Abstract
The secreted autotransporter toxin (Sat), found predominantly in uropathogenic Escherichia coli, is a member of the SPATE (serine protease autotransporters of Enterobacteriaceae) family and, as such, has serine protease activity and causes cytopathic effects on various cell types. To assess the contribution of the serine protease active site to the mechanism of action of Sat, mutations were made in the first (S256I), in the second (S258A), or in both (S256I/S258A) serine residues within the active site motif. Mutations in the first or both serines reduced protease activity to background levels (P < 0.001); a single mutation in the second serine reduced activity by 60% compared to wild type (P < 0.001). After reversion of the S256I mutation to wild type (I256S), we confirmed S256 as the catalytically active serine. None of these mutations affected secretion of the mature passenger domain or release into the supernatant. The S256I mutation, however, abrogated the cytotoxicity of Sat on human bladder (UM-UC-3) and kidney (HEK 293) epithelial cells, characterized by rounding and elongation, respectively, and a high level of cell detachment. Moreover, S256 is essential for Sat to mediate cytoskeletal contraction and actin loss in host cells as well as to degrade specific membrane/cytoskeletal (fodrin and leukocyte function-associated molecule 1) and nuclear [microtubule-associated proteins, LIM domain-only protein 7, Rap GTPase-activating protein, poly(ADP-ribose) polymerase] proteins in vitro. Lastly, Sat was internalized by host cells and localized to the cytoskeletal fraction where membrane/cytoskeletal target proteins reside.
Urinary tract infection (UTI) is a common extraintestinal infection (33), and Escherichia coli is by far the most common causative organism (30). UTI involves the urethra and the bladder, producing cystitis, or the kidneys, producing acute pyelonephritis. Uropathogenic E. coli strains that cause these infections display specific phenotypic traits. These include specific adhesins (P and type 1 fimbriae, necessary for attachment to uroepithelial cells, and other fimbriae including S and F1C, commonly produced by uropathogenic strains), aerobactin, cytotoxic necrotizing factor 1, and the pore-forming hemolysin (3, 14, 27, 53). Such isolates also typically carry large blocks of genes, called pathogenicity-associated islands (PAIs) (18), as well as numerous smaller inserts (52) not found in fecal isolates.
Uropathogens may also damage host epithelium by the export of autotransporter proteins. Studies of a number of autotransporters (reviewed in reference 23) have demonstrated that translocation across the inner membrane occurs via the sec-dependent pathway. Differing mechanisms for movement of the passenger domain across the outer membrane have been reported; however, all involve the β-barrel porin structure formed by the C-terminal autotransporter domain (23, 37, 49). Once transported to the bacterial cell surface, the passenger domain may remain attached to the outer membrane or be released by proteolytic cleavage (23).
Our laboratory previously identified a 107-kDa secreted protein, designated Sat (secreted autotransporter toxin), that is expressed significantly more often by E. coli strains associated with the clinical symptoms of acute pyelonephritis (68% of strains) than by fecal strains (14% of strains) (16). The polypeptide, isolated from E. coli CFT073, shares highest similarity to the subcategory of autotransporters termed SPATE (serine protease autotransporters of Enterobacteriaceae) proteins, which are produced by diarrheagenic E. coli and Shigella species isolates (23). The native Sat protein (142 kDa) includes the three characteristic domains of SPATE proteins: an unusually long N-terminal signal sequence, a secreted passenger domain (the mature protein) to which the phenotype of each protein is attributed, and a C-terminal autotransporter domain. The mature Sat protein (107 kDa) was shown to have a cytopathic effect on various cell lines (16, 17) and to elicit glomerular damage and a vigorous antibody response in mice transurethrally infected with E. coli CFT073 (16). In addition, the sat gene was shown to reside within PAI I of E. coli CFT073, implicating Sat as another possible PAI-encoded virulence determinant.
The activity of the serine protease motif of autotransporters has been characterized for a number of these proteins. Serine protease activity can catalyze autoproteolysis of the mature protein from the autotransporter domain at the bacterial surface (24) or have no such role (6, 21, 36, 46). Mutation of the serine protease motif can result in a loss of proteolytic or mucinase activity (21), cytotoxicity on target cells (36), or enterotoxic effects on rat jejunal tissue mounted on an Ussing chamber (36).
In this report, we demonstrate that the serine protease active site of Sat is necessary for protease and cytotoxic activities, contraction of the cytoskeleton, and loss of actin in cultured bladder and kidney cells but not for the processing or the release of the toxin from the bacterial surface. We show that wild-type Sat, but not its first-serine mutant, is able to degrade specific membrane/cytoskeletal and nucleus-associated proteins. Lastly, we demonstrate that Sat enters human bladder and kidney epithelial cells and localizes specifically to the cytoskeletal fraction where proposed protein targets reside.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli HB101(pDG4) is a wild-type Sat-expressing clone (16); E. coli HB101 pBluescript SK(−) (pBS SK−) is an empty vector control strain. Bacteria were cultured either in Luria-Bertani (LB) broth at 37°C with aeration in a shaking incubator (200 rpm) or on LB agar plates containing 1.5% agar at 37°C. Media were supplemented with ampicillin (100 μg/ml).
Site-directed mutagenesis.
Site-directed mutagenesis of the serine protease active site (GDS256GS258G) of Sat was performed using PCR overlap extension (25). In the first round of amplification for this two-step reaction, two DNA fragments were generated with overlapping ends which anneal during the second round of amplification, facilitating their use as a template. Point mutations were introduced into the overlapping regions using oligonucleotide primers B1 (5′-AGAGCCGATGTCTCC-3′) and C1 (5′-GGAGACATCGGCTCT-3′) to mutate the first serine to nonpolar isoleucine (S256I), B2 (5′-TAAGTATGCTCCAGCGCCG-3′) and C2 (5′-GGCGCTGGAGCATACTTA-3′) to mutate the second serine to nonpolar alanine (S258A) and to create a double-serine mutant (S256I/S258A), and B3 (5′-ATTCGGAGACAGTGGCTCTGGAGC-3′) and C3 (5′-GCTCCAGAGCCACTGTCTCCGAAT-3′) to make isoleucine revert to serine (I256S). Flanking primers A (5′-CCAGTCACGACGTTGTA-3′) and D (5′-AGTCCGTTCCACAAAGA-3′) hybridize upstream and downstream of the serine protease active site, respectively. By using pDG4, a plasmid expressing Sat S256I was generated (pDG4S256I) by A/B1 and D/C1 amplification. Second-serine and double-serine mutations were created by A/B2 and D/C2 amplification from pDG4 and pDG4S256I, respectively. Reversion mutation was produced by A/B3 and D/C3 amplification from pDG4S256I. All final mutated PCR products were generated by A/D amplification.
Cloning and sequencing of PCR products.
PCR products were ligated into pCR-BluntII-TOPO (Invitrogen, Carlsbad, California). Inserts were excised using the appropriate restriction enzymes (either HindIII-SphI or BamHI-NotI) and separated by agarose gel electrophoresis. Inserts were excised from the gel and purified using a QIAquick gel extraction kit (QIAGEN, Valencia, California) and ligated into similarly treated pDG4. Plasmids were introduced into the laboratory strain E. coli HB101 by electroporation (44). Sequencing of the first-, second-, and double-serine Sat mutant regions was done at the Biopolymer Core Facility at the University of Maryland, Baltimore, using an Applied Biosystems model 373A automated DNA sequencer using the Big Dye Terminator Cycle sequencing kit while the reverted mutant region was sequenced at the DNA Sequencing Core at the University of Michigan, Ann Arbor, according to protocols for Applied Biosystems DNA sequencers.
Mass spectrometry analysis.
All protein samples were digested overnight with trypsin, including alkylation and reduction. Peptides were extracted and analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry at the Protein Structure Facility at the University of Michigan, Ann Arbor. A three-pointed external calibration was performed, yielding a mass accuracy of 0.1%. Results were searched in the Protein Prospector database in both NCBI and Swiss-Prot.
Sat supernatant preparations.
Bacteria from overnight LB cultures of E. coli HB101 transformed with pDG4, pDG4S256I, pDG4S258A, pDG4S256I/S258A, or pDG4I256S were pelleted, and supernatants were filter sterilized. Supernatants were then concentrated 500-fold using 100,000-molecular-weight-cutoff (MWCO) Centricon Plus-80 filters (Millipore, Billerica, Massachusetts). Protein concentration was determined using a bicinchoninic acid assay (Pierce, Rockford, Illinois).
SDS-PAGE and Western blot analysis.
Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were either stained with Coomassie blue or electrotransferred onto a nitrocellulose membrane (Millipore) as described by Towbin et al. (48). Unless otherwise indicated, membranes were then incubated with primary rabbit anti-Sat serum (1:5,000 dilution) followed by incubation with secondary anti-rabbit-alkaline phosphatase conjugate (1:2,000 dilution).
Serine protease and protein substrate cleavage assays.
Protease activity was determined using the p-nitroanilide substrate assay (7). Concentrated supernatant containing wild-type Sat or mutant derivatives (20 μg) was added to 1 mM methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (Calbiochem, La Jolla, California) in a buffer containing 0.1 M morpholinepropanesulfonic acid (MOPS), pH 7.3, 0.2 M NaCl, and 0.01 mM ZnSO4. Samples were incubated at 37°C for 24 h, and substrate hydrolysis was monitored at 405 nm. All absorbance measurements were normalized to wild-type Sat values. Samples were also tested for serine protease activity after preincubation for 30 min at room temperature with 1 mM phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, St. Louis, Missouri). Reactions were conducted in quadruplicate, and the mean ± standard deviation was calculated. For protein substrate assays, spectrin (3 μg) (Sigma-Aldrich) and human coagulation factor V (2.5 μg) (Calbiochem) were combined with 5 μg of each concentrated supernatant in 20 μl of MOPS buffer (125 mM MOPS, 12.5 μM ZnSO4, 250 mM NaCl, pH 7.5) and incubated overnight at 37°C. Reaction products were separated by SDS-6% PAGE (31).
Tissue culture assays.
Cells were maintained in humidified 5% CO2-93% air at 37°C. UM-UC-3 human bladder epithelial cells (ATCC CRL-1749) and HEK-293 human kidney epithelial cells (ATCC CRL-1573) were cultured in Dulbecco's modified Eagle's medium (Gibco, Carlsbad, California) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine (Gibco), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Mediatech, Herndon, Virginia). Subconfluent cells were resuspended with trypsin-EDTA (Gibco), washed, and plated in eight-well Lab-Tek chamber slides (NNI, Naperville, IL). To perform the cytotoxicity assay, concentrated supernatants (100 μg/ml) containing wild-type and mutant derivatives of Sat were added directly to 80% confluent host cells in 200 μl of medium. Cells were incubated for 2 h at 37°C, washed twice with phosphate-buffered saline (PBS), and fixed with 3.7% (vol/vol) formaldehyde in PBS. Cells either were stained with Giemsa stain (Sigma-Aldrich) and visualized by light microscopy or were permeabilized by adding 0.1% Triton X-100 in PBS and stained with fluorescein isothiocyanate-phalloidin (0.5 μg/ml). Slides were mounted in Vectashield mounting medium with DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, California) and examined by fluorescence microscopy.
Isolation and cleavage of fodrin-enrichment and cellular fractions.
Fodrin-enrichment fractions were prepared according to the method of Villaseca et al. (51). Briefly, bladder and kidney cells were washed and lysed in phosphate buffer. Lysed cells were centrifuged, and the resulting pellet was washed three times in hypotonic phosphate buffer to obtain bladder and kidney cell membranes, which represent fodrin-enrichment fractions. Cellular fractions (cytosol, membrane, nuclear, and cytoskeleton) were prepared from bladder and kidney cells using the FractionPREP cell fractionation system (BioVision, Mountain View, California) and as directed by the manufacturer. Cytoskeletal fractions were solubilized by sonication in 0.2% SDS, 10 mM dithiothreitol. The same quantity of fodrin-enrichment or cellular fractions was incubated overnight at 37°C with each concentrated supernatant (5 μg) in MOPS buffer. Reaction products were separated by SDS-6% PAGE (31).
Purification of Sat.
Bacteria from overnight LB cultures of E. coli HB101(pDG4) were harvested by centrifugation (5,000 × g, 12 min, 4°C), and supernatants were filter sterilized. Sterile supernatants were concentrated 20-fold using a 30,000-MWCO Pellicon XL filter unit (Millipore) and then another 100-fold using a 100,000-MWCO Centricon Plus-80 filter (Millipore). This crude concentrate was subjected to ammonium sulfate precipitation as described elsewhere (54). Each cut was resuspended in anion-exchange buffer (0.025 M NaCl, 0.025 M Tris-HCl, pH 7.5). The appropriate cut (∼40% ammonium sulfate saturation) was dialyzed overnight against anion-exchange buffer. Dialyzed sample was applied over a 5-ml Econo-Pac High Q Cartridge (Bio-Rad, Hercules, California) which had been washed with 5 column volumes of anion-exchange buffer. Elution was carried out in 0.025 M Tris-HCl, pH 7.5, with a salt gradient from 25 to 500 mM NaCl at a flow rate of 2 ml/min. Fractions enriched for Sat (∼25% NaCl) were pooled, concentrated, and dialyzed overnight against gel filtration buffer (0.150 M NaCl, 0.025 M Tris-HCl, pH 7.5). Dialyzed sample was applied to a HiPrep 16/60 Sephacryl S-200 high-resolution column (GE Healthcare, Piscataway, New Jersey) previously equilibrated with gel filtration buffer according to the manufacturer's protocol. Fractions were collected using gel filtration buffer at a flow rate of 0.5 ml/min. Protein eluting in fractions consistent with the predicted 107-kDa-molecular-size Sat was pooled and concentrated. All column fractionation steps were conducted using the Biologic LP chromatography system and the BioFrac fraction collector (Bio-Rad).
Sat localization assay.
Confluent bladder and kidney epithelial cells were harvested by gentle rocking with glass beads in PBS. Cells were washed three times in PBS and resuspended in 1 ml supplemented Dulbecco's modified Eagle's medium (Gibco) without fetal bovine serum. Cells were intoxicated with purified Sat (25 μg/ml) or the corresponding volume of gel filtration buffer and incubated for 2 h at 37°C, 5% CO2. Viability was determined by trypan blue (Mediatech) staining. Supernatants from the intoxications were collected, and cells were washed three times in cold PBS. Cellular fractions were obtained as described above. Fraction proteins (200 μg) were resolved by SDS-6% PAGE. Immunoblotting was performed as described above with the following exceptions. Nitrocellulose membranes were incubated with primary rabbit anti-Sat serum (diluted 1:500). After analysis of the fractions for Sat, nitrocellulose membranes were stripped and immunoblotted with a cytoskeletal control primary antibody against vimentin (diluted 1:500) (Abcam, Cambridge, Massachusetts). Both primary incubations were followed by incubation with secondary anti-rabbit immunoglobulin G peroxidase conjugate (diluted 1:2,500) (Sigma-Aldrich).
RESULTS
S256 is required for Sat serine protease activity and Sat-mediated degradation of human coagulation factor V and spectrin.
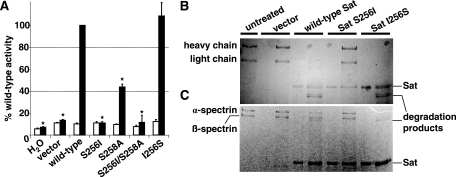
The serine protease active site of the passenger domain of Sat contains two serine residues (16). By using E. coli HB101 expressing wild-type Sat, single-nucleotide mutations were made that led to predicted amino acid changes in the first (Sat S256I), the second (Sat S258A), or both (Sat S256I/S258A) serine residues. The serine protease active site mutants were assessed for hydrolysis of methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (7), a colorimetric substrate optimal for Sat (12). Concentrated culture supernatants containing wild-type or mutant versions of Sat were incubated with the synthetic substrate, and protease activity was monitored by absorbance (Fig. 1A). Sat S256I and Sat S256I/S258A had negligible serine protease activity comparable to the vector control (P < 0.001), while Sat S258A had a 60% reduction in serine protease activity compared to wild-type Sat supernatant (P < 0.001). To confirm the significance of the first serine residue, we mutated the nonpolar isoleucine back to a serine (Sat I256S). The single mutation resulted in a distinct nucleotide sequence that nevertheless encoded the same amino acid sequence as wild type. The serine protease activity of Sat I256S was restored to wild-type levels. All enzymatically active proteins were completely inhibited by 30 min of preincubation with 1 mM PMSF, a potent serine protease inhibitor (Fig. 1A, open bars).
FIG. 1.
Serine protease activity and substrate specificity of wild-type and mutant versions of Sat. (A) Concentrated culture supernatants (20 μg protein) from E. coli HB101 transformed with empty vector or plasmids expressing wild-type Sat, Sat S256I, Sat S258A, Sat S256I/S258A, or revertant Sat I256S were incubated with 1 mM methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide at 37°C for 24 h, with (open bars) or without (filled bars) pretreatment with 1 mM PMSF. Absorbance was read at 405 nm. All absorbance measurements were normalized to those obtained for wild-type Sat. Asterisks indicate absorbance values significantly different from wild-type Sat (P < 0.001). (B and C) Purified human coagulation factor V (B) or purified spectrin (C) was incubated with supernatants containing wild-type or mutant derivatives of Sat. Reaction products were separated by SDS-6% PAGE. With the exception of the first lane containing untreated substrate, for each pair of lanes, the left lane contains supernatants alone while the right lane contains substrate protein incubated with each indicated supernatant.
In addition to synthetic substrates, Sat has been reported to cleave human coagulation factor V and spectrin (12). To evaluate whether the serine protease activity of Sat is responsible for degradation of these substrates, concentrated culture supernatants containing wild-type Sat or the serine mutant derivatives of Sat were incubated with these proteins. Only wild-type and revertant Sat I256S supernatants were able to degrade both chains of human coagulation factor V (Fig. 1B) and the heterodimeric subunits of spectrin (Fig. 1C). These data suggest that the first serine residue, S256, is the catalytically active serine of Sat.
Sat serine protease activity is not required for export and release of Sat.
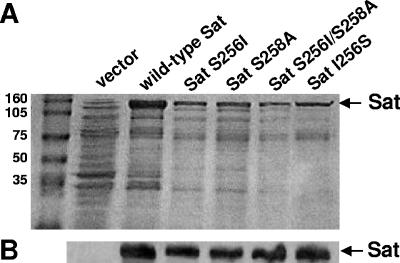
To study secretion of wild-type and mutated versions of Sat, concentrated culture supernatants were prepared from E. coli HB101 expressing the wild-type Sat, Sat S256I, Sat S258A, Sat S256I/S258A, and revertant Sat I256S and analyzed by SDS-10% PAGE (Fig. 2A) and Western blotting using anti-Sat serum (Fig. 2B). Sat (107 kDa) was present in every sample, indicating that none of the mutations made in the serine protease active site nor the reversion affected the translocation of the mature passenger domain and release of Sat into the culture supernatant.
FIG. 2.
Secretion of wild-type and mutant versions of Sat. (A) Coomassie blue-stained SDS-10% polyacrylamide gel of concentrated culture supernatants from E. coli HB101 expressing wild-type or mutant versions of Sat. Molecular masses (kDa) are shown on the left. (B) Detection of Sat by Western blotting using anti-Sat serum. Lanes are the same as indicated in panel A.
Sat serine protease activity is required for cytotoxicity of Sat.
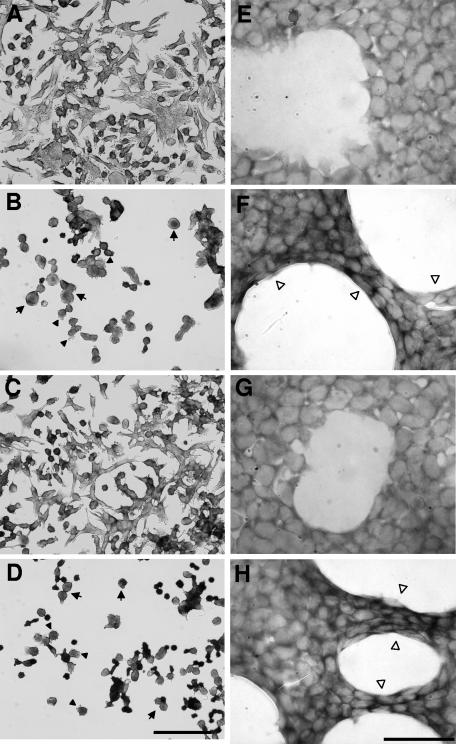
To determine if the S256I mutation abrogates the ability of Sat to cause cytopathic effects on urinary cells, concentrated supernatants from E. coli HB101 expressing wild-type Sat, Sat S256I, and the reverted mutant Sat I256S were applied to monolayers of human bladder and kidney epithelial cells (Fig. 3). Both cell lines treated with concentrated supernatants of wild-type Sat and Sat I256S showed a release of cellular focal contacts from the slide substratum, generating a high level of cell detachment from the epithelium (data not shown). In addition, host cells showed an elevated level of morphological alterations characterized by rounding (Fig. 3B and 3D, arrows) and membrane ruffling (Fig. 3B and 3D, filled arrowheads) of bladder cells and elongation of kidney cells (Fig. 3F and 3H, open arrowheads). In contrast, bladder and kidney cells treated with Sat S256I supernatant did not show morphological alterations and maintained their normal structure (Fig. 3C and 3G), similarly to cells treated with supernatant from the negative-control E. coli HB101 clone (Fig. 3A and 3E). We conclude that mutation of residue S256 abrogates the ability of Sat to cause cytopathic effects on urinary cells despite the fact that the same mutation has no effect on secretion and release of Sat into the supernatant.
FIG. 3.
Cytotoxic activity of Sat and its first-serine mutant for bladder and kidney cells. Bladder (A to D) and kidney (E to H) cells were incubated with concentrated culture supernatants of E. coli HB101 expressing empty vector (A and E), wild-type Sat (B and F), Sat S256I (C and G), and Sat I256S (D and H) for 2 h at 37°C. Cells were fixed and stained with Giemsa stain. Arrows indicate rounding of bladder cells, filled arrowheads point to membrane blebs on bladder cells, and open arrowheads point to the elongation of kidney cells. Bars, 100 μm for left panels (magnification, ×400) and 50 μm for right panels (magnification, ×1,000).
Sat serine protease activity is required for morphological alterations induced in the actin cytoskeleton of bladder and kidney cells.
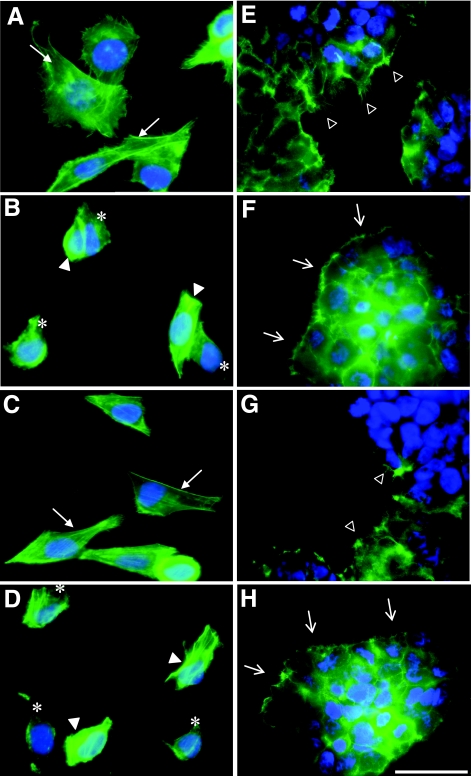
The rounding and membrane ruffling of bladder cells and elongation of kidney cells suggest that Sat serine protease activity disrupts the cytoskeleton or cytoskeleton-associated proteins. To further characterize these effects, kidney and bladder cells, incubated with Sat, were stained with fluorescein-labeled phalloidin and observed by fluorescence microscopy (Fig. 4). Bladder cells treated with negative-control supernatant revealed classic cytoskeletal actin structures, with linear F-actin stress fibers (Fig. 4A, arrows). After 2 h of exposure to wild-type Sat or to revertant mutant Sat I256S supernatants, the bladder cells revealed contraction of the cytoskeleton (Fig. 4B and 4D, filled arrowheads) and a loss of actin stress fibers (Fig. 4B and 4D, asterisks). However, supernatant containing the first-serine mutant Sat S256I did not produce any of these cytoskeletal effects (Fig. 4C), as those cells appeared similar to the control. Similarly, kidney cells treated with wild-type Sat and Sat I256S supernatants revealed a different actin structure than did kidney cells treated with negative-control supernatant (Fig. 4E). Indeed, the actin protrusions observed at the surface of the control cells were no longer present and were replaced by the formation of surface blebs (Fig. 4F and 4H, open arrows). Moreover, intoxicated kidney cells had a globular appearance and a loss of actin filaments normally present at the edge of control and Sat S256I supernatant-treated cells (Fig. 4E and 4G, open arrowheads). These data reveal that, in addition to serine protease activity and cytopathic effects, residue S256 is also necessary to cause characteristic cytoskeletal effects seen in bladder and kidney cells treated with Sat.
FIG. 4.
Effect of Sat and its first-serine mutant on the urinary epithelial cell cytoskeleton. Bladder (A to D) and kidney (E to H) cells were incubated with 100 μg protein/ml of concentrated supernatant from E. coli HB101 expressing the empty vector (A and E), wild-type Sat (B and F), Sat S256I (C and G), and revertant Sat I256S (D and H) for 2 h at 37°C. Actin was stained green while the nuclei were stained blue. For bladder cells, linear F-actin stress fibers are indicated by arrows, contraction of the cytoskeleton is indicated by filled arrowheads, and loss of actin stress fibers is shown with asterisks. For the kidney cells, actin protrusions are indicated by open arrowheads and formation of surface blebs in place of actin protrusions is indicated by open arrows. Left panels depict enlarged portions of images taken at a magnification of 400× while right panels show images taken at a magnification of 1,000×. Bar, 50 μm.
Identification of potential protein targets of Sat in the membrane fraction of bladder and kidney cells.
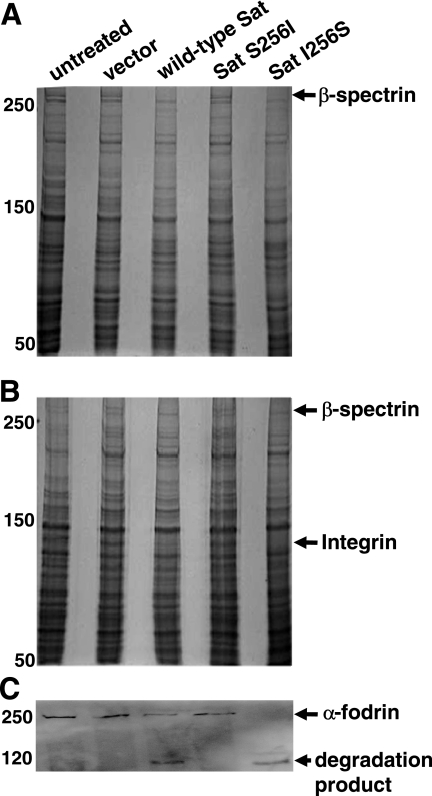
To identify potential membrane targets of Sat, isolated bladder and kidney cell membranes were incubated with each Sat supernatant and protein profiles were resolved by SDS-6% PAGE. The results showed degradation of a protein greater than 250 kDa in both bladder (Fig. 5A) and kidney (Fig. 5B) membrane fractions treated with wild-type Sat and revertant mutant Sat I256S. In contrast, membrane profiles treated with the first-serine protease Sat S256I appeared similar to untreated membranes and membranes treated with negative-control supernatant. A protein with homology to nonerythroid β-spectrin was identified by mass spectrometry (Table 1). Fodrin is nonerythroid spectrin, a heterodimeric cytoskeletal protein which serves to link actin filaments with other cytoskeletal proteins and the plasma membrane (1). To determine whether Sat cleaves fodrin as previously observed with erythrocyte spectrin (12), a Western blot assay of kidney cell fodrin-enrichment membrane fractions was performed using anti-α-fodrin chain antibodies (Fig. 5C). Degradation products were detected in samples incubated with wild-type Sat or Sat I256S. Similar results were seen with fodrin-enrichment fractions obtained from bladder cells (data not shown). The results confirmed that α-fodrin is degraded by wild-type Sat and revertant Sat I256S but not by active-site mutant Sat S256I.
FIG. 5.
Effect of Sat and its first-serine mutant on bladder and kidney membrane proteins. The same quantity of bladder (A) and kidney (B and C) membrane proteins was incubated overnight at 37°C with 5 μg protein of each concentrated supernatant. Reaction products were separated by SDS-6% PAGE. Molecular masses (kDa) are indicated on the left. The closest matches of proteins degraded by wild-type Sat and revertant Sat, but not by Sat S256I, identified by mass spectrometry are shown by the arrows. (C) Western blot of fodrin-enrichment fractions from kidney cells incubated with wild-type and mutant derivatives of Sat. Lanes for panels B and C are as listed for panel A.
TABLE 1.
Host cell membrane and nuclear proteins, identified by mass spectrometry, that are susceptible to proteolysis by Sat
| Band submitted (molecular mass [kDa]) | Database | Mass match (%) | Tolerance | Molecular mass (kDa) | Identified protein(s) |
|---|---|---|---|---|---|
| Bladder membrane (>250) | Swiss-Prot | 83 | 0.789 | 372 | Bullous pimphigoid antigen 1 (BPAG1); contains 30 beta-spectrin repeats (4) |
| 83 | 0.952 | 288 | Nonerythroid spectrin beta IV | ||
| NCBI | 83 | 0.789 | 372 | Bullous pimphigoid antigen 1 (BPAG1); contains 30 beta-spectrin repeats (4) | |
| 83 | 0.952 | 289 | Nonerythroid spectrin beta IV | ||
| Kidney membrane | |||||
| 1 (>250) | Swiss-Prot | 100 | 1.07 | 416 | Spectrin, nonerythroid beta-chain 4 |
| 80 | 0.875 | 288 | Spectrin, nonerythroid beta-chain 3 | ||
| NCBI | 100 | 1.45 | 372 | Bullous pimphigoid antigen 1 (BPAG1); contains 30 beta-spectrin repeats (4) | |
| 100 | 1.07 | 416 | Spectrin, nonerythroid beta-chain 5 | ||
| 2 (100-150) | Swiss-Prot | 66 | 1.78 | 128 | Leukocyte-function associated molecule 1 |
| NCBI | 66 | 1.78 | 128 | Leukocyte-function associated molecule 1 | |
| Bladder nucleus | |||||
| 1 (>250) | Swiss-Prot | 77 | 0.944 | 409 | Abnormal spindle-like microencephaly-associated protein = MAP |
| 77 | 1.37 | 453 | AKAP450 (centrosome- and Golgi complex-localized PKN-associated protein) = MAP | ||
| NCBI | 77 | 1.37 | 452 | AKAP450 (centrosome- and Golgi complex-localized PKN-associated protein) = MAP | |
| 2 (100-150) | Swiss-Prot | 66 | 1.33 | 192 | LIM domain-only protein 7 |
| NCBI | 66 | 1.33 | 192 | LIM domain-only protein 7 | |
| 3 (100-150) | Swiss-Prot | 66 | 1.20 | 199 | Signal-induced proliferation-associated 1-like protein 1 |
| NCBI | 66 | 1.20 | 199 | Signal-induced proliferation-associated 1-like protein 1 | |
| Kidney nucleus | |||||
| 1 (>250) | Swiss-Prot | 80 | 0.976 | 306 | MAP 1A (proliferation-related protein P80) |
| NCBI | 80 | 0.976 | 306 | MAP 1A (proliferation-related protein P80) | |
| 80 | 0.976 | 202 | MAP 1A (proliferation-related protein P80) | ||
| 2 (100-150) | Swiss-Prot | 85 | 0.818 | 113 | PARP |
| NCBI | 85 | 0.818 | 113 | PARP |
Interestingly, we found another target protein, specific to the kidney membrane, degraded by wild-type Sat but not by Sat S256I (Fig. 5B). The mass spectrometry results for this protein (Table 1) revealed homology with leukocyte function-associated molecule 1 (LFA-1), which is a member of the β2-integrin family of cell surface receptors. Integrins are receptors for extracellular matrix (ECM) molecules and are counterreceptors for surface proteins of apposed cells (26). These data show that Sat degrades target proteins in both bladder and kidney membranes and that S256 is necessary for Sat-mediated degradation of these putative host cell target proteins.
Identification of potential protein targets of Sat in the cytosol and nuclear fractions of bladder and kidney cells.
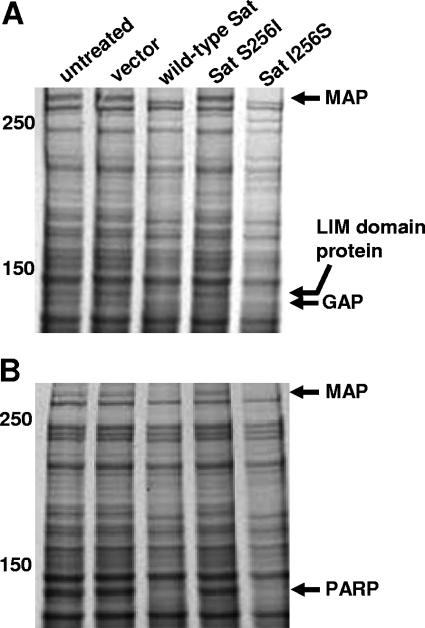
As we identified target proteins of Sat in the membrane fraction of host cells, we hypothesized that Sat may have protein targets in other cellular fractions. Thus, cytosolic and nuclear compartments of fractionated bladder and kidney cells were incubated with each Sat supernatant and the protein profiles were analyzed by SDS-6% PAGE (Fig. 6). No bladder and kidney cell cytosolic proteins were found degraded by Sat (data not shown). In contrast, we found susceptible substrate proteins degraded by wild-type Sat and revertant Sat I256S supernatants, including three in the nuclear fraction of bladder cells (Fig. 6A) and two in the nuclear fraction of kidney cells (Fig. 6B). Mass spectrometry analysis of these proteins (Table 1) revealed that the high-molecular-mass (>250-kDa) proteins degraded in both bladder and kidney nuclear fractions were homologous to microtubule-associated proteins (MAPs), which are involved in nuclear and cell division, organization of intracellular structure, and intracellular transport (2). The two other proteins degraded in the bladder nuclear fraction were found to be homologous to LIM domain-only protein 7 (28) and to signal-induced proliferation-associated 1-like protein 1, which is a GAP (GTPase-activating protein) of the Rap family of small GTPases (8). The second protein degraded in the nuclear fraction of kidney cells was found to be homologous to a poly(ADP-ribose) polymerase (PARP), an enzyme of central importance in a wide variety of biological processes including maintenance of genomic stability, DNA repair, transcriptional regulation, centromere function, modulation of telomere length, and regulation of protein degradation, endosomal vesicle trafficking, and apoptosis (11). These results show that in addition to membrane targets, Sat also degrades nuclear proteins with vital cellular roles. The relevance of these in vitro substrates depends, of course, on the ability of Sat to enter host cells and localize accordingly.
FIG. 6.
Effect of Sat and its first-serine mutant on bladder and kidney nuclear proteins. The same quantity of bladder (A) and kidney (B) nuclear proteins was incubated overnight at 37°C with 5 μg protein of each concentrated supernatant. Reaction products were separated by SDS-6% PAGE. Molecular masses (kDa) are indicated on the left. The proteins degraded by wild-type Sat but not by Sat S256I were identified by mass spectrometry. The closest matches are shown by the arrows. Lanes for panel B are as listed for panel A.
Sat localizes to the cytoskeletal fraction of intoxicated bladder and kidney cells.
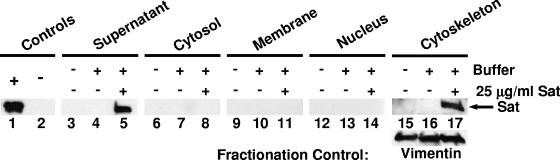
To successfully cleave putative target proteins, Sat must be internalized by host cells and reach specific intracellular locations. To assess this potential, bladder cells were intoxicated with biochemically purified Sat (25 μg/ml) for 2 h. It was determined that ≥80% of the bladder cells were viable after intoxication, as assessed by trypan blue staining. Cells were then washed and fractionated into cytosolic, membrane, nuclear, and cytoskeletal cellular components. Fractions were analyzed for the presence of Sat by Western blotting with anti-Sat serum (Fig. 7). As expected, mature Sat (107 kDa) was detected in the sample supernatant of intoxicated bladder cells (Fig. 7, lane 5). More interestingly, Sat was also detected in the cytoskeletal fraction of intoxicated bladder cells (Fig. 7, lane 17) but not in the cytoskeletal fraction of either untreated cells (Fig. 7, lane 15) or buffer-treated cells (Fig. 7, lane 16). Sat was not detected in cytosolic, membrane, or nuclear fractions of Sat-treated samples (Fig. 7, lanes 8, 11, and 14, respectively). Similar results were found with kidney epithelial cells (data not shown). Altogether, these data indicated that upon intoxication, Sat enters host cells by an unknown mechanism and localizes to the cytoskeletal fraction, where it can cleave target proteins such as spectrin and integrin.
FIG. 7.
Localization of Sat to the cytoskeletal fraction of intoxicated bladder epithelial cells. Lanes 1 and 2 contain 100 ng purified Sat protein or an equal volume of gel filtration buffer, respectively. Bladder cells were untreated (lanes 3, 6, 9, 12, and 15), treated with gel filtration buffer (lanes 4, 7, 10, 13, and 16), or treated with 25 μg/ml purified Sat (lanes 5, 8, 11, 14, and 17) and incubated at 37°C with 5% CO2 for 2 h. After fractionation, each intoxication supernatant (1 μg) and each cellular fraction (200 μg) were analyzed by Western blotting using anti-Sat serum. Vimentin (56 kDa), the major subunit of intermediate filaments of mesenchymal cells, served as a fractionation and loading control for the cytoskeletal fraction (bottom panel, lanes 15 to 17).
DISCUSSION
Autotransporters comprise a family of virulence factors (22, 32) characterized by a unique ability to promote their own secretion across the outer membrane of the bacterial envelope (20, 23, 45, 47). Sat is a representative member of the autotransporter family of secreted proteins and belongs to a subfamily termed SPATE featuring a conserved serine protease motif (GDSGSP) (16). This motif resides in an analogous position within Sat and other SPATE proteins (6, 9, 13, 19, 21, 38, 40, 41, 46). Sat was previously shown by our laboratory to elicit a vigorous antibody response in mice transurethrally infected with the pyelonephritogenic parent strain E. coli CFT073 (16) and to cause cytopathic effects on human bladder and kidney epithelial cells (17). In this study, we demonstrated that S256 is necessary for protease and cytopathic activity of wild-type Sat but is not involved in processing or release of Sat from the bacterial surface. Additionally, Sat is internalized by relevant host cells and localizes to the cytoskeletal fraction where cleavable target proteins reside.
The serine protease motif was observed to be solely responsible for the protease activity of mature Sat, and more precisely it was shown that S256 is the catalytically active residue within this motif. From the p-nitroanilide substrate data (Fig. 1A) it is clear that a single-nucleotide mutation that changes the first serine within the active site to an isoleucine (S256I) abolishes wild-type protease activity. To demonstrate that Sat S256I did not also carry a secondary mutation that was responsible for reduced protease activity, we made the isoleucine revert to a serine (I256S) and demonstrated for the first time a complete restoration of the wild-type serine protease activity of Sat. A single mutation in the second serine (S258A) reduced the protein activity by more than half. These results were not unexpected, as analogous mutants of Pet (36), Pic (21, 38), and EatA (39) lost their respective proteolytic activities.
Controversy exists over how cleavage of the passenger domain from the β-barrel occurs for many autotransporters, especially whether cleavage is a result of membrane-bound protease or an autoproteolytic event. Some members of the autotransporter family, such as immunoglobulin A1 protease and Hap from Haemophilus influenzae, rely on autoproteolysis involving the serine protease active site for processing and release of the passenger domain from the outer membrane (23, 24). In this study we clearly demonstrate that Sat is not autoprocessed by its serine protease active site, since mutants that lack serine protease activity are still secreted from E. coli in mature form (Fig. 2). This was also observed for Pet (36), SepA (6), EspC (46), Pic of enteroaggregative E. coli (21), Tsh (29), and EatA (39). These results suggest that another protease is required for Sat release. Navarro-Garcia et al. (34) have shown that normal processing of the Pet precursor occurs in the absence of DegP, OmpP, and OmpT proteases or DsbA isomerase. Due to the high degree of homology between Sat and Pet, we hypothesize that other endogenous membrane-associated enzymes are involved in release of the toxin from the bacterial surface.
Cytopathic activity of wild-type Sat on urinary cell lines is dependent on proteolytic activity. None of the cytopathic effects observed with supernatant containing wild-type Sat or Sat I256S were seen in cells treated with Sat S256I supernatant, demonstrating that the cytotoxic activity is conferred by the serine protease active site of Sat (Fig. 3). Our results appear similar to those previously reported for active site mutants of Pet (36). Additionally, the cell damage caused by EspC was characterized by cell contraction and cell detachment and was also due to disruption of the actin cytoskeleton, specifically perinuclear contraction of F actin and loss of stress fibers (35). These data combined with the morphological changes and detachment of Sat-treated bladder and kidney cells make it tempting to speculate that the cytopathic effects caused by Sat are associated with damage to the actin cytoskeleton or cytoskeleton-associated proteins, which ultimately may contribute to epithelial damage in urinary tract infections. Our results show contraction of the cytoskeleton and loss of actin stress fibers as early as 2 h after addition of wild-type and revertant Sat supernatants to bladder and kidney monolayers (Fig. 4). As expected, these alterations were absent from cells treated with Sat S256I, confirming the significance of this residue in the cytopathic activity of Sat.
The cytoskeletal effects mediated by Sat on urinary epithelial cells are likely associated with the degradation of fodrin (nonerythrocyte spectrin). Fodrin/spectrin is involved in stabilizing membrane structures, maintaining cell shape, and linking actin filaments with the plasma membrane (5, 10, 50). Our results affirmed that Sat is able to degrade both α- and β-spectrin chains (Fig. 1C) as previously demonstrated by Dutta et al. (12) and similarly shown for Pet and EspC. In addition, similarly to its closest homolog Pet (51), Sat was shown to cleave α-fodrin (Fig. 5C). Proteolytic attack on fodrin, thereby altering the cytoskeleton, may explain the rounding, elongation, membrane ruffling, and detachment observed when urinary cells are treated with wild-type and revertant Sat (Fig. 3).
Another protein target degraded by Sat was identified in the membrane of kidney epithelial cells. Mass spectrometry determination of this protein revealed homology with leukocyte function-associated molecule 1 (LFA-1), which is a member of the β2-integrin family of cell surface receptors. When cells come in contact with ECM, they extend filopodia to sample the terrain. Integrins at the tip of filopodia bind to the ECM and initiate formation of focal adhesion. Actin-rich lamellipodia are then generated, often between filopodia, as the cell spreads on the ECM (15). Extracellular ligand-bound integrins transduce a variety of signals which induce dramatic changes in the organization of the cytoskeleton (43). This could explain the lack of organized actin protrusions and formation of surface blebs seen in cells treated with wild-type and revertant Sat supernatants (Fig. 4). Moreover, these data might explain previous observations from Guyer et al. (17), who showed that Sat elicits significant morphological changes specific to the kidney, including dissolution of the glomerular membrane and destruction of tubular epithelial cells following an experimental UTI of CBA mice infected with wild-type E. coli CFT073 or an isogenic sat::pGP704 mutant.
In addition to membrane targets, in vitro analyses showed that wild-type and revertant Sat I256S degrade nuclear proteins that play vital roles in eukaryotic cells (Fig. 6). Proteins homologous to LIM domain proteins and Rap GAPs were found degraded in the nuclear fraction of bladder cells whereas a protein homologous to a PARP was degraded in nuclear kidney cell fractions. Proteins identified as MAPs were found to be degraded in both cell types. Although it is interesting to speculate on a role for the degradation of nuclear proteins in the pathogenesis of UTI, our localization data reveal that such targets may not be physiologically relevant and likely do not play a role in the cytopathic effects seen in urinary epithelial cells.
As mentioned above, proteins homologous to nonerythrocyte spectrin and β2-integrin were degraded in the membrane fraction of bladder and kidney cells in vitro. Although degradation was detected in the membrane fraction of urinary epithelial cells, these proteins are important for cytoskeletal integrity and function. Due to solubility issues with the cytoskeletal fraction in early experiments, distinct degradation of protein bands could not be detected as clearly as in the membrane fraction (Fig. 5 and data not shown). A later experiment showed that during intoxication, Sat is internalized and localizes specifically to the cytoskeletal fraction of bladder and kidney epithelial cells (Fig. 7 and data not shown). Localization of Sat to the cytoskeleton during intoxication of host cells combined with previous in vitro data showing host cell protein degradation indicates that, upon entry into bladder and kidney host cells, Sat likely cleaves proteins associated with the cytoskeleton. Future studies will focus on characterizing the proposed interaction between Sat and candidate cytoskeletal protein substrates.
Numerous bacterial toxins recognize and target the actin cytoskeleton. Actin-ADP-ribosylating toxins and the Vibrio cholerae RTX toxin directly affect structural proteins of the cytoskeleton. Others toxins alter the function of regulatory elements in control of the cytoskeleton. These toxins include Rho GTPases, proteins that belong to the superfamily of Ras proteins (Rho-activating and inactivating toxins), and another group that mimics eukaryotic master regulators (bacterial GAPs and guanine nucleotide exchange factors) (reviewed in reference 1). It was shown that the bacterial toxins acting on the cytoskeleton dramatically disturb cell morphology, intercellular junctions, the cell barrier permeability, and host cell processes dependent on actin (42). Despite differences in its structure and mode of action, Sat appears to attack identical or similar eukaryotic targets as other bacterial toxins. Alteration of the cytoskeleton appears to be a major mechanism for the host cell cytopathic effects caused by Sat. By virtue of cytopathic effects caused in urinary epithelial cells, Sat is proposed to play an important role in virulence during infection of the urinary tract by E. coli and may function to aid bacteria in breaching the protective epithelial cell barrier and invading the bloodstream, causing bacteremia in afflicted individuals.
Acknowledgments
This work was supported in part by Public Health Service grant AI43363 from the National Institutes of Health.
Editor: D. L. Burns
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. Watson. 1994. Molecular biology of the cell, 3rd ed. Garland Publishing, Inc., New York, N.Y.
- 2.Andersen, S. S. 2000. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 10:261-267. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 4.Baines, A. J. 2003. Comprehensive analysis of all triple helical repeats in beta-spectrins reveals patterns of selective evolutionary conservation. Cell. Mol. Biol. Lett. 8:195-214. [PubMed] [Google Scholar]
- 5.Beck, K. A., and W. J. Nelson. 1996. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am. J. Physiol. 270:C1263-C1270. [DOI] [PubMed] [Google Scholar]
- 6.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 7.Benjelloun-Touimi, Z., M. Si Tahar, C. Montecucco, P. J. Sansonetti, and C. Parsot. 1998. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology 144:1815-1822. [DOI] [PubMed] [Google Scholar]
- 8.Bernards, A. 2003. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603:47-82. [DOI] [PubMed] [Google Scholar]
- 9.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 10.Coleman, T. R., D. J. Fishkind, M. S. Mooseker, and J. S. Morrow. 1989. Functional diversity among spectrin isoforms. Cell Motil. Cytoskelet. 12:225-247. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach, J., and A. Burkle. 2005. Introduction to poly(ADP-ribose) metabolism. Cell. Mol. Life Sci. 62:721-730. [DOI] [PubMed] [Google Scholar]
- 12.Dutta, P. R., R. Cappello, F. Navarro-Garcia, and J. P. Nataro. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 70:7105-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foxman, B., L. Zhang, K. Palin, P. Tallman, and C. F. Marrs. 1995. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 171:1514-1521. [DOI] [PubMed] [Google Scholar]
- 15.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 16.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 17.Guyer, D. M., S. Radulovic, F. E. Jones, and H. L. Mobley. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 19.Heimer, S. R., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 21.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 24.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 25.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 26.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadrmas, J. L., and M. C. Beckerle. 2004. The LIM domain: from the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 5:920-931. [DOI] [PubMed] [Google Scholar]
- 29.Kostakioti, M., and C. Stathopoulos. 2004. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect. Immun. 72:5548-5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunin, C. M. 1987. Detection, prevention and management of urinary tract infections, 4th ed. Lea & Febiger, Philadelphia, Pa.
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Loveless, B. J., and M. H. Saier, Jr. 1997. A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol. Membr. Biol. 14:113-123. [DOI] [PubMed] [Google Scholar]
- 33.Mobley, H. L., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 34.Navarro-Garcia, F., A. Canizalez-Roman, J. Luna, C. Sears, and J. P. Nataro. 2001. Plasmid-encoded toxin of enteroaggregative Escherichia coli is internalized by epithelial cells. Infect. Immun. 69:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro-Garcia, F., A. Canizalez-Roman, B. Q. Sui, J. P. Nataro, and Y. Azamar. 2004. The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect. Immun. 72:3609-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oomen, C. J., P. van Ulsen, P. van Gelder, M. Feijen, J. Tommassen, and P. Gros. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parham, N. J., U. Srinivasan, M. Desvaux, B. Foxman, C. F. Marrs, and I. R. Henderson. 2004. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 230:73-83. [DOI] [PubMed] [Google Scholar]
- 39.Patel, S. K., J. Dotson, K. P. Allen, and J. M. Fleckenstein. 2004. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 72:1786-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajakumar, K., C. Sasakawa, and B. Adler. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richard, J. F., L. Petit, M. Gibert, J. C. Marvaud, C. Bouchaud, and M. R. Popoff. 1999. Bacterial toxins modifying the actin cytoskeleton. Int. Microbiol. 2:185-194. [PubMed] [Google Scholar]
- 43.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 302:1704-1709. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Stathopoulos, C., D. R. Hendrixson, D. G. Thanassi, S. J. Hultgren, J. W. St. Geme III, and R. Curtiss III. 2000. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microbes Infect. 2:1061-1072. [DOI] [PubMed] [Google Scholar]
- 46.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanassi, D. G., and S. J. Hultgren. 2000. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr. Opin. Cell Biol. 12:420-430. [DOI] [PubMed] [Google Scholar]
- 48.Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Bio/Technology 24:145-149. [Reprinted from Proc. Natl. Acad. Sci. USA 76:4350-4354, 1979.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veiga, E., E. Sugawara, H. Nikaido, V. de Lorenzo, and L. A. Fernandez. 2002. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. EMBO J. 21:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viel, A., and D. Branton. 1996. Spectrin: on the path from structure to function. Curr. Opin. Cell Biol. 8:49-55. [DOI] [PubMed] [Google Scholar]
- 51.Villaseca, J. M., F. Navarro-Garcia, G. Mendoza-Hernandez, J. P. Nataro, A. Cravioto, and C. Eslava. 2000. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 68:5920-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welch, R. A., E. P. Dellinger, B. Minshew, and S. Falkow. 1981. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature 294:665-667. [DOI] [PubMed] [Google Scholar]
- 54.Wood, W. I. 1976. Tables for the preparation of ammonium sulfate solutions. Anal. Biochem. 73:250-257. [DOI] [PubMed] [Google Scholar]