Abstract
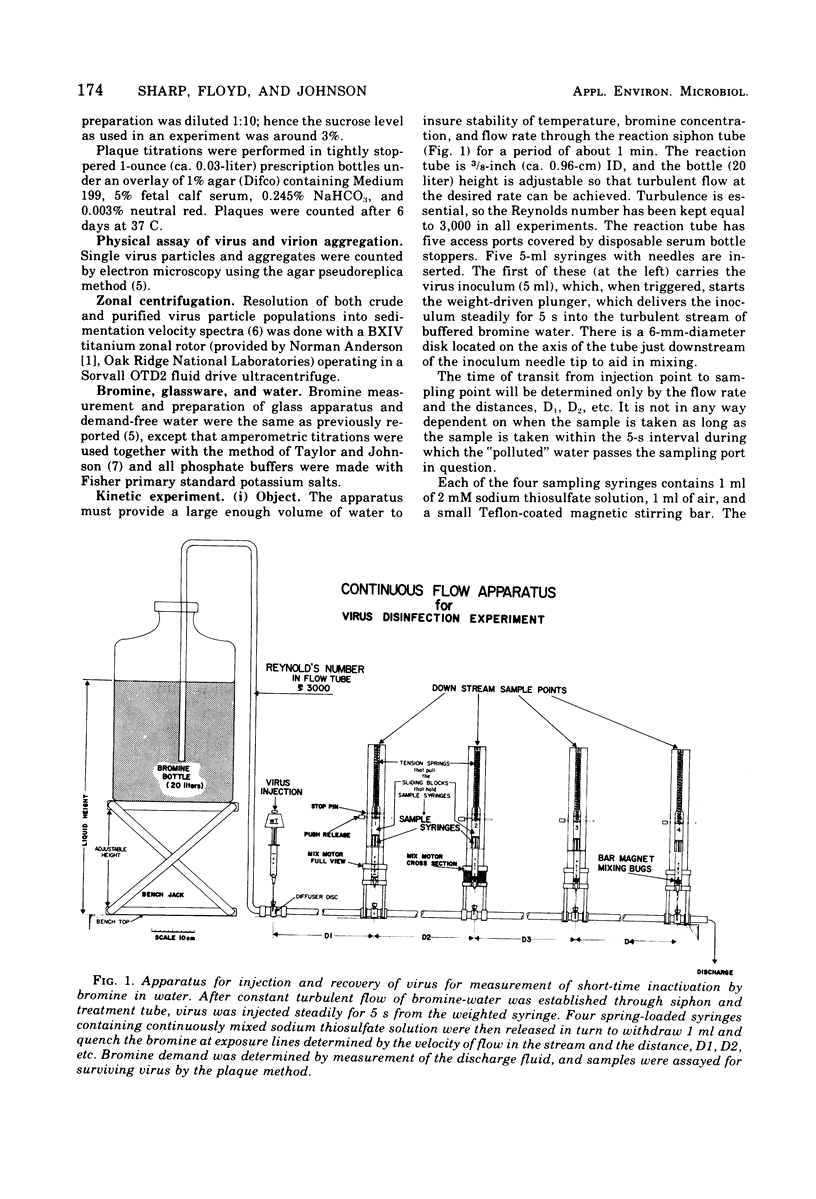
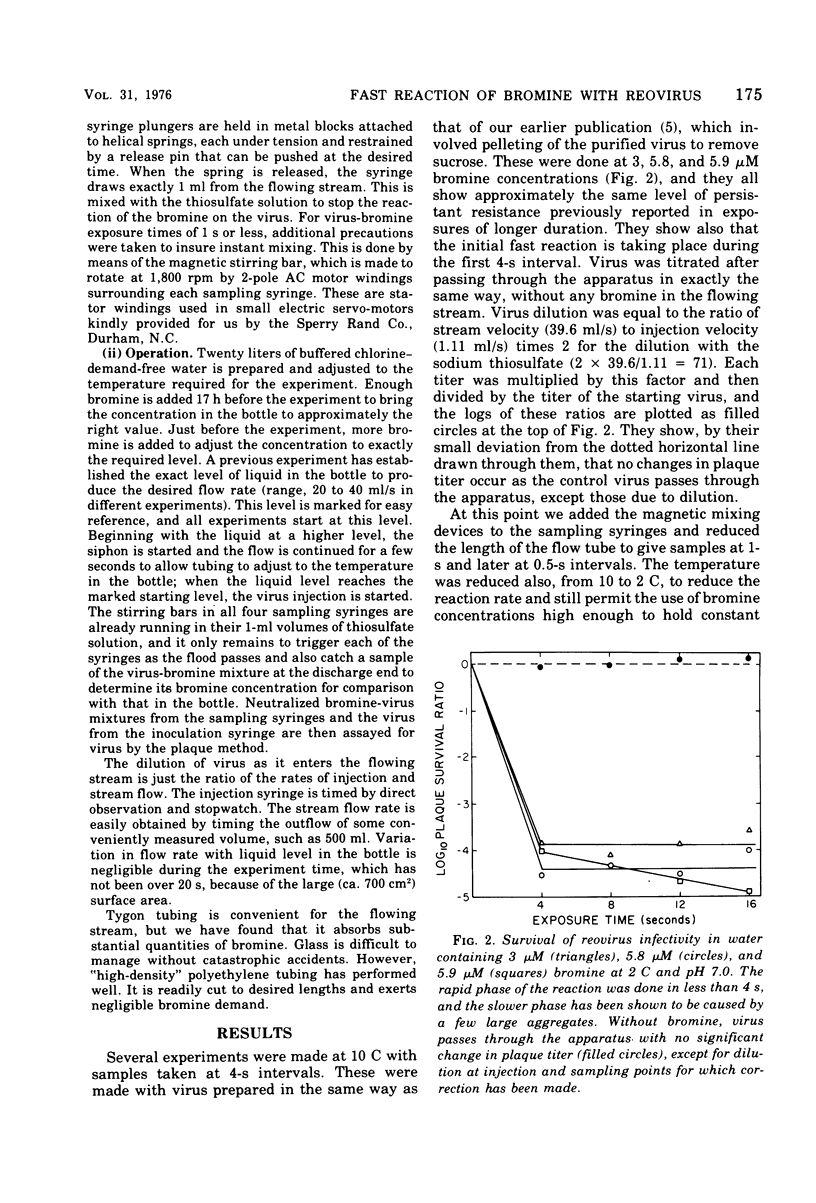
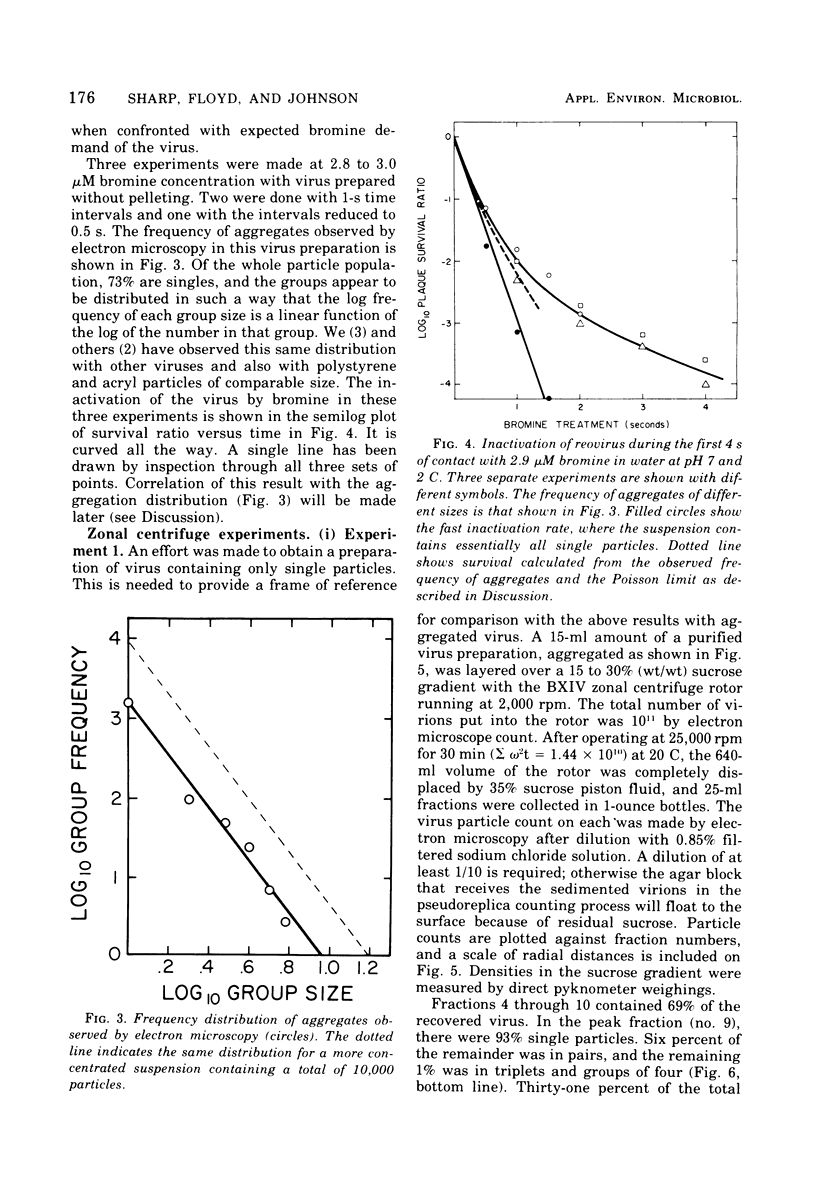
An apparatus is described for precise observation of the kinetics of the initial fast reaction of bromine with reovirus in turbulent flowing water. When quantitative electron microscopy shows that virus suspensions are essentially all single particles, the loss of infectivity follows first-order kinetics, the plaque titer falling at the rate of 3 log10 units/s at pH 7, 2 C, and at a 3-muM bromine concentration. Virus suspensions containing small aggregates (2 to 10/clump) exhibit a constantly decreasing disinfection rate with bromine. At a survival level of 10(-3) for single virions, the aggregated preparations have lost only 99% of their plaque titer and 10(-4) is reached only after 4 s of exposure. The disinfection rate does not appear to be a simple function of the size and frequency of aggregates in the virus suspension even when the aggregates contain no foreign material. Unpurified virus preparations (crude freeze-thaw lysates of infected cells) are shown, by zonal centrifugation, to contain 50% to over 90% of the infectivity in large, fast sedimenting aggregates. Such aggregates would strongly influence the bromine resistance of virus in polluted water.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEISTER R. G., PETERS D. H. QUANTITATIVE USE OF THE ELECTRON MICROSCOPE IN VIRUS RESEARCH. METHOD OF ANALYZING AND PREDICTING BIOLOGIC TITERS OF AGGREGATED VIRUS SUSPENSIONS WITH A NEW LAW OF AGGREGATION. Lab Invest. 1965 Jun;14:864–874. [PubMed] [Google Scholar]
- Kim K. S., Sharp D. G. Electron microscopic observations on the nature of vaccinia virus particle aggregation. J Immunol. 1966 Aug;97(2):197–202. [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Nature of the surviving plaque-forming unit of reovirus in water containing bromine. Appl Microbiol. 1975 Jan;29(1):94–101. doi: 10.1128/am.29.1.94-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., McGuire P. M. Spectrum of physical properties among the virions of a whole population of vaccinia virus particles. J Virol. 1970 Mar;5(3):275–281. doi: 10.1128/jvi.5.3.275-281.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDENKOPF S. J. Inactivation of type 1, poliomyelitis virus with chlorine. Virology. 1958 Feb;5(1):56–67. doi: 10.1016/0042-6822(58)90005-9. [DOI] [PubMed] [Google Scholar]


