Abstract
Medicinal plants are recognized as sources of natural antioxidants that can protect from biological system oxidative stress. The present cross-sectional before/after clinical trial was carried out to investigate the antioxidant properties of the decoction of the flowers of Echium amoenum Fisch & C.A. Mey in humans. A group of 38 healthy subjects was invited to use the E. amoenum (7 mg kg−1) twice daily for 14 days. Blood samples before and after entering the study were measured for lipid peroxidation level (LPO), total antioxidant capacity (TAC) and total thiol (SH) molecules. A significant reduction of blood LPO (24.65 ± 11.3 versus 19.05 ± 9.7, P = 0.029) was observed after 14 days of E. amoenum consumption. Blood TAC (1.46 ± 0.51 versus 1.70 ± 0.36, P = 0.018) and total thiol molecules (0.49 ± 0.11 versus 0.56 ± 0.12, P = 0.001) increased after 14 days of E. amoenum consumption. In conclusion, this antioxidative stress potential of E. amoenum may be due to its bioactive antioxidant components, especially rosmarinic acid and flavonoids. In recent years the importance of oxidative stress in the pathophysiology of many human disorders has been confirmed, thus use of this plant as a dietary supplement is highly recommended.
Keywords: antioxidant, decoction, Echium amoenum Fisch & C.A. Mey, human, oxidative stress
Introduction
Free radicals, like reactive oxygen species (ROS), nitrogen (RNS) and chlorine (RCS), are normal by-products of metabolism and they are introduced into the body from outside sources of harmful chemicals in the environment, unhealthy foods, stress, certain drugs, cigarette smoke, etc. Increasing the intake of antioxidants can neutralize free radicals and protect the body from cell damage. In the body, oxidative stress results from the imbalance between the extent of ROS formation and the antioxidant defense mechanisms. Links between oxidative stress and adverse health effects have been suggested for several groups of diseases, including cardiovascular, respiratory and neurological as well as for the general aging process. Such adverse effects are mediated by free radical damage to lipids, proteins and DNA. Protection from damage occurs through the action of multiple antioxidants, some endogenously produced and some provided through dietary intake (1–3). It is believed that medicinal plants are a potential source of antioxidants and ROS scavenger molecules (4). One of these plants is Echium amoenum Fisch & C.A. Mey that has been shown as a rich source of antioxidants, like rosmarinic acid (RA) and flavonoids. This plant belongs to the Boraginaceae family and is a biennial or perennial herb indigenous to the narrow zone of northern part of Iran and Caucasus, where it grows at highlands at the altitude ranging from 60 to 2200 m (5).
There has been an increased interest in Echium species, including E. amoenum (F.M.), because of their medicinal and nutritional properties. E. amoenum (F.M.) is one of the most important medicinal plants in Iranian traditional medicine (6). The flowers of this plant have been used as demulcent, anti-inflammatory and analgesic, anxiolytic, and sedative in folk medicine of Iran (5–7). Anxiolytic effect of the flower of this plant has been shown in two separate experimental studies in mice (8,9). In Western medicine, the flowers and the leaves of borage have been similarly used as antifebrile, antidepressant, anxiolytic, ameliorant of heart and pulmonary disturbances, poultice for inflammatory swellings, diuretic, laxative, emollient and demulcent, and recently as a possible protective factor against cancer (10–12). Extract of this plant has been shown to contain flavonoids, saponins, unsaturated terpenoids and sterols (8). Extract of Eqium was effective at intraperitoneal doses of 80–125 mg kg−1 in animals (10) and has not been toxic in doses as high as 6 g kg−1 (13).
Phytochemical studies on E. amoenum revealed the presence of many chemicals such as RA, anthocyanidine, flavonoids, γ-linolenic acid and trace amount of alkaloids (6,14,15). The antioxidant properties of flavonoids (16,17) and RA (18–22) have been well established.
Regarding the above-mentioned information, we were interested in performing a cross-sectional before/after clinical trial study to explore antioxidant influences of E. amoenum (F.M.) in human by evaluation of blood total antioxidant capacity (TAC), lipid peroxidation (LPO) and total thiol (SH) molecules.
Subjects and Methods
Study Design
A clinical trial study with a total of 38 subjects was designed. Subjects were volunteer students of Arak University of Medical Sciences, located in the south-west of Iran, who all lived in the university dormitory. Subjects were selected on a simple random basis from volunteers. The study was conducted in complete accordance with the declaration of Helsinki. All participants were provided with specific written consents obtained prior to entrance into the study. Each individual was extensively interviewed by a specialized physician who filled in a structured questionnaire specifying gender, smoking, dietary habits, sports habits and history of special disease, before obtaining blood. Then the subjects were administered E. amoenum (F.M.) flower decoction (7 mg kg−1) twice daily (morning and evening) for 2 weeks. The dose was selected on the basis of a pilot study and traditional use information. A supervisor carefully checked to make sure that the volunteers were taking the decoction properly. Demographic characteristics of the subjects are presented in Table 1.
Table 1.
Demographic characteristics of study subjects
| Using drug | Sport | Smoking | Age (year) | Sex | Number of subjects | ||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | Female | Male | ||
| 33 (86.8%) | 5 (13.2%) | 25 (65.8%) | 13 (34.2%) | 38 (100%) | 0 | 18–25 | 25 (65.8%) | 13 (34.2%) | 38 |
Materials
5,5′-Dithiobis-2-nitrobenzoic acid (DTNB), Tris base, 1,1,3,3′-tetraethoxypropane (MDA) from Sigma, UK, 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol from Merck, Germany, and 2,4,6-tripyridyl-s-triazine (TPTZ) from Fluka, Italy, were used in this study.
Plant Material
Flowers of E. amoenum (F.M.) were collected from a farm at 80 km north of Ghazvin (a city in western Iran) in June 2002. A total of 450 g air-dried flowers of E. amoenum was used to provide the decoction.
Plasma Preparation
Five milliliters of heparinized blood was collected from each subject at the end of the 2 week treatment, centrifuged at 1200 g for 10 min at 4°C and plasma freezed at −80°C until analysis. Blood samples were collected 12 h after the last dose of decoction was taken.
Measurement of Plasma TAC
Antioxidant capacity of plasma was determined by measuring the ability of plasma to reduce Fe3+ to Fe2+. The complex between Fe2+ and TPTZ gives a blue color with absorbance at 593 nm (23).
Measurement of Plasma Total Thiol Molecules
Total sulfhydryl content was determined in plasma by the method of Hu (24). A volume of plasma (0.20 ml) was mixed in a 10 ml test tube with 0.6 ml of Tris–EDTA buffer (Tris base 0.25 M, EDTA 20 mM, pH 8.2) followed by the addition of 40 μl of 10 mM of DTNB in methanol. The final volume of the reaction mixture was made up to 4.0 ml by adding 3.16 ml of methanol. The test tube was capped, and the color was developed for 15–20 min, followed by centrifugation at 3000 g for 10 min at ambient temperature. The absorbance of the supernatant was measured at 412 nm.
Measurement of LPO
LPO of plasma was determined by the reaction of TBA with MDA and other lipid peroxides. Briefly, plasma samples were mixed with TCA (20%) and the precipitate was dispersed in H2SO4 (0.05 M). TBA (0.2% in sodium sulfate) was added and heated for 30 min in a boiling water bath. LPO adducts were extracted by n-butanol and absorbance was measured at 532 nm (25).
Statistics
Paired t-test was used to analyze the significance of differences observed between study groups. F-test was used to determine the normal distribution of variances between groups. P-values >0.05 were considered insignificant.
Results and Discussion
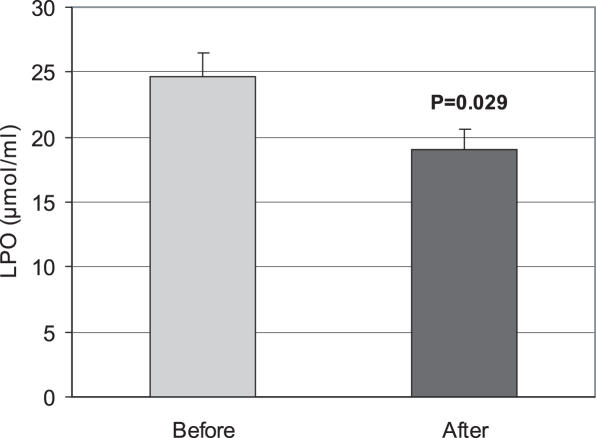
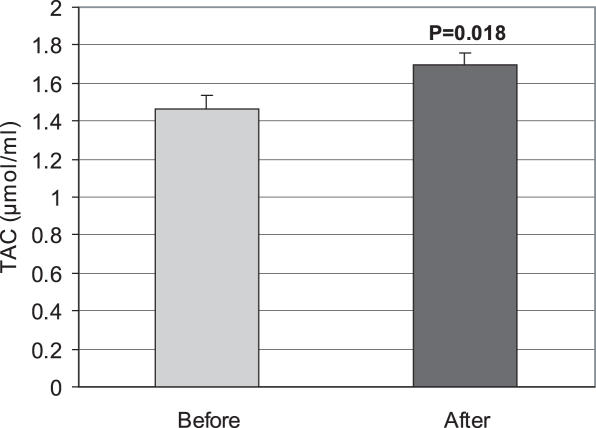
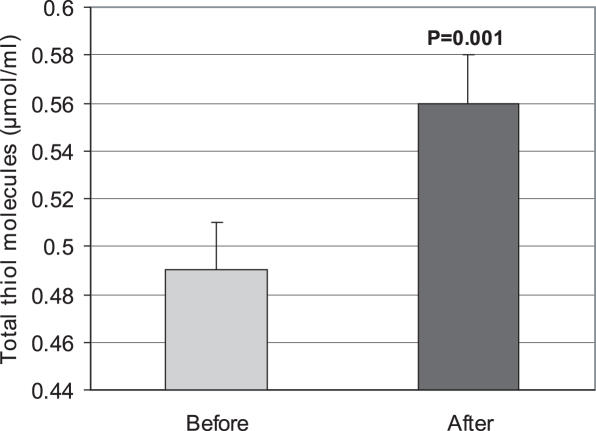
A significant decrease (P < 0.05) in LPO was observed by use of E. amoenum. The mean ± SD before and after using were 24.65 ± 11.33 and 19.05 ± 9.7 nmol ml−1 (Fig. 1). After use of the decoction, the TAC level increased significantly (P < 0.05). The mean ± SD values before and after were 1.46 ± 0.51 and 1.70 ± 0.36 μmol ml−1 (Fig. 2). A significant (P < 0.001) increase in total thiol molecules was observed after administration of the decoction (0.49 ± 0.11 μmol ml−1 before versus 0.56 ± 0.12 μmol ml−1 after (Fig. 3).
Figure 1.
Effect of the consumption of E. amoenum (F.M.) decoction (7 mg kg−1, twice per day for 14 days) on blood lipid peroxidation (LPO) level in healthy subjects (n = 38). Results are expressed as mean ± SE. P-value is represented in graph.
Figure 2.
Effect of the consumption of E. amoenum (F.M.) decoction (7 mg kg−1, twice per day for 14 days) on blood total antioxidant capacity (TAC) in healthy subjects (n = 38). Results are expressed as mean ± SE. P-value is represented in graph.
Figure 3.
Effect of the consumption of E. amoenum (F.M.) decoction (7 mg kg−1, twice per day for 14 days) on blood total thiol molecules in healthy subjects (n = 38). Results are expressed as mean ± SE. Significance difference in P-value is represented in graph.
The Consumption of E. amoenum Markedly Decreases ROS Concentrations
In this study, the influence of usage of the decoction (7 mg kg−1) twice daily (morning and evening) for 2 weeks on the oxidative stress status of healthy subjects was studied. Results indicate that TAC and thiol groups increased and LPO as a marker of ROS concentration markedly decreased after consumption of E. amoenum. In the body, antioxidants act as free radical scavengers and thus protect cells from being exposed to free radicals and further cellular damage. This is the mechanism by which they protect the human body from several diseases attributed to the reactions of radicals. Numerous substances have been suggested to act as antioxidants in this plant. Various phenolic antioxidants such as flavonoids, RA, tannins, coumarins, xanthenes and, more recently, procyanidins have been shown to scavenge radicals in a dose-dependent manner (26,27). In addition, flavonoids and RA have been introduced as the main constituents of E. amoenum (F.M.) in several phytochemistry studies (6,16,17,27,28).
The antioxidant potential of flavonoids has been well established (22,23). Flavonoids can highly scavenge most types of oxidizing molecules, including singlet oxygen and various free radicals, and thus act indirectly as an efficient antioxidant (29). They can also act directly by suppressing ROS formation (30).
RA Reduced Pro-inflammatory Molecule Expression and Enhanced Antioxidative Activity
RA, the other important constituent of this plant, is an ester of caffeic acid and 3,4-dihydroxyphenylacetic acid. It is commonly found in species of the Boraginaceae and the subfamily Nepetoideae of the Lamiaceae. There are a number of reports on the antioxidative activities of RA which all confirm that RA has strong antioxidant activity even higher than vitamin E. In this regard, the reported positive effects of RA include enhancement of superoxide and hydroxyl scavenging (20), inhibition of both low-density lipoprotein (21) and oil oxidation (19), suppression of arachidonate metabolism formation (31), inhibition of hemolysis (32), and having hyaluronidase and h-hexosaminidase activities (33). In addition, RA inhibited lung injury in mice that is regularly induced by diesel exhaust particles. RA showed this by reduction of pro-inflammatory molecule expression and enhanced antioxidative activity (34).
In conclusion, the present findings well indicate that E. amoenum (F.M.) decoction has very good potential to improve human antioxidant status and prevent normal oxidative stress that happens daily due to normal exposure to many causal chemicals and conditions. This potential of E. amoenum (F.M.) seems to be due to its bioactive antioxidant components, especially RA and flavonoids. In recent years the importance of oxidative stress in the pathophysiology of many human disorders has been highlighted (35–44), thus use of this plant as a dietary supplement is highly recommended (45,46). Trials to establish efficacy and optimum dosage of the present herbal product for treating human chronic diseases with pathophysiology of oxidative stress are essential.
Acknowledgements
This work was supported by a grant from Arak University of Medical Sciences.
References
- 1.Cochrane CG. Cellular injury by oxidant. Am J Med. 1991;91:23S–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- 2.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaiee A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:RA141–7. [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JMC. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 4.Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, et al. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 5.Rechinger KH. Flora Iranica. No. 48. Graz: Akademishe Druck-u.ver Lagsanstalt; 1967. p. 215. [Google Scholar]
- 6.Hooper D. Useful Plants and Drugs of Iran and Iraq. Chicago, USA: Field Museum of Natural History; 1937. p. 115. [Google Scholar]
- 7.Wretensjö I, Svensson L, Christie WW. Gas chromatographic mass spectrometric identification of the fatty acids in borage oil using the picolinyl ester derivatives. J Chromatogr A. 1990;521:89–97. [Google Scholar]
- 8.Shafaghi B, Naderi N, Tahmasb L, Kamelinejad M. Anxiolytic effect of Echium amoenum L. in mice. Iran J Pharm Res. 2002;1:37–41. [Google Scholar]
- 9.Kast RE. Borage oil reduction of rheumatoid arthritis activity may be mediated by increased cAMP that suppresses tumor necrosis factor-alpha. Int Immunopharmacol. 2001;1:2197–9. doi: 10.1016/s1567-5769(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor R, Klimaszewski A. Efficacy of borage oil in patients with atopic eczema. Br J Dermatol. 2000;143:200–1. doi: 10.1046/j.1365-2133.2000.03619.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez CA, Sanz JM, Marcos G, Pita P, Brullet E, Saigi E, et al. Borage consumption as a possible gastric cancer protective factor. Cancer Epidemiol Biomarkers Prev. 1993;2:157–8. [PubMed] [Google Scholar]
- 12.Iranian Herbal Pharmacopoeia (IHP) Vol. 2. Tehran: Ministry of Health Publication; 2002. pp. 25–6. 667–71. [Google Scholar]
- 13.Sayyah M, Sayyah M, Kammalinejad M. A preliminary randomized double blind clinical trial on the efficacy of aqueous extract of Echium amoenum in the treatment of mild to moderate major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:166–9. doi: 10.1016/j.pnpbp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Erdemoglua N, Kusmenoglua S, Vura M. Gamma-linolenic acid content and fatty acid composition of Boraginaceae seed oils. Eur J Lipid Sci Technol. 2004;106:160–4. [Google Scholar]
- 15.Delorme P, Jay M, Ferry S. Iventaire phytochimique des borraginacees indigenes. Planta Med. 1977;11:5–11. [Google Scholar]
- 16.Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19:481–6. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- 17.Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–22. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Ohto Y, Murakami A, Ohigashi H. Superoxide scavenging ability of rosmarinic acid from Perilla frutescens Britton Var. Acuta, F. viridis. J Agric Food Chem. 1998;46:4545–50. [Google Scholar]
- 19.Fuhrman B, Volkova N, Rosenblat M, Aviram M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin Eglabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal. 2000;2:491–506. doi: 10.1089/15230860050192279. [DOI] [PubMed] [Google Scholar]
- 20.Frankel EN, Huang S, Aeschbach R, Prior E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J Agric Food Chem. 1996;44:131–5. [Google Scholar]
- 21.Butterweck V, Hegger M, Winterhoff H. Flavonoids of St. John's wort reduce HPA axis function in the rat. Planta Med. 2004;70:1008–11. doi: 10.1055/s-2004-832631. [DOI] [PubMed] [Google Scholar]
- 22.Abdollahi M, Salehnia A, Mortazavi SHR, Ebrahimi M, Shafiee A, Fouladian F, et al. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of Satureja Khuzestanica essential oil as in rat in vivo; a toxicopharmacological study. Med Sci Monit. 2003;9:BR331–5. [PubMed] [Google Scholar]
- 23.Benzi IF, Strain S. Ferric reducing antioxidant assay. Methods Enzymol. 1999;292:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 24.Hu ML, Dillared CJ. Plasma SH and GSH measurement. Methods Enzymol. 1994;233:385–7. [Google Scholar]
- 25.Satoh K. Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 26.Czinner E, Hagymasi K, Blazovics A, Kery A, Szoke E, Lemberkovics E. In vitro antioxidant properties of Helichrysum arenarium (L.) Moench. J Ethnopharmacol. 2000;77:31–5. doi: 10.1016/s0378-8741(00)00304-4. [DOI] [PubMed] [Google Scholar]
- 27.D'Amelio FS. Botanics: A Phytocosmetic Desk Reference. London: CRC Press; 1999. p. 361. [Google Scholar]
- 28.Mehrabani M, Ghassemi N, Sajjadi E, Ghannadi AR, Shams-Ardakani MR. Main phenolic compound of petals of Echium amoenum Fish. and C.A. Mey., a famous medicinal plant of Iran. DARU. 2005;13:65–9. [Google Scholar]
- 29.Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56:317–33. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 30.van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, et al. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–42. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 31.Kimura Y, Okuda H, Okuda T, Hatano T, Arichi S. Studies on the activities of tannins and related compounds, X. Effects of caffeetannins and related compounds on arachidonate metabolism in human polymorphonuclear leukocytes. J Nat Prod. 1987;50:392–9. doi: 10.1021/np50051a009. [DOI] [PubMed] [Google Scholar]
- 32.Englberger W, Hadding U, Etschenberg E, Graf E, Leyck S, Winkelmann J, et al. Rosmarinic acid: a new inhibitor of complement C3-convertase with anti-inflammatory activity. Int J Immunopharmacol. 1988;10:729–37. doi: 10.1016/0192-0561(88)90026-4. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Miyazaki T, Ono M, Sakurai H. Antiallergic activities of rabdosiin and its related compounds: chemical and biochemical evaluations. Bioorg Med Chem. 1998;6:1051–6. doi: 10.1016/s0968-0896(98)00063-7. [DOI] [PubMed] [Google Scholar]
- 34.Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, et al. Rosmarinic acid inhibits lung injury induced by diesel exhaust particles. Free Radic Biol Med. 2003;34:1060–9. doi: 10.1016/s0891-5849(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 35.Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, et al. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol. 2005;24:439–45. doi: 10.1191/0960327105ht549oa. [DOI] [PubMed] [Google Scholar]
- 36.Mashayekhi F, Aghahoseini F, Rezaie A, Zamani MJ, Khorasani R, Abdollahi M. Alteration of cyclic nucleotides levels and oxidative stress in saliva of human subjects with periodontitis. J Contemp Dent Pract. 2005;4:46–53. [PubMed] [Google Scholar]
- 37.Malekirad AA, Ranjbar A, Rahzani K, Kadkhodaee M, Rezaie A, Taghavi B, et al. Oxidative stress in operating room personnel: occupational exposure to anesthetic gases. Hum Exp Toxicol. 2005;24:597–601. doi: 10.1191/0960327105ht565oa. [DOI] [PubMed] [Google Scholar]
- 38.Larijani B, Afshari M, Astanehi-Asghari F, Mojtahedi A, Rezaie A, Hosseinnezhad A, et al. Effect of short-term carvedilol therapy on salivary and plasma oxidative stress parameters and plasma glucose level in type II diabetes. Therapy. 2006;3:119–23. [Google Scholar]
- 39.Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Therapy. 2005;2:787–96. [Google Scholar]
- 40.Radfar M, Larijani B, Hadjibabaie M, Rajabipour B, Mojtahedi A, Abdollahi M. Effects of pentoxifylline on oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother. 2005;59:302–6. doi: 10.1016/j.biopha.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752–7. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- 42.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–73. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ranjbar A, Pasalar P, Sedighi A, Abdollahi M. Induction of oxidative stress in paraquat formulating workers. Toxicol Lett. 2002;131:191–4. doi: 10.1016/s0378-4274(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 44.Malekirad AA, Ranjbar A, Rahzani K, Pilehvarian AA, Rezaie A, Zamani MJ, et al. Oxidative stress in radiology staff. Environ Toxicol Pharmacol. 2005;20:215–8. doi: 10.1016/j.etap.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Azaizeh H, Ljubuncic P, Portnaya I, Said O, Cogan U, Bomzon A. Fertilization-induced changes in growth parameters and antioxidant activity of medicinal plants used in traditional Arab medicine. Evid Based Complement Alternat Med. 2005;2:549–56. doi: 10.1093/ecam/neh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2:301–8. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]