Abstract
This is the first study describing an experimental mastitis model using transgenic cows expressing recombinant human lactoferrin (rhLf) in their milk. The aim of the study was to investigate the concentrations in milk and protective effects of bovine and recombinant human lactoferrin in experimental Escherichia coli mastitis. Experimental intramammary infection was induced in one udder quarter of seven first-lactating rhLf-transgenic cows and six normal cows, using an E. coli strain isolated from cows with clinical mastitis and known to be susceptible to Lf in vitro. Clinical signs were recorded during the experimental period, concentrations of human and bovine Lf and indicators of inflammation and bacterial counts were determined for milk, and concentrations of acute-phase proteins and tumor necrosis factor alpha were determined for sera and milk. Serum cortisol and blood hematological and biochemical parameters were also determined. Expression levels of rhLf in the milk of transgenic cows remained constant throughout the experiment (mean, 2.9 mg/ml). The high Lf concentrations in the milk of transgenic cows did not protect them from intramammary infection. All cows became infected and developed clinical mastitis. The rhLf-transgenic cows showed milder systemic signs and lower serum cortisol and haptoglobin concentrations than did controls. This may be explained by lipopolysaccharide-neutralizing and immunomodulatory effects of the high Lf concentrations in their milk. However, Lf does not seem to be a very efficient protein for genetic engineering to enhance the mastitis resistance of dairy cows.
Lactoferrin (Lf), an iron-binding protein of the transferrin family, is found in the secondary granules of polymorphonuclear leukocytes and in mucosal secretions such as milk. Lf has a broad spectrum of antimicrobial properties and is believed to be a prominent component of the nonspecific host defense on the mucosal surface (7, 57). The bacteriostatic effect of Lf is based mainly on its ability to sequester iron (2). Bovine milk Lf (bLf) exhibits marked bacteriostatic activity against a wide range of bacteria, with the most susceptible species being Escherichia coli (32, 34). Susceptibility to Lf varies among bacterial species and strains (34). Lf binds lipopolysaccharide (LPS) on the outer cell membranes of gram-negative bacteria, causing the release of LPS by damaging the structural integrity of the membrane (14). Lf can also modulate the immunological response in vivo and in vitro by down-regulating LPS-induced cytokines (19, 37).
The concentration of bLf is high in colostrum (48) and in dry-period secretions (58). In healthy lactating cows, the bLf level in the milk varies from 0.02 to 0.45 mg/ml (58). During intramammary infection (IMI), the concentration of bLf in the milk increases (17, 18, 25), and Lf is considered one of the most important factors contributing to the defense of the mammary gland (52). For experimentally induced E. coli infection, the bLf concentration in milk was 30 times higher than that found in normal milk (18). The milk bLf concentration depends on the severity of the infection. The greatest increase in bLf concentration has been seen for acute coliform mastitis, whereas for subclinical mastitis much lower bLf values are detected (25, 29). In all studies, great individual variations in bLf concentration between cows have been reported. Exogenous Lf has been proposed to represent a potential nonantibiotic therapeutic approach for the treatment of IMI (11, 24). Some beneficial effect was reported for subclinical mastitis (26), but for experimental E. coli mastitis, no clear advantage could be demonstrated (33).
Mastitis caused by environmental coliform bacteria is an increasing problem for the dairy industries in many countries (12, 30). Coliform mastitis is often associated with severe clinical signs, extensive tissue damage, and considerable losses in milk yield (16, 51). In E. coli mastitis, the response of the host mainly determines the severity of the disease (8). Genetic engineering is one potential way to increase the host defense against mastitis (27, 39, 56). Increasing mastitis resistance through modifying the activities of genes or incorporating beneficial genes from other organisms into dairy cattle could have a positive impact on animal welfare and also on the economics of milk production. The transgenic approach to enhancing mastitis resistance has been studied in a mouse model (27). In the first published bovine model (56), cows carrying a gene coding for an antistaphylococcal peptide, lysostaphin, were shown to be resistant to Staphylococcus aureus-induced IMI. To increase the resistance of dairy cows to coliform IMI by transgenesis, the transfer of a gene encoding human lactoferrin (hLf) to the bovine mammary gland would, in theory, represent a good candidate approach. The first transgenic cows with the hLf gene were reported to express Lf in their milk, at levels from 0.3 to 2.8 mg/ml (55). Recombinant human Lf (rhLf) is structurally and functionally similar to natural hLf (44, 45). However, despite the sequence homology, there may be differences in activity, as shown for bovine and human lactoferricin (15).
The aim of this study was to investigate the response of rhLf-transgenic cows to experimentally induced E. coli IMI. The working hypothesis was that cows with elevated Lf concentrations in their milk would be less susceptible to mastitis than cows with physiological Lf concentrations.
MATERIALS AND METHODS
Animals and experimental data.
Seven primiparous Holstein-Friesian transgenic cows, produced and owned by Pharming Group NV, The Netherlands, and expressing human Lf in their milk, and six normal Holstein-Friesian dairy cows were used. The median age of the transgenic cows was 39 months (range, 38.5 to 39.9 months) at parturition and they had calved a median of 12 days before the experiment. The six normal cows used as control animals were 30 months old (range, 27.5 to 31.5 months) at parturition and had calved a median of 18 days before the experiment. Genomic insertion by microinjection of recombinant DNA into the pronucleus of a fertilized oocyte was the method used to generate a transgenic bull, and rhLf-transgenic cows were then produced by the embryo transfer technique (31). In brief, the donor heifers were superovulated and inseminated with transgenic sperm from the sire. The embryos were flushed from uteri, and multiplex PCR analysis was performed on biopsies to identify the males and the transgeneity. The female transgenic embryos were selected for transfer. The transgeneity of the calves was confirmed by hLf-transgenic-calf PCR analysis (6). The basic mean concentration of rhLf in the milk of transgenic cows during early lactation was 2.9 mg/ml, and that of bLf was 0.07 mg/ml (22).
The cows were fed according to their energy requirements with good-quality hay, silage, and concentrated grain. Water was available ad libitum. They were milked twice a day, at 8 a.m. and 4 p.m. The basic somatic cell count (SCC) in the milk of transgenic cows was, on average, 34,200/ml (range, 14,000 to 70,000/ml), and that in the milk of control cows was, on average, 99,600/ml (range, 38,000 to 177,000/ml). The cows were clinically healthy and had no bacterial growth in their milk samples before the experiment. One cow from the transgenic group and one from the control group were excluded from the trial because of abnormally high pretrial values for acute-phase proteins, which indicated some subclinical, concomitant disease.
E. coli mastitis was induced as described before (33, 47). On average, 1,700 CFU (range, 1,500 to 2,300 CFU) of E. coli strain FT238, isolated from cows with clinical mastitis, was infused into a single udder quarter of each cow. This bacterial strain is sensitive to bLf in vitro, with complete inhibition of growth being achieved at concentrations of >1.67 mg/ml (33).
The ethics committees of the University of Kuopio and the University of Helsinki approved the study protocol, and The Board for Gene Technology in Finland approved the use of transgenic animals.
Blood and milk samples.
Blood and aseptic milk samples from the challenged and contralateral udder quarters were collected 12 h and immediately before the challenge and every 4 hours postchallenge (p.c.) during the first 24 h. Thereafter, blood samples were drawn at 36, 60, 84, and 168 h (7 days) and at 14 days, and milk samples were taken before milking of cows at 36, 44, 60, 84, 132, 156, and 180 h and, finally, 14 days after the challenge. The jugular vein was used for blood sampling. Sera were separated and kept frozen at −70°C for later determinations of tumor necrosis factor alpha (TNF-α), serum amyloid A (SAA), haptoglobin, cortisol, urea, creatinine, albumin, total protein, alanine aminotransferase (ALAT), and alanine aminophosphatase (AFOS) levels. EDTA-treated blood was collected for leukocyte (WBC) counts and determinations of packed cell volume (PCV). Human and bovine lactoferrin levels, bacterial counts, milk SCCs, N-acetyl-β-d-glucosaminidase (NAGase) activities, LPS levels at 12 h p.c., and SAA, haptoglobin, and TNF-α levels were determined from the milk samples.
Clinical observations.
Systemic and local clinical signs were monitored throughout the experiment, every 4 h during the first 24 h, and subsequently every time the cows were milked. Heart rate, rectal temperature, rumen motility, appetite, and general attitude were recorded. Systemic signs were scored on a three-point scale (from 1 [no signs] to 3 [severe signs]), with half numbers also being used (47). The udder was palpated for soreness, swelling, hardness, and heat, and the appearance of the milk was assessed visually for clots and changes in color or composition every time the cows were milked. Local signs were scored on the same three-point scale as the systemic signs, as follows: for milk, 1 (normal) to 3 (serous or clotty milk); and for udders, 1 (no changes) to 3 (severe swelling and soreness in the quarter) (47). Cows showing scores of >1 but ≤1.5 were recorded as having mild mastitis, those with scores of >1.5 but ≤2.5 were recorded as having moderate mastitis, and those with scores of >2.5 to 3 were recorded as having severe mastitis. The milk yield from the infected udder quarter and the total milk yield were measured 4 days and 12 h before inoculation and thereafter every time the cows were milked until the seventh day and, finally, at 2 weeks p.c.
Human and bovine lactoferrin.
Quantitative recombinant human and natural bovine lactoferrin analyses were conducted by enzyme-linked immunosorbent assays (ELISAs). hLf was measured by an rhLf-specific ELISA according to the procedure recommended by Pharmingen, and anti-hLf was adsorbed with Sepharose to remove cross-reacting antibodies (54). bLf levels were measured using a bovine lactoferrin ELISA quantitation kit (Bethyl Laboratories, Inc.). The cross-reactivity of bLf with hLf was tested with bLf and hLf standards (Sigma, St. Louis, Mo.). The level of detection was 0.008 mg/ml. The interassay and intra-assay coefficients of variation (CVs) for the Lf analysis were <10% and <5%, respectively.
Bacterial counts and LPS in milk.
Bacterial counts in the milk were determined by preparing 10-fold dilution series of milk in sterile saline. Bacteria were cultured on blood agar at 37°C for 24 h, using serial dilutions, and were counted by a routine plate count method. The concentrations of LPS in the milk samples at 12 h p.c. were determined by using the Limulus amebocyte lysate test (BioWhittaker, Walkersville, MD) at the Regional Institute of Occupational Health, Kuopio, Finland.
Indicators of inflammation in milk and blood.
Milk SCCs were measured at Valio Ltd. Laboratories, Finland, by a fluoro-optical method using a Fossomatic instrument (Foss Electric, Hillerød, Denmark). SCC values over 20 × 106/ml were recorded as 20 × 106/ml. Milk NAGase activity was measured by the fluorogenic method of Kitchen and coworkers (28), using a microplate modification developed by Mattila and Sandholm (41). Interassay and intra-assay CVs for NAGase activity were <4.8% for the high control (mastitic milk [1.34 pmol]) and <6.6% for the low control (normal milk [0.147 pmol]).
An ELISA for the quantification of bovine TNF-α in plasma (9) was modified for serum and milk as described by Lehtolainen et al. (38). The milk samples were centrifuged twice at 25,000 × g for 40 min at 4°C, and the clear supernatants of the milk were used for ELISA analysis. The interassay (between days) and intraplate CVs for the serum TNF-α ELISA were below 10.1% and 8.6% for the high control (23.5 ng/ml) and the low control (1.9 ng/ml), respectively. For the milk TNF-α ELISA, the interassay and intraplate CVs for the high control (109.2 ng/ml diluted 1:32 to 1:128) were less than 9.8%, and those for the low control (6.4 ng/ml diluted 1:8) were below 14.0%. The detection limits of the ELISA were 0.5 ng/ml for serum and 1.0 ng/ml for milk. Serum cortisol was analyzed using a radioimmunoassay (Coat-A-Count cortisol; Diagnostic Product Corporation, Helsinki, Finland). The interassay and intra-assay CVs for serum cortisol were <6.4% and <5.1%, respectively.
The concentrations of SAA in sera and milk were determined by using a commercial ELISA test (Tridelta Development, Wicklow, Ireland). Serum and milk samples were initially diluted 1:500 and 1:50, respectively. For high SAA values, samples were diluted as necessary, up to 1:5,000 for serum samples and up to 1:10,000 for milk (maximal concentrations, 750 mg/liter and 1,500 mg/liter, respectively). The interassay and intra-assay CVs for the SAA analyses were <10% and <5%, respectively. Milk and serum haptoglobin concentrations were determined by a method based on the ability of haptoglobin to bind to hemoglobin (40), using tetramethylbenzidine as a substrate (1). The complex formed in the assay was determined photometrically (Multiskan MS; Labsystems, Vantaa, Finland). Interassay and intra-assay CVs for haptoglobin were <10% and <13%, respectively.
Hematological parameters (WBC counts and PCVs) were determined within 24 h of sampling, using an automated multiparameter analyzer with software for animal samples (Cell-Dyn 3700 system; Abbott Diagnostic Division, Abbott Park, IL). Serum urea, creatinine, albumin, and total protein were measured by enzymatic kinetic methods, using an automatic analyzer (KonePro; ThermoClinical Labsystems, Espoo, Finland). ALAT and AFOS activities were measured with an automatic analyzer (Kone Specific; ThermoClinical Labsystems, Espoo, Finland).
Statistical methods.
The effects of time postchallenge on concentrations of measured parameters and on clinical signs were analyzed statistically using a mixed-model analysis of variance (SPSS 11.0; SPSS Inc., Chicago, IL).
RESULTS
Clinical findings.
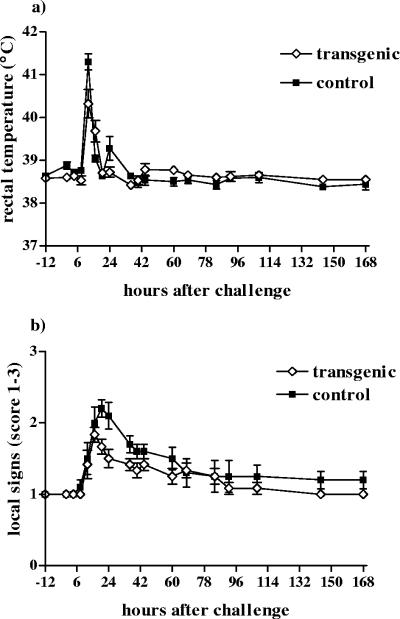
All cows in both groups became infected and developed clinical mastitis within 8 to 12 h p.c. The clinical response became visible 4 h earlier for the control group (P = 0.006). All transgenic cows and five cows in the control group showed mild to moderate systemic signs; only one cow in the control group exhibited a severe reaction. The body temperatures of the transgenic and control cows are presented in Fig. 1a. The mean temperature was lower for the transgenic cows than for the control cows (P = 0.031). The transgenic cows suffered significantly fewer severe systemic clinical signs than did control cows (P = 0.020), and they recovered faster (P = 0.008). Systemic signs in all cows in the transgenic group had returned to normal by 24 h, while the recovery of the control cows lasted over 48 h. The local signs for the infected udder quarters and changes in the appearance of milk disappeared within 7 days p.c. for both groups (Fig. 1b). No statistically significant differences between the local signs of the groups were found. The daily total milk yield during the experiment did not differ statistically between the groups.
FIG. 1.
Changes in responses of (a) rectal temperature and (b) local signs in transgenic (⋄) and control (▪) cows after an intramammary infusion of 1,700 CFU of E. coli into a single udder quarter. Data are presented as means ± SEM for six transgenic and five control cows.
Human and bovine lactoferrin.
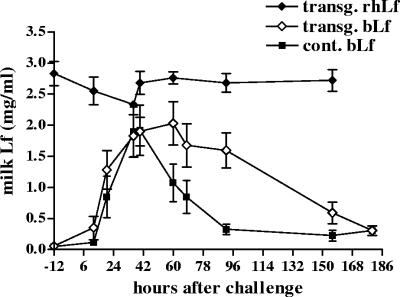
Total lactoferrin concentrations in the milk of the transgenic and control cows are presented in Fig. 2. Expression levels of rhLf remained rather constant during the experiment. The mean concentrations of rhLf in the milk ranged from 2.35 to 2.89 mg/ml. In the milk of the control udder quarters, mean bLf concentrations were slightly elevated and peaked at 36 to 40 h p.c., at 0.16 (standard error of the mean [SEM], ±0.03) mg/ml at 36 h p.c. and 0.14 (±0.02) mg/ml at 40 h p.c. for the transgenic group and at 0.20 (±0.06) and 0.24 (±0.08) mg/ml, respectively, for the control group. There was no statistically significant difference between concentrations of bLf in the challenged and control quarters.
FIG. 2.
Mean concentrations of rhLf (⧫) and bLf (⋄) in milk from challenged udder quarters of transgenic cows and of bLf (▪) in milk from control cows after an intramammary infusion of 1,700 CFU of E. coli into a single udder quarter during the experiment. Data are presented as means ± SEM for six transgenic and five control cows.
Bacterial counts and LPS in milk.
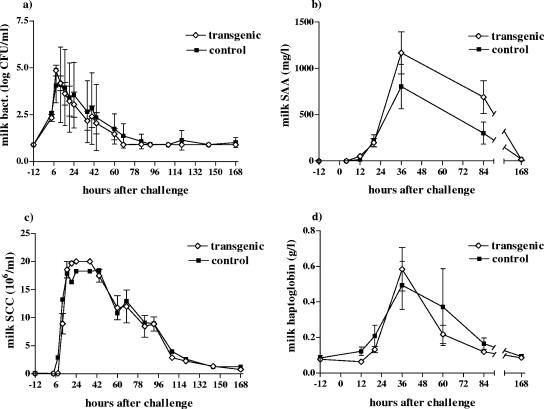
Bacterial counts in the milk of the challenged udder quarters peaked in both groups at 8 h p.c., and all bacteria were eliminated within 3.5 days p.c. (Fig. 3a). No significant differences were observed in the elimination times of bacteria between the two groups. The mean concentration of LPS in the milk of the transgenic cows at 12 h p.c. was 783 (SEM, ±437) endotoxin units/ml; the corresponding value for the control cows was 2,262 (±1,139) endotoxin units/ml. The difference in LPS concentrations between the groups was not statistically significant (data not shown).
FIG. 3.
Mean (a) bacterial counts (log CFU/ml), (b) milk SAA levels, (c) milk somatic cell counts, and (d) haptoglobin levels in milk from transgenic (⋄) and control (▪) cows during the experiment. Data are presented as means ± SEM for six transgenic and five control cows.
Indicators of inflammation in milk and blood.
Milk SCCs from the challenged udder quarters started to increase earlier (at 8 h p.c.) in the control cows, but for both groups they peaked at 16 h p.c. and started to decline after 44 h p.c. (Fig. 3c). No significant difference was found between the groups (P = 0.224). The NAGase activity of the milk was highest at 36 h p.c. for the transgenic group (on average, 2.49 pmol/min/μl) and at 40 h p.c. for the control group (on average, 2.46 pmol/min/μl). No significant difference was seen in the NAGase concentrations between the groups (data not shown). For both groups, SCCs and NAGase activities in the milk from challenged udder quarters returned to the baseline levels at 14 days p.c. Milk SCCs and NAGase activities in the contralateral control quarters remained at the prechallenge levels for both groups.
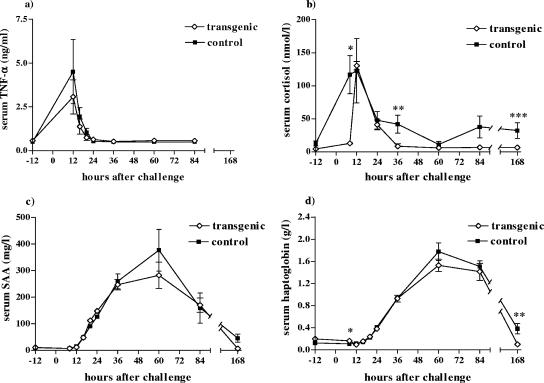
Experimental E. coli IMI induced both local and systemic TNF-α responses in the cows. A monophasic TNF-α response was present in the milk of the transgenic group, reaching a maximum level at 16 h p.c. (220.4 ± 50.3 ng/ml [mean ± SEM]). A biphasic TNF-α response was seen in the control group, with TNF-α concentrations in the milk peaking at 12 to 16 h p.c. (130.5 ± 24.2 and ± 14.9 ng/ml, respectively) and again at 24 h p.c. (162.5 ± 0.6 ng/ml). However, the second TNF-α peak for the control group was attributable to a single cow. Higher TNF-α concentrations were found in the milk of the transgenic group at hours 16 and 20 p.c. than in that from the control group, but the difference was not significant. The TNF-α concentrations returned to background levels within 60 h. In serum, the TNF-α concentrations peaked at 12 h p.c. and returned to the baseline level by 24 h p.c. (Fig. 4a). One of the control cows with severe systemic signs had the highest concentration of serum TNF-α at 12 h p.c. (10.9 ng/ml), but the mean concentrations of serum TNF-α did not differ statistically between groups.
FIG. 4.
Mean serum (a) TNF-α, (b) cortisol, (c) SAA, and (d) haptoglobin concentrations in transgenic (⋄) and control (▪) cows after an intramammary infusion of 1,700 CFU of E. coli into a single udder quarter during the experiment. Data are presented as means ± SEM for six transgenic and five control cows. The P values indicated in panel b are 0.003 (*), 0.031 (**), and 0.048 (***) for cortisol, and those in panel d are 0.035 (*) and 0.029 (**) for serum haptoglobin.
All cows responded to the challenge with increased serum cortisol concentrations, which were elevated in the control group at 8 h p.c. and peaked at 12 h p.c. (122.9 ± 48.7 nmol/liter [mean ± SEM]), whereas in the transgenic group they increased 4 h later, also peaking at the 12-h time point (130.4 ± 6.2 nmol/liter) (Fig. 4b). The serum cortisol concentrations of the transgenic group returned to normal by 36 h p.c., but in the control group they stayed elevated and did not return to baseline levels even by 7 days p.c. The differences in serum cortisol concentrations between the groups were significant at 8, 36, and 168 h p.c. (P = 0.003, P = 0.031, and P = 0.048, respectively).
Milk SAA concentrations started to increase at 12 h p.c. and were at their highest at 36 h p.c. for both groups (Fig. 3b). The mean peak value was 1,098.9 (±235.5 [SEM]) mg/liter for the transgenic cows and 891.3 (±246.3) mg/liter for the control cows. The milk haptoglobin concentration peaked at 36 h p.c. for both groups (Fig. 3d), with the average peak concentrations being 0.58 (±0.12) g/liter for the transgenic cows and 0.49 (±0.14) g/liter for the control cows. The milk SAA and haptoglobin concentrations did not differ statistically between the groups.
For both groups, serum SAA levels peaked at 60 h p.c. (Fig. 4c); the maximum concentrations were, on average, 282.4 mg/liter for the transgenic group and 376.9 mg/liter for the control group. The same pattern was seen for serum haptoglobin concentrations, but the differences were significant at 8 h (P = 0.035) and 168 h p.c. (P = 0.029) (Fig. 4d). The values were at their highest at 60 h p.c., at 1.53 g/liter, on average, for the transgenic cows and 1.77 g/liter, on average, for the control cows. Serum haptoglobin concentrations returned to the baseline level by 7 days p.c. for the transgenic group, but in the control group they were still elevated at this time point (P = 0.029). No differences were seen in serum concentrations of AFOS, ALAT, total protein, urea, creatinine, and albumin or in the WBC or PCV values between the groups (data not shown).
DISCUSSION
This is the first study describing an experimentally induced mastitis model using rhLf-transgenic dairy cows. The high concentration of Lf in the milk of the transgenic cows did not protect the udders from E. coli IMI, as all of the cows became infected. No differences were seen in the bacterial growth and times to bacterial elimination. The systemic clinical signs were milder in the transgenic group, but no difference was seen in the local signs. The mean concentration of endotoxin in the milk at 12 h p.c. was lower for the transgenic group than for the control group, but the difference was not statistically significant. No statistically significant differences were seen between the groups in any of the milk or blood inflammatory parameters, except for serum haptoglobin and cortisol levels. Genetic engineering of cows with the Lf gene to protect them against E. coli IMI was not as efficient as that reported for lysostaphin in a staphylococcal IMI model (56).
The total concentration of Lf in the milk of transgenic cows was markedly higher than the corresponding value for the control cows. The hLf gene is expressed under the control of the bovine αS1-casein promoter (6), and αS1-casein is expressed in bovine milk at a level of about 10 mg/ml. During IMI, the level of αS1-casein decreases in the milk, but the expression of casein does not change significantly (50), nor does the expression of rhLf. The regulation of bLf differs from that of the other milk proteins (49). bLf is released from the specific granules of neutrophils, and thus the concentration of bLf in milk is also related to the number of neutrophils present in the milk (17).
The rhLf present in the milk of transgenic animals, mice, and cows and natural hLf show very similar structural and functional properties in vitro (45, 53, 55). rhLf glycans may possess specific features that must be taken into account when interpreting results from in vitro and in vivo experiments (35). In spite of the structural similarities of rhLf and hLf, the differences in their iron-sequestering abilities and binding properties for microbial cell wall LPS may contribute significantly to the antimicrobial effects of lactoferrins and lactoferricins in vitro and in vivo (19, 46). According to Komine et al. (29), the antibacterial and immunostimulatory properties of Lf originating from the milk of mastitic cows were inferior to those of physiological Lf from healthy cows. rhLf is expressed under the control of bovine casein, and it remains to be determined whether the structure and function of rhLf change during IMI. In this study, the level of rhLf was rather constant during IMI, which suggests that rhLf may be largely independent of the regulation of bLf during inflammation.
In coliform mastitis, the clinical signs are attributed mainly to the effects of LPS, which induces the host response by stimulating an acute-phase response (8, 20). The abilities of Lf to bind LPS and to down-regulate LPS-induced cytokines have been hypothesized to be part of the immunomodulatory function of Lf (37). Lf is one of the proteins with high-affinity LPS binding, but it does not completely neutralize LPS activity, and lipid A can be still be active even after the Lf-LPS complex has been formed (43). Our finding of reduced clinical signs for transgenic animals may indicate that elevated Lf inhibited the activity of LPS to some extent, but not totally. Lf competes with LPS-binding protein for binding to LPS and might interfere with the interaction of LPS with CD14 (36). Lf is also believed to act like LPS-binding protein during the inflammatory activation of macrophages (43). The LPS-neutralizing activity of Lf may depend on the presence and concentration of other LPS-binding proteins.
At a concentration of <0.2 mg/ml, bLf cannot inhibit the growth of E. coli in vitro (13). Kutila et al. (32) found that E. coli isolates, our experimental strain included, were inhibited in vitro at a concentration of 1.67 mg/ml and that bacterial killing occurred at a relatively high initial concentration of bacteria (5,000 CFU/ml). In the present study, bacterial counts in the milk of the groups did not differ significantly, which means that the level of Lf was insufficient for a bacteriostatic effect. The concentration of Lf mRNA in the cisternal region and in ducts near the teat is higher than that in the epithelial ducts of the mammary parenchyma, and this may affect the prophylactic capacity of Lf against bacterial invasion via the teat (42).
In studies of experimentally induced LPS and E. coli mastitis, the increases in cortisol concentrations in mildly and moderately affected cows were transient (20, 23), but in the most severely affected cows the cortisol levels remained elevated (20). In our study, serum cortisol concentrations for the control cows rose earlier and remained elevated longer than those for the transgenic cows, indicating a stronger inflammatory reaction.
A rise in the serum TNF-α level reflects the severity of systemic signs (5, 20). In this study, the mean peak concentration was somewhat higher for the transgenic group, but the difference between groups was not significant. Lf may modulate immune responses by inhibiting cytokine activity, and this has been shown to be concentration dependent (10). Blum et al. (5) observed that peak plasma TNF-α concentrations were up to 20-fold lower than the maximal concentrations reached in milk. In our study, the mean serum TNF-α concentration for the transgenic group was almost 100-fold lower and that for the control group was 30-fold lower than the maximal concentrations in the milk.
SAA and haptoglobin have been reported to be sensitive inflammatory markers for acute E. coli mastitis, and their levels correlate with the severity of the cow's response (1). In the present study, SAA and haptoglobin concentrations in serum started to increase shortly after the challenge and remained lower in the transgenic cows than in the control cows. SAA and haptoglobin peaked earlier in the milk than in the serum for both groups. This was probably a result of the rapid local production of these proteins in the mammary gland, as described before (21). Haptoglobin binds harmful molecules, such as hemoglobin, as well as debris produced after tissue damage (4), and thus can help to restrict the spread of the infection by limiting the free iron available for E. coli bacteria, which is also one function of Lf; haptoglobin may thus complement the actions of Lf (21).
Our Lf transgenesis model did not provide protection against E. coli mastitis in dairy cows, in contrast to the findings for experimental S. aureus challenge in lysostaphin-transgenic cows (56). Lf reduced the severity of the inflammatory reaction, which could be seen in the systemic signs and in the serum cortisol and haptoglobin concentrations. Lactoferrin has broad or nonspecific antimicrobial activity, unlike lysostaphin, which is targeted against staphylococci and would thus have an advantage over lactoferrin. The inflammatory responses of the mammary gland to the two infections are different in that E. coli mainly causes acute IMI, whereas S. aureus tends to evoke a slowly developing chronic infection (3). This may partly explain the inconsistent results for these experiments. The inoculum size and challenge via the teat canal in the experimental E. coli mastitis model differ from natural infection, and the protective effect of additional Lf in milk could be better under natural conditions. However, it seems unlikely that Lf would be among the best candidate proteins for genetic engineering to enhance mastitis resistance in dairy cows if this approach is taken by the dairy industry in the future.
Acknowledgments
This work was supported by grants from the Walter Ehrström Foundation, the Finnish Veterinary Foundation, and the Research Foundation of Veterinary Medicine. We acknowledge Pharming NV, Holland, for the opportunity to carry out this unique experiment.
We thank Paavo Hujanen, Vehmersalmi, and the staff at Helsinki University for the care of the cows and for technical support. We also thank Reeta Pösö from the Department of Basic Veterinary Sciences and Satu Sankari from the Department of Clinical Veterinary Sciences for help with the blood chemistry analyses. The work of the laboratory staffs at the Department of Clinical Veterinary Sciences, Saari Unit, and at the Institute of Applied Biotechnology, University of Kuopio, is acknowledged. Finally, we thank the Veterinary Infectious Disease Organization, Saskatoon, University of Saskatchewan, Canada, for providing us with the mouse and recombinant anti-bovine TNF-α antibody.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Alsemgeest, S. P., H. C. Kalsbeek, T. Wensing, J. P. Koeman, A. M. van Ederen, and E. Gruys. 1994. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 16:21-23. [DOI] [PubMed] [Google Scholar]
- 2.Baker, H. M., and E. N. Baker. 2004. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals 17:209-216. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman, D. D., M. J. Paape, J. W. Lee, X. Zhao, J. C. Hope, and P. Rainard. 2004. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 11:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74-80. [DOI] [PubMed] [Google Scholar]
- 5.Blum, J. W., H. Dosogne, D. Hoeben, F. Vangroenweghe, H. M. Hammon, R. M. Bruckmaier, and C. Burvenich. 2000. Tumor necrosis factor-alpha and nitrite/nitrate responses during acute mastitis induced by Escherichia coli infection and endotoxin in dairy cows. Domest. Anim. Endocrinol. 19:223-235. [DOI] [PubMed] [Google Scholar]
- 6.Brink, M. F., M. D. Bishop, and F. R. Pieper. 2000. Developing efficient strategies for the generation of transgenic cattle which produce biopharmaceuticals in milk. Theriogenology 53:139-148. [DOI] [PubMed] [Google Scholar]
- 7.Brock, J. H. 2002. The physiology of lactoferrin. Biochem. Cell Biol. 80:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Burvenich, C., V. Van Merris, J. Mehrzad, A. Diez-Fraile, and L. Duchateau. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 34:521-564. [DOI] [PubMed] [Google Scholar]
- 9.Carstensen, L., C. M. Rontved, and J. P. Nielsen. 2005. Determination of tumor necrosis factor-alpha responsiveness in piglets around weaning using an ex vivo whole blood stimulation assay. Vet. Immunol. Immunopathol. 105:59-66. [DOI] [PubMed] [Google Scholar]
- 10.Crouch, S. P., K. J. Slater, and J. Fletcher. 1992. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 80:235-240. [PubMed] [Google Scholar]
- 11.Diarra, M. S., D. Petitclerc, and P. Lacasse. 2002. Effect of lactoferrin in combination with penicillin on the morphology and the physiology of Staphylococcus aureus isolated from bovine mastitis. J. Dairy Sci. 85:1141-1149. [DOI] [PubMed] [Google Scholar]
- 12.Dingwell, R. T., K. E. Leslie, Y. H. Schukken, J. M. Sargeant, L. L. Timms, T. F. Duffield, G. P. Keefe, D. F. Kelton, K. D. Lissemore, and J. Conklin. 2004. Association of cow and quarter-level factors at drying-off with new intramammary infections during the dry period. Prev. Vet. Med. 63:75-89. [DOI] [PubMed] [Google Scholar]
- 13.Dionysius, D. A., P. A. Grieve, and J. M. Milne. 1993. Forms of lactoferrin: their antibacterial effect on enterotoxigenic Escherichia coli. J. Dairy Sci. 76:2597-2606. [DOI] [PubMed] [Google Scholar]
- 14.Elass-Rochard, E., A. Roseanu, D. Legrand, M. Trif, V. Salmon, C. Motas, J. Montreuil, and G. Spik. 1995. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 312:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farnaud, S., A. Patel, E. W. Odell, and R. W. Evans. 2004. Variation in antimicrobial activity of lactoferricin-derived peptides explained by structure modelling. FEMS Microbiol. Lett. 238:221-226. [DOI] [PubMed] [Google Scholar]
- 16.Golodetz, C. L., and M. E. White. 1983. Prognosis for cows with severe clinical coliform mastitis. Vet. Rec. 112:402-403. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara, S., K. Kawai, A. Anri, and H. Nagahata. 2003. Lactoferrin concentrations in milk from normal and subclinical mastitic cows. J. Vet. Med. Sci. 65:319-323. [DOI] [PubMed] [Google Scholar]
- 18.Harmon, R. J., F. L. Schanbacher, L. C. Ferguson, and K. L. Smith. 1976. Changes in lactoferrin, immunoglobulin G, bovine serum albumin, and alpha-lactalbumin during acute experimental and natural coliform mastitis in cows. Infect. Immun. 13:533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haversen, L., B. G. Ohlsson, M. Hahn-Zoric, L. A. Hanson, and I. Mattsby-Baltzer. 2002. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell. Immunol. 220:83-95. [DOI] [PubMed] [Google Scholar]
- 20.Hirvonen, J., K. Eklund, A. M. Teppo, G. Huszenicza, M. Kulcsar, H. Saloniemi, and S. Pyorala. 1999. Acute phase response in dairy cows with experimentally induced Escherichia coli mastitis. Acta Vet. Scand. 40:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiss, S., M. Mielenz, R. M. Bruckmaier, and H. Sauerwein. 2004. Haptoglobin concentrations in blood and milk after endotoxin challenge and quantification of mammary Hp mRNA expression. J. Dairy Sci. 87:3778-3784. [DOI] [PubMed] [Google Scholar]
- 22.Hyvönen, P., L. Suojala, J. Haaranen, A. von Wright, and S. Pyörälä. 2006. Human and bovine lactoferrins in the milk of recombinant human lactoferrin-transgenic dairy cows during lactation. Biotechnol. J. 1:410-412. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, J. A., D. E. Shuster, W. J. Silvia, and R. J. Harmon. 1990. Physiological responses to intramammary or intravenous treatment with endotoxin in lactating dairy cows. J. Dairy Sci. 73:627-632. [DOI] [PubMed] [Google Scholar]
- 24.Kai, K., Y. Komine, K. Komine, K. Asai, T. Kuroishi, T. Kozutsumi, M. Itagaki, M. Ohta, and K. Kumagai. 2002. Effects of bovine lactoferrin by the intramammary infusion in cows with staphylococcal mastitis during the early non-lactating period. J. Vet. Med. Sci. 64:873-878. [DOI] [PubMed] [Google Scholar]
- 25.Kawai, K., S. Hagiwara, A. Anri, and H. Nagahata. 1999. Lactoferrin concentration in milk of bovine clinical mastitis. Vet. Res. Commun. 23:391-398. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, K., H. Nagahata, N. Y. Lee, A. Anri, and K. Shimazaki. 2003. Effect of infusing lactoferrin hydrolysate into bovine mammary glands with subclinical mastitis. Vet. Res. Commun. 27:539-548. [DOI] [PubMed] [Google Scholar]
- 27.Kerr, D. E., K. Plaut, A. J. Bramley, C. M. Williamson, A. J. Lax, K. Moore, K. D. Wells, and R. J. Wall. 2001. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat. Biotechnol. 19:66-70. [DOI] [PubMed] [Google Scholar]
- 28.Kitchen, B. J., G. Middleton, and M. Salmon. 1978. Bovine milk N-acetyl-beta-d-glucosaminidase and its significance in the detection of abnormal udder secretions. J. Dairy Res. 45:15-20. [DOI] [PubMed] [Google Scholar]
- 29.Komine, K., Y. Komine, T. Kuroishi, J. Kobayashi, Y. Obara, and K. Kumagai. 2005. Small molecule lactoferrin with an inflammatory effect but no apparent antibacterial activity in mastitic mammary gland secretion. J. Vet. Med. Sci. 67:667-677. [DOI] [PubMed] [Google Scholar]
- 30.Kossaibati, M. A., M. Hovi, and R. J. Esslemont. 1998. Incidence of clinical mastitis in dairy herds in England. Vet. Rec. 143:649-653. [DOI] [PubMed] [Google Scholar]
- 31.Krimpenfort, P., A. Rademakers, W. Eyestone, A. van der Schans, S. van den Broek, P. Kooiman, E. Kootwijk, G. Platenburg, F. Pieper, and R. Strijker. 1991. Generation of transgenic dairy cattle using ‘in vitro’ embryo production. Biotechnology (New York) 9:844-847. [DOI] [PubMed] [Google Scholar]
- 32.Kutila, T., S. Pyorala, H. Saloniemi, and L. Kaartinen. 2003. Antibacterial effect of bovine lactoferrin against udder pathogens. Acta Vet. Scand. 44:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutila, T., L. Suojala, T. Lehtolainen, H. Saloniemi, L. Kaartinen, M. Tahti, K. Seppala, and S. Pyorala. 2004. The efficacy of bovine lactoferrin in the treatment of cows with experimentally induced Escherichia coli mastitis. J. Vet. Pharmacol. Ther. 27:197-202. [DOI] [PubMed] [Google Scholar]
- 34.Lee, N. Y., K. Kawai, I. Nakamura, T. Tanaka, H. Kumura, and K. Shimazaki. 2004. Susceptibilities against bovine lactoferrin with microorganisms isolated from mastitic milk. J. Vet. Med. Sci. 66:1267-1269. [DOI] [PubMed] [Google Scholar]
- 35.Legrand, D., V. Salmon, B. Goddeville, M. Benaissa, Y. Plancke, and G. Spik. 1997. Structural determination of two N-linked glycans isolated from recombinant human lactoferrin expressed in BHK* cells, p. 111-117. In T. W. Hutchens and B. Lönnerdal (ed.), Lactoferrin. Interactions and biological functions. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 36.Legrand, D., E. Elass, M. Carpentier, and J. Mazurier. 2005. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol. Life Sci. 62:2549-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legrand, D., E. Elass, A. Pierce, and J. Mazurier. 2004. Lactoferrin and host defence: an overview of its immuno-modulating and anti-inflammatory properties. Biometals 17:225-229. [DOI] [PubMed] [Google Scholar]
- 38.Lehtolainen, T., C. Rontved, and S. Pyorala. 2004. Serum amyloid A and TNF-α in serum and milk during experimental endotoxin mastitis. Vet. Res. 35:651-659. [DOI] [PubMed] [Google Scholar]
- 39.Maga, E. A. 2005. Genetically engineered livestock: closer than we think? Trends Biotechnol. 23:533-535. [DOI] [PubMed] [Google Scholar]
- 40.Makimura, S., and N. Suzuki. 1982. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nippon Juigaku Zasshi 44:15-21. [DOI] [PubMed] [Google Scholar]
- 41.Mattila, T., and M. Sandholm. 1985. Antitrypsin and N-acetyl-beta-d-glucosaminidase as markers of mastitis in a herd of Ayrshire cows. Am. J. Vet. Res. 46:2453-2456. [PubMed] [Google Scholar]
- 42.Molenaar, A. J., Y. M. Kuys, S. R. Davis, R. J. Wilkins, P. E. Mead, and J. W. Tweedie. 1996. Elevation of lactoferrin gene expression in developing, ductal, resting, and regressing parenchymal epithelium of the ruminant mammary gland. J. Dairy Sci. 79:1198-1208. [DOI] [PubMed] [Google Scholar]
- 43.Na, Y. J., S. B. Han, J. S. Kang, Y. D. Yoon, S. K. Park, H. M. Kim, K. H. Yang, and C. O. Joe. 2004. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 4:1187-1199. [DOI] [PubMed] [Google Scholar]
- 44.Nuijens, J. H. 1993. A strategy to increase resistance in dairy cows. IDF Mastitis News 134:16-18. [Google Scholar]
- 45.Nuijens, J. H., P. H. van Berkel, M. E. Geerts, P. P. Hartevelt, H. A. de Boer, H. A. van Veen, and F. R. Pieper. 1997. Characterization of recombinant human lactoferrin secreted in milk of transgenic mice. J. Biol. Chem. 272:8802-8807. [DOI] [PubMed] [Google Scholar]
- 46.Prgomet, C., H. Sarikaya, R. M. Bruckmaier, and M. W. Pfaffl. 2005. Short-term effects on pro-inflammatory cytokine, lactoferrin and CD14 mRNA expression levels in bovine immunoseparated milk and blood cells treated by LPS. J. Vet. Med. A 52:317-324. [DOI] [PubMed] [Google Scholar]
- 47.Pyorala, S., L. Kaartinen, H. Kack, and V. Rainio. 1994. Efficacy of two therapy regimens for treatment of experimentally induced Escherichia coli mastitis in cows. J. Dairy Sci. 77:453-461. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez, L., P. Aranda, M. D. Perez, and M. Calvo. 1988. Concentration of lactoferrin and transferrin throughout lactation in cow's colostrum and milk. Biol. Chem. Hoppe-Seyler 369:1005-1008. [DOI] [PubMed] [Google Scholar]
- 49.Schanbacher, F. L., R. E. Goodman, and R. S. Talhouk. 1993. Bovine mammary lactoferrin: implications from messenger ribonucleic acid (mRNA) sequence and regulation contrary to other milk proteins. J. Dairy Sci. 76:3812-3831. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz, S., M. W. Pfaffl, H. H. Meyer, and R. M. Bruckmaier. 2004. Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis. Domest. Anim. Endocrinol. 26:111-126. [DOI] [PubMed] [Google Scholar]
- 51.Shpigel, N. Y., D. Levin, M. Winkler, A. Saran, G. Ziv, and A. Bottner. 1997. Efficacy of cefquinome for treatment of cows with mastitis experimentally induced using Escherichia coli. J. Dairy Sci. 80:318-323. [DOI] [PubMed] [Google Scholar]
- 52.Sordillo, L. M., and K. L. Streicher. 2002. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neoplasia 7:135-146. [DOI] [PubMed] [Google Scholar]
- 53.Thomassen, E. A., H. A. van Veen, P. H. van Berkel, J. H. Nuijens, and J. P. Abrahams. 2005. The protein structure of recombinant human lactoferrin produced in the milk of transgenic cows closely matches the structure of human milk-derived lactoferrin. Transgenic Res. 14:397-405. [DOI] [PubMed] [Google Scholar]
- 54.van Berkel, P. H., H. A. van Veen, M. E. Geerts, H. A. de Boer, and J. H. Nuijens. 1996. Heterogeneity in utilization of N-glycosylation sites Asn624 and Asn138 in human lactoferrin: a study with glycosylation-site mutants. Biochem. J. 319:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Berkel, P. H., M. M. Welling, M. Geerts, H. A. van Veen, B. Ravensbergen, M. Salaheddine, E. K. Pauwels, F. Pieper, J. H. Nuijens, and P. H. Nibbering. 2002. Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat. Biotechnol. 20:484-487. [DOI] [PubMed] [Google Scholar]
- 56.Wall, R. J., A. M. Powell, M. J. Paape, D. E. Kerr, D. D. Bannerman, V. G. Pursel, K. D. Wells, N. Talbot, and H. W. Hawk. 2005. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat. Biotechnol. 23:445-451. [DOI] [PubMed] [Google Scholar]
- 57.Ward, P. P., S. Uribe-Luna, and O. M. Conneely. 2002. Lactoferrin and host defense. Biochem. Cell Biol. 80:95-102. [DOI] [PubMed] [Google Scholar]
- 58.Welty, F. K., K. L. Smith, and F. L. Schanbacher. 1976. Lactoferrin concentration during involution of the bovine mammary gland. J. Dairy Sci. 59:224-231. [DOI] [PubMed] [Google Scholar]