Abstract
Herbal remedies are widely used for the treatment and prevention of various diseases and often contain highly active pharmacological compounds. Many medicinal herbs and pharmaceutical drugs are therapeutic at one dose and toxic at another. Toxicity related to traditional medicines is becoming more widely recognized as these remedies become popular in the Mediterranean region as well as worldwide. Most reports concerning the toxic effects of herbal medicines are associated with hepatotoxicity although reports of other toxic effects including kidney, nervous system, blood, cardiovascular and dermatologic effects, mutagenicity and carcinogenicity have also been published in the medical literature. This article presents a systematic review on safety of traditional Arab medicine and the contribution of Arab scholars to toxicology. Use of modern cell biological, biochemical, in vitro and in vivo techniques for the evaluation of medicinal plants safety is also discussed.
Keywords: Arab herbal medicine, complementary and alternative medicine (CAM), in vitro, in vivo, toxicity tests
Introduction
Parallel with recent increasing interest in alternative/herbal medicine for the prevention and treatment of various illnesses, there is increasing concern about the safety of medicinal plants. There are general and herb-specific concerns regarding medicinal plants and their ability to produce toxicity and adverse effects. Accidental herbal toxicity occurs not only as a result of a lack of pharmaceutical quality control in harvesting and preparation, but also because herbal remedies are believed to be harmless. Furthermore, a confusing nomenclature and lack of quality control and the accurate identification of plants are important concerns. There are no governmental regulations on the manufacture, purity, concentration, or labeling claims of herbal remedies and dietary supplements. The concentration, sample preparation, and the long-term stability of active compounds and other chemicals in plants varies by the part of the plant harvested and sold, and the maturity of the plant at the time of harvest. In addition, geography, soil composition and its contaminants, and year-to-year variations in soil acidity, water, weather conditions, and other growth factors have significant effects on the pharmaceutical properties of the medicinal plants. Therefore, the actual dose of active compounds being consumed is often variable, unpredictable or simply unknown. When compared with adults, children may be particularly susceptible to the effects of such dosage variations due to their smaller size and different capacity for detoxifying chemicals.
Early Arabic/Islamic Works on Poisons and Antidotes
Origins of the discussion on poisons and antidotes date back to the Greeks and Indians, as well as to the empiric knowledge of the indigenous population in the Arabic/Islamic world. One of the most important scholarly contributions is The Book on Poisons and Antidotes by the famous Arab alchemist, Abu Musa Jabir ben Hayyan. Based in Kufa, Iraq, he established himself as one of the leading scientists practicing medicine and alchemy in the eighth century. In the six chapters of his book, he identifies poisons by their traits and natural origins, modes of action, dosages, methods of administration and choice of drugs. He also identifies the target organ attacked by each poison, a proposition that is modern in its chemotherapeutic application.
Another example of an independent manual on toxicology is Kitab as-Sumum, written in five volumes by Shanaq, the Indian, and translated into Arabic by al-'Abbas bin Sa'id al-Jawhari in the ninth century. The work discusses how various poisons could be detected by sight, touch, taste or by toxic symptoms developed during the treatment. A similar analysis is found in a later book on toxicology by Ibn Wahshiyyah during the early tenth century. Many of the antidotes described by Arab scientists, like Abu Musa Jabir ben Hayyan and Ibn Wahshiyyah, are still used today by herbalists in our region (Table 1).
Table 1.
Poisons and antidotes used in the traditional Arab medicine
| Poison | Antidotes |
|---|---|
| Lead | Neuseant and then treatment with water extracts from seeds of Ficus carica (wild fig tree), Apium graveolens (wild celery), Anethum graveolens (dill), and then water extracts from Smilax officinalis (Sarsaparilla), Triticum vulgare (common wheat), Hyssopus officinalis (common hyssop) |
| Mercury | Neuseant and then treatment with water extracts of Smilax officinalis (Sarsaparilla) mixed with honey |
| Iron | Rosa canina (dog rose) and Viola odorata (sweet violet), Salix alba (white willow) mixed with small amounts of vinegar |
| Convolvulus scammonia L. | Extracts from Cydonia vulgaris (quince tree), Rheum ribes (current-fruited rhubarb) and Rhus coriaria (sumach) |
| Nerium oleander L. (Oleander) | Vitis vinifera (common grape), phoenix dactylifera (dates) and Ficus carica (wild fig tree) |
Herbal Medicine in the Mediterranean Region
Current Status
The Eastern region of the Mediterranean has been distinguished throughout the generations with a rich inventory of natural medicinal herbs used by local herbalists (1–8). A recent survey conducted by our group found only 31 professional Arab practitioners in Israel, Palestine and the Golan Heights still practicing their careers (9). This number is significantly less in comparison to the number of previous surveys (4,10). Each practitioner has his own methods of preparation, following his parent or teacher's tradition. A limited exchange of information takes place between healers in the same area. Furthermore, the data reveal that there is no systematic instruction of the next generation of healers, and that children of the practitioners have no interest in the subject. The knowledge and practice of traditional healing is a family matter and passed on by inheritance.
Knowledge of the Safety of Medicinal Plants
Based on results obtained from recent studies (4,9,11), the status of the knowledge of the safety and efficacy of medicinal plants used in our region can be summarized as follows: (i) Most practitioners have very limited training and knowledge. Some practitioners have even turned to ‘mystical’ or ‘magical methods of healing’. (ii) Related to this, traditional knowledge is not being passed down from generation to generation, as in the past. (iii) Lack of pharmaceutical quality control in harvesting and preparation procedures for medicinal remedies. Practitioners today buy readymade or partially prepared remedies from ‘Attarah’ shops, where plant materials are sold, rather than collecting the plants directly from nature. (iv) Plants used in certain regions are not used in others. For example, local practitioners from the Negev region of Israel use plant species found in the desert, overlooking plant varieties and knowledge spanning the entire Middle Eastern region.
Safety Concerns of Herbal Remedies
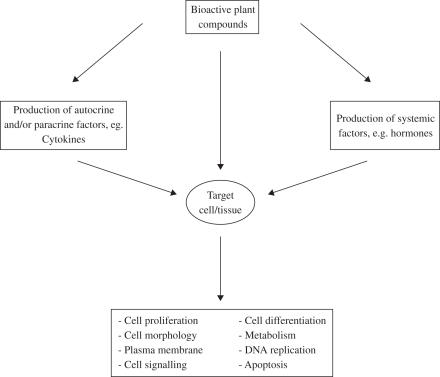
Toxicity of medicinal plants may be related to the mixtures of active compounds that they contain; their interactions with other herbs and drugs, contaminants, adulterants; or their inherent toxicity (Fig. 1). Plants have complex mixtures of terpenes, alkaloids, saponins and other chemicals, increasing the risk of adverse reactions to any one of them or to the additive or synergistic effects of chemical interactions. For example, more than 100 chemicals have been identified in tea tree oil (12).
Figure 1.
Direct and indirect herbal toxicity.
There are general and herb-specific concerns regarding toxicity and adverse effects. A confusing nomenclature and issues of quality control and the accurate identification of plants are important concerns. The common names of plants and herbal remedies can be outdated and variable depending on the geographic region (8,9). There is no governmental regulation on the manufacture, purity, concentration or labeling claims of herbal remedies and dietary supplements. Thus, it is always ‘buyer beware’ in this marketplace.
Dosage of the Active Compounds
The concentration of active ingredients and other chemicals in plants varies by the part of the plant harvested and sold; the maturity of the plant at the time of harvest; the time of year during harvest; geography and soil conditions; soil composition and its contaminants; and year-to-year variations in soil acidity, water, weather conditions and other growth factors. For example, Azaizeh and his co-workers (13) have reported in a recent study that fertilization affects the antioxidant activity of four medicinal plants used in traditional Arab medicine. They found that increasing the amount of fertilizer caused a significant concentration-dependent increase in antioxidant activity of cultivated Teucrium polium compared with the wild-type. In contrast, increasing the amount of fertilizer caused a significant concentration-dependent reduction in the antioxidant activity of powders prepared from cultivated Eryngium creticum when compared with wild plants. Therefore, the actual dose of active ingredients being consumed is often variable, unpredictable or simply unknown (14). Dosage-variation has greater effects on children due to their smaller size and different capacity for detoxifying chemicals (14,15).
Contaminations
Contaminants and adulterants of medicinal plants can be pharmacologically active and responsible for unexpected toxicity. Plants may be harvested from contaminated soils or cleaned improperly such that they may contain illness-producing microorganisms. Ayurvedic medications have been known to cause lead poisoning in children because of their contamination with this heavy metal and others, such as arsenic and mercury (16,17). Contamination of crop and medicinal plant samples by organic chemicals has become a pressing problem in many Arab countries. Low contamination levels were detected in cucumber and tomato in Palestine, Jordan and Egypt. Elevated levels of contamination were detected in vegetables from Pakistan, Egypt and in grapes from Jordan. Several poisonous cases and plant food contamination were reported in Morocco, Egypt, Iraq, Saudi Arabia, Sudan, Syria, Jordan, UAE, Pakistan and Yemen in the past years (18). For example, Selim and his co-workers (19) found that common Egyptian foods such as nuts and seeds, cereal grains were contaminated with aflatoxins. Twenty-nine percent of medicinal plants were found to be contaminated with aflatoxin B1. The highest mean concentration of aflatoxin B1 was in herb and medicinal plants (49 p.p.b.).
Quality Assurance
The increase in popularity has also brought some concerns and fears over professionalism of practitioners, quality, efficacy and safety of the ‘natural’ formulations available on the market. Over the past decade, several newsworthy episodes in developed as well as developing communities indicated adverse effects, sometimes life-threatening, allegedly arising as consequence of taking medicinal plants or traditional medicines from various ethnic groups (20,21). In some cases, adulteration, inappropriate formulation or lack of understanding of plant and drug interactions or uses has led to adverse reactions that are sometimes life-threatening or lethal to patients (11,22–24). Therefore, for safety and quality assurances, chemical analytical techniques should be applied at different stages for good practices in quality assurances of medicinal plants, including good agricultural practice by the farmers, good sourcing and laboratory practices by the pharmaceutical companies, good manufacturing practices and innovative clinical trial practices by researchers and physicians (21,25). We believe that any plant herb or ingredients taken from plants should be tested before being used as a remedy. Therefore, various advanced cell biological, biochemical, molecular biological and in vitro cell culture techniques are applied in our work with different medicinal herbs in order to test their safety before testing their efficacy (Table 2).
Table 2.
In vitro test methods used in order to evaluate the toxic effects of medicinal plants
| Test system | End points measured | Applications | References |
|---|---|---|---|
| MTT | Metabolic activity of a mitochondrial enzyme, succinate dehydrogenase | Cell viability, cell toxicity, cell number | 35,36,41,46–48 |
| LDH | Lactate dehydrogenase activity | Cell viability, toxicity | 35,36,41,49–51 |
| Cell counting | Cell number | Cell proliferation, cytostatic effects | 35,36 |
| Trypan blue | Trypan blue diffusion into the cells | Cell viability | 36,52 |
| DNA synthesis | 3H-thymidine incorporation or bromodeoxyuridine (BrdU) | Cell proliferation, cytostatic effects | 35,36,53,54 |
| Neutral red uptake | Dye uptake | Cell proliferation, cytostatic effects | 36,43,55 |
| Cell morphology | Morphological appearance of cells and tissues | Cytotoxicity, cell proliferation, cell differentiation | 36,56–58 |
| Protein synthesis | 35S-methionine and 3H-prolline; ELISA | Cell function | 36,39,40,59 |
Hepatotoxicity
Most reports of toxic effects due to the use of herbal medicines and dietary supplements are associated with hepatotoxicity (HT) although reports of other toxic effects including kidney, nervous system, blood, cardiovascular and dermatologic effects, mutagenicity and carcinogenicity have also been published in medical literature. Hepatic impairment resulting from the use of conventional drugs is widely acknowledged, but there is less awareness of the potential HT of herbal preparations and other botanicals, many of which are believed to be harmless and are commonly used for self-medication without supervision. Although regulation by the Food and Drug Administration may be part of the solution, increasing public awareness and education programs for healthcare professionals about the potential dangers of herbal preparations will need to be implemented (26,27). The reported toxicity of herbal formulations may be the result of several factors, including the contamination with pesticides, microbes, heavy metals, toxins or adulteration with orthodox drugs (18,24). On the basis of various case reports, the liver injury from herbal remedies has ranged from mild elevations of liver enzymes to fulminated liver failure requiring liver transplantation (14). For example, veno-occlusive disease may be caused by pyrrolizidine alkaloids, such as Senecio species, Heliotropium species and Comfrey (Symphytum officinale). Chapparal (Larrea divericata) leaf ingestion can lead to the development of either fulminant hepatic failure or cirrhosis. Kava (Piper methysticum) has been identified as causing an acute hepatitis. Many traditional Chinese herbal preparations have also been described to cause HT and rarely liver failure (14).
Teratogenic Effects
The effects of contaminations and dosage variations are higher in children than in adults (14). The teratogenic effects of herbs are not known in many cases. It is possible that herbal chemicals may be transported through the placenta to cause toxic effects on the sensitive growing fetus. For example, Roulet et al. (15) reported the case of a newborn whose mother drank senecionine-containing herbal tea daily for the duration of her pregnancy. The infant was born with hepatic vaso-occlusive disease and died; senecionine is one of the pyrrolizidine alkaloids associated with hepatic venous injury.
Safety Evaluation
Tissue Culture Systems
In order to determine the effects of plant extracts, cell culture model systems are used. In most of these culture systems, vertebrate cells cultured in vitro have been grown in monolayer on artificial substrate. However, it has long been realized that while growth in two dimensions is a convenient way of preparing and observing a culture and allows a high rate of cell proliferation, it lacks the cell–cell and cell–substrate interaction characteristic of whole tissue occurs in vivo. The interactions of a cell with substratum play an important role in the development, differentiation and regeneration of multicellular organisms. In cultured rat hepatocytes, morphology, basal functions such as growth (28,29) or protein synthesis and secretion (30) are affected by cell–substratum interactions (29). For toxicity testing in vitro and for studying mechanisms involved in the response of liver cells to xenobiotics, the maintenance, expression and regulation of P-450 isoenzymes is of primary interest (31–33).
Three Dimensional Tissue Cultures
Currently, the available conventional tissue culture polystyrene (TCPS) are not suitable to form tissue-like aggregates in vitro that require high cell density. In contrast to the in vivo situation, cells cultured on these flat TCPS built a monolayer of cells with flat cell morphology. In addition, all cell types, except those grown in suspension, are in contact with an environment with some type of topography. The substrate that shows this topography may be made of other cells or of extracellular material. Therefore, the engineering of a new generation of substrates that enables cultured cells to grow at a higher cell density and to maintain more in vivo like cell-to-cell interactions is very important in order to obtain more reliable results in vitro. Therefore, in our cell culture laboratories, we apply a newly developed cell carrier called DegraPol. DegraPol represents a versatile biodegradable class of multiblock copolymeric elastomers. This polymer has been developed for use as scaffolds for tissue engineering as well as for implantable medical devices (34). Using cell culture techniques, the newly developed, biodegradable, elastic and open porous (pore size 200–400 μm) polyesterurethane foams (DegraPol-foam) were found to be compatible for various cell types. Macrophages seeded on the DegraPol-foam were not activated and no cytotoxic effects were detected in these cells. Osteoblasts and fibroblasts seeded on the DegraPol-foam showed high cell adhesion and preserved their phenotype (35–37).
In Vitro Evaluations
For toxicological studies, some of the common standardized test methods are used in our laboratories. These methods are based on the extraction of the active compounds. The extracts are applied to the cells in different dilutions. After exposition of the cells to the extracts, cytotoxicity is assessed by various methods, including microscopic evaluation of cell morphology, the methyltetrazolium assay (MTT test), measurement of DNA and protein synthesis, lactate dehydrogenase activity (LDH), neutral red uptake, and apoptosis tests (36–39). The MTT and LDH assays are well-established methods used to assess mitochondrial competence and cell membrane integrity, respectively (40). The MTT is widely used to assess the viability and/or the metabolic state of the cells (40). In the LDH assay the leakage of the cytoplasm located enzyme LDH into the extracellular medium is measured. The presence of the exclusively cytosolic enzyme, LDH, in the cell culture medium is indicative of cell membrane damage (40,60). Using MTT and the LDH assays, eight plant species used in traditional Arabic medicine were tested to evaluate their cell integrity and cytotoxicity using two different types of cultured cells: rat pheochromocytoma PC12 cells and human hepatoblastoma HepG2 cells (41).
The results have indicated that the six aqueous plant extracts (Asphodelus microcarpus, E. creticum, Mercurialis annua, Rhamnus alaternus, T. polium, Urtica pilulifera) did not suppress mitochondrial respiration or increase LDH leakage in PC12 and HepG2 cultured cells with the exception of those prepared from Ecballium elaterium and Pistacia lentiscus and only at the higher concentrations, namely 500 and 1000 μg ml−1. In a recent study (42) the biosafety of three plant extracts were evaluated in vitro using co-cultures of cells from the human hepatocyte cell line (HepG2) and the human monocyte cell line (THP1). The hepatocyte monoculture was taken as the control. The monocultures and co-cultures were maintained under well-controlled in vitro cell culture conditions. Cells were treated with various concentrations of extracts from Pistacia palastina, Juglans regia and Quercus ithaburensis. All three plant extracts exhibited biosafe properties in the hepatocyte monoculture. In contrast, Pistacia palestina extract significantly reduced cell viability in co-cultures as measured with the MTT test and the LDH assay. It seems that the observed reduction in cell viability in co-culture is a result of monocyte-derived factors (Fig. 1).
Integration of Tradition with Modern Technology
Complementary and alternative medicine (CAM) has gained enormous popularity in our region and worldwide over the past 20 years (43–45,61). This increase in popularity has also brought some concerns and fears over professionalism of practitioners, quality, efficacy and safety of the ‘natural’ formulations available on the market. In some cases, adulteration, inappropriate formulation, or lack of understanding of plant and drug interactions or uses has led to adverse reactions that are life-threatening or lethal to patients.
Most reports of toxic effects due to the use of herbal medicines and dietary supplements are associated with HT. Hepatic impairment resulting from the use of conventional drugs is widely acknowledged, but there is less awareness of the potential HT of herbal preparations and other botanicals, many of which are believed to be harmless and are commonly used for self-medication without supervision. The reported toxicity of herbal formulations may be the result of several factors, including the contamination with pesticides, microbes, heavy metals, toxins or adulteration with orthodox drugs. Therefore, for safety and quality assurances, chemical analytical techniques should be applied at different stages for good practices in quality assurances of natural or herbal remedies, including good agricultural practice by the farmers, good sourcing and laboratory practices by the pharmaceutical companies, good manufacturing practices and innovative clinical trial practices by researchers and physicians.
We believe that any plant herb or ingredients taken from plants should be tested before being used as a remedy. Hence, various advanced cell biological, biochemical, molecular biological and in vitro cell culture techniques should be applied with different medicinal herbs in order to test their safety before testing their efficacy. In vitro cell culture methods have the advantage of relatively well-controlled variables and are generally accepted as a very effective method for safety testing. Advantages of such systems over classical methods (such as long-term studies on experimental animals) include decreased costs, a reduced time to completion and reduced numbers of animals necessary to complete the study. Because of the complicating secondary effects encountered in vivo, it is often difficult to evaluate the mechanism of action of an active compound of medicinal plant in a specific cell type or tissue. The fact that cells and tissues in vivo do not exist in isolation, but communicate with and are interdependent on neighboring tissue makes it essential in the research of herbal remedies and their active compounds in order to simulate the in vivo situation, where, for example, the microenvironment of the hepatocytes within the liver acinus involves gradients in oxygen tension, hormones, extracellular matrix components, non-parenchymal cells and effective exposure levels of xenobiotics from the periportal to the pericentral compartment. Hence, to simulate the in vivo situation a higher degree of complexity for in vitro methodology is required, such as the construct of co-cultures.
Future efforts in the development of biosafe and active medicinal plant compounds will probably be directed towards the application of modern techniques of cell and molecular biology to the field of medicinal plant research, such as the determination of gene activity at both the transcriptional and translational level is important in the context of mutagenesis and carcinogenesis. Furthermore, sample preparation is the crucial first step in the analysis of herbs. Currently, however, quality-related problems seem to be overshadowing the potential genuine health benefits of various medicinal plants. Thus, the development of ‘modern’ sample preparation techniques with significant advantages over conventional methods for the extraction and analysis of herbal remedies is likely to play an important role in the overall effort of ensuring and providing high-quality medicinal plants to consumers worldwide. In addition, advances in biotechnology, particularly methods for culturing plant cells and tissues, should provide new means for the commercial processing of even rare plants and the chemicals they produce.
Table 3.
LD50 values of traditional medicinal plants
| Plant species | Plant parts tested | LD50 (g kg−1 weight) |
|---|---|---|
| Alchemilla vulgaris L. (Rosaceae) | Leaves | 17.3 |
| Atriplex halimus L (Chenopodiaceae) | Leaves | 21.5 |
| Cichorium pumilum Jacq. (Asteraceae) | Leaves | 23.6 |
| Crataegus azarolus L. (Rosaceae) | Leaves | 23.4 |
| Eruca sativa Miller (Brassicaceae) | Leaves | 21.6 |
| Eryngium creticum Lam. (Apiaceae) | Leaves | 20.7 |
| Ferula hermonis Boiss (Apiaceae) | Roots | 8.8 |
| Hypericum triquetrifolium Turra | Leaves | 14.7 |
| Inula viscosa L. Ait. Inula (Asteraceae) | Leaves | 11.9 |
| Juglans regia L. (Juglandaceae) | Leaves | 16.9 |
| Mentha longofolia L. (Lamiaceae) | Leaves | 14.8 |
| Nigella sativa L. (Ranunculaceae) | Seeds | 19.8 |
| Olea europaea L. (Oleaceae) | Leaves | 19.3 |
| Portulaca oleracea L. (Portulacaceae) | Above ground parts | 23.8 |
| Saponaria officinalis L. (Caryophyllaceae) | Roots | 5.1 |
| Silene aegyptiaca L. L.f. (Caryophyllaceae) | Above ground parts | 25.2 |
| Urtica dioica L. (Urticaceae) | Leaves | 22.1 |
| Ziziphus spina-christi L. Desf. (Rhamnaceae) | Leaves | 22.2 |
Water extracts prepared from dried plant material were used. Values presented are means of 30–35 rats tested.
Acknowledgments
The authors would like to thank Ms Arisha Ashraf from the Galilee Society, Shefa-Amr, Israel for her constructive comments.
References
- 1.Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid Based Complement Alternat Med. 2005;2:475–9. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shams Aldeen A. Altadawy Fi Alashaab Kademan wa Hadethan (Healing with Herbs in the Past and Present) Beirut, Lebanon: Dar AlKutum Alelmeah; 1991. (in Arabic) [Google Scholar]
- 3.Munke L. AlTadawy Be Alashab Fi Masr Alkademeh (Healing with Herbs in Old Egypt) Cairo, Egypt: Maktabat Madbouly; 1993. (in Arabic) [Google Scholar]
- 4.Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73:221–32. doi: 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 5.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharmacol. 2000;72:191–205. doi: 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 6.Palevitch D, Yaniv Z. Medicinal Plants of the Holy Land. Tel Aviv, Israel: Modan Publishing House; 2000. [Google Scholar]
- 7.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J Ethnopharmacol. 2002;82:131–45. doi: 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 8.Said O, Khalil K, Fulder S, Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ethnopharmacol. 2002;83:251–65. doi: 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 9.Azaizeh H, Fulder S, Khalil K, Said O. Ethnobotanical survey of local practitioners of the Middle Eastern region: the status of traditional Arabic medicine. Fitoterapia. 2003;74:98–108. doi: 10.1016/s0367-326x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 10.Dafni A, Yaniv Z, Palevitch D. Ethnobotanical survey of medicinal plants in northern Israel. J Ethnopharmacol. 1984;10:295–310. doi: 10.1016/0378-8741(84)90017-5. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;89:193–7. doi: 10.1016/s0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 12.Carson CF, Riley TV. Toxicity of the essential oil of Melaleuca alternifolia or tea tree oil. J Toxicol Clin Toxicol. 1995;33:193–4. doi: 10.3109/15563659509000474. [DOI] [PubMed] [Google Scholar]
- 13.Azaizeh H, Ljubuncic P, Portnaya L, Said O, Cogan U, Bomzon A. Fertilization-induced changes in growth parameters and antioxidant activity of medicinal plants used in traditional Arab medicine. Evid Based Complement Alternat Med. 2005;2:549–56. doi: 10.1093/ecam/neh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf AD. Herbal remedies and children: Do they work? Are they harmful? Pediatrics. 2003;112:240–6. [PubMed] [Google Scholar]
- 15.Roulet M, Laurini R, Rivier L, Calame A. Hepatic veno-occlusive disease in newborn infant of a woman drinking herbal tea. J Pediatr. 1988;112:433–6. doi: 10.1016/s0022-3476(88)80330-5. [DOI] [PubMed] [Google Scholar]
- 16.Moore C, Adler R. Herbal vitamins: lead toxicity and developmental delay. Pediatrics. 2000;106:600–2. doi: 10.1542/peds.106.3.600. [DOI] [PubMed] [Google Scholar]
- 17.Kew J, Morris C, Aihie A, Fysh R, Jones S, Brooks D. Arsenic and mercury intoxication due to Indian ethnic remedies. Br Med J. 1993;306:506–7. doi: 10.1136/bmj.306.6876.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Nahhal Y. Contamination and safety status of plant and food in Arab countries. J Appl Sci. 2004;4:411–7. [Google Scholar]
- 19.Selim MI, Popendorf W, Ibrahim SM, El Sharkawy S, El Kashory SE. Aflatoxin B1 in common Egyptian foods. J Assoc Off Anal Chem. 1996;79:1124–9. [PubMed] [Google Scholar]
- 20.Elvin-Lewis M. Should we be concerned about herbal remedies? J Ethnopharmacol. 2001;75:141–64. doi: 10.1016/s0378-8741(00)00394-9. [DOI] [PubMed] [Google Scholar]
- 21.Chan K. Some aspects of toxic contaminants in herbal remedies. A review. Chemosphere. 2003;52:1361–71. doi: 10.1016/S0045-6535(03)00471-5. [DOI] [PubMed] [Google Scholar]
- 22.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–8. doi: 10.1016/s0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 23.Ernst E. Herbal medications for common ailments in the elderly. Drugs Aging. 1999;15:423–8. doi: 10.2165/00002512-199915060-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–8. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 25.Rousseaux CG, Schachter H. Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Res. 2003;68:505–10. doi: 10.1002/bdrb.10053. [DOI] [PubMed] [Google Scholar]
- 26.Chitturi S, Farrell GC. Herbal hepatotoxicity: an expanding but poorly defined problem. J Gastroenterol Hepatol. 2000;15:1093–9. doi: 10.1046/j.1440-1746.2000.02349.x. [DOI] [PubMed] [Google Scholar]
- 27.Pak E, Esrason KT, Wu VH. Hepatotoxicity of herbal remedies: an emerging dilemma. Prog Transplant. 2004;14:91–6. doi: 10.1177/152692480401400203. [DOI] [PubMed] [Google Scholar]
- 28.Enat R, Jefferson DM, Ruiz-Opazo N, Gatmaitan Z, Leinwand LA, Reid LM. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci USA. 1984;81:1411–5. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawada N, Tomomura A, Satter CA, Satter GL, Kleinman HK, Pitot HC. Effects of extracellular matrix components on the growth and differentiation of cultured rat hepatocytes. In Vitro Cell Dev Biol. 1987;23:267–73. doi: 10.1007/BF02623709. [DOI] [PubMed] [Google Scholar]
- 30.Sudhakaran PR, Stamatoglou SC, Hughes RC. Modulation of protein synthesis and secretion by substratum in primary cultures of rat hepatocytes. Exp Cell Res. 1986;167:505–16. doi: 10.1016/0014-4827(86)90190-4. [DOI] [PubMed] [Google Scholar]
- 31.Schulz V, Hansel R, Tyler VE. Berlin: Springer-Verlag; 1998. Rational phytotherapy: a physician's guide to herbal medicine. [Google Scholar]
- 32.Saad B, Schawalder HP, Maier P. Crude liver membrane fractions maintain liver specific functions in long term, serum free rat hepatocyte cultures. In Vitro Cell Dev Biol. 1993;29:32–40. doi: 10.1007/BF02634369. [DOI] [PubMed] [Google Scholar]
- 33.Saad B, Scholl FA, Thomas H, Schawalder HP, Streit V, Waechter F, et al. Crude liver membrane fractions and extracellular matrix components as substrata regulate differentially the preservation and inducibility of P-450 isoenzymes in cultured rat hepatocytes. Eur J Biochem. 1993;213:805–14. doi: 10.1111/j.1432-1033.1993.tb17823.x. [DOI] [PubMed] [Google Scholar]
- 34.Saad B, Matter S, Ciardelli G, Uhlschmid GK, Welti M, Neuenschwander P, et al. Interactions of osteoblasts and macrophages with biodegradable, and highly porous polyesterurethane foam and its degradation products. J Biomed Mater Res. 1996;32:355–66. doi: 10.1002/(SICI)1097-4636(199611)32:3<355::AID-JBM8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.Saad B, Kuboki Y, Welti M, Uhlschmid GK, Neuenschwander P, Suter UW. DegraPol-foam: a degradable and highly porous polyesterurethane foam as a new substrate for bone formation. Artif Organs. 2001;24:939–45. doi: 10.1046/j.1525-1594.2000.06664.x. [DOI] [PubMed] [Google Scholar]
- 36.Saad B, Abu-Hijleh G, Suter UW. Cell culture techniques for assessing tissue compatibility of biomaterials. In: Arshady R, editor. Polymers in Medicine and Biotechnology. Polymer Chemistry and Biodegradation. 2003. pp. 263–99. [Google Scholar]
- 37.Saad B, Abu-Hijleh G, Neuenschwander P, Suter UW. DegraPol-foam: a new biodegradable material for tissue engineering: in vitro evaluations of the cell compatibility. Emirates Med J. 2004;22:127–34. [Google Scholar]
- 38.Saad B. In vitro evaluation of tissue compatibility of biomaterials. Euro-Asian J Appl Sci. 2005;3:33–52. [Google Scholar]
- 39.Saad B, Dakuar S, Aziazeh H, Abu-Hijleh G. Development of New 3D Test System for the Evaluation of Biosafety and Effects of Medicinal Plants. 3rd International Symposium on natural Drugs. Naples: Italy; 2003. [Google Scholar]
- 40.Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. New York, USA: John Wiley & Sons Inc.; 2000. [Google Scholar]
- 41.Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, et al. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;99:43–7. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 42.Saad B, Dakwar S, Said O, Abu Hijleh G, Albattah F, Kmeel AS, et al. Evaluation of medicinal plants hepatotoxicity using co-cultures of hepatocytes and monocytes. Evid Based Complement Alternat Med. 2006;3:93–8. doi: 10.1093/ecam/nel002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Liu Y, Meng Y, Yang M, He K. Structure of polysaccharide from Polygonatum cyrtonema Hua and the antiherpetic activity of its hydrolyzed fragments. Antiviral Res. 2004;63:183–9. doi: 10.1016/j.antiviral.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Cooper EL. Drug discovery, CAM and natural products. Evid Based Complement Alternat Med. 2004;1:215–7. doi: 10.1093/ecam/neh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper EL. Complementary and alternative medicine, when rigorous, can be science. Evid Based Complement Alternat Med. 2004;1:1–4. doi: 10.1093/ecam/neh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, et al. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003;84:131–8. doi: 10.1016/s0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 47.Kan SF, Huang WJ, Lin LC, Wang PS. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int J Cancer. 2004;10:641–51. doi: 10.1002/ijc.20138. [DOI] [PubMed] [Google Scholar]
- 48.Thanh PN, Jin W, Song G, Bae K, Kang SS. Cytotoxic coumarins from the root of Angelica dahurica. Arch Pharm Res. 2004;27:1211–5. doi: 10.1007/BF02975883. [DOI] [PubMed] [Google Scholar]
- 49.Al-Ghaithi F, El-Ridi MR, Adeghate E, Amiri MH. Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol Cell Biochem. 2004;261:143–9. doi: 10.1023/b:mcbi.0000028749.63101.cc. [DOI] [PubMed] [Google Scholar]
- 50.Benedi J, Arroyo R, Romero C, Martin-Aragon S, Villar AM. Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci. 2004;23:1263–76. doi: 10.1016/j.lfs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 51.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Siva Sundara Kumar D, Cheung HY, Lau CS, Chen F, Hyde KD. In vitro studies of endophytic fungi from Tripterygium wilfordii with anti-proliferative activity on human peripheral blood mononuclear cells. J Ethnopharmacol. 2004;94:295–300. doi: 10.1016/j.jep.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Leem K, Park SY, Lee DH, Boo YM, Cho KH, Lim J, et al. Effects of Jaoga-Yukmiwon(R), a Korean herbal medicine, on chondrocyte proliferation and longitudinal bone growth in adolescent male rats. Phytother Res. 2003;17:1113–6. doi: 10.1002/ptr.1321. [DOI] [PubMed] [Google Scholar]
- 54.Son YO, Lee KY, Lee JC, Jang HS, Kim JG, Jeon YM, et al. Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua. Stokes on normal versus transformed hepatic cell lines. Toxicol Lett. 2005;155:15–25. doi: 10.1016/j.toxlet.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Liu JJ, Huang RW, Lin DJ, Peng J, Wu XY, Pan XL, et al. Anti-proliferative effects of oridonin on SPC-A-1 cells and its mechanism of action. J Int Med Res. 2004;32:617–25. doi: 10.1177/147323000403200606. [DOI] [PubMed] [Google Scholar]
- 56.Aslam MN, Fligiel H, Lateef H, Fisher GJ, Ginsburg I, Varani J. PADMA 28: a multi-component herbal preparation with retinoid-like dermal activity but without epidermal effects. J Invest Dermatol. 2005;124:524–9. doi: 10.1111/j.0022-202X.2004.23596.x. [DOI] [PubMed] [Google Scholar]
- 57.Bei W, Peng W, Ma Y, Xu A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005;76:1975–88. doi: 10.1016/j.lfs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Mijatovic S, Maksimovic-Ivanic D, Radovic J, Miljkovic DJ, Harhaji LJ, Vuckovic O, et al. Anti-glioma action of aloe emodin: the role of ERK inhibition. Cell Mol Life Sci. 2005;62:589–98. doi: 10.1007/s00018-005-4425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saad B, Frei K, Scholl F, Fontana A, Maier P. Hepatocyte-derived IL-6 and TNF-a mediate the LPS-induced acute phase response and NO-release by cultured rat hepatocytes. Eur J Biochem. 1995;229:349–5. doi: 10.1111/j.1432-1033.1995.0349k.x. [DOI] [PubMed] [Google Scholar]
- 60.Reen RK, Karan M, Singh K, Karan V, Johri RK, Singh J. Screening of various Swertia species extracts in primary monolayer cultures of rat hepatocytes against carbon tetrachloride- and paracetamol-induced toxicity. J Ethnopharmacol. 2001;75:239–47. doi: 10.1016/s0378-8741(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 61.Azaizeh H, Saad B, Khaleel K, Said O. The state of the art of traditional Arab herbal medicine in the Eastern Region of the Mediterranean: a review. Evid Based Complement Alternat Med. 2006;3:229–35. doi: 10.1093/ecam/nel034. [DOI] [PMC free article] [PubMed] [Google Scholar]