Abstract
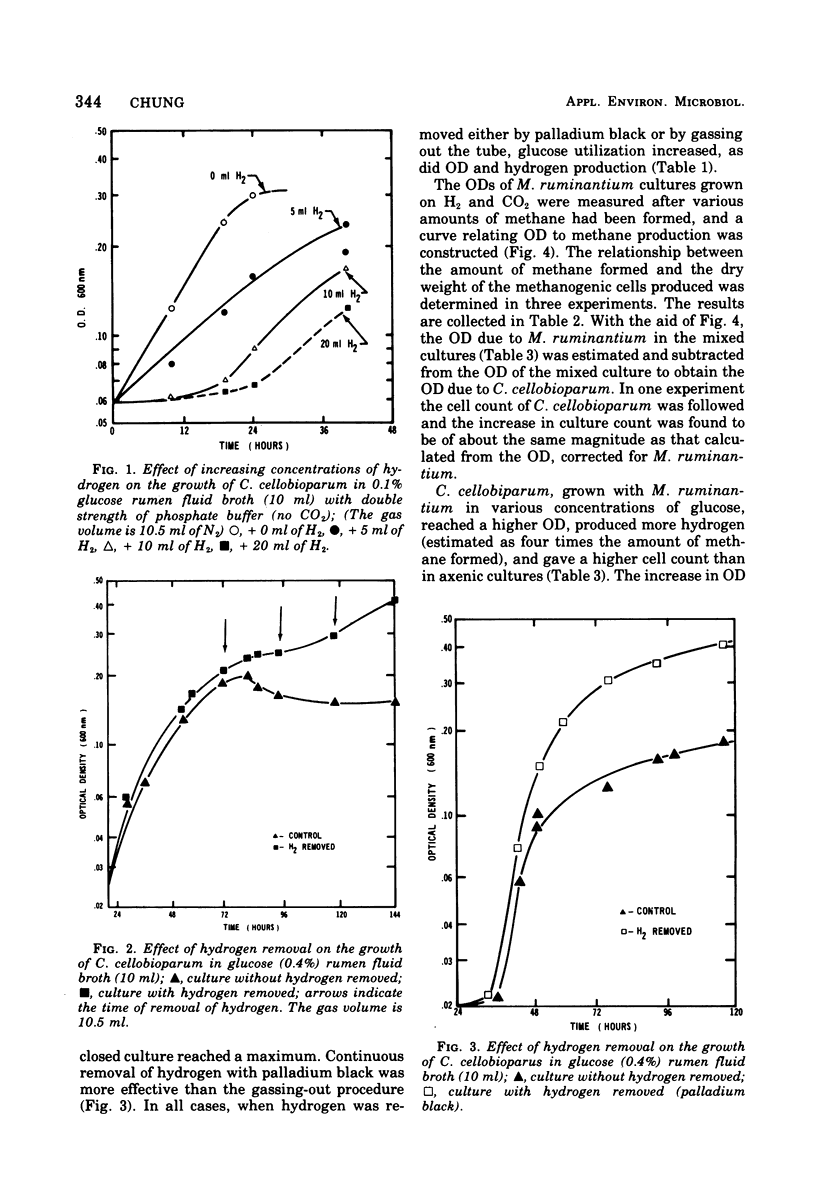
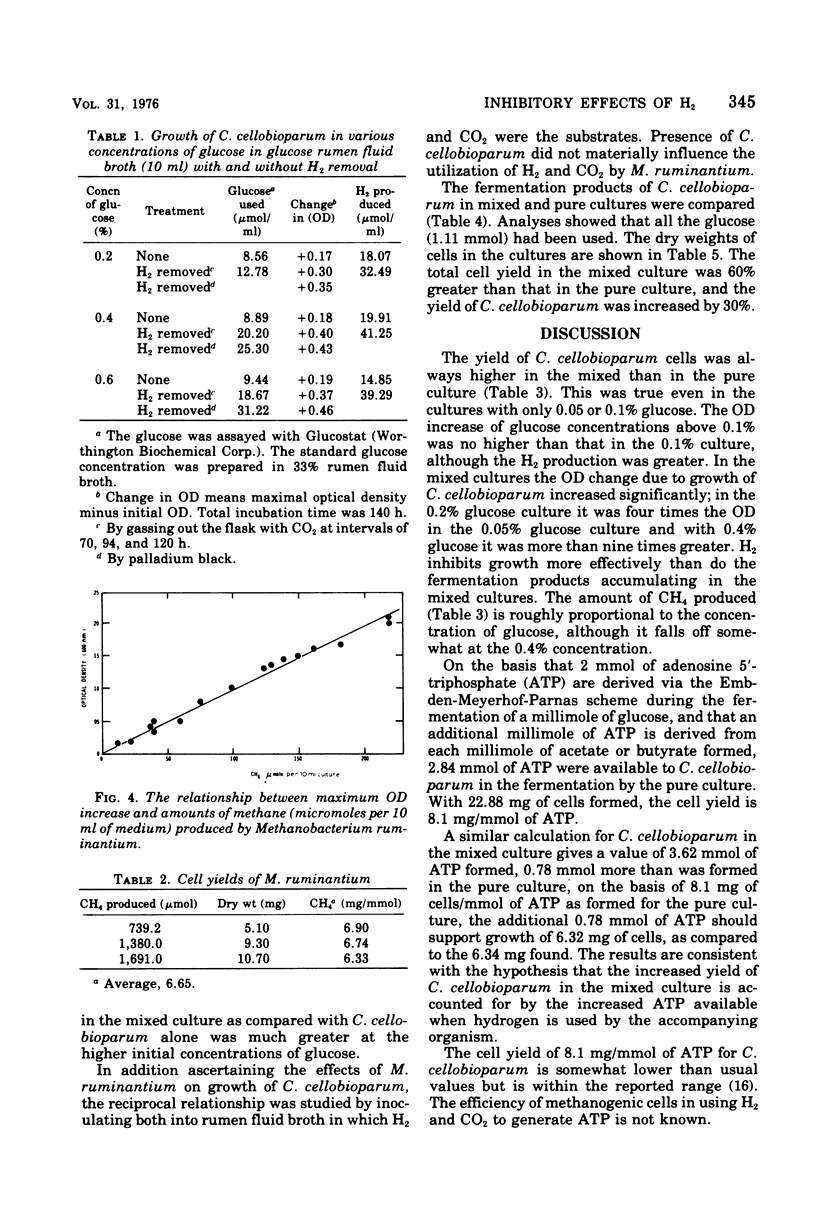
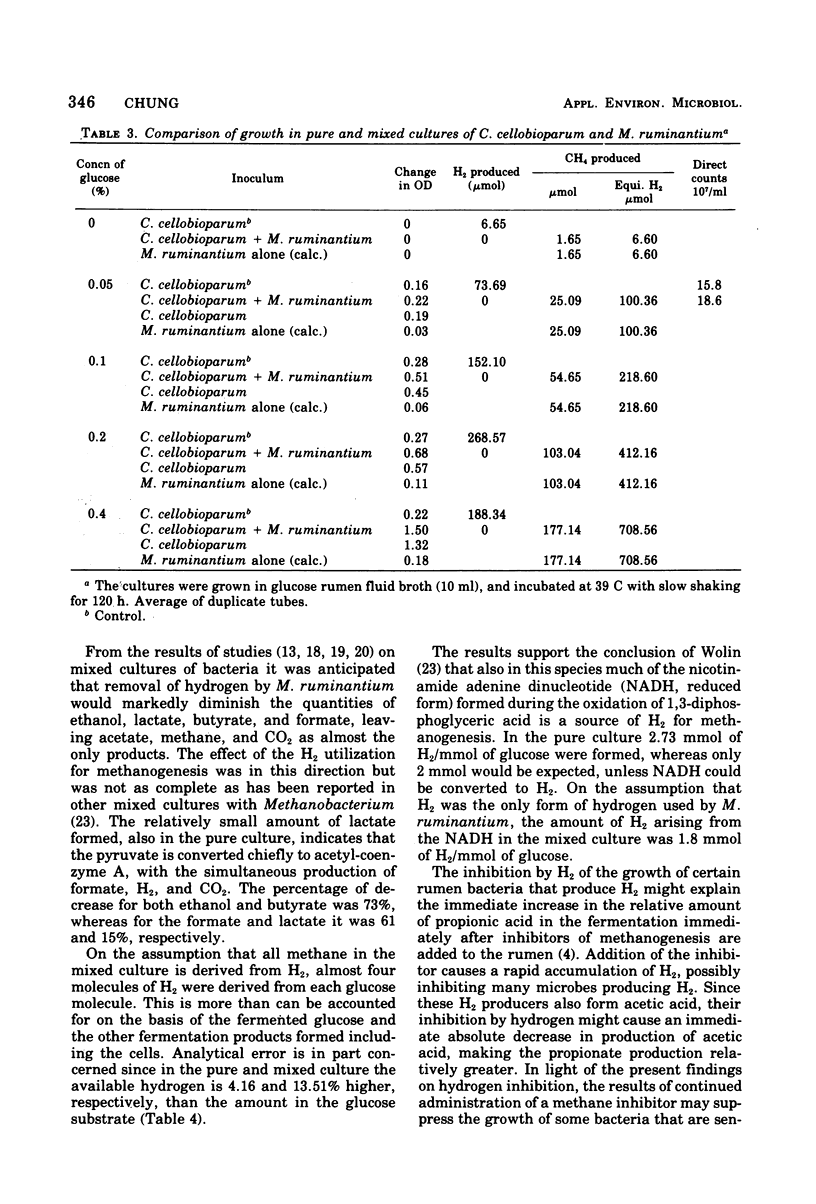
Hydrogen inhibits the growth of hydrogen-producing Clostridium cellobioparum, but not of Escherichia coli or Bacteroides ruminicola. The inhibition is reversible. When hydrogen was removed either by palladium black or by gassing out the tube, glucose utilization increased as did optical density and hydrogen production of C. cellobioparum. Removal of the H2 by methanogenic bacteria favors the growth of C. cellobioparum. Grown with Methanobacterium ruminantium in various concentrations of glucose, the Clostridium reaches a higher optical density and produces more H2 and a higher viable cell count. The cell yield is also higher than in pure culture. In mixed culture, C. cellobioparum produces more acetic acid and less lactic acid, ethanol, and butyric acid than in pure culture. The significance of this metabolic shift and hydrogen utilization in methanogenesis is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYERS W. A. Phosphorolysis and synthesis of cellobiose by cell extracts from Ruminococcus flavefaciens. J Biol Chem. 1959 Nov;234:2819–2822. [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer D. I., Henderickx H. K. The effect of C18 unsaturated fatty acids of methane production in vitro by mixed rumen bacteria. Biochim Biophys Acta. 1967 Jun 6;137(3):484–497. doi: 10.1016/0005-2760(67)90130-0. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Reichl J., Prins R. Parameters of rumen fermentation in a continuously fed sheep: evidence of a microbial rumination pool. Appl Microbiol. 1971 Dec;22(6):1104–1113. doi: 10.1128/am.22.6.1104-1113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Smith W., Bauchop T., Yu I., Rabinowitz J. C. Formate as an intermediate in the bovine rumen fermentation. J Bacteriol. 1970 May;102(2):389–397. doi: 10.1128/jb.102.2.389-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: I. The Culture and Physiology of an Anaerobic Cellulose-digesting Bacterium. J Bacteriol. 1944 Nov;48(5):499–513. doi: 10.1128/jb.48.5.499-513.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGHERITA S. S., HUNGATE R. E. SEROLOGICAL ANALYSIS OF BUTYRIVIBRIO FROM THE BOVINE RUMEN. J Bacteriol. 1963 Oct;86:855–860. doi: 10.1128/jb.86.4.855-860.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki K., Hungate R. E., Lotter L., Hofmann R. R., Maloiy G. Stomach fermentation in East African Colobus monkeys in their natural state. Appl Microbiol. 1974 Apr;27(4):713–723. doi: 10.1128/am.27.4.713-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS R. A. ACTION OF CHLORAL HYDRATE ON RUMEN MICROORGANISMS IN VITRO. J Dairy Sci. 1965 Jul;48:991–993. doi: 10.3168/jds.s0022-0302(65)88376-x. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Linehan B., Wolin M. J. H2 production by Selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):480–483. doi: 10.1128/am.29.4.480-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Wolin M. J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973 Nov;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Metabolic interactions among intestinal microorganisms. Am J Clin Nutr. 1974 Nov;27(11):1320–1328. doi: 10.1093/ajcn/27.11.1320. [DOI] [PubMed] [Google Scholar]


