Abstract
Japanese herbal (Kampo) medicine, Hochuekkito (Bu-Zhong-Yi-Qi-Tang in Chinese, TJ-41) and Juzentaihoto (Shi-Quan-Da-Bu-Tang in Chinese, TJ-48) are well-known Kampo formulas used as tonic. Although these medicines have separately been applied to the patients clinically depending on their symptoms, the differences of the pharmacological activities for these medicines have not been fully understood. TJ-48 and TJ-41 were compared for their effects on antibody response in upper respiratory mucosal immune system in vivo. Oral administration of TJ-41 (100 mg kg−1 per day) to early aged BALB/c mice, which were nasally sensitized with influenza hemagglutinin vaccine, significantly enhanced influenza virus-specific IgA and IgG antibody titers in nasal cavity and sera, respectively. However, oral administration of TJ-48 (100 mg kg−1 per day) failed to show the enhancing activity. TJ-41 increased not only influenza virus-specific IgA antibody titer but also total IgA antibody titer in nasal cavity. The stimulating activity of TJ-41 disappeared after treatment with methotrexate. The present study strongly suggests that TJ-41 can stimulate the mucosal immune system of upper respiratory tract, and results in enhancement of antigen-specific antibody response in upper respiratory mucosal and systemic immune systems.
Keywords: Hochuekkito, influenza virus, Japanese herbal (Kampo) medicine, mucosal immune system of upper respiratory tract, specific antibody response
Introduction
Formulas of traditional Japanese herbal (Kampo) medicines, Juzentaihoto (Shi-Quan-Da-Bu-Tang in Chinese, TJ-48) and Hochuekkito (Bu-Zong-Yi-Qi-Tang in Chinese, TJ-41) each comprise totally 10 kinds; 5 each same and 5 each different component herbs, and are used well clinically as tonic. TJ-48 has been applied clinically for recuperation after surgery or chronic diseases with symptoms such as exhaustion, fatigue, loss of appetite, pale face, dry skin and anemia whereas TJ-41 has been used for the treatment of weak patients, who have chronic diseases, tuberculosis, surgery with loss of appetite, mild fever, night sweat, palpitation, fear, restlessness, weak feeble voice, slurred speech and disturbance of vision (1). TJ-48 is also used for the treatments of anemia, dry skin and erosion of skin or mucosa whereas TJ-41 is used for the patients having respiratory disorder (2).
Pharmacological activities of these medicines have been evaluated by several research groups from the various aspects as follows: anticancer effects (3–7), inhibition of metastasis (8), suppression of recurrence of cancer (9), suppression of toxicity of anticancer drugs (10,11), hematoprotective activity (12,13), radioprotective activity (14–16), anti-infectious activity (17–21), antiallergic activity (22,23). However, few researches on evaluation of differences in mechanisms of action of these medicines have been tried in order to explain the differences of the clinical efficacy of these formulas from basic research. Although it has been reported that both medicines inhibit liver metastasis of colon 26-L5 cells, TJ-41 shows the activity by the activation of NK cells whereas TJ-48 expresses activity through activation of macrophages (8). TJ-48 also has been shown to exhibit anti-infectious activity against Candida albicans in cyclophosphamide-treated mice (20). Although TJ-41 could not have the activity for this Candida-infected mouse model, it could inhibit predonisolone-induced Candida-infection (20,21). It is thought that more studies are necessary to elucidate the differences of action mechanisms of these medicines in order to understand the differences of clinical efficacy between these Kampo medicines.
The mucosal immune system consists of an integrated network of tissue, lymphoid and mucus membrane-associated cells for regulations of host-protection, allergic diseases and oral tolerance. Mucosal immune systems in each local site such as gastrointestinal, pulmonary and genitourinary tracts and the exocrine glands connect each other through lymphocyte homing, and this intertissue communication is named as common mucosal immune system (CMIS) (24,25). The Peyer's patches in the small intestine play important roles for CMIS as one of the inductive sites. The lymphocytes in Peyer's patches are rapidly eliminated from the patches, and migrate into systemic circulation through the mesentric lymph nodes following to the thoracic duct. The lymphocytes finally home to the effecter sites of local mucosa such as lamina propria of intestinal and upper respiratory tracts (25). It has been suggested that the inductive sites of CMIS also communicate closely with systemic immune system through lymphocyte homing (24,25), and that the mucosal immune system regulates both local mucosal and systemic immune systems.
In classical theory of traditional herbal remedies on Chinese, Japanese, Korean and Ayurvedic medicines, the concept of ‘spleen’ has been expressed as a functional unit for spleen and intestine (1), which plays many roles such as nutritional absorption, and blood and energy supplies. Therefore, it has been considered that the dysfunction of ‘spleen’ affects the functions of whole body (1). Classical literatures in Chinese medicine describe that some of the herbal medicines may have several regulating activities against the concept of ‘spleen’. These considerations can assume that a part of the functions of ‘spleen’ resembles those of mucosal immune system in intestine. Because traditional herbal medicines are generally administered orally, there is a possibility that these medicines express their clinical effects through the action on mucosal immune system in intestine such as Peyer's patches. Matsumoto and Yamada, and Hong et al. have found that TJ-48 modulates the functions of lymphocytes in Peyer's patches and mesenteric lymph nodes, and intraepithelial lymphocytes (IEL), which belong to mucosal immune system in intestine (26,27), and the combination of some component herbs in the formula of TJ-48 is essential for expression of the modulating activity against immunocompetent cells of Peyer's patches (28). It has also been reported that lignin–carbohydrate complexes and arabino-3,6-galactans-containing pectic polysaccharides in TJ-48 are the active ingredients, which enhance the function of the lymphocytes in Peyer's patches (intestinal immune system modulating activity) (29,30). Recently, we have also found that TJ-41 stimulates the function of immunocompetent cells in Peyer's patches (Kiyohara, Matsumo, Yamada, unpublished results). These results postulate the possibility that these medicines may affect mucosal immune systems in the local sites by the same or different mechanisms.
In the present study, we attempted to compare the efficacy of TJ-41 and TJ-48 on the function of mucosal immune system in upper respiratory tract, and found that these medicines have the different effects for the mucosal immune system of upper respiratory tract.
Materials and Methods
Materials
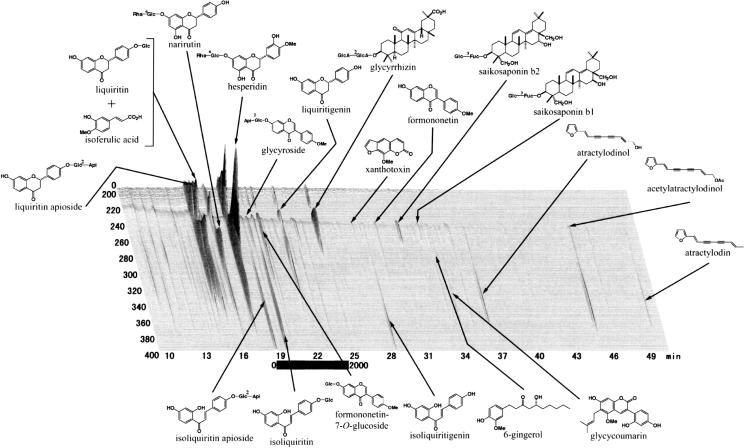
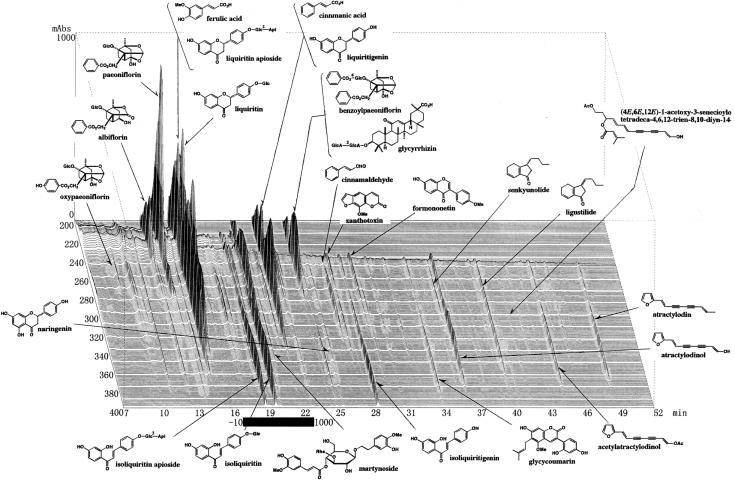
Spray-dried extract preparations of Juzentaihoto (Shi-Quan-Da-Bu-Tang in Chinese, TJ-48) and Hochuekkito (Bu-Zhong-Yi-Qi-Tang in Chinese, TJ-41) were kindly supplied by Tsumura & Co. (Tokyo, Japan). TJ-48 for 1 day dose was prepared as follows: a mixture of single herbs of Astragali Radix (3 g, roots of Astragalus membranaceus Bunge), Cinnamomi Cortex (3 g, barks of Cinnamomum cassia Blume), Rehmanniae Radix (3 g, roots of Rehmania glutinosa Libosch var. purpurea Makino), Paeonia Radix (3 g, roots of Paeonia lactiflora Pall), Cnidii Rhizoma (3 g, rhizomes of Cnidium officinale Makino), Atractylodis lanceae Rhizoma (3 g, rhizomes of Atractylodes lancea DC.), Poria (3 g, fungi of Poria cocos Wolf), Angelicae Radix (3 g, roots of Angelica acutiloba Kitagawa), Ginseng Radix (3 g, roots of Panax ginseng C.A. Meyer) and Glycyrrhizae Radix (1.5 g, roots of Glycyrrhiza uralensis Fisch et DC.) was added to water and extracted at 100°C for 1 h. The extracted solution was filtered and spray-dried to obtain dry extract powder. For the preparation of TJ-41, a mixture of Astragali Radix (4 g, roots of A. membranaceus Bunge), Atractylodis lanceae Rhizoma (4 g, rhizomes of A. lancea DC.), Ginseng Radix (4 g, roots of P. ginseng C.A. Meyer), Angelicae Radix (3 g, roots of A. acutiloba Kitagawa), Bupleuri Radix (2 g, roots of Bupleurum falcatum L.), Zizyphi Fructus (2 g, fruits of Zizyphus jujuba Miller var. inermis Rehder), Aurantii Bobilis Pericarpium (2 g, pericarps of ripe fruits of Citrus unshu Markovich), Glycyrrhizae Radix (1.5 g, roots of G. uralensis Fisch et DC.), Cimicifugae Rhizoma (1 g, rhizomes of Cimicifuga simplex Wormskjord) and Zingiberis Rhizoma (0.5 g, rhizomes of Zingiber officinale Roscoe) was also extracted by the above same procedure to obtain dry extract powder. Ministry of Health, Labour and Welfare in Japan chose each 5 g of TJ-48 and TJ-41 as a dosage for 1 day for adult human. In the present study, the dosages of the medicines were calculated from their 1 day dosages for adult human. Three-dimensional HPLC (3D-HPLC) analyses were carried out to know broad chemical profiles of TJ-48 and TJ-41, and the spray-dried extract preparations of TJ-41 and TJ-48 showing 3D-HPLC profiles as in Figs 1 and 2 were used for the present study.
Figure 1.
3D-HPLC profiles of Hochuekkito (TJ-41).
Figure 2.
3D-HPLC profiles of Juzentaihoto (TJ-48).
Vaccines
Influenza A/PR/8 hemagglutinin (HA) vaccine was prepared from mouse-adapted influenza virus A/PR/8/34 according to the methods of Nagai and Yamada (31). Mouse-adapted influenza viruses A/PR/8/34 (H1N1), which were preserved in The Kitasato Institute (Tokyo, Japan), were grown in allantoic cavity of 10 days old embryonated eggs for 48 h at 34°C. The allantoic fluid containing live viruses was harvested and centrifuged at 1000 g for 20 min. The virus was recovered from the resulting supernatant by sucrose density centrifugation, and the vaccine was prepared by inactivation with ether, according to the method of Davenport et al. (32).
A split vaccine of influenza HA vaccine produced from influenza virus A/New Caledonia/20/99 (A/NC/20) was kindly supplied from Center for Biologicals of The Kitasato Institute (Saitama, Japan) (influenza A/NC/20 HA vaccine).
Mice
Specific pathogen-free female BALB/c mice of 7 weeks old (young) and 6 months old (early aged) were purchased from Japan SLC (Shizuoka, Japan). The animals were housed in plastic cages in an air-conditioned room at 23 ± 2°C with a relative humidity of 55 ± 10% under a 12 h light/dark cycle, fed a standard laboratory diet and given water ad libitum. Animal experiments were performed according to the animal care guidelines of The Kitasato Institute and Kitasato University.
Immunization
Mice were anesthetized by intraperitoneal injection of sodium amobarbital (Sigma, 0.2 ml per mouse in a sterilized saline solution at 11 mg ml−1), and then immunized by a primary intranasal inoculation of 1, 2.5 or 5 μg of HA vaccines in sterilized phosphate-buffered saline (PBS) (10 μl each) at Day 0. The mice were given a secondary intranasal inoculation of the vaccine at the same dose as the primary immunization at 28 days (Day 28) after the primary immunization by the above same procedure.
Administrations of Medicines
The mice were administered orally aqueous solutions of TJ-48 and TJ-41 at doses of 100 mg kg−1 per day (0.5 ml per mouse) from 7 days before the primary immunization (Day −7) to 14 days after the secondary immunization (Day 42) once a day. Methotrexate (20 mg kg−1, Takeda Pharmaceutical Co. Ltd, 200 μl per mouse) was also administered orally to the early aged mice twice at 3 days each before the primary and secondary immunizations (Day −3 and Day 25).
Specimens
The procedure was performed according to the method of Nagai et al. (33). Briefly, at 21 days after the primary immunization (Day 21), the blood sample was taken from the retroorbital plexus of mice by a glass capillary, and the serum sample was separated from the blood by centrifugation after standing for 2 h at room temperature. At 42 days after the primary immunization, mice were sacrificed using ether and then bled from the heart with syringe to prepare sera as described above. After bleeding, the head of the mouse was removed, and the lower jaw of the mouse was excised. A hypodermic needle was inserted into the posterior opening of the nasopharynx, and 2 ml of sterilized PBS containing 0.1% bovine serum albumin (BSA) was injected to collect the nasal wash. The nasal washes were centrifuged to remove cellular debris and stored at −80°C prior to use for antibody analysis.
ELISA for Influenza Virus-specific and Total IgA Antibodies
The titers of influenza virus-specific antibodies in the sera (IgG class) and nasal washes (IgA class) were measured by ELISA according to the procedure of Nagai et al. (33). Briefly, the wells of a 96-well ELISA plate (ImmunomMaxisorp, Nunc) were coated with 100 μl of the influenza HA vaccines (5 μg ml−1) in 10 mM carbonate/bicarbonate buffer, pH 9.6. The plate was incubated for 2 h at room temperature, and then washed three times with 200 μl of PBS containing 0.05% Tween 20 and 0.1% NaN3 (PBS–Tween). PBS containing 0.5% skim milk and 0.1% NaN3 (blocking buffer) was placed in the wells (200 μl), and incubated overnight at 4°C. After washing the plate with PBS–Tween three times, each sample of serum or nasal wash (100 μl) was added to a set of three wells. Because IgA and IgG antibodies in the samples of nasal washes and sera recognize the same antigenic epitope of the influenza HA vaccines, the IgA and IgG antibodies in the samples were separated by Protein G-Sepharose in advance. Samples for ELISA were prepared as follows: Protein G–Sepharose (about 300–400 μl, Sigma) packed into Ultra-free-MC centrifuge column units with low binding Durapore membrane (pore size 0.45 μm; Millipore) was equilibrated with 20 mM sodium phosphate buffer, pH 7.0 (binding buffer). The nasal wash (200 μl) or serum (5 μl) was applied to the Protein G-Sepharose column and centrifuged at 1000 r.p.m. until all the solutions were loaded into the gel. The column was washed with the binding buffer (200 μl each) twice to obtain the unabsorbed fraction as IgA antibody fraction. The bound IgG antibody in the column was further eluted from the column with 200 μl each of 0.1 M glycine–HCl buffer, pH 2.7, twice into the plastic tubes containing 40 μl of 1 M Tris–HCl buffer, pH 9.0, immediately. The unabsorbed and absorbed fractions each were diluted with the blocking buffer (25 times for the samples from the sera; twice for those from the nasal washes) and placed on the wells of influenza HA vaccine-immobilized ELISA plate. The plates were incubated for 2 h at room temperature and washed three times with PBS–Tween. Alkaline phosphatase coupled goat anti-mouse IgA (Zymed) or IgG (Sigma) antibody diluted with the blocking buffer (1:1000) was added to each well (100 μl per well). The plates were incubated overnight at room temperature, and then washed four times with PBS–Tween. Finally, p-nitrophenylphosphate (Wako Pure Chemical Co. Ltd, 1 mg ml−1) in 10% diethanolamine buffer, pH 9.8 (150 μl), was added to each well. After incubation at room temperature, the absorbance of the well was measured at 405 nm by a Microplate Reader (Model 450, Bio-Rad).
Total IgA in nasal wash was also measured by ELISA according to the procedure of Nagai et al. (33). Goat anti-mouse IgA antibody (Pharmingen (5 μg ml−1, 100 μl)) in 10 mM carbonate/bicarbonate buffer (pH 9.6) containing 0.01% BSA was incubated in a 96-well ELISA plate for 3 h at 37°C, and the plate was washed four times with PBS–Tween. After the plate was incubated with the above same blocking buffer (250 μl) for 1 h at 37°C, the plate was washed four times with PBS–Tween. The nasal washes were diluted with SuperBlock blocking buffer (Pierce) containing 0.05% Tween 20 (1:40), and the wells were incubated with the diluted nasal washes (100 μl) for 1 h at 37°C. After the plate was washed four times with PBS–Tween, the wells were incubated with alkaline phosphatase-coupled anti-mouse IgA antibody (1:1000) in the blocking buffer for 1 h at 37°C, and the plate was washed four times with PBS–Tween. The bound alkaline phosphatase activity was measured by the same procedure as described above.
Statistics
All results are expressed as the mean ± SEM. The significance of differences between experimental groups was analyzed with ANOVA followed by Fisher's PLSD procedure. The probability (P) values < 0.05 were considered significant.
Results
Do TJ-41 and TJ-48 Enhance Primary Antibody Response in the Systemic Immune System?
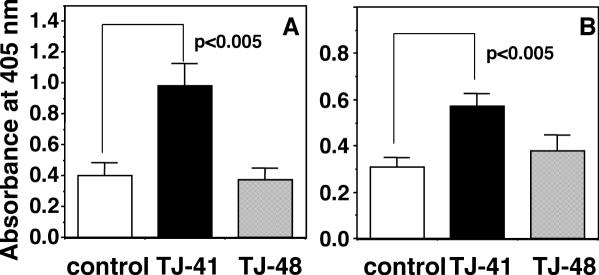
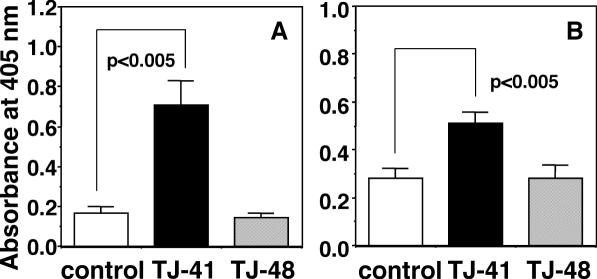
It has been known that primary intranasal inoculation of the antigen induces antigen-specific antibody response in systemic immune system through upper respiratory mucosal immune system to result in the elevation of antigen-specific antibody titer in blood stream, and that secondary intranasal inoculation of antigen additively induces the elevation of antigen-specific antibody level of secretory IgA class in nasal cavity (34). In the present study, we attempted to evaluate whether TJ-41 and TJ-48 can stimulate antibody response in systemic immune system through upper respiratory mucosal immune system by using primary intranasal inoculation of influenza HA vaccine. Young female BALB/c mice of 7 weeks old (young mice) were intranasally inoculated primarily with the influenza A/PR/8 HA vaccine (5 μg per mouse) at Day 0. The mice were also orally administered with TJ-41 or TJ-48 at a dose of 100 mg kg−1 per day from 7 days before (Day −7) to 21 days (Day 21) after the vaccine inoculation. Then the influenza virus-specific IgG antibody titers in the sera of the mice at Day 21 were measured. Oral administration of TJ-41 significantly enhanced the influenza virus-specific IgG antibody titers whereas TJ-48 had no effect (Fig. 3A). TJ-41 and TJ-48 have been often used for the patients having debilitated conditions. It has been reported that BALB/c mice of 6 months old show the significantly lower antibody response especially of antigen-specific IgG class antibody against T cell-dependent antigen than young BALB/c mice of 7 weeks old (35). Koga et al. and Hagiwara et al. also have found that both mucosal immune functions in intestine and upper respiratory tract of mice are diminished during aging (36,37). Therefore, the effects of TJ-41 and TJ-48 on the antibody response against primary intranasal inoculation of the influenza HA vaccine were also studied by using BALB/c mice of 6 months old (early aged mice). TJ-41 and TJ-48 each were orally administered by the above same schedule to the early aged BALB/c mice, which were given primary intranasal inoculation of the influenza A/PR/8 HA vaccine. TJ-41 also enhanced significantly the titer of anti-influenza virus-specific IgG antibody in the sera at Day 21 as observed in the young mice; however, TJ-48 did not affect the titer even in the early aged mice (Fig. 3B).
Figure 3.
Comparison of effects of oral administrations of TJ-41 and TJ-48 (100 mg kg−1 per day each) on influenza virus-specific IgG antibody titers in sera of young (A) and early aged BALB/c mice (B) against primary intranasal inoculation of influenza A/PR/8 HA vaccine (5 μg per mouse). Influenza virus-specific IgG antibody titer was measured for the sera collected on Day 21 by ELISA as described in Materials and Methods.
TJ-41 Enhances IgA Antibody Response in Early Aged Mice
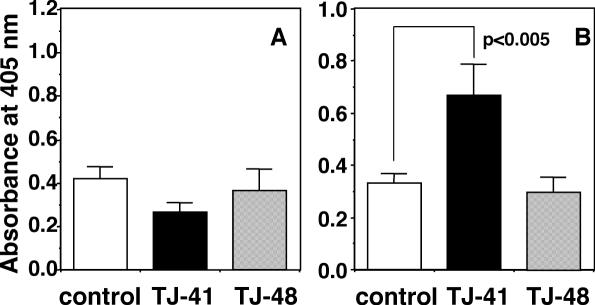
In order to evaluate the effects of TJ-48 and TJ-41 on antigen-specific IgA antibody titer in upper respiratory tract, the model mice that received secondary intranasal immunization with influenza HA vaccine was employed. Early aged and young BALB/c mice were intranasally inoculated twice with the influenza A/PR/8 HA vaccine at Day 0 and Day 28, and TJ-41 and TJ-48 each were orally administered to the mice from 7 days (Day −7) before to 42 days (Day 42) after the primary inoculation of the vaccine. The nasal washes were collected at Day 42 from the mice, and the titer of influenza virus-specific IgA antibody was measured. TJ-41 significantly enhanced influenza virus-specific IgA antibody titer in nasal washes of the early aged BALB/c mice whereas TJ-48 showed no activity (Fig. 4B). However, neither TJ-41 nor TJ-48 had any effect on the IgA antibody titer in the nasal washes of the young mice (Fig. 4A). The influenza virus-specific IgG antibody titer was also measured for the sera from secondary immunized BALB/c mice at Day 42, and oral administration of TJ-41 increased the influenza virus-specific IgG antibody titers in the sera of both young and early aged mice (Fig. 5). However, oral administration of TJ-48 had no effect in both mice (Fig. 5).
Figure 4.
Comparison of effects of oral administrations of TJ-41 and TJ-48 (100 mg kg−1 per day each) on influenza virus-specific IgA antibody titers in nasal washes of young (A) and early aged BALB/c mice (B) against secondary intranasal inoculation of influenza A/PR/8 HA vaccine (5 μg per mouse). Influenza virus-specific IgA antibody titers were measured for the nasal washes collected at Day 42 by ELISA.
Figure 5.
Comparison of effects of oral administrations of TJ-41 and TJ-48 (100 mg kg−1 per day each) on influenza virus-specific IgG antibody titers in sera of young (A) and early aged BALB/c mice (B) against secondary intranasal inoculation of influenza A/PR/8 HA vaccine (5 μg per mouse). Influenza virus-specific IgG antibody titer was measured for the sera collected at Day 42 by ELISA.
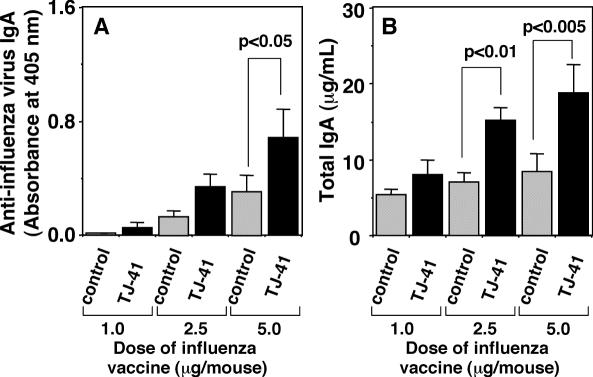
In order to know whether TJ-41 enhances a basic level of secretory IgA production in upper respiratory mucosal immune system, total IgA titer in nasal wash of early aged mice, who were administered TJ-41 and intranasally immunized with different amounts of influenza HA vaccine, was analyzed. TJ-41 (100 mg kg−1 per day) was administered orally to the early aged BALB/c mice, which were secondary immunized with different doses (1.0, 2.5 and 5.0 μg per mouse) of intranasal inoculations of influenza A/NC/20 HA vaccine, and the total and influenza virus-specific IgA antibody titers were measured for the nasal washes. TJ-41 significantly enhanced total IgA titer as well as the influenza virus-specific IgA antibody titer in the nasal washes of the mice, which were immunized with 5.0 μg per mouse of the influenza vaccine (Fig. 6), suggesting that TJ-41 enhances basic level of secretory IgA production in upper respiratory mucosal immune system of the early aged mice.
Figure 6.
Effect of oral administration of TJ-41 (100 mg kg−1 per day) on influenza virus-specific IgA antibody titer (A) and total IgA antibody titer (B) in nasal washes of early aged BALB/c mice which were immunized with secondary intranasal inoculation of different doses (1.0, 2.5 and 5.0 μg per mouse) of influenza A/NC/20 HA vaccine.
Methotrexate Treatment Suppressed the Stimulating Activity of TJ-41
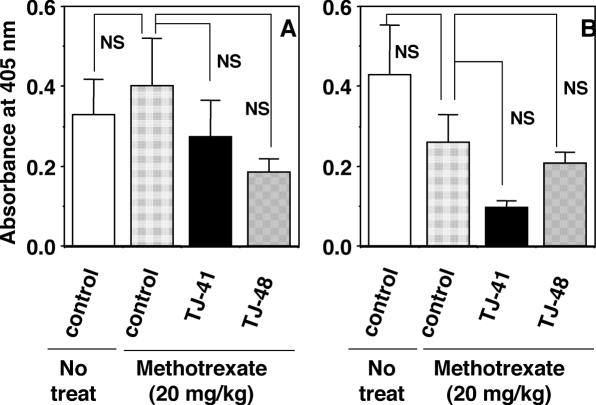
Methotrexate is a well-known anticancer drug, and usually used for inductions of acute injury in small intestine and impairment of intestinal immune system in animal studies (38,39). Administration of methotrexate (20 mg kg−1) has been reported to reduce total IgA titer in the intestinal fluids of rats (39). We also reconfirmed that the administration of methotrexate to BALB/c mice also diminished total IgA titers in mouse feces (data not shown). By this condition, methotrexate did not seem to decrease notably antigen-specific antibody responses in systemic immune system and upper respiratory mucosal immune system against intranasally inoculated influenza HA vaccine (Fig. 7). Effect of methotrexate on the stimulating activity of TJ-41 for influenza viral antigen-specific antibody responses in both immune systems was examined. The early aged BALB/c mice, which were secondary immunized with intranasal inoculation of influenza A/PR/8 HA vaccine, were administered orally with methotrexate (20 mg kg−1) twice at 3 days before the primary and secondary immunizations (Day −3 and Day 25), and the mice were administered with TJ-41 or TJ-48 at a dose of 100 mg kg−1 per day from Day −7 to Day 42. TJ-41 failed to increase the influenza virus-specific antibody titers in both sera and nasal washes at Day 42 (Fig. 7).
Figure 7.
Effect of pretreatment with methotrexate on enhanced activity of oral administration of TJ-41 against influenza virus-specific IgG antibody response in sera (A) and of influenza virus-specific IgA antibody response in nasal washes (B) of early aged BALB/c mice. Early aged BALB/c mice, which were secondary immunized with intranasal inoculation of influenza A/PR/8 HA vaccine, were administered orally with methotrexate (20 mg kg−1) twice at Day −3 and Day 25, and the mice were also administered with TJ-41 or TJ-48 (100 mg kg−1 per day) from Day −7 to Day 42. Influenza virus-specific IgG and IgA antibodies were measured for sera and nasal washes collected at Day 42, respectively.
Discussion
The present study clearly indicates that Hochuekkito (Bu-Zong-Yi-Qi-Tang, TJ-41) potentiates the function of mucosal immune system in upper respiratory tract especially for the early aged mice, whereas Juzentaihoto (Shi-Quan-Da-Bu-Tang, TJ-48) failed to stimulate the same immune system although TJ-48 modulates the function of immunocompetent cells in Peyer's patches as one of the inductive sites for mucosal immune system (26,27). TJ-41 has been often used for patients having respiratory symptoms, especially those patients who cannot recover well from cold syndrome; however, TJ-48 has not been applied for treatment of cold syndrome clinically (2). Mori et al. (40) have reported that TJ-41 increases IFN-α level in bronchoalveolar lavage fluid (BALF) of influenza virus-infected mice in early stages of the infection. Therefore, the stimulating actions of TJ-41 on both IFN-α production and antibody responses in upper respiratory mucosal immune system are able to explain well its clinical effect on patients having cold syndrome. Although TJ-41 and TJ-48 have separately been applied clinically for recovery from fatigue of whole body depending on the symptoms of patients, differences of the action mechanism of these medicines have little been understood. The present study postulates a possibility that differences in the action mechanisms between TJ-41 and TJ-48 can be resolved from clarification of their actions on upper respiratory mucosal immune system.
The present study clearly indicated that TJ-41 potentiated total and the antigen-specific IgA production in upper respiratory mucosal immune system of only early aged mice but not in young mice. Hagiwara et al. (37) have reported that aged mice have diminished antigen-specific IgA responses in upper respiratory mucosal immune system compared to young mice in the presence of little dosage of an adjuvant, cholera toxin. Therefore, it is postulated that TJ-41 may be able to potentiate only the downregulated upper respiratory mucosal immune system.
It has been proposed that upper respiratory mucosal immune systems are regulated partially by lymphocyte homing from intestinal Peyer's patches through CMIS (25,34). The stimulating activity of TJ-41 on upper respiratory mucosal immune system disappeared by impairment of intestinal immune system with methotrexate treatment. However, methotrexate alone seemed not to reduce the upper respiratory mucosal immune function of BALB/c mice at the dose used in the present study (Fig. 7). Because TJ-41 stimulates immunocompetent cells of Peyer's patches in vitro (Kiyohara, Matsumo, Yamada, unpublished results), there is a possibility that TJ-41 may express stimulating activity on upper respiratory mucosal immune system through modulation of functions of immunocompetent cells in intestinal Peyer's patches. As another action mechanisms of TJ-41, the possibility also still remains that the active ingredients of TJ-41 may stimulate the upper respiratory mucosal immune function directly after its absorption from digestive system.
The present study indicated that TJ-41 strongly enhanced antigen-specific IgG response in systemic immune system against the intranasally administered antigen in both young and early aged mice, but TJ-48 did not. TJ-48 as well as TJ-41 has been reported to enhance antigen-specific IgG response when the antigens were administered intraperitoneally (41). It has been proposed that upper respiratory mucosal immune system contributes to the induction of antibody responses on not only upper respiratory tract but also spleen and peripheral lymph nodes as systemic immune system (34). Therefore, TJ-41 may enhance the antigen-specific IgG response in systemic immune system against intranasally inoculated antigen by potentiating the function of upper respiratory mucosal immune system toward systemic immune system. Since the stimulating activity of TJ-41 on systemic immune system against intranasally inoculated antigen was observed even in young mice, the action mechanism of TJ-41 for expression of this stimulating activity is considered to be different from those for the stimulating activity on secretory IgA antibody response in upper respiratory tract. However, the mechanism of regulation of immunocompetent cells in systemic immune system through upper respiratory mucosal immune system has not been well known (34). Therefore, the comprehensive analysis on the effects of TJ-41 against intestinal immune system as well as upper respiratory mucosal immune system is required for understanding action mechanism of TJ-41 on mucosal immune system.
Acknowledgments
A part of the present work was supported by The 21st Century COE Program, Ministry of Education, Culture, Sports, Science and Technology of Japan, and Grants-in Aid for Scientific Research (B) from Japan Society for the Promotion of Science (grant ID: 16390201). A part of this work was also supported by a fund from Tsumura & Co. Ltd, Japan. We would like to thank Dr I. Sakakibara (Research Division, Tsumura & Co.) and Tsumura & Co. for analyses of TJ-41 and TJ-48 by 3D-HPLC, and Ms M. Inoue for her technical assistance.
References
- 1.Terasawa K. Kampo. Japanese-Oriental Medicine: Insight from Clinical Case. Tokyo: KK Standard McIntyre; 1993. [Google Scholar]
- 2.Hanawa T. Series of Contemporary Medicine 20: Lesson of Clinical Practice for Kampo Medicine. Tokyo: Kinbara Publisher; 1995. (in Japanese) [Google Scholar]
- 3.Tagami K, Niwa K, Lian Z, Gao J, Mori H, Tamaya T. Preventive effect of Juzen-taiho-to on endometrial carcinogenesis in mice is based on Shimotsu-to constituent. Biol Pharm Bull. 2004;27:156–61. doi: 10.1248/bpb.27.156. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Kato M, Takeda K, Kawamoto Y, Akhand AA, Hossain K, et al. T-cell-immunity-based inhibitory effects of orally administered herbal medicine Juzen-taiho-to on the growth of primarily developed melanocytic tumors in RET-transgenic mice. J Invest Dermatol. 2001;117:694–701. doi: 10.1046/j.0022-202x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi Y, Fujii H, Kimura F, Mishima T, Murata J, Tazawa K, et al. Inhibitory effect of a traditional Chinese medicine, Juzen-taiho-to, on progressive growth of the weakly malignant clone cells derived from murine fibrosarcoma. Jpn J Cancer Res. 1996;87:1039–44. doi: 10.1111/j.1349-7006.1996.tb03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada M, Seta K, Ito O, Tamada K, Li T, Terao H, et al. Concomitant immunity against tumor development is enhanced by the oral administration of a Kampo medicine, Hochu-ekki-to (TJ-41: Bu-Zhong-Yi-Qi-Tang) Immunopharmacol Immunotoxicol. 1995;17:687–703. doi: 10.3109/08923979509037189. [DOI] [PubMed] [Google Scholar]
- 7.Cho JM, Sato N, Kikuchi K. Prophylactic anti-tumor effect of Hochu-ekki-to (TJ41) by enhancing natural killer cell activity. In Vivo. 1991;5:389–91. [PubMed] [Google Scholar]
- 8.Saiki I. A Kampo medicine “Juzen-taiho-to”—prevention of malignant progression and metastasis of tumor cells and the mechanism of action. Biol Pharm Bull. 2000;23:677–88. doi: 10.1248/bpb.23.677. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama H, Takemoto N, Maruyama N, Komatsu Y, Kawamura H. Antitumor effect of Juzen-taiho-to, a kampo medicine, combined with surgical excision for transplanted Meth-A fibrosarcoma. Int J Immunother. 1993;9:117–25. [Google Scholar]
- 10.Sugiyama K, Ueda H, Ichio Y. Protective effect of juzen-taiho-to against carboplatin-induced toxic side effects in mice. Biol Pharm Bull. 1995;18:544–8. doi: 10.1248/bpb.18.544. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama K, Ueda H, Ichio Y, Yokota M. Improvement of cisplatin toxicity and lethality by juzen-taiho-to in mice. Biol Pharm Bull. 1995;18:53–8. doi: 10.1248/bpb.18.53. [DOI] [PubMed] [Google Scholar]
- 12.Hisha H, Yamada H, Sakurai MH, Kiyohara H, Li Y, Yu CZ, et al. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-Taiho-To. Blood. 1997;90:1022–30. [PubMed] [Google Scholar]
- 13.Kawamura H, Maruyama H, Takemoto N, Komatsu Y, Aburada M, Ikehara S, et al. Accelerating effect of Japanese kampo medicine on recovery of murine hemopoietic stem cells after administration of mitomycin C. Int J Immunother. 1989;5:35–42. [Google Scholar]
- 14.Kim SH, Lee SE, Oh H, Kim SR, Yee ST, Yu YB, et al. The radioprotective effects of bu-zhong-yi-qi-tang: a prescription of traditional Chinese medicine. Am J Clin Med. 2002;30:127–37. doi: 10.1142/S0192415X02000144. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda S, Kaneko M, Kumazawa Y, Nishimura C. Protective activity of a Chinese medicine, hochu-ekki-to, to impairment of hematopoietic organs and to microbial infection. Yakugaku Zasshi. 1990;110:682–7. doi: 10.1248/yakushi1947.110.9_682. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi Y, Yasumizu R, Ikehara S. Preventive effect of TJ-48 on recovery from radiation injury. Gan To Kagaku Ryoho. 1989;16:1494–9. (in Japanese) [PubMed] [Google Scholar]
- 17.Yamaoka Y, Kawakita T, Nomoto K. Protective effect of a traditional Japanese medicine Hochu-ekki-to (Chinese name: Bu-Zhong-Yi-Qi-Tang), on the susceptibility against Listeria monocytogenes in infant mice. Int Immunopharmacol. 2001;1:1669–77. doi: 10.1016/s1567-5769(01)00076-5. [DOI] [PubMed] [Google Scholar]
- 18.Kido T, Mori K, Daikuhara H, Tsuchida H, Ishige A, Sasaki H. The protective effect of hochu-ekki-to (TJ-41), a Japanese herbal medicine, against HSV-1-infection in mitomycin C-treated mice. Anticancer Res. 2000;20:4109–13. [PubMed] [Google Scholar]
- 19.Hossain MS, Takimoto H, Hamano S, Yoshida H, Ninomiya T, Minamishima Y, et al. Protective effects of hochu-ekki-to, a Chinese traditional herbal medicine against murine cytomegalovirus infection. Immunopharmacology. 1999;41:169–81. doi: 10.1016/s0162-3109(98)00066-6. [DOI] [PubMed] [Google Scholar]
- 20.Abe S, Tansho S, Ishibashi H, Inagaki N, Komatsu Y, Yamaguchi H. Protective effect of oral administration of a traditional medicine, Juzen-taiho-to, and its components on lethal Candida albicans infection in immunosuppressed mice. Immunopharmacol Immunotoxicol. 1998;20:421–31. doi: 10.3109/08923979809034824. [DOI] [PubMed] [Google Scholar]
- 21.Abe S, Tansho S, Ishibashi H, Akagawa G, Komatsu Y, Yamaguchi H. Protection of immunosuppressed mice from lethal Candida infection by oral administration of a Kampo medicine, hochu-ekki-to. Immunopharmacol Immunotoxicol. 1999;21:331–42. doi: 10.3109/08923979909052766. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Mizuno N, Kutsuna H, Teramae H, Ueoku S, Onoyama J, et al. Hochu-ekki-to suppresses development of dermatitis and elevation of serum IgE level in NC/Nga mice. Drugs Exp Clin Res. 2003;29:81–4. [PubMed] [Google Scholar]
- 23.Nakada T, Watanabe K, Matsumoto T, Santa K, Toriizuka K, Hanawa T. Effect of orally administered Hochu-ekki-to, a Japanese herbal medicine, on contact hypersensitivity caused by repeated application of antigen. Int Immunopharmacol. 2002;2:901–11. doi: 10.1016/s1567-5769(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 24.McGhee JR, Lamm ME, Strober W. Mucosal immune response. An overview. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Blemenstock J, McGhee JR, editors. Mucosal Immunology. 2nd edition. London: Academic Press; 1999. pp. 485–506. [Google Scholar]
- 25.Kiyono H, Fukuyama S. NALT-versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong T, Matsumoto T, Kiyohara H, Yamada H. Enhanced production of hematopoietic growth factors through T cell activation in Peyer's patches by oral administration of Kampo (Japanese herbal) medicine, Juzen-Taiho-To. Phytomedicine. 1998;5:353–60. doi: 10.1016/S0944-7113(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T, Yamada Orally administered Kampo (Japanese herbal) medicine “Juzen-taiho-to” modulates cytokine secretion in gut-associated-lymphoreticular tissues in mice. Phytomedicine. 19992000;6:425–30. doi: 10.1016/S0944-7113(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 28.Kiyohara H, Matsumoto T, Yamada H. Combination effects of herbs in a multi-herbal formula: Expression of Juzen-taiho-to's immuno-modulatory activity on the intestinal immune system. Evid Based Complement Alternat Med. 2004;1:83–91. doi: 10.1093/ecam/neh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyohara H, Matsumoto T, Yamada H. Lignin-carbohydrate complexes: intestinal immune system modulating ingredients in kampo (Japanese herbal) medicine, Juzen-Taiho-To. Planta Med. 2000;66:20–4. doi: 10.1055/s-2000-11116. [DOI] [PubMed] [Google Scholar]
- 30.Kiyohara H, Matsumoto T, Yamada H. Intestinal immune system modulating polysaccharides in a Japanese herbal (Kampo) medicine, Juzen-Taiho-To. Phytomedicine. 2002;9:614–24. doi: 10.1078/094471102321616427. [DOI] [PubMed] [Google Scholar]
- 31.Nagai T, Yamada H. In vivo anti-influenza virus activity of Kampo (Japanese herbal) medicine “Sho-seiryu-to”: Stimulation of mucous immune system and effect on allergic pulmonary inflammation model mice. Immunopharmacol Immunotoxicol. 1998;20:267–81. doi: 10.3109/08923979809038544. [DOI] [PubMed] [Google Scholar]
- 32.Davenport FM, Hennessy AV, Brandon FM, Webster RG, Barrett CD, Jr, Lease GO. Comparisons of serologic and febrile responses in humans to vaccination with influenza A viruses on their hemagglutinations. J Lab Clin Med. 1964;65:5–13. [PubMed] [Google Scholar]
- 33.Nagai T, Kiyohara H, Munakata K, Shirahata T, Sunazuka T, Harigaya Y, et al. Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int Immunopharmacol. 2002;2:1183–93. doi: 10.1016/s1567-5769(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 34.Sminia T, Kaal G. Nasal-associated lymphoid tissue. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Blemenstock J, McGhee JR, editors. Mucosal Immunology. 2nd edition. London: Academic Press; 1999. pp. 357–364. [Google Scholar]
- 35.Kiyohara H, Matsumoto T, Takemoto N, Kawamura H, Komatsu Y, Yamada H. Effect of oral administration of a pectic polysaccharide fraction from a Kampo (Japanese herbal) medicine “Juzen-taiho-to” on antibody response of mice. Planta Med. 1993;61:429–34. doi: 10.1055/s-2006-958130. [DOI] [PubMed] [Google Scholar]
- 36.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J Immunol. 2000;165:5352–9. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- 37.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J Immunol. 2003;170:1754–62. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 38.Chu KU, Higashide S, Evers BM, Rajaraman S, Ishizukla J, Townsend CM, Jr, et al. Bombesin improves survival from methotrexate-induced enterocolitis. Ann Surg. 1994;220:570–7. doi: 10.1097/00000658-199410000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao Y, Yu JL, Ljungh A, Molin G, Jeppsson B. Intestinal immune response to oral administration of Lactobacillus reuteri R2LC, Lactobacillus platarum PSM9843, pectin and oatbase on methotrexate-induced enterocolitis in rats. Microb Ecol Health Dis. 1996;9:261–70. [Google Scholar]
- 40.Mori K, Kido T, Daikuhara H, Sakakibara I, Sakata T, Shimuzu K, et al. Effect of Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on the survival of mice infected with influenza virus. Antiviral Res. 1999;44:103–11. doi: 10.1016/s0166-3542(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 41.Iwama Y, Amagaya S, Ogihara Y. Effects of Japanese herbal (Kampo) medicines on immune responses. Inflammation. 1984;4:566–8. [Google Scholar]