Abstract
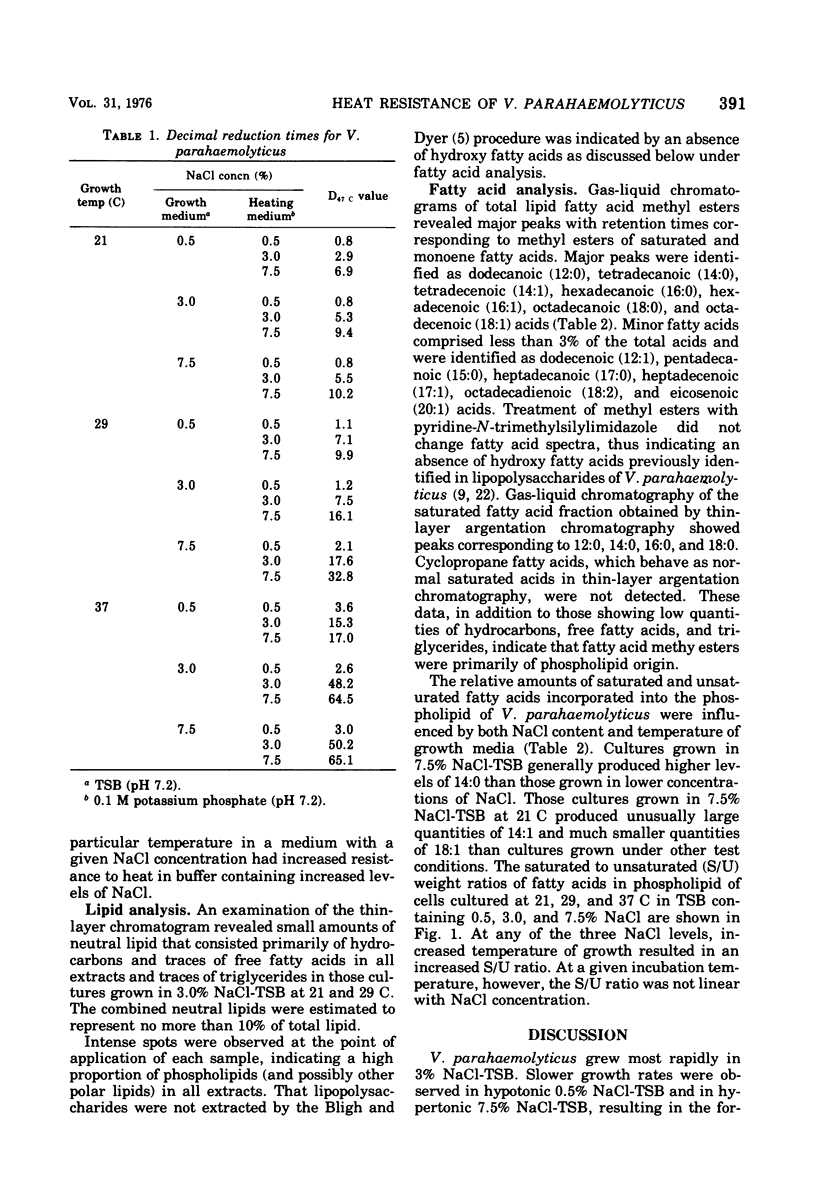
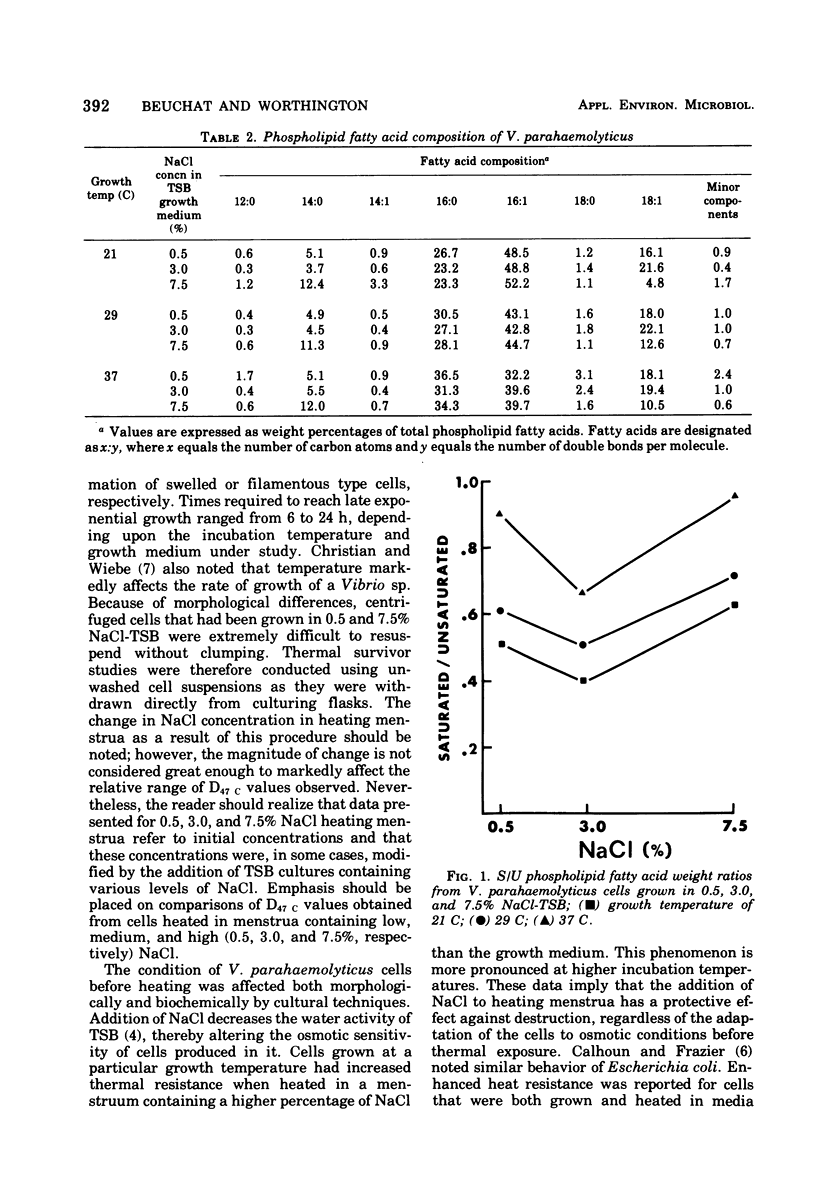
Vibrio parahaemolyticus was grown in tryptic soy broth (TSB) containing NaCl levels of 0.5, 3.0, and 7.5% (wt/vol). Cultures incubated at 21, 29, and 37 C were harvested in late exponential phases and thermal death times at 47 C (D47 c; time at 47 C required to reduce the viable population by 90%) were determined in phosphate buffer containing 0.5, 3.0, and 7.5% NaCl. At a given NaCl concentration in the growth medium, D47 c values increased with elevated incubation temperatures and with elevated levels of NaCl in the heating menstrua. Differences in thermal resistance of cells cultured at a particular temperature were greater between those grown in TSB containing 0.5 and 3.0% NaCl than between those grown in TSB containing 3.0 and 7.5% NaCl. D47c values ranged from 0.8 min (grown at 21 C in TSB with 0.5% NaCl) to 6.5 min (grown at 37 C in TSB with 7.5%, heated in 7.5% NaCl buffer). Methyl esters of major phospholipid fatty acids extracted from cells were quantitated. The ratio of saturated to unsaturated fatty acids in cells grown at a given NaCl concentration increased with elevated incubation temperature. At a particular growth temperature, however, saturated to unsaturated fatty acids ratios were lowest for cells grown in TSB containing 3.0% NaCl.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A. C., Boothroyd M., Jones E. The effect of water activity on the heat resistance of heat sensitive and heat resistant strains of salmonellae. J Appl Bacteriol. 1970 Sep;33(3):515–522. doi: 10.1111/j.1365-2672.1970.tb02228.x. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R. Combined effects of water activity, solute, and temperature on the growth of Vibrio parahaemolyticus. Appl Microbiol. 1974 Jun;27(6):1075–1080. doi: 10.1128/am.27.6.1075-1080.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchat L. R. Interacting effects of pH, temperature, and salt concentration on growth and survival of Bibrio parahaemolyticus. Appl Microbiol. 1973 May;25(5):844–846. doi: 10.1128/am.25.5.844-846.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun C. L., Frazier W. C. Effect of available water on thermal resistance of three nonsporeforming species of bacteria. Appl Microbiol. 1966 May;14(3):416–420. doi: 10.1128/am.14.3.416-420.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covert D., Woodburn M. Relationships of temperature and sodium chloride concentration to the survival of Vibrio parahaemolyticus in broth and fish homogenate. Appl Microbiol. 1972 Feb;23(2):321–325. doi: 10.1128/am.23.2.321-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke C. F., Colwell R. R. Studies of the cell envelope of Vibrio parahaemolyticus. Can J Microbiol. 1973 Feb;19(2):241–245. doi: 10.1139/m73-036. [DOI] [PubMed] [Google Scholar]
- Galanos D. S., Kapoulas V. M., Voudouris E. C. Detection of adulteration of olive oil by argentation thin layer chromatography. J Am Oil Chem Soc. 1968 Dec;45(12):825–829. doi: 10.1007/BF02540162. [DOI] [PubMed] [Google Scholar]
- Hansen E. W. Correlation of fatty acid composition with thermal resistance of E. coli. Dan Tidsskr Farm. 1971;45(10):339–344. [PubMed] [Google Scholar]
- Hansen E. W., Skadhauge K. The influence of growth temperature on the thermal resistance of E. coli. Dan Tidsskr Farm. 1971;45(1):24–28. [PubMed] [Google Scholar]
- Heinen W., Klein H. P., Volkmann C. M. Fatty acid composition of Thermus aquaticus at different growth temperatures. Arch Mikrobiol. 1970;72(2):199–202. doi: 10.1007/BF00409525. [DOI] [PubMed] [Google Scholar]
- Jones D., Bowyer D. E., Gresham G. A., Howard A. N. An improved spray reagent for detecting lipids on thin-layer chromatograms. J Chromatogr. 1966 Jun;23(1):172–174. doi: 10.1016/s0021-9673(01)98665-0. [DOI] [PubMed] [Google Scholar]
- Kito M., Aibara S., Kato M., Hata T. Differences in fatty acid composition among phosphatidylethanolamine, phosphatidylglycerol and cardiolipin of Escherichia coli. Biochim Biophys Acta. 1972 Mar 23;260(3):475–478. doi: 10.1016/0005-2760(72)90062-8. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak M. J., Nair S., Venkataraman A. Studies on the fatty acid composition of some salmonellas. J Gen Microbiol. 1970 Feb;60(2):151–157. doi: 10.1099/00221287-60-2-151. [DOI] [PubMed] [Google Scholar]
- Ray P. H., White D. C., Brock T. D. Effect of temperature on the fatty acid composition of Thermus aquaticus. J Bacteriol. 1971 Apr;106(1):25–30. doi: 10.1128/jb.106.1.25-30.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Palin W. J., Watson D. W. Nature and linkages of the fatty acids present in lipopolysaccharides from Vibrio metchnikovii and Vibrio parahemolyticus. Eur J Biochem. 1973 Aug 1;37(1):116–120. doi: 10.1111/j.1432-1033.1973.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderzant C., Nickelson R. Survival of Vibrio parahaemolyticus in shrimp tissue under various environmental conditions. Appl Microbiol. 1972 Jan;23(1):34–37. doi: 10.1128/am.23.1.34-37.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. E., Beuchat L. R. Alpha-galactosidase activity of fungi on intestinal gas-forming peanut oligosaccharides. J Agric Food Chem. 1974 Nov-Dec;22(6):1063–1066. doi: 10.1021/jf60196a011. [DOI] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]



