Abstract
Ichthyotherapy (therapy with the so-called ‘Doctorfish of Kangal’, Garra rufa) has been shown to be effective in patients with psoriasis in the Kangal hot springs in Turkey. This study evaluates the efficacy and safety of ichthyotherapy in combination with short-term ultraviolet A sunbed radiation in the treatment of psoriasis under controlled conditions. We retrospectively analyzed 67 patients diagnosed with psoriasis who underwent 3 weeks of ichthyotherapy at an outpatient treatment facility in Lower Austria between 2002 and 2004. Main outcome measures are as follows: overall relative reduction in Psoriasis Area Severity Index (PASI) score; proportion of patients with an improvement in their PASI score of ≥75% (PASI-75) and ≥50% (PASI-50); patient-reported outcomes assessed with a custom questionnaire; and patient follow-up with a questionnaire sent out in March 2005. Safety was evaluated by reviewing adverse events and vital signs. Overall there was a 71.7% reduction in PASI score compared to baseline (P < 0.0001). Of the 67 patients studied, 31 (46.3%) achieved PASI-75 and 61 patients (91%) achieved at least PASI-50. Patients reported substantial satisfaction with the treatment. The reported mean remission period was 8.58 months [95% confidence interval (CI) 6.05–11.11]. A total of 87.5% of patients reported a more favorable outcome with ichthyotherapy, when asked to compare ichthyotherapy to other previously tried therapies. Sixty-five percent stated that after the relapse their symptoms were less severe than before treatment. There were no significant adverse events. The benefit demonstrated in this study along with the favorable safety profile suggests that ichthyotherapy could provide a viable treatment option for patients with psoriasis.
Keywords: Garra rufa, ichthyotherapy, Kangal fish, psoriasis treatment, ultraviolet A
Introduction
Psoriasis is a common skin disorder with a worldwide distribution; the average prevalence in Europe and the USA has been estimated at 2% (1). Whilst considerable advances have been made in the management of this disease in recent years, there is no cure, and no simple, safe and invariably effective treatment (2). The disease carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction (3). A wide range of treatments is offered for psoriasis. Some patients rely on conventional pharmacological methods; others try alternative and complementary treatment modalities (4).
Surely one of the most unusual alternative treatments is the so-called ‘Doctorfish of Kangal’ in the Central Anatolia region of Turkey. This treatment was first mentioned in The Lancet in 1989 (5) but the details of the treatment were published only recently by Özcelik et al. (6) According to the authors two different types of fish live in the pools of the Kangal hot spring: Cyprinion macrostomus and Garra rufa. Both fish are members of the carp and minnow family (Cyprinidae). Garra rufa is regarded as the main therapeutic. Garra rufa is normally a bottom dweller, where it adheres by suction to rocks with its ventral crescent-shaped mouth to feed on phyto- and zooplankton (7). However, in the hot pools of Kangal, where phyto- and zooplankton are scarce, these fish feed on the skin scales of bathers, reportedly reducing illnesses such as psoriasis and atopic dermatitis (6).
Whether this remarkable treatment is also effective outside of the Kangal hot spring in Turkey is unknown. Since there have been many unscientific and misleading names for this kind of therapy, we suggest the term ‘ichthyotherapy’, in accordance with other so-called biotherapy concepts such as maggot therapy (use of sterile fly larvae), hirudotherapy (use of leeches) and apitherapy (use of bee venom). In this retrospective study, we set out to evaluate the efficacy and safety of ichthyotherapy when used in combination with short-term ultraviolet (UV) A radiation in patients with psoriasis vulgaris in an outpatient treatment facility.
Patients and Methods
Patients
We retrospectively analyzed 67 patients diagnosed with psoriasis who underwent 3 weeks of ichthyotherapy combined with a short course of UVA sunbed treatment at an outpatient treatment facility in Lower Austria between 2002 and 2004. All patients had moderate to severe chronic plaque psoriasis. Patients referred themselves to the treatment facility to find relief from their symptoms. The costs of this treatment were not covered by the patients' health care insurance. All patients had signed informed consent and were under constant medical supervision throughout the course of treatment.
Treatment Regimen
Treatment lasted 3 weeks. A daily 2 h ‘fish bath’ (Fig. 1) was taken in a bathtub at a comfortably warm temperature (36–37°C). Patients with no contraindication to UV exposure (reported history of UV-related skin malignancies) used a commercially available stand-up rapid-tan facility (Cyclone 60 with 60 Cosmolux VHR160 lamps, CMC Sun Capsule USA) for 3–5 min after each bath session according to skin type. The spectral emission of the lamps is shown in Fig. 2. After UV exposure, the patients applied a generic skin lotion (Pharmacy Neunkirchen, Austria) containing glycerine, Butyrospermum parkii (Shea butter) and Aloe vera extract. If psoriasis lesions severely involved the scalp, the patient's head was shaved before treatment.
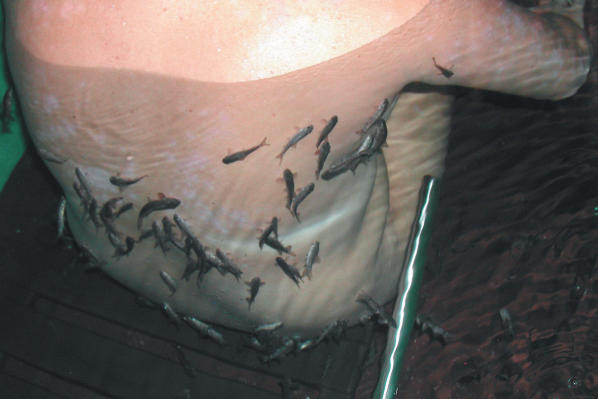
Figure 1.
Patient sitting in standard treatment tub during therapy with the Kangal fish G. rufa.
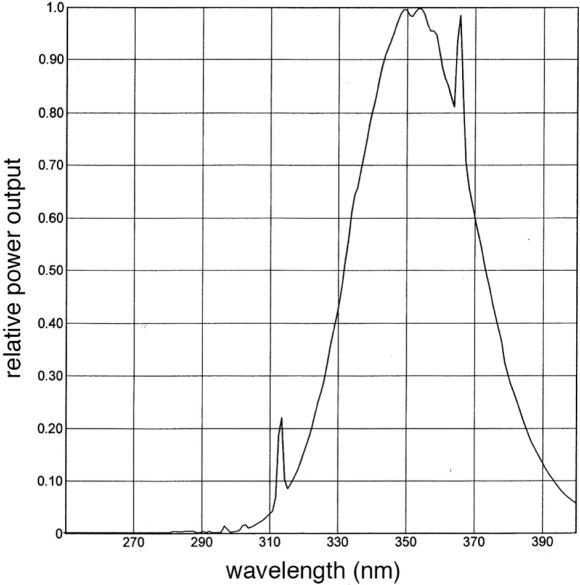
Figure 2.
Spectral emission of employed UVA lamps.
Treatment Tubs
The treatment tubs, made from food-safe plastic (Chemo®, Weinstadt, Germany) had a capacity of 1100 l, were filled to ∼80% capacity. Between 250 and 400 fishes were used, depending on the size and severity of the skin lesions. The bathtubs were equipped with an elaborate fresh water system, the technical details of which will be published elsewhere. The water in the tubs was constantly filtered and sterilized (700 liters per h) by a filter pump and a UVC water sterilization device and was simultaneously enriched with oxygen. The water was exchanged completely 3 to 4 times a day. A thermostat was used to maintain a comfortable water temperature between 36 and 37°C.
The fish, reared in an adjacent breeding facility, ranged from 5 to 10 cm in length (∼1.5 years old) when used for treatment. In contrast to the Kangal hot spring, where they lack nutrients owing to the high temperatures of the spring, the fish were fed commercially available fish food (TetraMin®, Tetra GmbH, Melle, Germany) daily after the treatment sessions.
Water samples were tested monthly for Legionella species and Pseudomonas aeruginosa. The fish were examined for Aeromonas hydrophila, Aeromonas sobria, Aeromonas caviae, Mycobacterium marinum and Mycobacterium piscium to rule out any risk for potential zoonotic infections. Since the fishes were given only commercially available fish food, the risk of Diphyllobothrium latum (fish tapeworm) infestation could be ruled out based on the absence of an intermediate host.
Each patient was allocated to a single bathing tub for the duration of the 3 week treatment, and no two patients shared the same tub at any time during that period. After the 3 week treatment, each tub along with the technical equipment was disinfected (2% solution Dodarcana® Rapid, Schülke & Mayr, Austria) for 1 h.
Assessment
Clinical Assessment
The primary efficacy outcome measure was the overall total reduction in Psoriasis Area Severity Index (PASI) score and the proportion of patients with 50 and 75% improvement in PASI score (PASI-50 and PASI-75) at week 3, relative to baseline. The PASI is a physician-accessed score, recognized by the US Food and Drug Administration to assess efficacy of psoriasis therapies in clinical trials. The PASI score takes into account the extent of involved skin surface and the severity of erythema, desquamation, and plaque induration. The composite score range from 0 to 72, with higher numbers indicating more severe disease and a reduction in score representing improvement. The PASI-75 is the currently recognized benchmark of end points used in psoriasis clinical trials. PASI-50 is also regarded as a clinically significant end point in the assessment of psoriasis (8). PASI was assessed on high-resolution digital color photographs taken at baseline and at the end of the 3 week treatment period. Baseline measurements were those made closest but prior to the beginning of treatment.
Additionally, response to treatment was defined according to the rate of improvement in PASI score, as follows: complete response, >95% improvement; good or marked, 75–94%; moderate, 50–74%; slight, 25–49%; and none, <25%.
Patient-reported Outcomes
Patient-reported outcomes were evaluated by a short custom questionnaire immediately after the 3 week course of treatment (Table 1) and by a follow-up questionnaire sent to all patients after treatment in March 2005 to assess the duration of remission, the number of different treatment regimens prior to ichthyotherapy, the severity of a possible relapse and the personal satisfaction with ichthyotherapy when compared with previous treatments (Tables 2 and 3). At this point the time elapsed since the end of therapy was from 3 months to almost 3 years.
Table 1.
Answers to the short custom questionnaire immediately after 3 weeks of therapy (n = number of completed questions)
| Extremely | Considerable | A little | Not at all | |
|---|---|---|---|---|
| How much did your skin condition improve after the treatment considering | ||||
| Itching? (n = 63) | 46 (73.0%) | 15 (23.8%) | 2 (3.2%) | 0 |
| Pain? (n = 54) | 48 (88.9%) | 6 (11.1) | 0 | 0 |
| Skin tension? (n = 62) | 51 (82.3%) | 11 (17.7%) | 0 | 0 |
| Induration? (n = 64) | 49 (76.6%) | 14 (21.9%) | 1 (1.6%) | 0 |
| Scaling? (n = 66) | 56 (84.8%) | 9 (13.6%) | 1 (1.5%) | 0 |
| Psychological problems?* (n = 66) | 31 (47.0%) | 18 (27.3%) | 4 (6.1%) | 1 (1.5%) |
| Have you attained your treatment goal? (n = 66) | 44 (66.7%) | 21 (31.8%) | 1 (1.5%) | 0 |
| Yes | Probably yes | Probably no | No | |
| Would you use this kind of therapy again? (n = 66) | 59 (89.4%) | 6 (9.1%) | 1 (1.5%) | 0 |
*Twelve patients (18.2%) stated that they had no psychological problems prior to treatment.
Table 2.
Patient-reported psoriasis treatments used prior to ichthyotherapy as assessed by the mailed follow-up questionnaire
| N | % | |
|---|---|---|
| Cortison | 29 | 72.5 |
| UVA/UVB | 21 | 52.5 |
| Vit. D3 | 19 | 47.5 |
| Sole therapy | 18 | 45 |
| PUVA | 17 | 42.5 |
| Oilbath | 16 | 40 |
| Homeopathy | 12 | 30 |
| Dead Sea | 9 | 22.5 |
| Salicylic acid | 9 | 22.5 |
| Dithranol | 9 | 22.5 |
| Tar preparations | 9 | 22.5 |
| Fumaric acid | 8 | 20 |
| Acupuncture | 7 | 17.5 |
| Balneophototherapy | 6 | 15 |
| Vit. A | 4 | 10 |
| MTX | 1 | 2.5 |
| Cyclosporin | 0 | 0 |
| Other (including new biologicals) | 11 | 27.5 |
Table 3.
Results of the mailed follow-up questionnaire (n = number of completed questions)
| More | Equally | Less | |
|---|---|---|---|
| Compared to other tried treatments this kind of therapy helped me (n = 40) | 35 (87.5%) | 4 (10%) | 1 (2.5%) |
| If a relapse occurred, how severe were the symptoms compared to baseline?* (n = 39) | 3 (7.7%) | 10 (25.6%) | 24 (61.5%) |
*Two patients (5.1%) still in remission at the time the questionnaire were received.
Safety Evaluation
The safety and tolerability of the therapy were evaluated by reviewing adverse events (assessed weekly), vital signs and a weekly physical examination.
Statistical Analyses
The primary end point (efficacy of treatment) was evaluated by comparing the mean PASI score before and after 3 weeks of treatment. The PASI scores were analyzed using a Wilcoxon's signed rank test for paired comparison. P-values ≤ 0.05 were considered statistically significant. Analysis was carried out using Prism 4 for Macintosh (GraphPad Software, Inc., San Diego, CA).
Results
Patients' Characteristics
Sixty-seven patients, 39 male and 28 female, were included in this retrospective study. All 67 patients completed the 3 week treatment. Ages ranged between 10 and 75 years [mean 41.01 years, 95% confidence interval (CI) 37.51, 44.52]. The mean duration of psoriasis at baseline was 13.9 years (range 1–35 years; 95% CI 11.6, 16.21).
Treatment Efficacy
Physician-assessed Outcomes
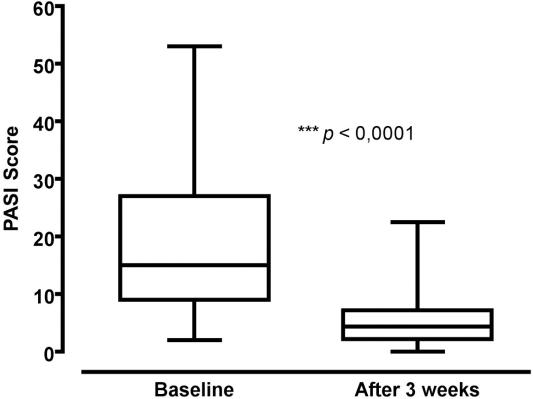
At the end of the 3 week treatment course, 31 of the 67 patients (46.3%) achieved PASI-75 and 30 other patients (44.8%) achieved PASI-50. The mean PASI score at baseline for the entire study cohort was 18.9 ± 12.37 (95% CI 15.89, 21.9). PASI score at the end of the treatment period was 5.34 (95% CI 4.27, 6.42). Overall there was a 71.7% reduction in PASI score compared to baseline (P < 0.0001) (Fig. 3).
Figure 3.
Box plot of PASI scores before and after treatment. Horizontal lines show 75th percentile, median and 25th percentile, whiskers indicate range.
Response to treatment was complete in three patients (4.5%), marked in 29 (43.3%), moderate in 29 (43.3%) and slight in 6 (8.9%). No patient failed to respond at all.
Patient-reported Outcomes
In the short questionnaire given immediately after the 3 weeks of treatment, patients reported substantial satisfaction with the treatment (Table 1).
Of the 67 follow-up questionnaires sent to the patients in March 2005, 64 were deliverable. Forty questionnaires were returned, giving a response rate of 60%. The mean time since the end of treatment was 21.8 (95% CI 18.99, 24.68). The reported mean remission period was 8.58 months (range 1–30 months; 95% CI 6.05, 11.11) with two patients (5.1%) still in remission at the time the questionnaire was received (time elapsed since the end of therapy was 12 and 26 months, respectively). The mean number of previously used other therapies was 5 ± 3 (± SD) (range 0–12; 95% CI 4.02, 5.93) (Table 2). When asked to compare their treatment results with all previously used therapies, 87.5% of patients reported a more favorable outcome with ichthyotherapy. A total of 65.1% stated that after the relapse their symptoms were less severe than compared with baseline (Table 3).
Safety Evaluation, Side effects
No severe side effects were recorded during the treatment period. Mild, transient bleeding from open crusted lesions was reported by one patient with eczema, and UV radiation-related erythema by two others. No clinically significant pattern of changes in vital signs were observed during the study period. Ichthyotherapy in combination with UVA treatment was generally very well tolerated.
Discussion
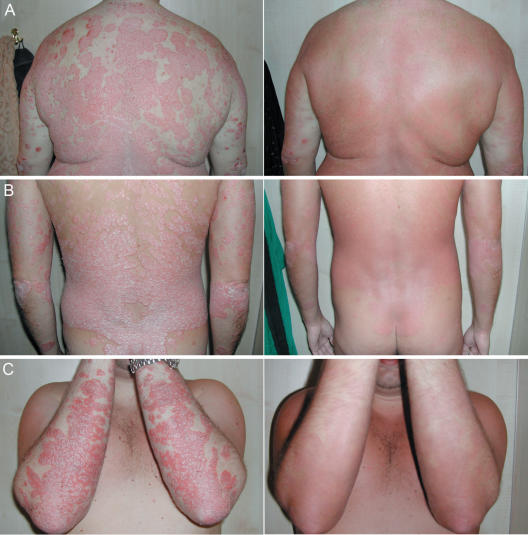
This retrospective study indicates that ichthyotherapy used in combination with short-term UVA radiation is an effective and safe treatment for psoriasis vulgaris. A total of 46.3% of the 67 patients achieved PASI-75 and an additional 44.8% patients achieved at least PASI-50 after a 3 week course of treatment with the ‘Doctorfish of Kangal’ G. rufa (Fig. 4). It is well established that a 75% improvement in PASI score is a clinically meaningful end point for clinical trials, and there is strong evidence demonstrating that a 50% improvement in PASI score is also associated with a significant improvement in the patients quality of life (7). These findings are further supported by the results of the questionnaire completed by the patients immediately after treatment (Table 1).
Figure 4.
Three patients before and after a 3 week combined treatment with ichthyotherapy and UVA radiation.
Follow-up Survey
The results of the follow-up survey indicate that most of the patients were more satisfied with ichthyotherapy than with any other previously tried treatment. This satisfaction might be explained, at least in part, by the rather long-reported mean remission period of 8.6 months. The response rate of the survey is comparable to those of other mail-based surveys (9). However, we are aware that this approach leaves room for potential non-response bias; patients with unfavorable results (i.e. early relapse) may have been less likely than others to respond to our survey.
Combination with UV Radiation
In this study it was also shown that ichthyotherapy combined with UVA radiation was generally well tolerated with only three patients experiencing mild side effects. Two of these three patients showed UVA treatment-related erythema. Whereas a prolonged UVA treatment course, or frequently repeated courses, may have an, as yet, undefined risk with regard to melanoma and aging changes, a recent study suggests that UVB but not UVA radiation initiates melanoma (10). However, we are aware of the risks as identified by the British Photodermatology Group Workshop (11), and therefore suggest to further evaluate ichthyotherapy in combination with broadband UVB and narrowband UVB treatment.
Possible Mechanisms of Action
Several mechanisms have been suggested regarding the observed efficacy of ichthyotherapy in Turkey (6). One obvious mechanism is the physical contact with the fish, which feed on the desquamating skin, thus leading to a rapid reduction of the scales. This phenomenon was also consistently observed in our patients, who reported a pleasing micromassage like feeling while the fish nibbled at their skin. The fish seem to prefer affected to healthy skin, possibly it is easier to nibble at this surface. Another suggested mechanism is the direct effect of natural UV radiation owing to the high altitude (1650 m) of the Kangal spa (6). Phototherapy is a well-recognized option for patients with widespread psoriasis lesions with climatotherapy being the simplest form (2). However, a simultaneous removal of scales by the fish probably facilitates the penetration of UV rays to the dermis. This exposure of the lesions may explain the better outcome of combined ichthyotherapy/UVA treatment when compared with the poor results of UVA sunbed treatment alone (12). A third suggested mechanism of ichthyotherapy in Kangal is the presence of a high level of selenium (1.3 mg l−1) in the hot spring water (6). While Özcelik et al. (6) argue that selenium constitutes an important factor to treatment success in Kangal, an analysis of the water used in our study revealed a selenium concentration of <5 μg l−1. Therefore, selenium is not likely to have contributed to the observed efficacy of ichthyotherapy reported in the present study. A fourth mechanism suggested by Özcelik et al. (6) is the reverse Koebner phenomenon. The reverse Koebner phenomenon is seen when an area of psoriasis clears following injury (13). However, it had been shown that destruction of the dermal papillae within the injured tissue plays the key role in preventing epidermal hyperplasia of psoriasis (14). Therefore, it seems unlikely that the reverse Koebner phenomenon contributes to the observed efficacy of ichthyotherapy. Finally, psychological factors such as stress are regarded a causal or exacerbating factor in psoriasis (1). Thus, the 2 h daily fish bath, which most patients referred to as relaxing and pleasing, might have contributed to the observed treatment effect by reducing patients' stress and enhancing psychological well-being.
Treatment Protocol
There are two important key differences between this study and the study by Özcelik et al. (6) concerning treatment protocol. First, in our study the patients were required to stay in the treatment tubs for only 2 h per day, whereas the mean stay in the pools in Kangal was 7.4 ± 1.1 h a day (6). This shortened treatment time makes ichthyotherapy more acceptable for the patients and considerably improves compliance. Second, at the treatment facility described herein, each patient was allocated a personal bathing tub, and the fish only came into contact with a single patient. Conversely, in the pools of the Kangal hot spring patients are required to take their bath with 10–20 patients simultaneously (M.G., personal observation). This approach might be unacceptable for some patients and a possible hygienic risk cannot be entirely excluded. It is evident that many questions remain unanswered concerning the optimal protocols for ichthyotherapy. In the absence of studies to address these issues, we recommend that the described protocol may be used for guidance.
Conclusion
The present study is limited by the relatively small number of patients treated and by lack of a control group. Randomized studies would be needed to compare the ichthyotherapy treatment with controls (e.g. water with the UV therapy alone) and to assess treatment with standardized health-related quality of life questionnaires.
In summary, the observed benefit, along with the favorable safety profile, suggests that ichthyotherapy combined with a short course UVA treatment could provide a viable treatment option for patients with psoriasis vulgaris. Prospective controlled studies are now warranted to validate the efficacy of this unusual treatment modality.
Acknowledgments
The authors are indebted to R. Sherman for valuable comments on an earlier version of the manuscript.
References
- 1.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebwohl M. A clinician's paradigm in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1 Suppl 1):S59–69. doi: 10.1016/j.jaad.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 4.Olalde Rangel JA, Magarici M, Amendola F, del Castillo O. The systemic theory of living systems. Part IV: systemic medicine—the praxis. Evid Based Complement Alternat Med. 2005;2:429–39. doi: 10.1093/ecam/neh139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warwick D, Warwick J. The doctor fish—a cure for psoriasis? Lancet. 1989;335:1093–4. [Google Scholar]
- 6.Özcelik S, Polat HH, Akyol M, Yalcin AN. Kangal hot spring with fish and psoriasis treatment. J Dermatol. 2000;27:386–90. doi: 10.1111/j.1346-8138.2000.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 7. Accessed at: http://www.fishbase.org/Summary/speciesSummary.php?ID=26483&genusname=Garra&speciesname=rufa (last accessed May 1st 2006)
- 8.Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Score (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50:859–66. doi: 10.1016/j.jaad.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Asch DA, Jedrziewsky MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–36. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 10.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 11.British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatol Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327–30. [PubMed] [Google Scholar]
- 12.Turner RJ, Walshaw D, Diffey BL, Farr PM. A controlled study of ultraviolet A sunbed treatment of psoriasis. Br J Dermatol. 2000;143:957–63. doi: 10.1046/j.1365-2133.2000.03827.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241–8. doi: 10.1046/j.1473-2165.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 14.Grekin DA, Van Scott EJ. Dermal role and control in psoriasis. Arch Dermatol. 1972;105:292–3. [PubMed] [Google Scholar]