Abstract
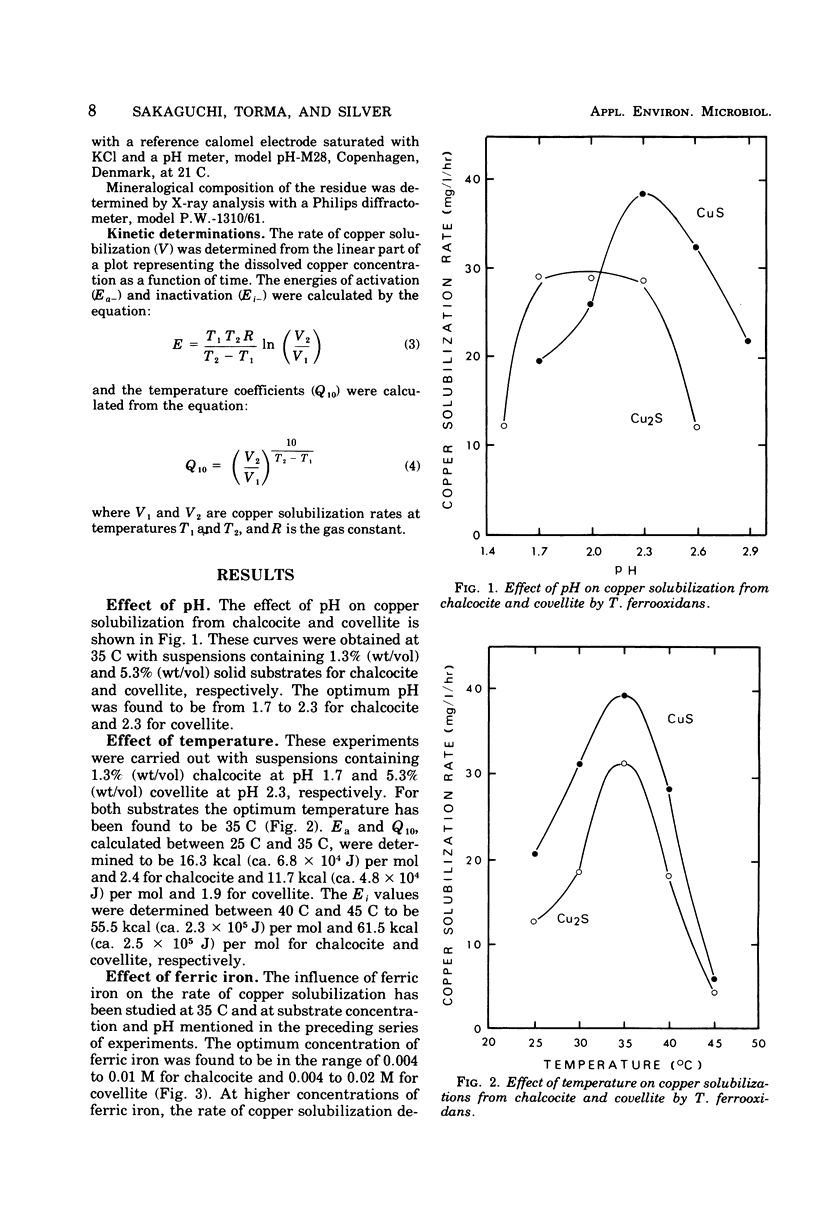
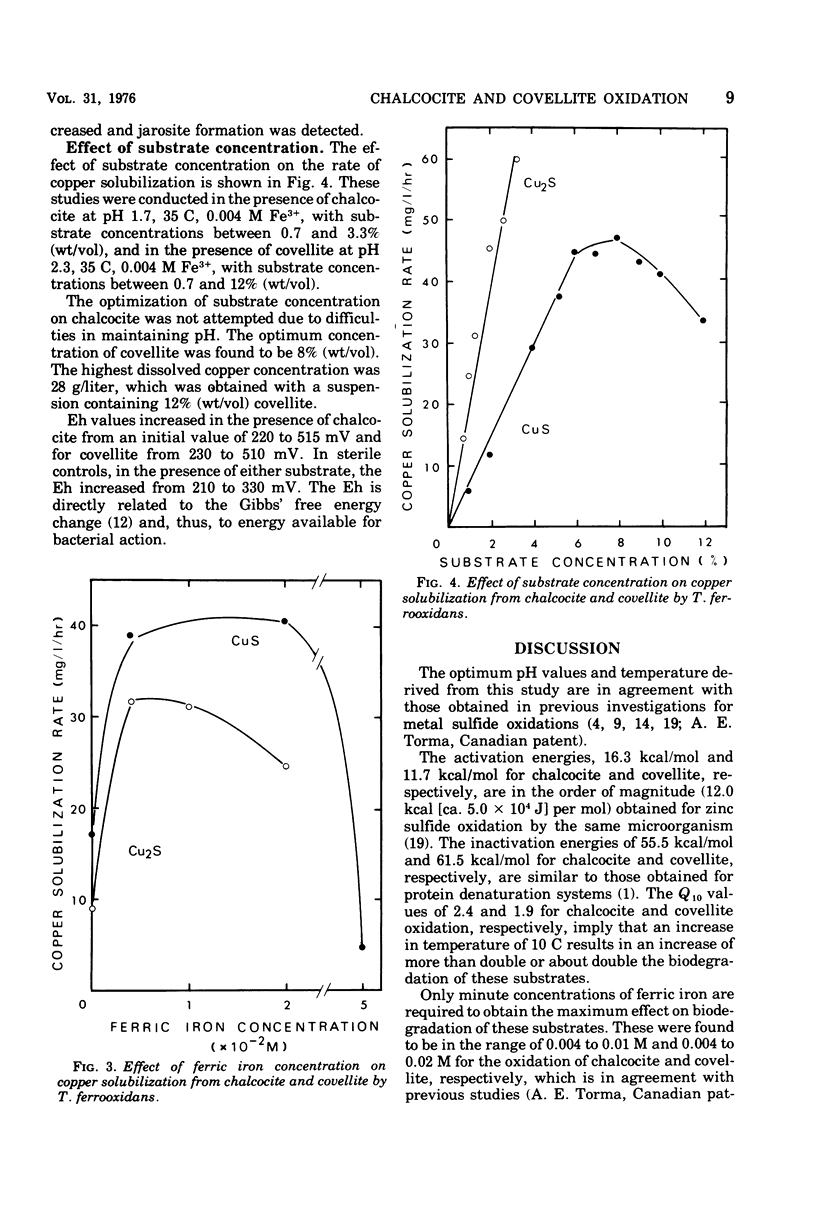
The microbiological oxidation of synthetic chalcocite and covellite has been investigated using an adapted strain of Thiobacillus ferrooxidans. Biodegradation of chalcocite was found to be 90 to 100% and that of covellite 45 to 60%. Optimum conditions for the oxidation of chalcocite were: pH, 1.7 to 2.3; temperature, 35 C; and ferric iron concentration in the range of 0.004 to 0.01 M. For covellite, the optimum conditions were: pH 2.3; temperature, 35 C; and ferric iron concentration in the range of 0.004 to 0.02 M. The energies of activation were determined to be 16.3 kcal (ca. 6.8 X 10(4) J) per mol and 11.7 kcal (ca. 4.8 X 10(4) J) per mol for chalcocite and covellite, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colmer A. R., Hinkle M. E. The Role of Microorganisms in Acid Mine Drainage: A Preliminary Report. Science. 1947 Sep 19;106(2751):253–256. doi: 10.1126/science.106.2751.253. [DOI] [PubMed] [Google Scholar]
- Nielsen A. M., Beck J. V. Chalcocite Oxidation and Coupled Carbon Dioxide Fixation by Thiobacillus ferrooxidans. Science. 1972 Mar 10;175(4026):1124–1126. doi: 10.1126/science.175.4026.1124. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., TRUSELL P. C. ISOLATION AND PROPERTIES OF AN IRON-OXIDIZING THIOBACILLUS. J Bacteriol. 1963 Mar;85:595–603. doi: 10.1128/jb.85.3.595-603.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Torma A. E. Oxidation of metal sulfides by Thiobacillus ferrooxidans grown on different substrates. Can J Microbiol. 1974 Feb;20(2):141–147. doi: 10.1139/m74-023. [DOI] [PubMed] [Google Scholar]
- Silverman M. P. Mechanism of bacterial pyrite oxidation. J Bacteriol. 1967 Oct;94(4):1046–1051. doi: 10.1128/jb.94.4.1046-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torma A. E. Microbiological oxidation of synthetic cobalt, nickel and zinc sulfides by Thiobacillus ferrooxidans. Rev Can Biol. 1971 Sep;30(3):209–216. [PubMed] [Google Scholar]
- Torma A. E., Walden C. C., Branion R. M. Microbiological leaching of a zinc sulfide concentrate. Biotechnol Bioeng. 1970 Jul;12(4):501–517. doi: 10.1002/bit.260120403. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ores. Z Allg Mikrobiol. 1972;12(4):311–346. doi: 10.1002/jobm.3630120406. [DOI] [PubMed] [Google Scholar]



