Abstract
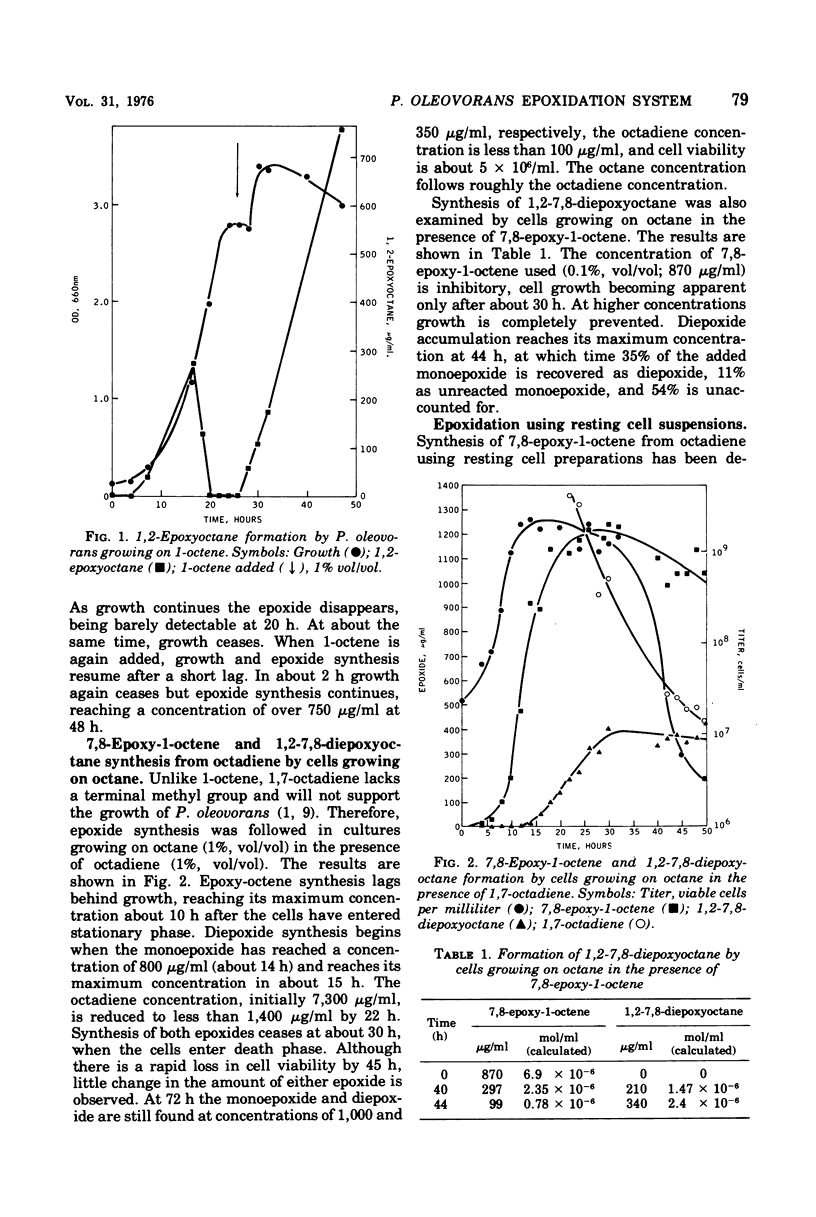
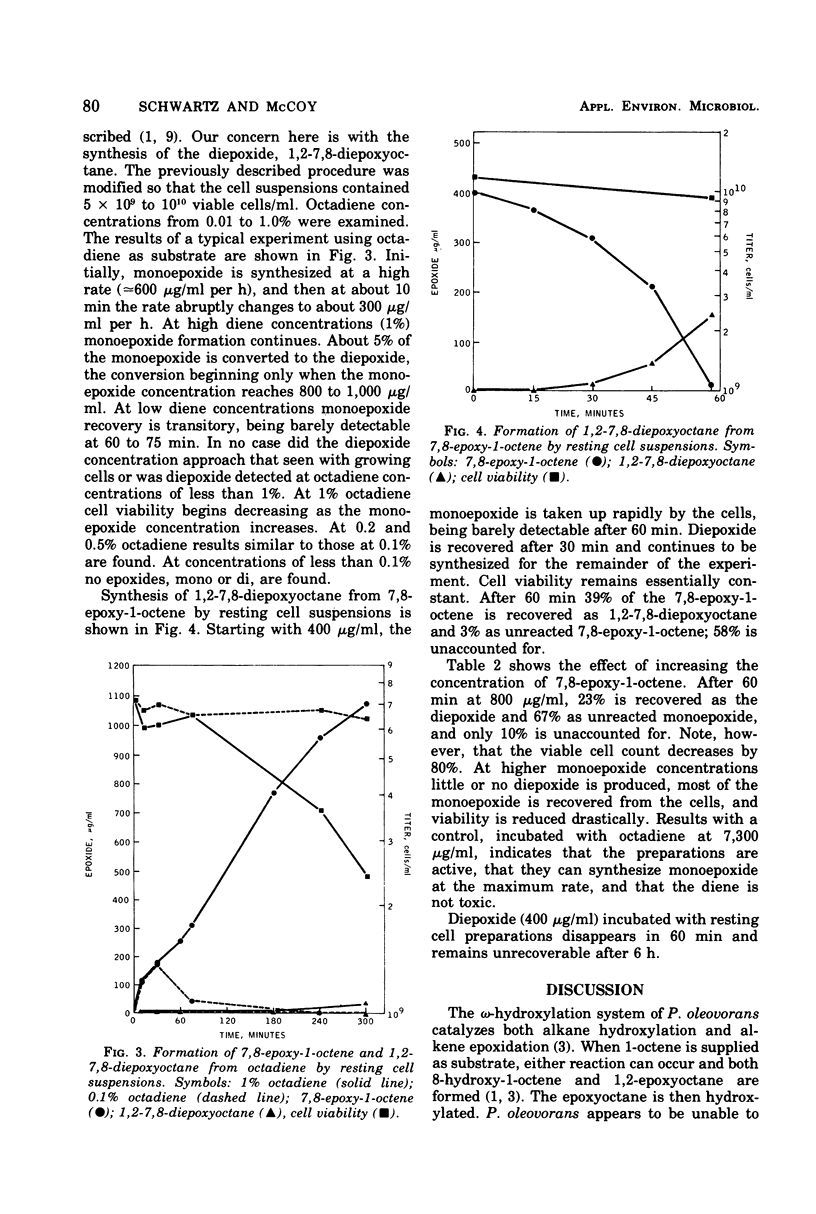
The kinetics of the enzymatic formation of 7,8-epoxy-1-octene, 1,2-7,8-diepoxyoctane, and 1,2-epoxyoctane by growing and resting cell suspensions of Pseudomonas oleovorans are described. Formation of 1,2-epoxyoctane occurs concurrently with exponential growth on 1-octene, providing that 1-octene is in excess. Conversion of 1,7-octadiene to 7,8-epoxy-1-octene by cells growing on octane lags behind exponential growth and continues into the stationary phase, terminating upon cell death. Formation of 1,2-7,8-diepoxyoctane does not begin until the cells are well into the stationary phase and also continues until cell death. Results with growing and resting cell suspensions suggest that the various substrates compete for the same enzyme system; that viable cells are essential for substrate transport and epoxidation by whole cells; and that whole cells may concentrate and sequester the epoxides, rendering them unrecoverable by our current methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Hou C. T. Oxidation of 1-alkenes to 1,2-epoxyalkanes by Pseudomonas oleovorans. Appl Microbiol. 1973 Jul;26(1):86–91. doi: 10.1128/am.26.1.86-91.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. I. Alkene epoxidation by the -hydroxylation system of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1230–1234. doi: 10.1016/0006-291x(72)90842-x. [DOI] [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. II. Comparison between the epoxidation and hydroxylation reactions catalyzed by the -hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1973 Mar 10;248(5):1725–1730. [PubMed] [Google Scholar]
- May S. W., Abbott B. J., Felix A. On the role of superoxide in reactions catalyzed by rubredoxin of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1540–1545. doi: 10.1016/0006-291x(73)91161-3. [DOI] [PubMed] [Google Scholar]
- May S. W., Schwartz R. D. Stereoselective epoxidation of octadiene catalyzed by an enzyme system of Pseudomonas oleovorans. J Am Chem Soc. 1974 Jun 12;96(12):4031–4032. doi: 10.1021/ja00819a060. [DOI] [PubMed] [Google Scholar]
- Ong T. M., De Serres F. J. Mutagenicity of chemical carcinogens in Neurospora crassa. Cancer Res. 1972 Sep;32(9):1890–1893. [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Pseudomonas oleovorans hydroxylation-epoxidation system: additional strain improvements. Appl Microbiol. 1973 Aug;26(2):217–218. doi: 10.1128/am.26.2.217-218.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D. Octene epoxidation by a cold-stable alkane-oxidizing isolate of Pseudomonas oleovorans. Appl Microbiol. 1973 Apr;25(4):574–577. doi: 10.1128/am.25.4.574-577.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



