the present study, we investigated the nature of the in vitro interactions between cells derived from the human meninges and Neisseria lactamica, which has also been associated with intracranial inflammation, like the closely related bacterium Neisseria meningitidis. It is generally accepted that N. lactamica associates primarily with cells of mucosal epithelia (5, 22, 30, 53, 55), but significantly, we now demonstrate that N. lactamica also shows a predilection for adhering to human meningeal cells. Moreover, the dynamics of association were similar to those observed for N. meningitidis, although the overall levels of association with N. lactamica were generally lower. In a previous study, pili were identified as the major ligand that mediated adherence of N. meningitidis to meningeal cells (32). Pilin (pilE) genes are present in a wide range of Neisseria species (3), and analysis of pilE loci from different Neisseria species has shown the presence of two distinct structural classes: one class (class II) includes the pilin genes from N. lactamica and some strains of N. meningitidis, and the other (class I) contains gonococci and the remaining N. meningitidis strains (2). Expression of class I or class II pili by different strains of N. meningitidis has been shown not to influence adherence to meningeal cells (32), and since N. lactamica also expresses class II pili, it is unlikely that the class of pilus expressed accounts for the observed differences in levels of adherence of this bacterium compared with N. meningitidis. It is possible that adherence is influenced by differences between the species in expression of the pilin-associated protein PilC, which is present in N. meningitidis but absent in N. lactamica (44, 45). However, other potential adhesins, such as the Opa and Opc proteins (16, 61), which are both present in N. meningitidis but absent in the N. lactamica species used in this study, may also contribute to the observed differences in adherence between the two species. In addition, the fact that challenge with a saturating MOI of N. lactamica, compared with the same concentrations of N. meningitidis, still resulted in lower levels of N. lactamica association with meningioma cells than N. meningitidis at each time point suggests that a difference in host cell receptors used by these species and/or receptor density may also influence adherence.
The range of bacterial concentrations (ratio of MOI of bacteria to cells of 0.002:1 to 2,000:1) used to infect meningioma cells showed a correlation with the concentration of bacteria found in the CSF during natural infection. Although N. lactamica and N. meningitidis can be cultured from CSF, estimates of bacterial numbers in untreated patients with meningitis are unreliable even if care is taken to optimize sample collection and culture procedures (7). In addition, reported numbers are likely to be underestimates that do not take into account bacterial cell death occurring in the CSF. In the case of N. meningitidis, the levels of meningococcal LPS in the CSF accurately reflect the bacterial growth rate and the ability to release LPS (7). Moreover, quantification of LPS content in meningococci showed that LPS at 100 ng/ml was equivalent to approximately 108 bacteria (57). The study of Brandtzaeg et al. (8) reported median LPS levels in the CSF of patients with meningitis at 2.5 ng/ml (range, 0.025 to 500 ng/ml), and this converts to approximately 2.5 × 106 CFU/ml (range, 2.5 × 104 to 2.5 × 108 CFU/ml). Thus, these median values are in general agreement with our concentrations of meningococci (102 to 108 CFU) used to infect meningioma cells. Although to our knowledge there are no published numbers for N. lactamica recovered from the CSF of patients with meningitis, it is possible that large bacterial numbers could occur through direct inoculation from surgery or from other trauma.
An important finding from the present study was that cells derived from the meninges were involved in the innate host defense response to N. lactamica. Meningioma cells challenged with this organism secreted a specific subset of proinflammatory (IL-6), chemoattractant (IL-8, MCP-1, and RANTES), and growth-factor-related (GM-CSF) cytokines. This profile of cytokine secretion was also observed following infection with N. meningitidis (13, 26), suggesting that N. lactamica and N. meningitidis share similar modulins capable of stimulating the meninges. However, although the levels of IL-8, MCP-1, and GM-CSF secretion induced by N. lactamica and N. meningitidis were essentially similar for each cytokine measured, some differences were observed in IL-6 and RANTES secretion. N. lactamica induced lower levels of IL-6 secretion by meningeal cells than N. meningitidis. Interestingly, OM from both bacteria were poor stimulators of IL-6 production, suggesting that components of the bacteria other than OM modulins are largely responsible for induction of this cytokine. This conclusion is consistent with our previous study, which demonstrated that OM and also pure LPS from meningococci had a minimal effect on IL-6 secretion (13). Recent studies have shown that both immunoglobulin A (IgA) protease and peptidoglycan fragments, which are both secreted by pathogenic Neisseria (20, 39), up-regulate IL-6 production by peripheral blood mononuclear cells. However, N. lactamica does not produce IgA protease (44, 45), and it is possible that the observed induction of IL-6 secretion is due to peptidoglycan release, although the involvement of additional uncharacterized secreted components cannot be excluded (49). In the case of RANTES, it was of particular interest that while low concentrations of both bacteria induced similar levels of secretion, high concentrations of N. meningitidis significantly down-regulated chemokine production during infection. This was also observed with the highest concentration of N. lactamica during infection, but the effect was less pronounced compared to that with N. meningitidis, and such differences may be important contributions to the pathogenesis of infection caused by meningococci.
Knowledge of the modulins expressed by N. lactamica and N. meningitidis is important for understanding how both organisms are likely to interact with human meningeal cells. In the present study, the phenotype of the meningococcus was similar to that of strains isolated from the CSF of patients with meningitis (19, 52) in expressing capsule, pili, LPS, and the major outer membrane proteins PorA, PorB, Opa, Opc, and Rmp. To our knowledge, no information is available on the antigens expressed by Neisseria lactamica isolated from the CSF of patients with meningitis. However, N. lactamica does express pili, LPS, PorB, and Rmp but not capsule, PorA, or Opc. Recently, comparative genomic studies of N. lactamica and N. meningitidis have demonstrated further differences between the two bacteria (44, 45). Conserved DNA sequences encoding virulence-associated genes were identified in N. meningitidis that were absent in N. lactamica, including those genes encoding capsule synthesis, secreted Frp and RTX toxins, PilC, and IgA1 protease, as well as several genes whose products are homologous with virulence-associated proteins found in other bacteria. In addition, several genes encoding enzymes associated with metabolic pathways, transporter proteins, integral membrane proteins, and lipoproteins were present in N. meningitidis and absent in N. lactamica (44, 45). Interestingly, an in vitro study of gene expression in N. lactamica and N. meningitidis on contact with epithelial cells demonstrated changes in expression of 285 genes in N. lactamica and 347 genes in N. meningitidis, with 167 genes common to both organisms (30). Dissimilarities in gene expression between the bacteria, identified by both comparative genomics (44, 45) and following host cell interaction (30), may account in part for differences in the pathogenicity of N. lactamica and N. meningitidis, particularly with respect to the ability of the latter to invade the nasopharyngeal mucosa, survive in the blood, and penetrate the blood-CSF barrier.
Based on the data in the present study, a model by which N. lactamica induces intracranial inflammation can be proposed. Despite the obvious genetic and phenotypic differences between the bacteria, following entry into the SAS, N. lactamica is able to proliferate in the CSF, adhere to meningeal cells, and induce an inflammatory response similar to that observed with N. meningitidis. The interactions of N. lactamica, bacterial products, and, to a lesser extent, released OM induce the secretion of IL-6, IL-8, MCP-1, RANTES, and GM-CSF by leptomeningeal cells. This pattern of secretion is consistent with the elevated levels of these and other cytokines found in the CSF of patients with meningitis caused by N. meningitidis and other bacteria (12). Roles for IL-8 in the in vivo chemotaxis of polymorphonuclear leukocytes (PMNL) into the CSF, abetted by up-regulation of cell adhesion molecules on blood vessel endothelium, a consequence in part of RANTES secretion, have been demonstrated (1, 23). However, the ability of large numbers of N. lactamica cells to down-regulate RANTES production to some extent suggests that N. lactamica may influence the ability of PMNL to invade the SAS, thereby allowing bacterial persistence in the CSF. Moreover, down-regulation of RANTES was even more pronounced following N. meningitidis infection, suggesting that this bacterium shows a greater capacity for manipulating the host PMNL response. Clearance of bacteria in the SAS is also dependent on MCP-1 secretion, which results in monocyte accumulation in the SAS, with GM-CSF acting as a maturation factor (27). Although our data suggest that lower levels of IL-6 production by the meninges in response to N. lactamica may lead to a reduction in leukocytosis and induction of the fever response, this is likely to be outweighed by the proinflammatory cytokine production by infiltrating PMNL and monocytes and resident macrophages.
During the course of leptomeningitis, significant cell and tissue injury is observed in the SAS. Both N. lactamica and N. meningitidis have been reported to kill blood vessel endothelial cells in vitro (21), exacerbating further entry into the CSF of bacteria and inflammatory cells. However, leptomeningitis is an acute, compartmentalized inflammatory response, and both the bacterial and inflammatory cell exudate are largely contained within the SAS. In particular, the inability of N. lactamica to invade meningeal cells, a property shared with N. meningitidis (32), suggests that the pia mater provides a barrier to direct penetration of either bacterium to the sub-pial space and brain cortex below. However, a significant finding from the present study was that whereas meningeal cells remained viable on prolonged challenge with N. lactamica, cell death was induced by N. meningitidis and the mechanism was overwhelming necrosis with no significant apoptosis. Our observations on the lack of an apoptotic phenotype following meningococcal challenge are consistent with the reports from Wells et al. (64) and Robinson et al. (49), who demonstrated that meningococci induced elevated gene expression of antiapoptotic factors in meningioma cells and a small but significant resistance effect to staurosporine-induced apoptosis. Thus, the ability to kill meningeal cells suggests that meningococci and components of the pathogen may be able to interact with the underlying glia limitans superficial to the brain cortex, which is consistent with astrocyte activation that is often observed during bacterial meningitis (28). It is possible that this sequence of events may result in a higher degree of neurological damage and death from N. meningitidis meningitis compared with infection caused by N. lactamica.
In summary, the interactions of N. lactamica with cells derived from the meninges share many similarities with N. meningitidis, suggesting that when Neisseria species enter the CSF the innate response of the human meninges is remarkably conserved. However, it is likely that differential expression of modulins between the bacteria contributes to the observed differences in pathogenic potential, such as cytokine regulation and induction of cell death, and consequently may influence the eventual prognosis for the patient with leptomeningitis. Identifying the nature of the bacterial components involved may suggest new targets for therapeutic intervention during infection.
Acknowledgments
The work was supported by the Meningitis Research Foundation and by a Medical Research Council studentship awarded to Mark Fowler.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Adams, D. H., and A. R. Lloyd. 1997. Chemokines: leucocyte recruitment and activation of cytokines. Lancet 349:490-495. [DOI] [PubMed] [Google Scholar]
- 2.Aho, E. L., A. M. Keating, and S. M. McGillivray. 2000. A comparative analysis of pilin genes from pathogenic and nonpathogenic Neisseria species. Microb. Pathog. 28:81-88. [DOI] [PubMed] [Google Scholar]
- 3.Aho, E. L., R. Urwin, A. E. Batcheller, A. M. Holmgreng, K. Havi, A. M. Kulakoski, E. E. Vomhof, N. S. Longfors, C. B. Erickson, Z. K. Anderson, J. M. Dawlaty, and J. J. Mueller. 2005. Neisserial pilin, genes display extensive interspecies diversity. FEMS Microbiol. Lett. 249:327-334. [DOI] [PubMed] [Google Scholar]
- 4.Alcolado, R., R. O. Weller, E. P. Parrish, and D. Garrod. 1988. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol. Appl. Neurobiol 14:1-17. [DOI] [PubMed] [Google Scholar]
- 5.Bergman, P., L. Johansson, V. Asp, L. Plant, G. H. Gudmundsson, A. B. Jonsson, and B. Agerberth. 2005. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell. Microbiol. 7:1009-1017. [DOI] [PubMed] [Google Scholar]
- 6.Bowler, L. D., Q. Y. Zhang, J. Y. Riou, and B. G. Spratt. 1994. Interspecies recombination between the PenA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis - natural events and laboratory simulation. J. Bacteriol. 176:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P. 1996. Systemic meningococcal disease: clinical pictures and pathophysiological background. Rev. Med. Microbiol. 7:63-72. [Google Scholar]
- 8.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166:650-652. [DOI] [PubMed] [Google Scholar]
- 9.Braun, J. S., R. Novak, P. J. Murray, C. M. Eischen, S. A. Susin, G. Kroemer, A. Halle, J. R. Weber, E. I. Tuomanen, and J. L. Cleveland. 2001. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J. Infect. Dis. 184:1300-1309. [DOI] [PubMed] [Google Scholar]
- 10.Brown, N. M., N. K. Ragge, and D. C. E. Speller. 1987. Septicemia due to Neisseria lactamica initial confusion with Neisseria meningitidis. J. Infect. 15:243-245. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright, K. A. V. 1995. Meningococcal carriage and disease, p. 115-146. In K. A. V. Cartwright (ed.), Meningococcal disease. John Wiley & Sons, Chichester, United Kingdom.
- 12.Christodoulides, M., J. E. Heckels, and R. O. Weller. 2002. The role of the leptomeninges in meningococcal meningitis, p. 1-37. In C. Ferreiros, M. T. Criado, and J. Vazquez (ed.), Emerging strategies in the fight against meningitis. Horizon Press, Norfolk, United Kingdom.
- 13.Christodoulides, M., B. L. Makepeace, K. Partridge, D. Kaur, M. I. Fowler, R. O. Weller, and J. E. Heckels. 2002. Interaction of Neisseria meningitidis with human meningeal cells induces the secretion of a distinct group of chemotactic, proinflammatory, and growth factor cytokines. Infect. Immun. 70:4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christodoulides, M., E. Rattue, and J. E. Heckels. 1999. Influence of adjuvants on the humoral immune response towards a synthetic peptide containing a B-cell epitope from meningococcal class 1 protein. Vaccine 18:131-139. [DOI] [PubMed] [Google Scholar]
- 15.Degterev, A., M. Boyce, and J. Y. Yuan. 2003. A decade of caspases. Oncogene 22:8543-8567. [DOI] [PubMed] [Google Scholar]
- 16.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 17.Del Rio, C., D. S. Stephens, J. S. Knapp, R. J. Rice, and W. O. Schalla. 1989. Comparison of isolates of Neisseria gonorrhoeae causing meningitis and report of gonococcal meningitis in a patient with C8 deficiency. J. Clin. Microbiol. 27:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning, D. W., and S. S. Gill. 1991. Neisseria lactamica meningitis following skull trauma. Rev. Infect. Dis. 13:216-218. [DOI] [PubMed] [Google Scholar]
- 19.Devoe, I. W., and J. E. Gilchrist. 1975. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J. Exp. Med. 141:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(Anh)Mtetra, a natural bacterial-cell wall breakdown product, induces interleukin-1-beta and interleukin-6 expression in human monocytes - a study of the molecular mechanisms involved in inflammatory cytokine expression. J. Biol. Chem. 269:4201-4206. [PubMed] [Google Scholar]
- 21.Dunn, K. L. R., M. Virji, and E. R. Moxon. 1995. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb. Pathog. 18:81-96. [DOI] [PubMed] [Google Scholar]
- 22.El Ahmer, O. R., M. W. Raza, M. M. Ogilvie, D. M. Weir, and C. C. Blackwell. 1999. Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol. Med. Microbiol. 23:331-341. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender, K., U. Schminke, S. Ries, A. Ragoschke, U. Kischka, M. Fatar, and M. Hennerici. 1997. Endothelial-derived adhesion molecules in bacterial meningitis: association to cytokine release and intrathecal leukocyte- recruitment. J. Neuroimmunol. 74:130-134. [DOI] [PubMed] [Google Scholar]
- 24.Feil, E., J. J. Zhou, J. M. Smith, and B. G. Spratt. 1996. A comparison of the nucleotide sequences of the adk and recA genes of pathogenic and commensal Neisseria species: evidence for extensive interspecies recombination within adk. J. Mol. Evol. 43:631-640. [DOI] [PubMed] [Google Scholar]
- 25.Feurer, D. J., and R. O. Weller. 1991. Barrier functions of the leptomeninges: a study of normal meninges and meningiomas in tissue culture. Neuropathol. Appl. Neurobiol. 17:391-405. [DOI] [PubMed] [Google Scholar]
- 26.Fowler, M. I., R. O. Weller, J. E. Heckels, and M. Christodoulides. 2004. Different meningitis-causing bacteria induce distinct inflammatory responses on interaction with cells of the human meninges. Cell. Microbiol. 6:555-567. [DOI] [PubMed] [Google Scholar]
- 27.Geissler, K., M. Harrington, C. Srivastava, T. Leemhuis, G. Tricot, and H. E. Broxmeyer. 1989. Effects of recombinant human colony stimulating factors (Csf) (granulocyte-macrophage Csf, granulocyte Csf, and Csf-1) on human monocyte macrophage differentiation. J. Immunol. 143:140-146. [PubMed] [Google Scholar]
- 28.Gray, F., and P. Nordmann. 1997. Bacterial infections, p. 113-156. In D. I. Graham and P. L. Lantos (ed.), Greenfields neuropathology: infections. Arnold, London, United Kingdom.
- 29.Greenberg, L. W., and E. Kleinerman. 1978. Neisseria lactamica meningitis. J. Pediatr. 93:1061-1062. [DOI] [PubMed] [Google Scholar]
- 30.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, F. Randazzo, and G. Grandi. 2002. Gene expression profile in Neisseria meningitidis and Neisseria lactamica upon host-cell contact: from basic research to vaccine development. Ann. N. Y. Acad. Sci. 975:202-216. [DOI] [PubMed] [Google Scholar]
- 31.Hansman, D. 1978. Meningitis caused by Neisseria lactamica. N. Engl. J. Med. 299:491. [PubMed] [Google Scholar]
- 32.Hardy, S. J., M. Christodoulides, R. O. Weller, and J. E. Heckels. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36:817-829. [DOI] [PubMed] [Google Scholar]
- 33.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollis, D. G., G. L. Wiggins, and R. E. Weaver. 1969. Neisseria lactamica sp.n., a lactose-fermenting species resembling Neisseria meningitidis. Appl. Microbiol. 17:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphries, H. E., M. Triantafilou, B. L. Makepeace, J. E. Heckels, K. Triantafilou, and M. Christodoulides. 2005. Activation of human meningeal cells is modulated by lipopolysaccharide (LPS) and non-LPS components of Toll-like receptor (TLR)4 and TLR2 signalling. Cell. Microbiol. 7:415-430. [DOI] [PubMed] [Google Scholar]
- 36.Lauer, B. A., and C. E. Fisher. 1976. Neisseria lactamica meningitis. Am. J. Dis. Child. 130:198-199. [DOI] [PubMed] [Google Scholar]
- 37.Lin, C. C., Y. C. Yeh, C. Y. Yang, C. L. Chen, G. F. Chen, C. C. Chen, and Y. C. Wu. 2002. Selective binding of mannose-encapsulated gold nanoparticles to type 1 pili in Escherichia coli. J. Am. Chem. Soc. 124:3508-3509. [DOI] [PubMed] [Google Scholar]
- 38.Linz, B., M. Schenker, P. X. Zhu, and M. Achtman. 2000. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol. Microbiol. 36:1049-1058. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzen, D. R., F. Dux, U. Wolk, A. Tsirpouchtsidis, G. Haas, and T. F. Meyer. 1999. Immunoglobulin A1 protease, an exoenzyme of pathogenic neisseriae, is a potent inducer of proinflammatory cytokines. J. Exp. Med. 190:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massari, P., Y. Ho, and L. M. Wetzler. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. USA 97:9070-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 42.Orden, B., and M. A. Amerigo. 1991. Acute otitis media caused by Neisseria lactamica. Eur. J. Clin. Microbiol. Infect. Dis. 10:986-987. [DOI] [PubMed] [Google Scholar]
- 43.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrin, A., S. Bonacorsi, E. Carbonnelle, D. Talibi, P. Dessen, X. Nassif, and C. Tinsley. 2002. Comparative genomics identifies the genetic islands that distinguish Neisseria meningitidis, the agent of cerebrospinal meningitis, from other Neisseria species. Infect. Immun. 70:7063-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrin, A., X. Nassif, and C. Tinsley. 1999. Identification of regions of the chromosome of Neisseria meningitidis and Neisseria gonorrhoeae which are specific to the pathogenic Neisseria species. Infect. Immun. 67:6119-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitrak, D. L., H. C. Tsai, K. M. Mullane, S. H. Sutton, and P. Stevens. 1996. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J. Clin. Investig. 98:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pong, A., and J. S. Bradley. 1999. Bacterial meningitis and the newborn infant. Infect. Dis. Clin. N. Am. 13:711-733. [DOI] [PubMed] [Google Scholar]
- 48.Reutelingsperger, C. P. M., and W. L. vanHeerde. 1997. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 53:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson, K., M. Taraktsoglou, K. S. J. Rowe, K. G. Wooldridge, and D. A. A. Ala'Aldeen. 2004. Secreted proteins from Neisseria meningitidis mediate differential human gene expression and immune activation. Cell. Microbiol. 6:927-938. [DOI] [PubMed] [Google Scholar]
- 50.Schagger, H., and G. von Jagow 1987. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 51.Schifman, R. B., and K. J. Ryan. 1983. Neisseria lactamica septicemia in an immunocompromised patient. J. Clin. Microbiol. 17:934-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephens, D. S., K. M. Edwards, and F. M. G. Morris. 1982. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 146:568. [DOI] [PubMed] [Google Scholar]
- 53.Telferbrunton, W. A., H. Young, and D. R. K. Fraser. 1980. Isolation of Neisseria Lactamica from the female genital tract - a case report. Br. J. Vener. Dis. 56:325-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tinsley, C. R., and J. E. Heckels. 1986. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J. Gen. Microbiol. 132:2483-2490. [DOI] [PubMed] [Google Scholar]
- 55.Townsend, R., L. Goodwin, T. M. Stevanin, P. B. Silcocks, A. Parker, M. C. J. Maiden, and R. C. Read. 2002. Invasion by Neisseria meningitidis varies widely between clones and among nasopharyngeal mucosae derived from adult human hosts. Microbiology 148:1467-1474. [DOI] [PubMed] [Google Scholar]
- 56.Tuomanen, E. 1999. Molecular and cellular biology of pneumococcal infection. Curr. Opin. Microbiol. 2:35-39. [DOI] [PubMed] [Google Scholar]
- 57.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. van der Ley, M. van Deuren, R. E. Callard, and N. Klein. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Deuren, M., P. Brandtzaeg, and J. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virji, M., C. Alexandrescu, D. J. P. Ferguson, J. R. Saunders, and E. R. Moxon. 1992. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol. Microbiol. 6:1271-1279. [DOI] [PubMed] [Google Scholar]
- 60.Virji, M., H. Kayhty, D. J. P. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 61.Virji, M., K. Makepeace, D. J. P. Ferguson, M. Achtman, and E. R Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 62.Virji, M., K. Zak, and J. E Heckels. 1987. Outer-membrane protein-iii of Neisseria-gonorrhoeae - variations in biological properties of antibodies directed against different epitopes. J. Gen. Microbiol. 133:3393-3401. [DOI] [PubMed] [Google Scholar]
- 63.Weller, R. O. 1995. Fluid compartments and fluid balance in the central nervous system, p. 1202-1204. In P. L. Williams (ed.), Gray's anatomy. Churchill Livingstone, Edinburgh, United Kingdom.
- 64.Wells, D. B., P. J. Tighe, K. G. Wooldridge, K. Robinson, and D. A. A. A. Aldeen. 2001. Differential gene expression during meningeal-meningococcal interaction: evidence for self-defense and early release of cytokines and chemokines. Infect. Immun. 69:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willingham, M. C. 1999. Cytochemical methods for the detection of apoptosis. J. Histochem. Cytochem. 47:1101-1109. [DOI] [PubMed] [Google Scholar]
- 66.Wilson, H. D., and T. L. Overman. 1976. Septicemia due to Neisseria lactamica. J. Clin. Microbiol. 4:214-215. [DOI] [PMC free article] [PubMed] [Google Scholar]