Abstract
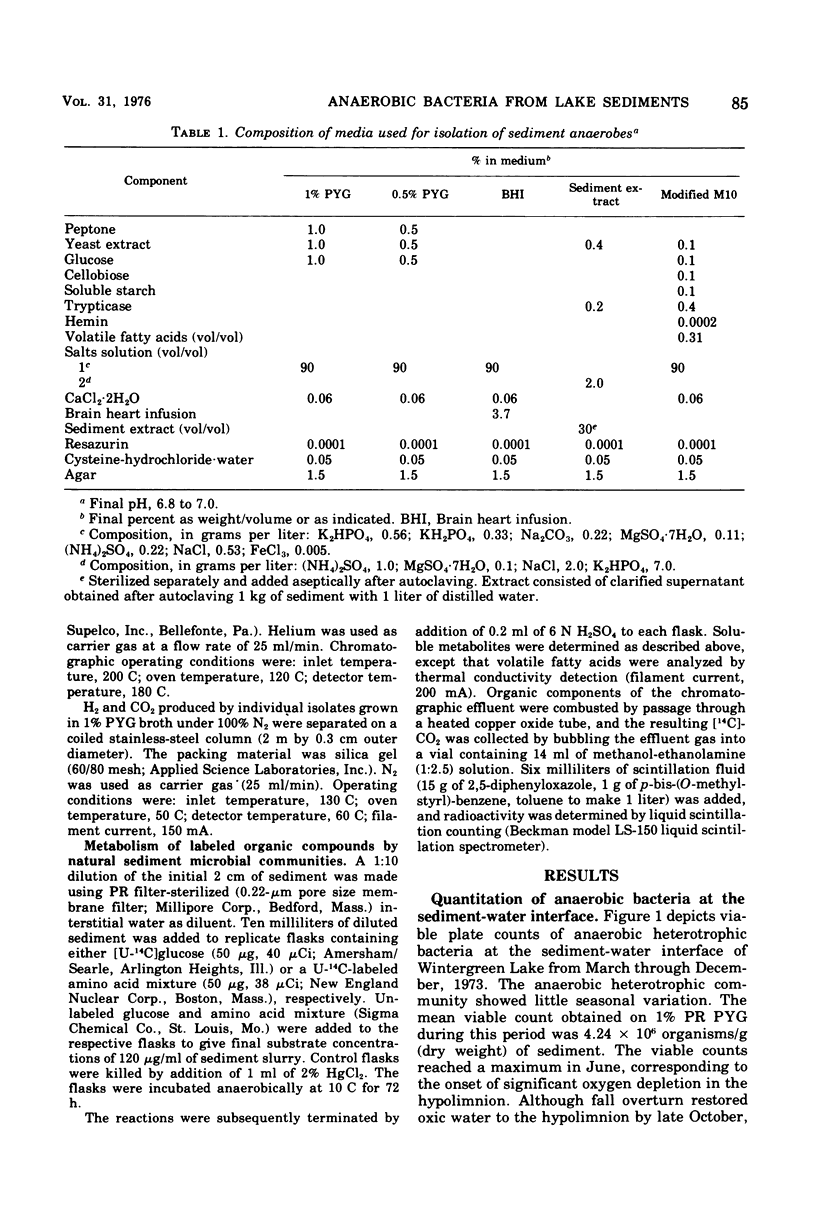
Strict anaerobic culture techniques were used to quantitatively and qualitatively evaluate the anaerobic heterotrophic bacteria present at the sediment-water interface of hyperutrophic Wintergreen Lake (Augusta, Mich.). Anaerobic plate counts remained constant from March through December, 1973, ranging from 2.4 X 10(6) to 5.7 X 10(6) organisms/g (dry weight) of sediment. The isolatable bacteria represented a small percentage of the total microbial community, which was shown by direct microscopic counts to be 2.0 X 10'' organisms/g (dry weight) of sediment during June and July. Bacteria of the genus Clostridium dominated the isolates obtained, accounting for 71.8% of the 960 isolates examined. A single species, Clostridium bifermentens, comprised 47.7% of the total. Additional bacterial groups and the percentage in which they were isolated included: Streptococcus sp. (10.8%), unidentified curved rods (9.5%y, gram-positive nonsporing rods (5.6%), and motile gram-negative rods (1.9%). Temperature growth studies demonstrated the ability of all the isolates to grow at in situ sediment temperatures. Gas-liqid radiochromatography was used to determine the soluble metabolic end products from [U-14C]glucose and a U-14C-labeled amino acid mixture by representative sedimentary clostridial isolates and by natural sediment microbial communities. At in situ temperatures the natural sediment microflora produced soluble fermentative end products characteristic of those elaborated by the clostridial isolates tested. These results are considered strong presumptive evidence that clostridia are actively metabolizing in the sediments of Wintergreen Lake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Finne G., Matches J. R. Low-temperature-growing clostridia from marine sediments. Can J Microbiol. 1974 Dec;20(12):1639–1645. doi: 10.1139/m74-255. [DOI] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Holdeman L. V., Moore W. E. Roll-tube techniques for anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1314–1317. doi: 10.1093/ajcn/25.12.1314. [DOI] [PubMed] [Google Scholar]
- Mah R. A., Sussman C. Microbiology of anaerobic sludge fermentation. I. Enumeration of the nonmethanogenic anaerobic bacteria. Appl Microbiol. 1968 Feb;16(2):358–361. doi: 10.1128/am.16.2.358-361.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matches J. R., Liston J., Curran D. Clostridium perfringens in the environment. Appl Microbiol. 1974 Oct;28(4):655–660. doi: 10.1128/am.28.4.655-660.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matches J. R., Liston J. Mesophilic clostridia in Puget Sound. Can J Microbiol. 1974 Jan;20(1):1–7. doi: 10.1139/m74-001. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



