Abstract
The Clostridium perfringens alpha-toxin has previously been implicated as the major virulence factor in necrotic enteritis in chickens, although definitive proof has not been reported. In this study an alpha-toxin mutant was constructed in a virulent chicken isolate and shown to retain full virulence in a chicken disease model. These results demonstrated that alpha-toxin is not an essential virulence factor in the pathogenesis of necrotic enteritis in chickens.
Avian necrotic enteritis (NE) was first described by Parish in 1961 (33), and since then it has been reported to occur in almost all poultry-producing countries (8, 17, 24, 29). NE is an enteric disease that is caused predominantly by Clostridium perfringens type A and to a lesser extent by type C strains (41). Clinical NE is thought to occur when C. perfringens proliferates to high numbers in the small intestine and produces extracellular toxins that damage the intestine. The major toxin believed to be involved is the alpha-toxin, but its precise role in the disease process is not completely understood. The alpha-toxin is a secreted zinc-metalloenzyme which has both phospholipase C and sphingomyelinase activity and is the major toxin involved in the pathogenesis of human gas gangrene (5, 42). All five toxin types of C. perfringens (A to E) carry and express the alpha-toxin structural gene, plc.
Early studies on the reproduction of the disease involved intraduodenal infusion of large volumes of broth culture (2) or crude toxin (3) into chickens. Typical lesions of NE were seen as early as 5 h after infusion of C. perfringens cells. From these studies the authors concluded that because alpha-toxin was the major toxin secreted by C. perfringens, alpha-toxin must be the major virulence factor causing NE in chickens. Another study used oral inoculation with broth culture to cause characteristic necrotic lesions (20) and found that although many germfree birds died, no commercial birds died. A third of the birds inoculated with semipurified alpha-toxin died, but no bird died after receiving culture supernatant neutralized by antiserum raised against the semipurified alpha-toxin. Over the years, these studies have been the principle evidence given for the proposed major role of alpha-toxin in NE in chickens. The limitation of this interpretation is that it does not take into account other secreted toxins that the bacteria may have produced. While C. perfringens isolates are toxin typed by the presence of four major toxins, α, β, ɛ, and ι, various strains can also produce a range of other toxins (CPE, β2 toxin, perfringolysin O [θ-toxin], collagenase [κ-toxin], etc.).
Other studies call into doubt the causative role of alpha-toxin in NE. In one report, no difference in alpha-toxin levels was found when in vitro alpha-toxin levels were compared between isolates from diseased and healthy birds (22). Yet another study found that the intestinal level of alpha-toxin was not correlated with disease lesion scores (46). Another inconsistency in the assumptions regarding the role of alpha-toxin is the extent of heterophil, lymphocyte, and plasma cell infiltration in infected tissues (2, 21, 36). In clostridial myonecrosis (gas gangrene), a disease primarily mediated by the same alpha-toxin, there is marked leukostasis and lack of inflammatory infiltrate in tissues infected by C. perfringens cells (19). By contrast, in NE there is a typically extensive immune cell infiltration, indicating quite a different etiology compared to classical alpha-toxin-induced disease. The construction and virulence testing of plc mutants (5, 12, 31) have been important in determining the role of alpha-toxin in gas gangrene, but to date there have been no reports of the construction of a defined plc mutant in a C. perfringens chicken isolate.
In this study, alpha-toxin levels of chicken isolates were compared to virulence data obtained from the same isolates in commercial birds. We also report the construction of chromosomal plc mutants of a virulent poultry isolate. Virulence testing of the resultant defined chromosomal mutants showed that alpha-toxin was not an essential virulence factor in NE.
Isolation and characterization of C. perfringens from diseased flocks.
Eighteen isolates (Table 1) were obtained from six broiler flocks from birds that suffered from NE. These strains were isolated from gut contents, intestinal samples, liver, and kidney. Multiplex PCR toxin typing (10, 28) carried out using reference strains (Table 1) as positive controls showed that all 18 isolates were C. perfringens toxin type A (Table 2), which agrees with surveys carried out in Europe (16, 22, 30), Korea (48), and North America (28). Two isolates were positive for the cpb2 gene, which encodes the β2-toxin. While the β2-toxin has been implicated in NE in other animals, for example, in pigs (45), no correlation between NE disease isolates and β2-toxin has been established.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Plasmids | ||
| pSM20 | Knrgfp+ | 27 |
| pJIR750 | CmroriCP oriEC | 9 |
| pALK1 | pSM20 Ω (BamHI-SpeI:pJIR750, 0.9 kb) Δgfp (Cmr Knr) | Recombinant |
| pALK2 | pALK1 Ω (1.8-kb upstream plc fragment, catP, 2.1-kb downstream plc fragment) (Cmr Knr) | Recombinant |
| Strains | ||
| JIR325 | Strain 13 Rifr Nalr | 25 |
| JIR4107 | JIR325 plc::erm(B) | 4 |
| ATCC 13124 | Type A (plc+) | |
| CN 1884 | Type B (plc+cpb+etx+) | R. G. Wilkinson (Univ. Melbourne) |
| CN 2109 | Type C (plc+cpb+) | R. G. Wilkinson (Univ. Melbourne) |
| CN 462 | Type D (plc+etx+) | R. G. Wilkinson (Univ. Melbourne) |
| ATCC 27324 | Type E (plc+cpi+cpe+cpb2+) | |
| NE18-M1 | EHE-NE18 Δplc::catP | This work |
| NE18-M4 | EHE-NE18 Δplc::catP | This work |
| NAG-NE1 | Isolated from gut contents of chicken with NE, flock 1 | 37 |
| EHE-NE3 | Isolated from liver of chicken with NE, flock 2 | 37 |
| EHE-NE4 | Isolated from liver of chicken with NE, flock 2 | 37 |
| EHE-NE5 | Isolated from kidney of chicken with NE, flock 2 | 37 |
| EHE-NE7 | Isolated from gut wall of chicken with NE, flock 2 | 37 |
| EHE-NE9 | Isolated from gut wall of chicken with NE, flock 2 | 37 |
| EHE-NE13 | Isolated from gut wall of chicken with NE, flock 2 | 37 |
| EHE-NE14 | Isolated from gut contents of chicken with NE, flock 2 | 37 |
| EUR-NE15 | Isolated from gut contents of chicken with NE, flock 3 | 37 |
| EHE-NE16 | Isolated from gut contents of chicken with NE, flock 4 | 37 |
| EHE-NE17 | Isolated from gut contents of chicken with NE, flock 4 | 37 |
| EHE-NE18 | Isolated from gut contents of chicken with NE, flock 4 | 37 |
| EHE-NE20 | Isolated from gut contents of chicken with NE, flock 5 | This work |
| EHE-NE21 | Isolated from gut contents of chicken with NE, flock 5 | This work |
| EHE-NE22 | Isolated from gut contents of chicken with NE, flock 5 | This work |
| NAG-NE23 | Isolated from gut wall of chicken with NE, flock 6 | 37 |
| NAG-NE24 | Isolated from gut wall of chicken with NE, flock 6 | 37 |
| NAG-NE25 | Isolated from gut wall of chicken with NE, flock 6 | 37 |
Cmr, chloramphenicol resistant; Knr, kanamycin resistant; Nalr, nalidixic acid resistant; Rifr, rifampin resistant.
TABLE 2.
Alpha-toxin and θ-toxin production and PCR-based toxin typing of C. perfringens isolates derived from chickens diagnosed with NE
| Strain no. | Toxin type | Alpha-toxinb (U mg−1 protein) | θ-toxinb (log2 [titer]) |
|---|---|---|---|
| NAG-NE1 | A | (2.3 ± 1.0) × 10−3 | 6.3 ± 0.2 |
| EHE-NE3 | A | (1.9 ± 0.6) × 10−3 | 5.2 ± 1.1 |
| EHE-NE4 | A | (2.0 ± 0.7) × 10−3 | 5.8 ± 0.1 |
| EHE-NE5 | A | (13.3 ± 3.4) × 10−3 | 6.6 ± 0.1 |
| EHE-NE7 | A | (4.3 ± 3.8) × 10−3 | 6.6 ± 0.3 |
| EHE-NE9 | A | (8.3 ± 1.1) × 10−3 | 6.4 ± 0.1 |
| EHE-NE13 | A | (4.0 ± 0.8) × 10−3 | 5.7 ± 0.1 |
| EHE-NE14 | A | (4.0 ± 0.9) × 10−3 | 4.9 ± 0.1 |
| EUR-NE15 | A | (2.1 ± 0.2) × 10−3 | 4.4 ± 0.4 |
| EHE-NE16 | A | (5.2 ± 2.0) × 10−3 | 6.0 ± 0.2 |
| EHE-NE17 | A | (4.8 ± 0.8) × 10−3 | 7.9 ± 0.4 |
| EHE-NE18 | A | (1.9 ± 0.3) × 10−3 | 7.5 ± 0.1 |
| EHE-NE20 | A | (1.1 ± 0.3) × 10−3 | 4.7 ± 0.1 |
| EHE-NE21 | A | (2.7 ± 0.1) × 10−3 | 5.4 ± 0.3 |
| EHE-NE22 | A | (2.3 ± 0.6) × 10−3 | 6.0 ± 0.2 |
| NAG-NE23 | A (cpb2+) | (2.6 ± 1.4) × 10−3 | 6.2 ± 0.4 |
| NAG-NE24 | A (cpb2+) | (2.0 ± 0.5) × 10−3 | 6.0 ± 0.2 |
| NAG-NE25 | A | (3.6 ± 1.2) × 10−3 | 5.6 ± 0.5 |
| JIR325a | A | (16.6 ± 3.3) × 10−3 | 7.5 ± 0.3 |
A derivative of strain 13, a human gas gangrene isolate, used as a control.
Results represent averages of duplicate assays carried out on preparations from at least three separate cultures of each strain, ± standard deviations.
A selection of these isolates was evaluated in a chicken virulence model and found to cause lesions characteristic of NE, although the severity of disease varied among the isolates. Quantitative in vitro alpha-toxin (38) and θ-toxin (4) assays (Table 2) showed that there was no correlation between severity of disease and alpha-toxin or θ-toxin production. The amount of alpha-toxin produced by most of the NE disease isolates was considerably lower than that from the human gas gangrene strain JIR325. Among type A C. perfringens strains, JIR325 is regarded as producing a low level of alpha-toxin (11). Therefore, the alpha-toxin levels found in this collection of disease isolates must be regarded as very low. This result is similar to those of recent studies where no significant difference was found between the levels of alpha-toxin produced from disease isolates and healthy bird isolates (22), and there was no correlation between in vivo toxin levels and lesion scores (46). In one study, in vitro alpha-toxin levels of diseased birds were found to be higher than in uninfected birds (23), but the growing weight of evidence indicates that this is not a common finding.
Construction of plc mutants in the chicken C. perfringens isolate EHE-NE18.
Isolate EHE-NE18 was chosen for subsequent studies because, unlike many of the isolates, it could be transformed (35) with plasmid DNA (pJIR750) and it caused significant disease in the NE induction model. The suicide plasmid pALK2 was constructed by cloning fragments of the plc gene region on either side of the catP cassette in pALK1 (Table 1), which led to an 890-bp deletion of the plc gene. This suicide plasmid was used to transform strain EHE-NE18 to thiamphenicol resistance (10 μg/ml). Two independently derived plc mutants, NE18-M1 and NE18-M4, were isolated from 20 independent transformation experiments, and PCR analysis was used to confirm that both mutants were derived from double reciprocal crossover events between pALK2 and the plc region on the EHE-NE18 chromosome. SmaI pulsed-field gel electrophoresis patterns were determined by using previously described methods (13, 40). EHE-NE18 and its mutants had identical profiles, which confirmed these strains were isogenic mutant isolates.
Quantification of the levels of alpha-toxin and θ-toxin produced by the plc mutants showed that neither mutant synthesized active alpha-toxin (the mutant levels were below the detection limit of the assay; EHE-NE18 produced [1.9 ± 0.3] × 10−3 U · mg−1). Both mutants produced similar levels of perfringolysin O (NE18-M1, 7.5 ± 0.1; NE18-M4, 7.6 ± 0.8; EHE-NE18, 7.5 ± 0.2; units are log2[titer]).
The plc mutants still produce NE in a chicken disease model.
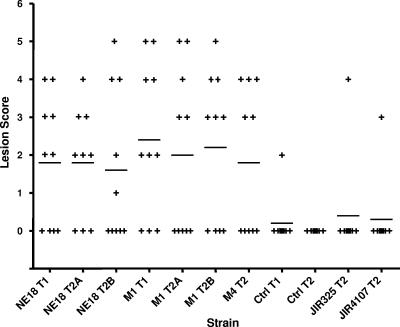
Chickens were challenged with the two plc mutants and the isogenic wild-type strain. Other strains tested included the strain 13 derivative JIR325 and its plc mutant, JIR4107 (4). Commercial Ross 308 broiler chickens were fed an antibiotic-free chicken starter diet containing 20% protein for 13 days. On day 14 feed was changed to a wheat-based feed containing 50% fish meal. On day 20, feed was withdrawn and each bird was orally challenged with 1.5 ml of C. perfringens stationary-phase culture. On day 21, birds were again orally challenged and feed was returned infected with C. perfringens (20 ml culture in first 100 g of feed per group). On day 24, chickens were euthanized with inhaled carbon dioxide gas and their small intestines (duodenum to ileum) were examined for gross necrotic lesions. In each group the survival rate was 100%. The results (Fig. 1) showed that the lesion scores in birds infected by the two independent plc mutants were not significantly different from those in birds infected by the wild-type parent strain. These results were reproducible, since the same results were obtained in independent virulence trials. The unchallenged negative control group from trial 1 had a single bird with a single 1-mm necrotic lesion, presumably resulting from infection by a fortuitous environmentally derived strain. The negative control group in the second trial had no lesions. C. perfringens strains were reisolated directly from the lesions of affected birds. Twenty colonies from each bird were subcultured onto sheep blood agar with or without thiamphenicol. As expected, the lesions sampled from birds challenged with the isolates from the wild-type strain were susceptible to thiamphenicol and produced both alpha-toxin and θ-toxin, whereas birds infected with the mutants yielded only thiamphenicol-resistant C. perfringens colonies that produced θ-toxin but not alpha-toxin. PCR analysis confirmed that the plc gene in the latter isolates remained insertionally inactivated. These results clearly demonstrate that the alpha-toxin-negative mutants produce lesions of equal severity to those in the wild-type strain from which they were derived.
FIG. 1.
NE challenge model. Lesion scores of individual 24-day-old broiler chickens challenged with C. perfringens are shown. Each group consisted of 10 birds. The solid bars represent the average lesion score in each group. Intestinal lesions in the small intestine (duodenum to ileum) were scored as follows: 0, no gross lesions; 1, thin or friable walls; 2, focal necrosis or ulceration (1 to 5 foci); 3, focal necrosis or ulceration (6 to 15 foci); 4, focal necrosis or ulceration (16 or more foci); 5, patches of necrosis 2 to 3 cm long; 6, diffuse necrosis typical of field cases. The results are from two trials (T1 and T2), with some strains tested in duplicate in the second trial (T2A and T2B). Birds not challenged with C. perfringens acted as negative controls (Ctrl). The C. perfringens isolates tested and their abbreviations are as follows: NE18, EHE-NE18; M1, NE18-M1; M4, NE18-M4. One-tailed, nonparametric t test analyses of the challenge (EHE-NE18) and mutant derivatives against their respective controls all showed a statistical difference (P < 0.05), but no statistical significance was seen between the mutant and the wild-type strains.
The human C. perfringens isolate JIR325, which produced significantly higher levels of alpha-toxin than EHE-NE18, did not produce an appreciable level of disease; just a single bird in the treatment group had lesions. There was no difference in disease incidence between the wild-type JIR325 strain and its alpha-toxin mutant, JIR4107 (4).
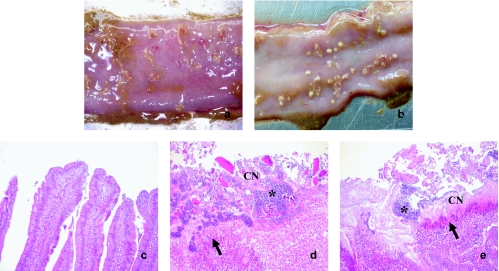
At necropsy, segments of ileum or jejunum measuring approximately 2 to 4 cm were collected into 10% sodium phosphate-buffered formalin. The small intestine samples were cross-sectioned at 4-mm intervals, and segments were processed to paraffin-embedded blocks for routine histology, cut at 4 to 5 μm, and stained with hematoxylin and eosin (H&E). A comparison of the gross pathology of the lesions resulting from infection with EHE-NE18 and NE18-M1 (Fig. 2a and b) showed that there were no observable macroscopic differences between the infected groups. The macroscopic lesions had the typical signs of NE in that the lesions were sharply demarcated from the surrounding mucosa. The histopathology of unchallenged, wild-type, and mutant gut tissue sections was compared. Microscopic examination of the infected tissue (Fig. 2d and e) revealed a small intestine with a thick lumenal lining of acellular coagulative necrotic debris covered by a thick layer of rod-shaped bacteria, with a layer of degenerate and necrotic debris separating the underling viable lamina propria and a heavy invasion of immune cells in the underlying tissue. This typical innate inflammatory response is in contrast with the alpha-toxin-mediated inhibition of neutrophil invasion that is the hallmark of gas gangrene (15). While there was no histologically observable difference between the microscopic lesions from the wild-type and mutant infections, there were significant differences between the challenged and unchallenged birds (Fig. 2c).
FIG. 2.
Gross pathology and histopathology of infected birds. The small intestines of broiler chickens challenged with either EHE-NE18 (a and d) or NE18-M1 (b and e) are shown. (a) Gross pathology of the small intestine of a 24-day broiler chicken challenged with EHE-NE18 (lesion score, 4). (b) Gross pathology of the small intestine of a 24-day broiler chicken challenged with NE18-M1 (lesion score, 4). (c) Histopathology of the small intestine from a control chicken. (d) Histopathology of the small intestine with a thick diffuse layer of necrotic cellular debris mixed with large numbers of bacteria of an H&E-stained section. Magnification, ×100. (e) Histopathology of the small intestine with diffuse coagulative necrosis of the mucosa covered by a thick layer of bacteria/bacilli. The underlying viable tissue is separated from the necrotic mucosa by degenerate heterophils and cellular debris of an H&E-stained section. Magnification, ×100. Arrow, leukocyte or heterophils; *, bacterial cells; CN, coagulative necrosis.
The in vivo analysis of the isogenic plc mutants has provided clear evidence that the alpha-toxin is not an essential virulence factor in the pathogenesis of NE in chickens. The inactivation of the chromosomal plc gene had no effect on the virulence of the resultant strains in chickens. As the wild-type isolate was obtained from a clinical case of chicken NE and produced a disease pathology similar to that reported in many other disease induction models (6, 14, 32, 34, 44, 47), we conclude that our results can be readily extrapolated to other virulent strains.
The observation that the NE18-M1 and NE18-M4 mutants were still completely virulent contrasts with a recent study in which isolated spontaneous alpha-toxin mutants were isolated by repeated passaging and found to be avirulent in their model (43). However, the alpha-toxin mutants were not complemented, failing to rule out the possibility that mutations in other regions of the genome resulted in the strain becoming avirulent.
All toxin types of C. perfringens produce alpha-toxin, yet only some type A and C strains cause disease in chickens. Clearly, alpha-toxin cannot be sufficient to cause disease, as otherwise all C. perfringens strains would be capable of inducing disease, which is clearly not so, as shown by the results obtained with JIR325 in this study. The NE-causing isolates must have other attributes that confer a virulence phenotype. The long-held belief that alpha-toxin is the main virulence factor for NE in chickens has been based on conclusions that extrapolate too much from the data provided. Early studies used culture supernatants to reproduce the disease (3), but these supernatants potentially contained many secreted proteins. Subsequent studies (1, 20) using antibodies prepared against culture supernatants or partially purified toxin preparations did not explore the possibility that toxins other than alpha-toxin may be produced by these bacteria. Since that time, there have been many studies investigating the many other toxins that C. perfringens can produce and their involvement in causing animal disease (7, 18, 26, 39, 45). However, no other candidate toxin that has been found in a majority of isolates that cause NE in chickens has been identified.
In conclusion, this study presents definitive evidence that alpha-toxin is not an essential causative agent of NE in chickens and provides the basis for further work to identify virulence factors that do play a crucial role in the development of this disease. Such studies are currently under way in our laboratories.
Acknowledgments
We thank the Australian Poultry Cooperative Research Centre and the Rural Industries Research and Development Corporation for their support. Anthony Keyburn was the recipient of an Australian Poultry Cooperative Research Centre Postgraduate Scholarship. The research was also supported by a grant from the Australian Research Council to the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
Editor: J. T. Barbieri
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Al-Sheikhly, F., and R. B. Truscott. 1977. The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens. Avian Dis. 21:256-263. [PubMed] [Google Scholar]
- 2.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of broth cultures of Clostridium perfringens into the duodenum. Avian Dis. 21:230-240. [PubMed] [Google Scholar]
- 3.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum. Avian Dis. 21:241-255. [PubMed] [Google Scholar]
- 4.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191-202. [DOI] [PubMed] [Google Scholar]
- 5.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba, E., A. L. Fuller, J. M. Gilbert, S. G. Thayer, and L. R. McDougald. 1992. Effects of Eimeria brunetti infection and dietary zinc on experimental induction of necrotic enteritis in broiler chickens. Avian Dis. 36:59-62. [PubMed] [Google Scholar]
- 7.Bacciarini, L. N., P. Boerlin, R. Straub, J. Frey, and A. Grone. 2003. Immunohistochemical localization of Clostridium perfringens β2-toxin in the gastrointestinal tract of horses. Vet. Pathol. 40:376-381. [DOI] [PubMed] [Google Scholar]
- 8.Bains, B. S. 1968. Necrotic enteritis of chickens. Aust. Vet. J. 44:40. [DOI] [PubMed] [Google Scholar]
- 9.Bannam, T. L., and J. I. Rood. 1993. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 29:233-235. [DOI] [PubMed] [Google Scholar]
- 10.Bueschel, D. M., B. H. Jost, S. J. Billington, H. T. Trinh, and J. G. Songer. 2003. Prevalence of cpb2, encoding β2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121-129. [DOI] [PubMed] [Google Scholar]
- 11.Bullifent, H. L., A. Moir, M. M. Awad, P. T. Scott, J. I. Rood, and R. W. Titball. 1996. The level of expression of α-toxin by different strains of Clostridium perfringens is dependent on differences in promoter structure and genetic background. Anaerobe 2:365-371. [Google Scholar]
- 12.Chen, Y., B. A. McClane, D. J. Fisher, J. I. Rood, and P. Gupta. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ Microbiol. 71:7542-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collie, R. E., and B. A. McClane. 1998. Evidence that the enterotoxin gene can be episomal in Clostridium perfringens isolates associated with non-food-borne human gastrointestinal diseases. J. Clin. Microbiol. 36:30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowen, B. S., L. D. Schwartz, R. A. Wilson, and S. I. Ambrus. 1987. Experimentally induced necrotic enteritis in chickens. Avian Dis. 31:904-906. [PubMed] [Google Scholar]
- 15.Ellemor, D. M., R. N. Baird, M. M. Awad, R. L. Boyd, J. I. Rood, and J. J. Emmins. 1999. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect. Immun. 67:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engstrom, B. E., C. Fermer, A. Lindberg, E. Saarinen, V. Baverud, and A. Gunnarsson. 2003. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet. Microbiol. 94:225-235. [DOI] [PubMed] [Google Scholar]
- 17.Ficken, M. D., and D. P. Wages. 1997. Necrotic enteritis, p. 261-264. In B. W. Calnek (ed.), Diseases of poultry, 10th ed. Mosby-Wolfe, London, England.
- 18.Fisher, D. J., K. Miyamoto, B. Harrison, S. Akimoto, M. R. Sarker, and B. A. McClane. 2005. Association of β2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747-762. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Diaz, M., and A. Alape-Giron. 2003. Role of Clostridium perfringens phospholipase C in the pathogenesis of gas gangrene. Toxicon 42:979-986. [DOI] [PubMed] [Google Scholar]
- 20.Fukata, T., Y. Hadate, E. Baba, T. Uemura, and A. Arakawa. 1988. Influence of Clostridium perfringens and its toxin in germ-free chickens. Res. Vet. Sci. 44:68-70. [PubMed] [Google Scholar]
- 21.Gazdzinski, P., and R. J. Julian. 1992. Necrotic enteritis in turkeys. Avian Dis. 36:792-798. [PubMed] [Google Scholar]
- 22.Gholamiandekhordi, A. R., R. Ducatelle, M. Heyndrickx, F. Haesebrouck, and F. Van Immerseel. 2006. Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status. Vet. Microbiol. 113:143-152. [DOI] [PubMed] [Google Scholar]
- 23.Hofshagen, M., and H. Stenwig. 1992. Toxin production by Clostridium perfringens isolated from broiler chickens and capercaillies (Tetrao urogallus) with and without necrotizing enteritis. Avian Dis. 36:837-843. [PubMed] [Google Scholar]
- 24.Long, J. R. 1973. Necrotic enteritis in broiler chickens. I. A review of the literature and the prevalence of the disease in Ontario. Can. J. Comp. Med. 37:302-308. [PMC free article] [PubMed] [Google Scholar]
- 25.Lyristis, M., A. E. Bryant, J. Sloan, M. M. Awad, I. T. Nisbet, D. L. Stevens, and J. I. Rood. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761-777. [DOI] [PubMed] [Google Scholar]
- 26.Manteca, C., G. Daube, T. Jauniaux, A. Linden, V. Pirson, J. Detilleux, A. Ginter, P. Coppe, A. Kaeckenbeeck, and J. G. Mainil. 2002. A role for the Clostridium perfringens β2 toxin in bovine enterotoxaemia? Vet. Microbiol. 86:191-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKean, S., J. Davies, and R. Moore. 2005. Identification of macrophage induced genes of Corynebacterium pseudotuberculosis by differential fluorescence induction. Microbes Infect. 7:1352-1363. [DOI] [PubMed] [Google Scholar]
- 28.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58:702-705. [PubMed] [Google Scholar]
- 29.Nairn, M. E., and V. W. Bamford. 1967. Necrotic enteritis of broiler chickens in western Australia. Aust. Vet. J. 43:49-54. [DOI] [PubMed] [Google Scholar]
- 30.Nauerby, B., K. Pedersen, and M. Madsen. 2003. Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens. Vet. Microbiol. 94:257-266. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien, D. K., and S. B. Melville. 2004. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 72:5204-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olkowski, A. A., C. Wojnarowicz, M. Chirino-Trejo, and M. D. Drew. 2006. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 81:99-108. [DOI] [PubMed] [Google Scholar]
- 33.Parish, W. E. 1961. Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J. Comp. Pathol. 71:377-393. [PubMed] [Google Scholar]
- 34.Riddell, C., and X. M. Kong. 1992. The influence of diet on necrotic enteritis in broiler chickens. Avian Dis. 36:499-503. [PubMed] [Google Scholar]
- 35.Scott, P. T., and J. I. Rood. 1989. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 82:327-333. [DOI] [PubMed] [Google Scholar]
- 36.Shane, S. M., J. E. Gyimah, K. S. Harrington, and T. G. Snider III. 1985. Etiology and pathogenesis of necrotic enteritis. Vet. Res. Commun. 9:269-287. [DOI] [PubMed] [Google Scholar]
- 37.Sheedy, S. A., A. B. Ingham, J. I. Rood, and R. J. Moore. 2004. Highly conserved alpha-toxin sequences of avian isolates of Clostridium perfringens. J. Clin. Microbiol. 42:1345-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan, J., T. A. Warner, P. T. Scott, T. L. Bannam, D. I. Berryman, and J. I. Rood. 1992. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27:207-219. [DOI] [PubMed] [Google Scholar]
- 39.Smedley, J. G., III, D. J. Fisher, S. Sayeed, G. Chakrabarti, and B. A. McClane. 2004. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 152:183-204. [DOI] [PubMed] [Google Scholar]
- 40.Smith, C. L., and C. R. Cantor. 1987. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 155:449-467. [DOI] [PubMed] [Google Scholar]
- 41.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Songer, J. G. 1997. Bacterial phospholipases and their role in virulence. Trends Microbiol. 5:156-161. [DOI] [PubMed] [Google Scholar]
- 43.Thompson, D. R., V. R. Parreira, R. R. Kulkarni, and J. F. Prescott. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 113:25-34. [DOI] [PubMed] [Google Scholar]
- 44.Truscott, R. B., and F. Al-Sheikhly. 1977. Reproduction and treatment of necrotic enteritis in broilers. Am. J. Vet. Res. 38:857-861. [PubMed] [Google Scholar]
- 45.Waters, M., A. Savoie, H. S. Garmory, D. Bueschel, M. R. Popoff, J. G. Songer, R. W. Titball, B. A. McClane, and M. R. Sarker. 2003. Genotyping and phenotyping of β2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J. Clin. Microbiol. 41:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Wilkie, D. C., A. G. Van Kessel, T. J. Dumonceaux, and M. D. Drew. 2006. The effect of hen-egg antibodies on Clostridium perfringens colonization in the gastrointestinal tract of broiler chickens. Prev. Vet. Med. 74:279-292. [DOI] [PubMed] [Google Scholar]
- 47.Williams, R. B., R. N. Marshall, R. M. La Ragione, and J. Catchpole. 2003. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol. Res. 90:19-26. [DOI] [PubMed] [Google Scholar]
- 48.Yoo, H. S., S. U. Lee, K. Y. Park, and Y. H. Park. 1997. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 35:228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]