Abstract
This paper presents the novel domain of evidence-based research (EBR) in the treatment of patients with Alzheimer's disease (AD) from the perspective of traditional medicine and of complementary and alternative medicine. In earlier lectures we have described the process of evidence-based medicine as a methodological approach to clinical practice that is directed to aid clinical decision-making. Here, we present a practical example of this approach with respect to traditional pharmacological interventions and to complementary and alternative treatments for patients with AD.
Keywords: Alzheimer's disease, evidence-based medicine, systematic review, treatment interventions
Clinical Evidence in Alzheimer's Disease (AD)
Epidemiological Evidence
Clinical Characteristics
Alzheimer's disease (AD) is a progressive disease of the brain. It is a common type of dementia in the elderly, which can have devastating outcomes on the diagnosed patient, on the caregiver and family, and on society at large. Many other conditions can lead to similar memory loss, confusion, agitation and metabolic disturbances. Therefore, rushing to give a diagnosis of AD is unwise and is not common practice. Owing to the absence of an absolute diagnostic test for AD, diagnosis must depend on observing trends as the disease evolves over time.
Patients with AD show loss of cognitive, intellectual, functional and social abilities, and therefore become fully dependent on their caregiver. It is estimated that in 2010 over five million people will be diagnosed with probable AD in the United States alone. Increasing age is the greatest risk factor for AD; one-tenth of elderly over 65 years of age develop AD, whereas nearly half of those over age 85 are diagnosed with probable AD. Certain people in the population are at greater risk of developing AD due to various genetic risk factors associated with AD such as apolipoprotein (APO) polymorphism. The allele frequency for APO-E4 is significantly higher in patients with AD compared to control subjects (1). A person with AD is expected to live an average of 8 years and up to 20 years after the onset of symptoms (1–3).
Psychosocial Concerns
The social and the medical costs to care for patients with AD are mounting rapidly. National estimates of annual costs of caring for individuals with AD today total close to $100 billion (estimates by the Alzheimer's Association and the National Institute on Aging), and business costs approach $61 billion per year in the United States alone. Over 40% of this budget is dedicated to health care for patients with probable AD. Among them, 7 out of 10 live at home, where almost 75% of their care is provided by family and friends. The remaining 60% of the cost of AD is associated with expenditures related to caregivers of patients with AD (e.g. family and friends, nurse and other professional allied health staff), and include loss of productivity, absenteeism, worker replacement, etc. (4–6).
About half of all nursing home residents carry the diagnosis of probable AD, or AD-related dementia. The average cost for nursing home care is $42 000 per year but can exceed $70 000 per year in some areas of the country, which leads to an estimation of $174 000 for the average lifetime cost of care for a family member with AD. Whereas the family absorbs these costs, to a large extent, the Federal government estimated spending approximately $640 million for AD research in fiscal year 2003 alone. An accurate diagnosis is a key factor in insuring the highest benefit to the patient and the caregiver, while minimizing the cost.
Biomedical Evidence
Neuropsychopathology
Since ancient times, it has been clear that some people lose mental sharpness (cognitive function) as they age. It was in 1906 that AD was first described by Alois Alzheimer (1864–1915) in an autopsy on the brain of a 56-year-old woman, Augusta D. of Frankfurt. Ms D. had died after several years of progressive mental deterioration marked by increasing confusion and memory loss. The German neurologist described an odd disorganization of the nerve cells in Ms D.'s cerebral cortex, the part of the brain responsible for reasoning and memory. The cells contained clusters suggestive of a rope tied in knots. Alzheimer named them ‘neurofibrillary tangles’. There also was an unexpected accumulation of cellular debris around the affected nerves, which are now recognized as the ‘senile plaques’. Alzheimer speculated that the nerve tangles and plaques were responsible for the woman's dementia (7). Several independent cases soon revealed similar patterns, which led the German psychiatrist Emil Kraepelin (1856–1926) to name the disease in honor of his mentor.
AD progressively destroys the ability to reason, remember, imagine and learn
We now know that in AD, tangles and plaques eventually take over healthy brain tissue, devastating the areas of the brain associated with intellectual function, and progressively destroying the ability to reason, remember, imagine and learn. AD characteristically is a progressive condition marked, at its onset, by simple forgetfulness of instances such as recent events, telephone numbers or directions to familiar places. Patients with AD experience personality changes, such as poor impulse control and judgment, distrust, increased stubbornness and restlessness. The disease progresses into difficulty in executing tasks that require planning, decision-making and judgment, such as working, balancing a cheque book or driving a car. A person with probable AD typically has trouble finding the right word, and often substitutes unusual words, making comprehension of speech or writing difficult. It is quite common for a person with probable AD to become confused or lost in a familiar neighborhood, to demonstrate poor or decreased judgment about social behavior, clothing, money and abstract thinking. A person with probable AD may misplace items, and put them in unusual places (e.g. placing a writing pen in the freezer). Patients with probable AD may show rapid mood swings, personality changes, confusion, suspicious behavior, fearfulness, anger, or dependence on a family member or caregiver. They may become passive, apathetic and uninterested in performing usual activities.
Post-mortem examination reveals two abnormal structures in the brain associated with AD. Amyloid plaques are clumps formed by the β-amyloid protein (Aβ; 42 amino acids) that accumulate outside of cells. Neurofibrillary tangles are clumps of altered τ (tau) proteins inside cells. Although it is known that these structures are toxic to neurons, the exact role plaques and tangles play in the onset and progression of AD-dementia is not fully determined (2,8–10).
Progression of AD-dementia symptoms corresponds in a general way to the underlying neuronal cell degeneration that takes place in AD. Nerve cell damage typically begins with cells involved in learning and memory, and gradually spreads to cells that control every aspect of thinking, judgment and behavior. Neuropathology eventually impairs cells that control and coordinate movement.
Apoptosis (programmed cell death) may be the mechanism of neuronal death in AD since DNA fragmentation, cell shrinkage, membrane swelling and caspase activation all occur in involved neurons. Aβ appears to be one, if not the main trigger of neuronal apoptosis, and extracellular Aβ has been shown to activate c-Jun-N-terminal kinase, which leads to transcription of Fas ligand (FasL). The binding of FasL to Fas leads to caspase activation, which directs the apoptotic process. Aβ also induces apoptosis of lymphocytes, and renders phagocytic cells of innate immunity unresponsive (11–18).
Clinical Evidence
Disease Progression and Stages of Social Withdrawal
Everyday skills, such as personal grooming or a lifelong hobby, are eventually affected, gradually leading to social withdrawal. Simple tasks of independent daily living (e.g. eating, bathing, using the toilet) become impossible, and patients often lose interest in personal hygiene and appearance, as well as social sexual inhibitions. Communication of all kinds becomes difficult as written and spoken language ability dwindles. Withdrawal from family members often occurs as patients at this stage become agitated, belligerent and deny the illness. At the later stage of the disease, patients are mostly bedridden, and await death, which results from pneumonia or related complications. In brief, signs of clinical impairment include changes in memory, which are normal in aging, but that are exacerbated in patients with probable AD by symptoms of difficulties in communicating, learning, thinking and reasoning. These symptoms are severe enough to impact the person's work performance, social activities and family life. (3,19–21).
Staging provides useful frames of reference for the process of diagnosis
The diagnosis of probable AD is obtained by clinical assessment. Early diagnosis permits time to make choices that maximize quality-of-life, lessens anxieties about unknown problems, provides a better chance of benefiting from treatment and allows more time to plan for the future (3,19,21–25).
Staging systems have been developed to provide useful frames of reference for the process of diagnosis by exclusion, and for understanding how the disease unfolds, and for clinical decision-making. It is recognized that the stages are artificial benchmarks in a continuous process that can vary greatly from one person to another. Nevertheless, the Global Deterioration Scale and other similar instruments have proven to be a reliable diagnostic system to generate clinical evidence toward an outline of key symptoms characterizing seven stages ranging from unimpaired function to very severe cognitive decline (21).
Agitation Often Reflects an Underlying Infection or Medical Illness
Above and beyond the general symptomatology, a person with probable AD typically manifests what is commonly referred to as agitation. In the early stages of the disease, agitation accompanies memory loss, thinking problems, personality changes, irritability, anxiety, depression, sleep disturbances, delusions (firmly held belief in things that are not real), hallucinations (seeing, hearing or feeling things that are not there), pacing, repetitive and restless movement, general emotional distress, and cursing or threatening language.
Agitation often reflects an underlying infection or medical illness, pain or discomfort, including loss of hearing or eyesight. Prescription medications for the treatment of AD-associated or non-AD dementias can cause agitation, especially when multiple medications are used. Agitation may be exacerbated by drug interactions, or by circumstances that worsen the person's ability to think, including moving to an unfamiliar environment or variable caregivers. Agitation can disrupt patient care, and interfere with the ability of the patient or the caregiver to carry out activities of independent daily living. The treatment of agitation depends on a careful diagnosis, determination of the possible causes and the types of agitated behavior the person is experiencing. With proper pharmacological treatment and intervention, significant reduction or stabilization of the symptoms can often be achieved (21,26,27). Atypical anti-psychotic and anti-convulsant medications with mood-stabilizing properties are most commonly used to treat agitation (20,25,27–30).
Treatment of Patients with AD
Traditional Pharmacological Intervention for Patients with AD
Pharmacological Interventions
There is no cure for AD, but several drug treatments are available that improve or stabilize symptoms. Certain strategies and activities may minimize or prevent behavioral problems. Early initiation of treatment can delay the need for nursing home care.
Current interventions for AD include acetylcholinesterase inhibitors (AchI), which are indicated for patients with mild to moderate symptoms. Treatment with memantine interferes with the glutamate neurotransmitter receptor system and is the sole intervention recommended for moderate to severe cases of AD. A spectrum of alternative treatments for AD has also been proposed, and must be examined judiciously in preclinical, clinical and evidence-based research (EBR) studies.
The US Food and Drug Administration (FDA) has approved drugs to treat cognitive symptoms of AD. Cholinesterase inhibitors [donepezil (Aricept®), approved in 1996; rivastigmine (Exelon®), approved in 2000; galantamine (Reminyl®), approved in 2001; and tacrine (Cognex®), approved in 1993], aim at inhibiting cholinesterase, the enzyme in brain neurons that regulates the levels of acetylcholine. The drugs keep levels of the chemical messenger high, even while the cells that produce the messenger continue to die. About half of the patients who take cholinesterase inhibitors experience a modest improvement in cognitive symptoms. Patients who receive tacrine may suffer from serious side effects, including liver damage (21).
Memantine–HCl (aka, Namenda™) was FDA-approved in October 2003. It has a reported effectiveness for the treatment of moderate to severe AD. Memantine was tested in two placebo-controlled Phase III clinical trials in the United States, and one earlier trial in Europe. Typically, patients treated with memantine scored higher on measures of cognition, daily function (i.e. activities of daily living such as eating, walking, toileting, bathing and dressing) and global performance, with limited side effects (dizziness, confusion, headache and constipation), compared to those on placebo. Memantine has a mechanism of action distinct from other approved treatments for AD, which, as noted, are acetylcholinesterase inhibitors and are indicated for the treatment of mild to moderate AD. In contrast, memantine is a low-affinity antagonist for N-methyl-d-aspartate (NMDA) receptor, which binds the neurotransmitter glutamate. Glutamate plays an integral role in the neural pathways associated with learning and memory. Abnormal levels of glutamate may lead to neuronal cell dysfunction, and memantine may blunt these deleterious effects (21,30,31).
Pharmacological Side Effects
Medications given to patients with probable AD-related dementia increase the risk for tooth root caries and periodontal disease due to the drugs' side effects. For example, the anti-convulsant drug phenytoin can cause gingival hyperplasia specially in the presence of plaque, while many antipsychotic agents such as phenothiazines used to control behavioral problems, especially aggression and emotional instability, can cause xerostomia, a lack of saliva (32).
Complementary and Alternative Intervention in AD
Certain herbal remedies and alternative dietary supplements have been suggested as effective treatments for AD. Claims about the safety and effectiveness of these products lack scientific proof. Concerns about these alternative strategies include lack of knowledge and assurance about safety, purity, side effects and potential interactions with prescribed medications. Supplement or alternative treatment should not be recommended without consulting a physician.
CAM and Anti-Oxidants such as Gingko biloba May Protect Cell Membranes from Inflammatory Processes
Among the alternative treatments, Ginkgo biloba, a plant extract rich in compounds that may have positive effects on cells within the brain and the body, is believed to have antioxidant and anti-inflammatory properties. Thus, it may protect cell membranes from inflammatory processes associated with plaque and tangle formation (vide infra), and help regulate neurotransmitter production, function and metabolism. Research has established no measurable difference, however, in the overall benefit in patients with probable AD treated with this traditional Chinese medicine (TCM) herb (33). Although few side effects are associated with its use, it may reduce the ability of blood to clot, and thus lead to serious internal bleeding, when taken in combination with aspirin or warfarin (34–36). The moss extract, Huperzine A, is also not FDA-approved, and appears to mimic cholinesterase inhibitors. It has not been associated with risks of serious side effects to date (37). Finally, it has been proposed that ‘Coral’ calcium supplements may be a cure for AD, because it is a form of calcium carbonate derived from the shells of formerly living organisms that once made up coral reefs, and hence rich in other minerals. Research has failed to support these claims to date (21).
Promising Alternative Strategies for AD Involve Preventing Neuronal Toxicity
Phosphatidylserine is one among the many specialized lipids in neuronal cell membranes. Given the fact that neurons degenerate in AD, the strategy behind phosphatidylserine dietary supplements is to prevent neuron toxicity and death by providing excess of this lipid. Results of clinical trials to date appear encouraging, but larger carefully controlled trials are needed to determine the viability of this treatment (38). In addition, the natural antioxidant coenzyme Q10 (i.e. ubiquinone), required for normal ‘household’ cell metabolism, is under testing as well. Its synthetic equivalent, idebenone, when tested in clinical trials with patients with AD, failed to show favorable results.
Alternative treatments are based on the observation that AD develops and progresses as a result of the production of the βA protein. Since accumulation of this protein is associated with oxidative and inflammatory damage, promising alternative strategies for treating patients with AD involve the use of anti-oxidants (e.g. vitamin E) and anti-inflammatory drugs.
Ecam and Preventing Cognitive Decline
It has long been recognized that patients with AD present an irreversible decline of cognitive functions as a consequence of cell deterioration in the forebrain cholinergic projection system. It is now believed that the reduction of the number of cholinergic cells at this cerebral site disrupts not just its functions locally and direct connections, but also significantly alters the modulation of related systems, leading to interference in several aspects of behavioral performance, arousal, attention, learning and emotion (39–41). Therefore, concerted efforts in alternative treatments for this condition have used supplements of choline.
In brief, given the fact that patients with AD will present an ever-increasing fiscal onus to society as their number climbs to over 10 million in the United States in the next decade, it is imperative to develop, test and establish successful treatment interventions. It is also evident that supplementing current pharmacological treatment (i.e. cholinesterase inhibitors) with alternative medicine, a popular trend in the current ‘self-help’ societal paradigm requires stringent and rigorous control, such as that provided by evidence-based medicine (EBM).
EBR in the Treatment of Patients with AD
As noted before, EBR is the break-open avenue for future research in the health sciences in general and in AD preclinical and clinical research in particular (42). Systematic research on research seeks to establish and to determine what is the best available evidence for treatment for each individual patient. This critical approach is key particularly in the case of AD, when one considers the sharp rise in the aging population and the subjects at-risk for AD in the next decades, in relation to the often under-tested, unreliable and sometimes unfounded ‘popular’ alternative treatments. EBR, which is the best tool presently to examine systematically the strength of clinical data to mold, as it were, novel and improved modes of intervention to meet the criteria of excellence we demand for the benefit of the patients.
Reviewing the Evidence about Pharmacological Intervention
Due to the rising number of patients with AD, several modes of treatment interventions exist. In general, two among the pharmacological treatments have shown more promising results in treating AD: acetylcholinesterase inhibitors (AChI) and NMDA antagonists (30,42).
Stating the Question
A best-case study was designed to evaluate the current published literature on both AChI and the NMDA antagonist (memantine). The PICO question was formulated as follows: in a patient population over the age of 45, with moderate AD, are acetylcholinesterase inhibitors the treatment of choice over NMDA antagonists, in effectively increasing the quality-of-life? The outcome of interest, quality-of-life, was measured based on three domains of AD that are known to deteriorate as the disease progresses and worsens:
Cognitive function,
Global performance and
Activities of daily living.
Obtaining the Sample
The search was restricted to articles relevant to the PICO question within the PubMed Database. Only articles in English were considered, and authors were not contacted regarding original data. Review articles, abstracts, unpublished reports and publications in press were not considered. The search used a combination of the search terms ‘moderate Alzheimer's disease’, ‘Alzheimer's disease’, ‘acetylcholinesterase inhibitors’, ‘daily living’, ‘quality of life’, ‘NMDA antagonist’, ‘tacrine’, ‘donepezil’, ‘rivastigmine’, ‘galantamine’, ‘memantine’ and ‘treatment’. The search was limited to clinical trials, and to subjects between the ages of 45–64.
The titles and abstracts of all published articles obtained from this search were examined in order to determine if it were applicable to the study's purpose/PICO question. An initial screening was carried out based on the following inclusion criteria:
The study was a clinical trial published in the English language.
Patients met the criteria for AD-associated dementia [as per Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)], and/or the probable AD criteria based on the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorder's Association [reports of patients with other dementias (e.g. vascular dementia) were excluded].
Patients were older than 45.
Treatment fell in either one of the two categories: acetylcholinesterase inhibitors or NMDA antagonists.
Quality-of-life was assessed in one or more of the three given domains of AD—cognitive function, global performance and activities of daily living.
Men and women were included, as well as patients of any race and/or ethnicity.
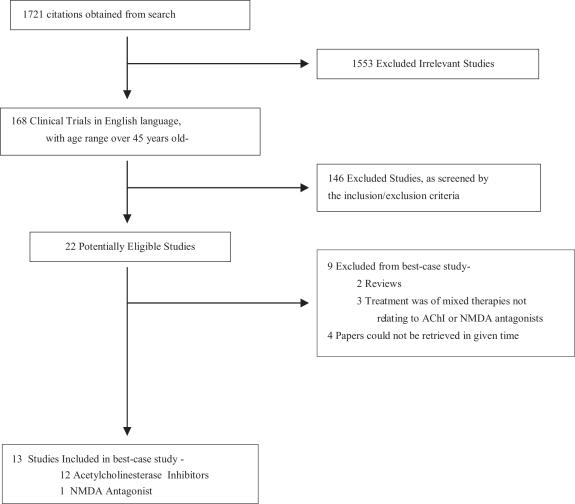
Using the PubMed database, the search conducted brought in an initial lot of 1721 papers. Of these papers, 168 articles were clinical trials published in the English language, with subjects falling in the age range of 45–64 (as specified in the advanced search/limitations of PubMed). As described, a screening was done to filter out trials failing to meet the inclusion and exclusion criteria of the search strategy. These irrelevant studies were thus omitted from the best-case study (Scheme 1). A final lot of 13 papers were included in this best-case study (31,43–54).
Scheme 1.
Search Process: flow diagram of included and excluded studies. A search for relevant studies was performed using the PubMed database, and subsequently filtered out based on the inclusion/exclusion described. Thirteen reports (12 acetylcholinesterase inhibitors and 1 NMDA antagonist) were included in the best-case study examining pharmacological interventions for AD, and thus evaluated individually on its quality.
Critical Evaluation
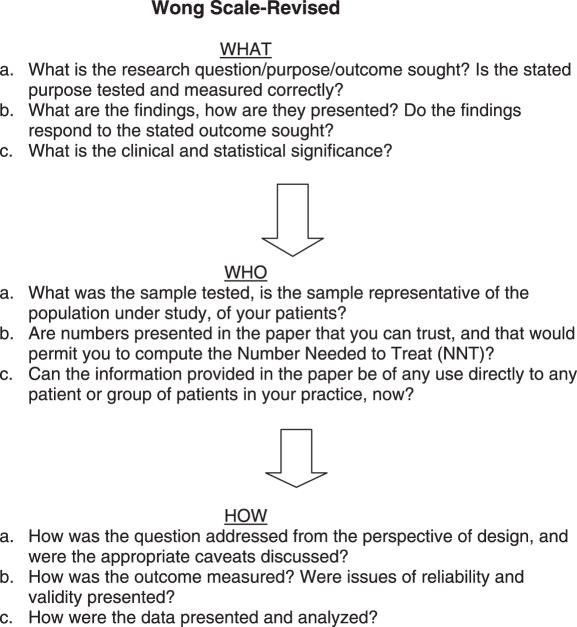
Reports were evaluated for quality of methodology, design and data analysis by the Wong Scale-Revised, and the data analyzed statistically (Analyze-It, version 1.72) (Fig. 1 and Table 1). This scale is based on reviewer responses of nine questions concerning the research quality of each individual paper; with a score of 1, 2 or 3 (best) provided for each query, as well as a comprehensive total score ranging from 9 to 27) (55). Papers falling under a total Wong score of 18 indicates that the quality of the methodology, design and data analysis fail to support the reliability of the author's conclusions and were thus omitted from the evidence supporting a consensus statement. This ‘acceptable sampling’ approach aims to determine whether the papers examined are acceptable, based on the features posed by the Wong Scale-Revised (42).
Figure 1.
Wong Scale-Revised. The Wong Scale-Revised consists of nine questions used to evaluate the quality of a study. Once applied, various scores are generated that determine the validity of the paper based on a scale of 1–3, with 1 = inappropriate, 2 = mediocre, 3 = appropriate. A comprehensive score falls in the range of 9–27 points. Studies whose scores sum a total of 18 or less are rejected while those scoring 19 or over are accepted [modified from (21)].
Table 1.
Acceptable sampling analysis of pharmacological interventions: acetylcholinesterase inhibitors and NMDA antagonists
| Paper | Question Wong Scale | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| What A | What B | What C | Who A | Who B | Who C | How A | How B | How C | ||
| 1 | 3.00 | 3.00 | 2.00 | 2.00 | 2.00 | 3.00 | 2.00 | 2.00 | 3.00 | 22.00 |
| 2 | 3.00 | 3.00 | 2.00 | 1.00 | 3.00 | 2.00 | 3.00 | 2.00 | 2.00 | 21.00 |
| 3 | 2.00 | 2.00 | 3.00 | 1.00 | 2.00 | 2.00 | 1.00 | 2.00 | 3.00 | 18.00 |
| 4 | 2.00 | 3.00 | 3.00 | 2.00 | 1.00 | 3.00 | 2.00 | 1.00 | 2.00 | 19.00 |
| 5 | 2.00 | 3.00 | 2.00 | 2.00 | 2.00 | 3.00 | 1.00 | 3.00 | 2.00 | 20.00 |
| 6 | 2.00 | 3.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 15.00 |
| 7 | 2.00 | 3.00 | 2.00 | 2.00 | 1.00 | 3.00 | 2.00 | 2.00 | 2.00 | 19.00 |
| 8 | 2.00 | 2.00 | 2.00 | 1.00 | 2.00 | 3.00 | 3.00 | 2.00 | 3.00 | 20.00 |
| 9 | 1.00 | 2.00 | 2.00 | 2.00 | 2.00 | 3.00 | 1.00 | 1.00 | 1.00 | 15.00 |
| 10 | 2.00 | 3.00 | 2.00 | 1.00 | 2.00 | 2.00 | 2.00 | 3.00 | 3.00 | 20.00 |
| 11 | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 3.00 | 2.00 | 3.00 | 17.00 |
| 12 | 2.00 | 3.00 | 3.00 | 2.00 | 1.00 | 3.00 | 1.00 | 2.00 | 3.00 | 20.00 |
| 13 | 3.00 | 3.00 | 2.00 | 2.00 | 1.00 | 3.00 | 2.00 | 2.00 | 3.00 | 21.00 |
| Mean | 2.15 | 2.69 | 2.15 | 1.54 | 1.62 | 2.54 | 1.92 | 2.00 | 2.38 | 18.75 |
| SD | 0.55 | 0.48 | 0.55 | 0.52 | 0.65 | 0.66 | 0.76 | 0.58 | 0.77 | 2.09 |
| 95% CI | 1.82–2.49 | 2.40–2.98 | 1.82–2.49 | 1.23–1.85 | 1.22–2.01 | 2.14–2.94 | 1.46–2.38 | 1.65–2.35 | 1.92–2.85 | 17.67–20.33 |
| P | ||||||||||
| Strong evidence | What B, Who C | 0.503 | ||||||||
| Adequate evidence | How C | 1.000 | ||||||||
| Moderate evidence | What A, What C, How B | 0.495 | ||||||||
| Weak evidence | Who A, Who B, How A | 0.14 | ||||||||
| Variation | SSq | DF | MSq | F | P | Coefficient | P | |||
| ANOVA analysis of Wong scores | Distribution of total scores | |||||||||
| Paper | 15.86 | 8.00 | 1.98 | 5.14 | <0.0001 | Shapiro-Wilk | 0.8977 | 0.1245 | ||
| Within cells | 41.69 | 108.00 | 0.39 | Skewness | −0.8341 | 0.1676 | ||||
| Total | 57.56 | 116.00 | Kurtosis | −0.1145 | – | |||||
Scores obtained from the Wong Scale-Revised were statistically analyzed using a one-way ANOVA. Data were used to determine the acceptability of the 13 applicable studies as a whole.
The literature regarding treatment of AD by the two modes of pharmacological intervention under comparison was reliable [mean ± standard deviation attribute score (i.e. total Wong scale score) of 18.75 ± 2.09; 95% CI = 17.67–20.33]. Three papers obtained a total Wong scale score of less than 18; implying that the quality of the methodology, design and data analysis of these few papers failed the minimum cut-off requirement of acceptability. Further analysis of the scores led to the establishment of criterion of acceptability for each of the individual domains of research assessed by the Wong scale.
Analyses Indicate that an AChI or NMDA Antagonist Was Beneficial in Terms of Increasing Patients' Overall Global Performance
Following the acceptable sampling analysis, meta-analyses were conducted (BioStat Comprehensive Meta-Analysis software, version 1.0.25). Studies, which provided descriptive statistics, were used to calculate the effect size for the meta-analysis. Therefore, those papers that failed to report exact statistical values (mean ± SD) were omitted. Five trials provided data on 1033 patients with mild to moderate AD, aged 45 or older (422 patients randomized to the treatment group and 611 to the placebo group). Duration of treatment also varied among the studies. One trial reported testing the NMDA-antagonist memantine, whereas the remaining four investigated acetylcholinesterases: tacrine (one report), galantamine (one report) and rivastigmine (two reports). The AchI eptastigmine has not yet been fully approved by the US FDA, and was thus excluded from the analysis.
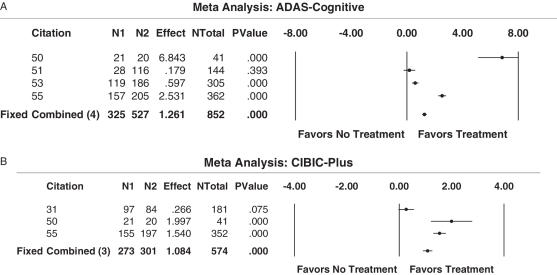
A meta-analysis was carried out analyzing the AD assessment scale—cognitive subscale (ADAS-cog) as the outcome measure. This test assesses cognition based on various fields, such as memory, language, orientation and praxis (56). Another meta-analysis was performed on the results obtained from the Clinician Interview Based Impression of Change Scale plus caregiver information (CIBIC-plus). This assesses the global performance of AD patients, based specifically on changes occurred due to the treatment (57) (Table 2). The overwhelming findings of these analyses indicate that all treatments, whether an AChI or NMDA antagonist, were beneficial in terms of increasing patients' overall global performance, assessed as ADAS-cog (Fig. 2A), or as CIBIC-plus (Fig. 2B). Our data also indicate that AchI compared more favorably than mean time with respect to the CIBIC-plus treatment outcome of global performance (Fig. 2B).
Table 2.
Instruments for assessing quality-of-life in patients with AD
| Domains assessed | Instrument | Source | Scale |
|---|---|---|---|
| Cognitive function | Alzheimer's disease assessment scale (cognitive)—ADAS-cog | Patient | 0–70 points |
| 0 = no errors | |||
| 70 = severe impairment | |||
| Cognitive function | Syndrom–Kurz test (SKT) | ||
| Global performance | Clinician Interview based Impression of Change Scale (plus caregiver information) CIBIC-Plus | Patient and caregiver during interview with clinician | 1–7 points |
| 1, 2, 3 = marked, moderate, or improvement | |||
| 4 = no change | |||
| 5, 6, 7 = minimal, moderate or marked deterioration | |||
| Activities of daily living | Progressive Deterioration Scale (PDS) | Caregiver | 29 items, with a score range of 0–100 |
| 100 = less able to carry out activities of daily living | |||
| Activities of daily living | Geriatric Evaluation by Relative's Rating Instrument (GERRI) | Caregiver |
The three domains of quality-of-life (cognition, global performance, activities of daily living) were assessed by the ADAS-cognitive scale, SKT, CIBIC-Plus, PDS and GERRI tests. Meta-analyses were generated using the results of the five stated tests.
Figure 2.
(A) Results from meta-analysis of ADAS-cognitive outcome (assessment of cognition) for pharmacological interventions (acetylcholinesterase inhibitors versus NMDA antagonists). A meta-analysis was carried out to evaluate the efficacy of AChI and NMDA antagonists in increasing the cognitive performance of patients with Alzheimer's disease, based on ADAS-cognitive scores. All four studies favored the active treatment over placebo. (B) Results from meta-analysis of CIBIC-Plus score (assessment of global performance) for pharmacological interventions best-case study (acetylcholinesterase inhibitors versus NMDA antagonists). A meta-analysis was carried out to evaluate the efficacy of AChI and NMDA antagonists in increasing the global performance of patients with Alzheimer's disease, based on scores obtained from CIBIC-Plus. All three studies favored the active treatment over placebo.
Reviewing the Evidence about Complementary and Alternative Treatment
Stating the Question
By the same approach we formulated a PICO question with respect to complementary and alternative treatment for patients with AD. In brief, it stated that ‘in a patient population over the age of 45 with moderate AD, are antioxidants more effective in increasing the quality-of-life than no treatment?’ The outcome of interest (quality-of-life) was measured based on three domains of AD
Cognitive function,
Global performance and
Activities of daily living.
Obtaining the Sample
As above, this search was restricted to articles relevant to the PICO question within the PubMed database. Authors were not contacted regarding original data. Review articles, abstracts, unpublished reports and publications in press were not considered. The PubMed search used a combination of the following terms: ‘moderate Alzheimer's disease’, ‘Alzheimer's disease’, ‘treatment’, ‘antioxidants’, ‘daily living’ and ‘quality of life’. The search was limited to clinical trials and to subjects between the ages of 45–64; as indicated by the limited/advanced search feature of the PubMed database. The titles and abstracts of all published reports obtained from the PubMed search were further examined in order to determine its applicability to the study's aim. The literature was screened to filter out all irrelevant papers based on the same inclusion/exclusion criteria as mentioned in the previous example. In this example, however, antioxidants were used as the active treatment rather than AChI or NMDA antagonists.
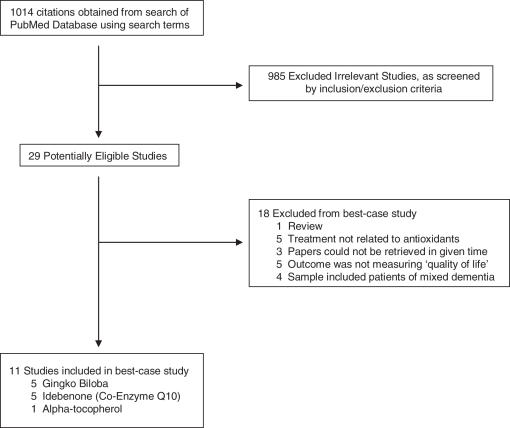
The search was conducted and initially provided a lot of 1014 papers to be screened according to the criteria previously described. A total of 985 papers were excluded due to their irrelevancy to the PICO question. Of the papers remaining, there were 29 potentially eligible studies, which were further examined using the exclusion/inclusion criteria. Ultimately, the majority of the published studies on antioxidants was eliminated from this study largely because most samples were not exclusively AD patients, and included other types of dementias (e.g. vascular dementia). The 11 studies that met the exclusion/inclusion criteria of this best-case study are listed in Table 3 (58–68). The search process is represented in Scheme 2.
Table 3.
Acceptable sampling analysis of Wong Scores analyzing complementary and alternative medicine approaches
| Paper | Wong Scores | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 1C | 2A | 2B | 2C | 3A | 3B | 3C | ||
| Hofferberth (58) | 2 | 3 | 2 | 1 | 3 | 3 | 1 | 1 | 2 | 18 |
| Kanowski et al. (59) | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 1 | 3 | 21 |
| Kanowski et al. (60) | 3 | 3 | 3 | 1 | 1 | 2 | 2 | 2 | 2 | 19 |
| Le Bars et al. (61) | 3 | 3 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 21 |
| Maurer et al. (62) | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 23 |
| Bergamasco et al. (63) | 2 | 3 | 3 | 2 | 1 | 2 | 2 | 1 | 2 | 18 |
| Gutzmann and Hadler (64) | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 2 | 2 | 19 |
| Senin et al. (65) | 2 | 3 | 2 | 2 | 1 | 3 | 1 | 2 | 1 | 17 |
| Thal et al. (68) | 3 | 2 | 3 | 1 | 1 | 3 | 2 | 2 | 2 | 20 |
| Weyer et al. (66) | 3 | 3 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 17 |
| Sano et al. (67) | 3 | 3 | 2 | 3 | 1 | 3 | 3 | 2 | 2 | 22 |
| Mean | 2.73 | 2.82 | 2.27 | 1.73 | 1.73 | 2.45 | 2.00 | 1.73 | 2.00 | 19.55 |
| SD | 0.47 | 0.40 | 0.47 | 0.79 | 1.01 | 0.69 | 0.63 | 0.65 | 0.45 | 2.02 |
| 95% CI | 2.41–3.041 | 2.55–3.09 | 1.96–2.59 | 1.20–2.26 | 1.05–2.41 | 1.99–2.92 | 1.58–2.42 | 1.29–2.16 | 1.70–2.30 | 18.19–20.90 |
| Source of variation | SSq | DF | MSq | F | P | |||||
| ANOVA analysis of Wong Scores | ||||||||||
| Wong Scores | 16.141 | 8 | 2.018 | 4.87 | <0.01 | |||||
| Within cells | 37.273 | 90 | 0.414 | |||||||
| Total | 53.414 | 98 | ||||||||
| Coefficient | P | |||||||||
| Distribution of total Wong Scores | ||||||||||
| Shapiro-Wilk | 0.9424 | 0.55 | ||||||||
| Skewness | 0.3208 | 0.614 | ||||||||
| Kurtosis | −1.0359 | – | ||||||||
Scores obtained from the Wong Scale-Revised were statistically analyzed using a one-way ANOVA. Data were used to determine the acceptability of the 13 applicable studies as a whole.
Scheme 2.
Search Process: flow diagram of included and excluded studies. A search for relevant studies was performed using the PubMed database, and subsequently filtered out based on the inclusion/exclusion described. Eleven reports (5 Ginkgo biloba, 5 idebneone and 1 alpha-tocopherol) were included in the best-case study examining antioxidants as a treatment for AD, and thus each study was evaluated individually on its quality.
Critical Evaluation and Analysis and Interpretation
As previously described, each individual paper was then evaluated entirely for its quality on methodology, design and data analysis by the implementation of the Wong Scale-Revised (42) (Fig. 1); followed by statistical analysis, as above, using a one-way ANOVA (Analyse-It, version 1.72). All papers were critically examined, and rated by one trained evaluator. Scores and statistical analysis are shown in Table 3. Two of the papers received a score falling below the cut-off score of 18 and were therefore rejected. The conclusions of these two studies were not included in the evidence supporting the consensus statement. Only 9 out of the 11 papers (84.6%) were included in the generation of the consensus statement.
This best-case study shows that the available literature regarding the treatment of AD using antioxidants compared to no treatment was reliable [mean ± SD attribute score (i.e. total Wong scale score) of 19.55 ± 2.02; 95% CI = 18.19–20.90). This is further supported by the fact that the majority of included papers received a total attribute Wong score above the cut-off line of 18 (only two studies were omitted). Additional analysis of the scores, as examined for each domain of the Wong Scale-Revised (42), indicates both adequacies and deficiencies in the satisfaction of the queries addressed.
Five trials provided enough data to run a meta-analysis examining the outcome of the quality-of-life in Alzheimer's patients. It was necessary that quality-of-life (outcome measured) was assessed in one or more of the three given domains of AD (cognitive function, global performance and daily living activities) using the appropriate psychometric tests described in Table 4. Data were provided for 1017 patients with mild to moderate AD, aged 45 or older; with 650 patients randomized to the antioxidant treatment group and 367 to the placebo group. The duration of treatment varied from study to study, ranging from 24 weeks to 12 months; with the majority reporting data for ∼24 weeks. In this meta-analysis, four studies tested an extract of Gingko biloba referred to as EGb 761, while one report examined the efficacy of idebenone (a compound of the antioxidant coenzyme Q10).
Table 4.
Summary of studies included in best-case study
| Author | Year | Treatment | Dosage/method of administration |
|---|---|---|---|
| 60 | 1994 | Ginkgo biloba: Extract Egb 761 | 80 mg daily |
| 61 | 2003 | Ginkgo biloba: Extract Egb 761 | 240 mg EGb 761 daily |
| 62 | 1996 | Ginkgo biloba: Extract Egb 761 | 240 mg EGb 761 daily |
| 63 | 1997 | Ginkgo biloba: Extract Egb 761 | 120 mg EGb 761 daily |
| 64 | 1997 | Ginkgo biloba: Extract Egb 761 | 240 mg EGb 761 daily |
| 65 | 1994 | Idebenone | 90 mg daily |
| 66 | 1998 | Idebenone | 90 and 120 mg daily |
| 67 | 1992 | Idebenone | 45 mg daily |
| 68 | 1997 | Idebenone | 30 and 90 mg daily |
| 69 | 1997 | Alpha-tocopherol (vitamin E) | 2000 IU daily |
| 70 | 2003 | Idebenone | 120, 240, or 360 mg Egb 761 daily |
The 11 studies included in the best-case study examining antioxidants (Ginkgo biloba, idebenone and alpha-tocopherol) as a complementary and alternative treatment for AD were then analyzed by an ‘acceptable sampling’ approach.
Meta-analyses were conducted (BioStat Comprehensive Meta-Analysis software, version 1.0.25) using data from the Alzheimer's Disease Assessment Scale-cognitive subset (ADAS-cog) and Syndrom–Kurztest, also known as the Short Cognitive Performance Test (SKT). The Syndrom–Kurztest test focuses on the patient's cognitive performance as well. This test has been shown to be validated to measure attention and memory functions (69).
The literature shows that the effect of antioxidant treatment for mild to moderate AD on cognitive function, as it was assessed by the ADAS-cog scale and SKT, support the use of antioxidants. Moreover, from these results, there is promising evidence to speculate the potential benefits of Ginkgo biloba as a treatment option. More clinical trials need to be performed on both Ginkgo biloba and idebenone to determine their advantages and treatment effects.
Consensus Statement
Traditional Treatment of Choice for Moderate AD Is AChI Inhibitors, in Terms of QOL
AD is a devastating disorder of the brain's nerve cells that impairs memory, thinking and behavior, which leads, ultimately, to death. Its certain diagnosis can be secured by post-mortem brain biopsies only, and diagnoses obtained from inpatients before death are best reported as ‘probable AD’. Accuracy of pre-morbid diagnosis approximates 90%. The impact of the disease on individuals, families and our health care system makes AD one of the greatest medical, social and fiscal challenges for the 21st century.
Taken together, the best available evidence derived from the best-case study examining pharmacological interventions suggests that the treatment of choice for individuals with moderate AD is AChI inhibitors, over NMDA antagonists, in terms of quality-of-life. This evidence-based analysis also uncovered the fact that adverse effects occurred as a result of each treatment, which may affect the overall tolerability of the drug.
Studies and research on memantine (the only NMDA antagonist approved by the US FDA as of yet) is rather new compared to the drugs classified as AChI. Thus, it is not surprising that there exist a larger number of reports on AChI versus that of NMDA antagonists. This imbalance, unfortunately, may create a selection bias in the analytical aspects of this best-case study. It is therefore self-evident that, as more studies are conducted on the efficacy of various drugs for the treatment of AD, the consensus statement will require regular revisions and updates with the inclusion of the latest available evidence.
CAM Intervention: Antioxidant Treatment for Mild to Moderate AD Potentially Increases QOL
From the viewpoint of CAM, the best-case study presented here in the context of complementary and alternative intervention in patients with AD attempts to present the overall reliability of the best available evidence related to treating AD with the use of antioxidants. This approach is more complementary when compared with the more traditional pharmacological therapies (acetylcholinesterase inhibitors and NMDA antagonists). It is important to note also that other substances having antioxidant activity do exist, and have been studied in relation to AD, but simply have not been included due to the criteria of this study. Furthermore, there is an extensive area of treatments categorized as CAM such as, massage, acupuncture, trans-cutaneous electric nerve stimulation, music therapy, counseling, psychotherapy and exercise that were not studied in this best-case study.
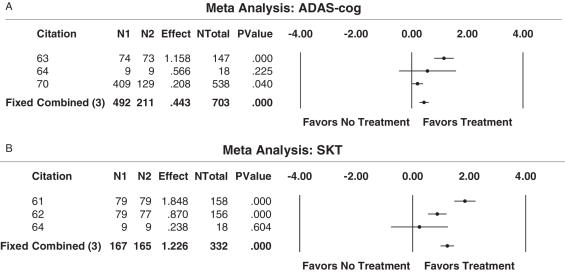
Via the ‘acceptable sampling’ technique (42), the given lot of 11 papers were analyzed for their research quality, and the best available evidence from these studies indicates that at this moment there is no precise answer to whether the use of antioxidants should be used to treat patients with AD. Overall, the effect of antioxidant treatment compared with no treatment is beneficial; as based on the ability of this therapy approach to increase the quality-of-life in the three domains of cognition, global performance and daily living functioning. However, doubts about the effectiveness of idebenone are evident in the literature (68). The meta-analyses conducted supports the use of antioxidants compared with no treatment in terms of data obtained from the SKT, as well as when examining data from the ADAS-cognitive scale (Fig. 3A and 3B). It is important to note though that the studies included in the meta-analyses examined the effects of Ginkgo biloba in four reports, versus idebenone, which constituted data from one report. This difference potentially creates a selection bias in the analysis of the data. Moreover, a large number of the studies using antioxidants as a form of complementary and alternative medicine assessed a sample of patients with a wide range of dementia, and thus were not included in this best-case study as determined by the inclusion/exclusion criteria.
Figure 3.
(A) Results from meta-Analysis of ADAS-cognitive scores (assessment of cognitive performance) for a best-case study on complementary and alternative approaches (antioxidants). A meta-analysis was carried out to evaluate the efficacy of antioxidants in increasing the global performance of patients with Alzheimer's disease, as determined by scores from the ADAS-cognitive scale. Three studies on Ginkgo biloba favored treatment, whereas one study on idebenone favored the placebo. (B) Results from meta-analysis of SKT scores (assessment of cognitive performance) for a best-case study on complementary and alternative approaches (antioxidants). Using data from the SKT, a meta-analysis was carried out to evaluate the efficacy of antioxidants in increasing the global performance of patients with AD. All three studies favored the use of antioxidants to increase cognitive ability in AD patients.
Taking the results from both approaches utilized, the CAM best-case study suggests that antioxidant treatment for individuals with mild to moderate AD does have the potential to beneficially increase quality-of-life, although there are some reports that disagree. Evidence also revealed that the side effects observed were minor: mainly consisting of headaches, nausea, insomnia and anxiety. Furthermore, no detrimental consequences such as a decrease in the quality-of-life occurred as a result of antioxidant administration. The use of antioxidant treatment appears to have a positive outcome, although it is clear that more clinical trials need to be carried out in order to fully support the use of antioxidants as a primary treatment for AD. Other concerns that must be addressed by clinical trials should also examine its potential reaction with other modes of interventions, including already established pharmaceuticals.
Limitations
The research approach performed in this best-case study exemplifies the importance of critically analyzing the evidence available, such that one can determine if the results presented are trustworthy to support clinical actions to improve the status of the patient. As a result, the consensus statement must be regularly updated to represent a culmination of all of the newly published literature.
As with every methodology, biases and problems exist in EBR in medicine. Not possessing the capacity for critical analysis of the research methodology would preclude correct data analysis, ultimately preventing appropriate decision-making in the clinical realm (70). Specifically in the context of the topic of this paper, systematic review of the literature, one of the tenets of EBM, can show biases and limitations: the review parameters may be incorrectly or poorly drawn, thus affecting conclusions and findings (71).
The process of systematic review of the research evidence, the raison d'être of EBM, is a process of critical research on research. As we noted above, the merit and strength of EBM lies in the rigor of its scientific method, and in the quality and clinical use of its product. The product of this process is valuable firstly because it identifies the best available evidence for intervention, and secondly because it generates a cost-effectiveness analysis, which is a process of decision analysis that incorporates risks as well as cost. Effectiveness and utilities of these clinical data and information are estimated to aid the final clinical decision-making process for the benefit of each individual patient. However, in order to be reliable, the EBM outcome for any given clinical condition needs to be updated at regular 6–12 month intervals (42). The challenge of staying current with the ever-changing literature field can be aided by the tools provided by EBM, such as critically and systematically appraising evidence, and incorporating it into clinical practice (72). In short, it can be argued that guiding clinical practice by EBM postulates is necessary to improve quality of care by the utilization of efficacious methods, and by extension, the elimination of the ineffective and harmful ones (73).
Divergent findings could suggest fundamental methodological issues, which may lead to substantial misinterpretations in the meta-analyses. In a fixed-model meta-analysis, the assumption is that there is some overall common difference that can be estimated. In order to test for homogeneity. To test this assumption, the Q or the I2 statistics often ensure that the population difference is the same across all the studies. Neither test was applied in the analyses described above, thus ignoring potential differences among the studies, such as population differences that may not be constant across the studies (i.e., random-model). Another caveat of these analyses is the pervasive inherent bias (cf., “publication bias”) we identified but could not explore in depth due to the paucity of the available reports. A useful graphical representation of this bias could have been the traditional funnel plot, in which the magnitude of the effect is plotted against the sample size. The true mean, μ, is taken as 0, and the standard deviation as 1. The difference between two ideally equal groups that show both significant and non-significant results, should form a funnel-like shape that extends to infinity along the 95% confidence intervals (74). Taken together, these methodological issues seriously hamper the interpretation of the meta-analysis presented here. In conclusion, the state of our research and of the literature to date does not permit an unequivocal and fully satisfactory EBR determination of the best available evidence in terms of the efficacy and effectiveness of CAM in general and of anti-oxidants in particular for patients with sDAT. Rather, it emphasizes several important caveats and deficiencies of the current research that must now be addressed, lest EBR yield to misinterpretations of the literature and erroneous inferences for the detriment to the patients.
Acknowledgments
The authors thank the students and colleagues of the UCLA Evidence-based research group for their contribution. The author is indebted to Dr Michael Newman and Dr Janet Bauer for the discussions leading to this work. This study was supported in part by funds of the UCLA School of Dentistry, the University of Ancona, the Neurology Section Health District Urbino, and the Alzheimer's Association (http://www.alz.org/).
References
- 1.Scacchi R, Gambina G, Ruggeri M, Martini MC, Ferrari G, Silvestri M, et al. Plasma levels of apolipoprotein E and genetic markers in elderly patients with Alzheimer's disease. Neurosci Lett. 1999;259:33–6. doi: 10.1016/s0304-3940(98)00889-1. [DOI] [PubMed] [Google Scholar]
- 2.Roses AD. Apolipoprotein E affects the rate of Alzheimer's disease expression: Beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994;53:429–37. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Little JW. Dental management of patients with Alzheimer' disease. Gen Dent. 2005;53:289–96. [PubMed] [Google Scholar]
- 4.Evans DA. Estimated prevalence of Alzheimer's disease in the United States. Milbank Q. 1990;68:267–89. [PubMed] [Google Scholar]
- 5.Hendrie HC. Epidemiology of Alzheimer's disease. Geriatrics. 1997;52:S4–8. [PubMed] [Google Scholar]
- 6.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Zentralbl Nervenheilkd Psychiatr. 1907;18:1777–9. [Google Scholar]
- 8.Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid-β peptide. Cell. 1993;75:1039–42. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 9.Cotman CW. The β-amyloid peptide, peptide self-assembly, and the emergence of biological activities. A new principle in peptide function and the induction of neuropathology. Ann NY Acad Sci. 1997;814:1–16. doi: 10.1111/j.1749-6632.1997.tb46140.x. [DOI] [PubMed] [Google Scholar]
- 10.Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, et al. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh VK. Studies of neuroimmune markers in Alzheimer's disease. Mol Neurobiol. 1994;9:73–81. doi: 10.1007/BF02816106. [DOI] [PubMed] [Google Scholar]
- 12.Kalaria RN, Cohen DL, Premkumar DR. Cellular aspects of the inflammatory response in Alzheimer's disease. Neurodegeneration. 1996;5:497–503. doi: 10.1006/neur.1996.0069. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi VR, García M, Rey L, Cacabelos R. Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer's Disease (AD) individuals. J Neuroimmunol. 1999;97:163–71. doi: 10.1016/s0165-5728(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini L, Passer BJ, Tabaton M, Ganjei JK, D'Adamio L. Alternative, non-secretase processing of Alzheimer's beta-amyloid precursor protein during apoptosis by caspase-6 and -8. J Biol Chem. 1999;274:21011–6. doi: 10.1074/jbc.274.30.21011. [DOI] [PubMed] [Google Scholar]
- 15.Reilly CE. Beta-amyloid of Alzheimer's disease activates an apoptotic pathway via caspase-8. J Neurol. 2000;247:155–6. doi: 10.1007/pl00007801. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer I, Puig B, Krupinsk J, Carmona M, Blanco R. Fas and Fas ligand statement in Alzheimer's disease. Acta Neuropathol(Berl) 2001;102:121–31. doi: 10.1007/s004010000325. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura I, Uetsuki T, Kuwako K, Hara T, Kawakami T, Aimoto S, et al. Cell death induced by a caspase-cleaved transmembrane fragment of the Alzheimer amyloid precursor protein. Cell Death Differ. 2002;9:199–208. doi: 10.1038/sj.cdd.4400931. [DOI] [PubMed] [Google Scholar]
- 18.Fiala M, Lin J, Kermani-Arab V, Ringman J, Gustavson A, Sayre J, et al. Ineffective phagocytosis of amyloid-β by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2005;7:221–32. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth E, Ritzline PD. An overview of the etiology, diagnosis and treatment of Alzheimer's disease. Phys Ther. 1998;78:1325–31. doi: 10.1093/ptj/78.12.1325. [DOI] [PubMed] [Google Scholar]
- 20.Fillit H, Cummings J. Practice guidelines for the diagnosis and treatment of Alzheimer's Disease in a managed care settings. II—Pharmacologic therapy. Manag care Interface. 2000;13:51–6. [PubMed] [Google Scholar]
- 21.Chiappelli F, Prolo P, Iribarren J, Neagos N, Fiala M, Concepcion E, et al. Alzheimer's disease: new frontiers for the XXI century. In: Frank Columbus, editor. Advances in Psychology Research, Progress in Alzheimer's Disease Research. Nova Science Publisher; 2006. pp. 233–5. Chapter 9; [Google Scholar]
- 22.Provinciali L, Minciotti P, Ceravolo G, Angeleri F, Sanguinetti CM. Transcranial doppler sonography as a diagnostic tool in vascular dementia. Eur Neurol. 1990;30:98–103. doi: 10.1159/000117320. [DOI] [PubMed] [Google Scholar]
- 23.Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafstrom M, Holmen K, et al. Prevalence of Alzheimer's disease and other dementias in an elderly urban population. Relationship with age, sex and education. Neurology. 1991;41:1886–92. doi: 10.1212/wnl.41.12.1886. [DOI] [PubMed] [Google Scholar]
- 24.Bracco L, Gallato R, Grigoletto F, Lippi A, Lepore V, Bino G, et al. Factors affecting course and survival in Alzheimer disease. Arch Neurol. 1994;51:1213–5. doi: 10.1001/archneur.1994.00540240057016. [DOI] [PubMed] [Google Scholar]
- 25.Cummings JL. Treatment of Alzheimer's disease. Clin Cornerstone. 2001;3:27–39. doi: 10.1016/s1098-3597(01)90046-8. [DOI] [PubMed] [Google Scholar]
- 26.Reichman WE. Alzheimer's disease: clinical treatment options. Am J Manag Care. 2000;6:S1125–32. [PubMed] [Google Scholar]
- 27.Hake AM. The treatment of Alzheimer's disease: the approach from clinical specialist in the trenches. Semin Neurol. 2002;22:71–4. doi: 10.1055/s-2002-33050. [DOI] [PubMed] [Google Scholar]
- 28.McGuffey EC. Alzheimer's disease: an overview for the pharmacist. J Am Pharm Assoc. 1997;NS37:347–52. doi: 10.1016/s1086-5802(16)30205-4. [DOI] [PubMed] [Google Scholar]
- 29.De La Graza VW. Pharmacologic treatment of Alzheimer's disease. An update. Am Fam Physician. 2003;68:1365–72. [PubMed] [Google Scholar]
- 30.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine study group memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 31.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine study group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 32.Chapman PJ, Shaw RM. Normative dental treatment needs of Alzheimer's patients. Aust Dent J. 1991;36:141–4. doi: 10.1111/j.1834-7819.1991.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 33.Geng J. Treatment of 50 cases of senile dementia by acupuncture combined with inhalation of herbal drugs and oxygen. J Tradit Chin Med. 1999;19:287–9. [PubMed] [Google Scholar]
- 34.Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, et al. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–82. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–32. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagmur E, Piatkowski A, Groger A, Pallua N, Gressner AM, Kiefer P. Bleeding complication under Gingko biloba medication. Am J Hematol. 2005;79:343–4. doi: 10.1002/ajh.20346. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- 38.Engel RR, Satzger W, Gunther W, Kathmann N, Bove D, Gerke S, et al. Double-blind cross-over study of phosphatidylserine vs. placebo in patients with early dementia of the Alzheimer type. Eur Neuropsychopharmacol. 1992;2:149–55. doi: 10.1016/0924-977x(92)90025-4. [DOI] [PubMed] [Google Scholar]
- 39.Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–7. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 40.Shen ZX. Brain cholinesterases: III. Future perspectives of AD research and clinical practice. Med Hypotheses. 2004;63:298–307. doi: 10.1016/j.mehy.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira AA, Jr, Hodges HM. Alzheimer's disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res. 2005;2:79–95. doi: 10.2174/1567205052772759. [DOI] [PubMed] [Google Scholar]
- 42.Chiappelli F, Prolo P, Cajulis OS. Evidence-based research in complementary and alternative medicine I—history. Evid Based Complement Alternat Med. 2006;2:1–10. doi: 10.1093/ecam/neh106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomized double-blind trial. Lancet. 2004;363:2105–15. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 44.Boada-Ramirez M, Brodaty H, Cras P, Baloyannis S, Emre M, 322 Study Group et al. Efficacy and safety of donepexil in patients with Alzheimer's disease. Drugs Aging. 2004;21:45–53. doi: 10.2165/00002512-200421010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology. 2001;57:613–20. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 46.Hancock G, Charlesworth G. Donepezil slows decline in daily life activities in people with moderate to severe Alzheimer's disease and alleviates caregiver burden. Evid Based Ment Health. 2004;7:20–1. doi: 10.1136/ebmh.7.1.20. [DOI] [PubMed] [Google Scholar]
- 47.Imbibo BP, Lucca U, Luchelli F, Alberoni M, Thal LJ, Epastigmine Study Group A 25-week placebo-controlled study of eptastigmine in patients with Alzheimer Disease. Alzheimer Dis Assoc Disord. 1998;12:313–22. doi: 10.1097/00002093-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Jones RW, Soininen H, Hager K, Aarsland D, Passmore P, DONGAL Study Group et al. A multinational, randomized, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:58–67. doi: 10.1002/gps.1038. [DOI] [PubMed] [Google Scholar]
- 49.Karaman Y, Erdogan F, Koseoglu E, Turan T, Ersoy AO. A 12-month study of the efficacy of rivastigmine in patients with advances moderate Alzheimer's Disease. Dement Geriatr Cogn Disord. 2005;19:51–6. doi: 10.1159/000080972. [DOI] [PubMed] [Google Scholar]
- 50.Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI, et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. JAMA. 1994;271:985–91. [PubMed] [Google Scholar]
- 51.Patterson CE, Passmore AP, Crawford VLS. A 6-month open-label study of the effectiveness and tolerability of galantamine in patients with Alzheimer's disease. Int J Clin Pract. 2004;58:144–8. doi: 10.1111/j.1368-5031.2004.0107.x. [DOI] [PubMed] [Google Scholar]
- 52.Raskind MA, Peskind ER, Truyen L, Kershaw P, Damarju CV. The cognitive benefits of galantamine are sustained for at least 36 months. Arch Neurol. 2004;61:252–6. doi: 10.1001/archneur.61.2.252. [DOI] [PubMed] [Google Scholar]
- 53.Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomized controlled trial. Br Med J. 1999;318:633–8. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–95. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 55.Potyk D. Treatments for Alzheimers disease. Southern Med J. 2005;98:628–35. doi: 10.1097/01.SMJ.0000166671.86815.C1. [DOI] [PubMed] [Google Scholar]
- 56.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 57.Knopman DS, Knapp MJ, Gracon SI, Davis CS. The clinical interview-based impression (CIBI): a clinician's global change rating scale in Alzheimer's disease. Neurology. 1994;44:2315–21. doi: 10.1212/wnl.44.12.2315. [DOI] [PubMed] [Google Scholar]
- 58.Hofferberth B. The efficacy of EGb 761 in patients with senile dementia of the Alzheimer type: a double-blind, placebo-controlled study on different levels of investigation. Human Psychopharmacol. 1994;9:215–22. [Google Scholar]
- 59.Kanowski S, Hoerr R. Gingko biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry. 2003;36:297–303. doi: 10.1055/s-2003-45117. [DOI] [PubMed] [Google Scholar]
- 60.Kanowski S, Herrmann WM, Stephan K, Wieirich W, Horr R. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild tomdoerate primary degenerative dementia of the Alzheimer type of multi-farct dementia. Pharmacopsychiatry. 1996;29:47–56. doi: 10.1055/s-2007-979544. [DOI] [PubMed] [Google Scholar]
- 61.Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo Biloba for dementia. JAMA. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 62.Maurer K, Ihl R, Frolich L. Clinical efficacy of Ginkgo Biloba special extract EGb 761 in dementia of the Alzheimer type. J Psychiat Res. 1997;31:645–55. doi: 10.1016/s0022-3956(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 63.Bergamasco B, Scarzella L, La Commare P. Idebenone, a new drug in the treatment of cognitive impairment in patients with dementia of the Alzheimer type. Funct Neurol. 1994;9:161–8. [PubMed] [Google Scholar]
- 64.Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer's disease: update on a 2-year double-blind multicentre study. J Neural Transm. 1998;54:301–10. doi: 10.1007/978-3-7091-7508-8_30. [DOI] [PubMed] [Google Scholar]
- 65.Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A, et al. Arch Gerontol Geriatr. Vol. 15. 1992. Idebenone in senile dementia of Alzheimer type: a multicentre study; pp. 249–60. [DOI] [PubMed] [Google Scholar]
- 66.Weyer G, Babej-Dolle RM, Hadler D, Hofmann S, Herrman WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer's disease. Neuropsychobiology. 1997;36:73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- 67.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegine, alpha-tocopherol, or both as treatment for Alzheimer disease. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 68.Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, Weiner MF, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer's disease. Neurology. 2003;61:1498–502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 69.Kim YS, Nibbelink DW, Overall JE. Factor structure and scoring of the SKT test battery. J Clin Psychol. 1993;49:61–71. doi: 10.1002/1097-4679(199301)49:1<61::aid-jclp2270490109>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 70.Landry MD, Sibbald WJ. From data to evidence: evaluative methods in evidence-based medicine. Respir Care. 2001;46:1226–35. [PubMed] [Google Scholar]
- 71.Timio M, Antiseri D. Evidence-based medicine: reality and illusions. Extension of epistemological reflexions. Ital Heart J Suppl. 2000;1:411–4. [PubMed] [Google Scholar]
- 72.Akobeng AK. Principles of evidence based medicine. Arch Dis Child. 2005;90:837–40. doi: 10.1136/adc.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q. 2003;74:387–99. doi: 10.1023/a:1026091611425. [DOI] [PubMed] [Google Scholar]
- 74.Moradi DR, Moy PK, Chiappelli F. Evidence-based research in alternative protocols to dental implantology: a closer look at publication bias. Cal Dental Association J. 2006. pp. 877–86. [PubMed]