Abstract
The effects of variation in ionic levels on the stability and replication of two bacteriophages (nt-1 and nt-6) host specific for the marine bacterium Beneckea natriegens were examined. Monovalent cations influenced the adsorption of the nt-1 but not the nt-6 phage; however, one-step growth studies showed that NaCl was required for replication of both phage. The NaCl optimum for nt-1 production was 0.25 M NaCl, the same as the growth optimum for B. natriegens. However, the optimum for nt-6 production was 0.16 M NaCl. These NaCl optima for host and phage are at estuarine rather than oceanic levels. The nt-1 phage was better suited to replicate at NaCl levels typical of higher salinity areas (18-35%) and the nt-6 phage was better suited to replicate at lower salinities (5-18%). The nt phage were more resistant to low NaCl levels than their host bacterium and appeared limited to marine waters by the lower survival salinity of B. natriegens coupled with phage inactivation processes occurring in natural estuarine waters.
Full text
PDF

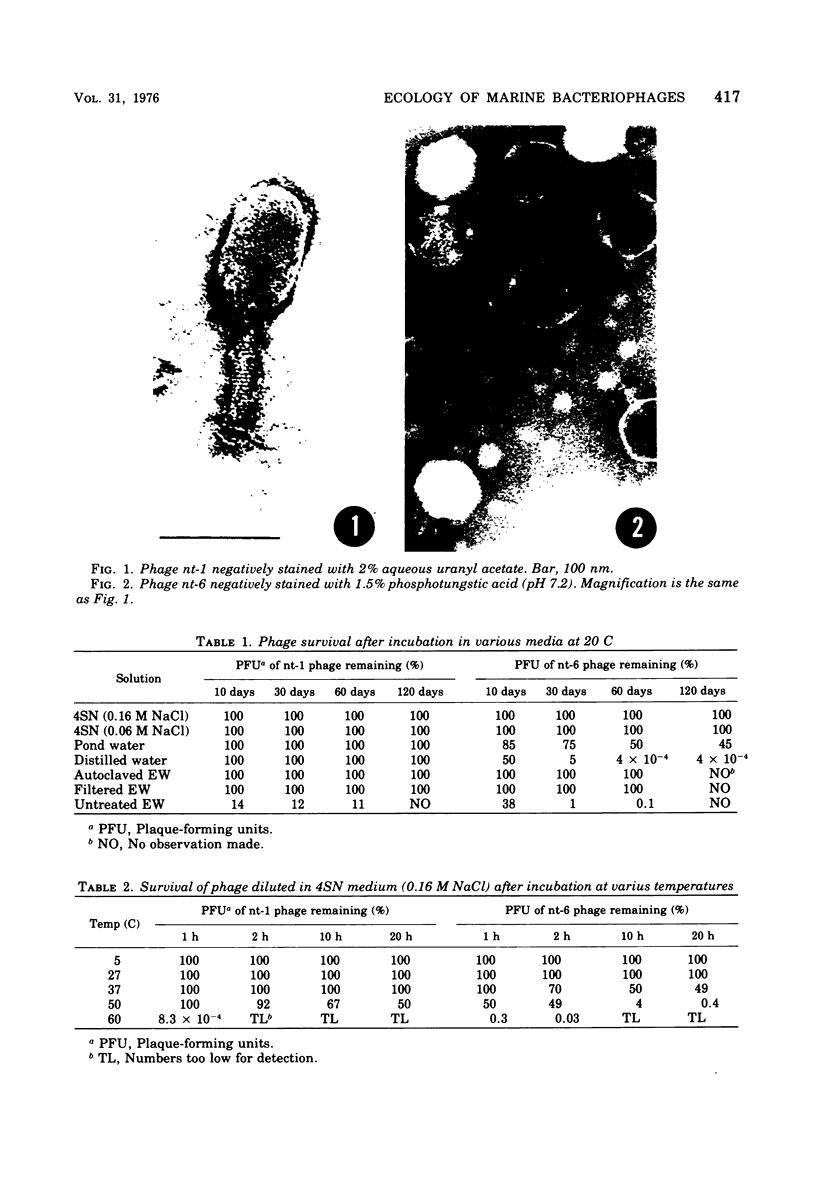
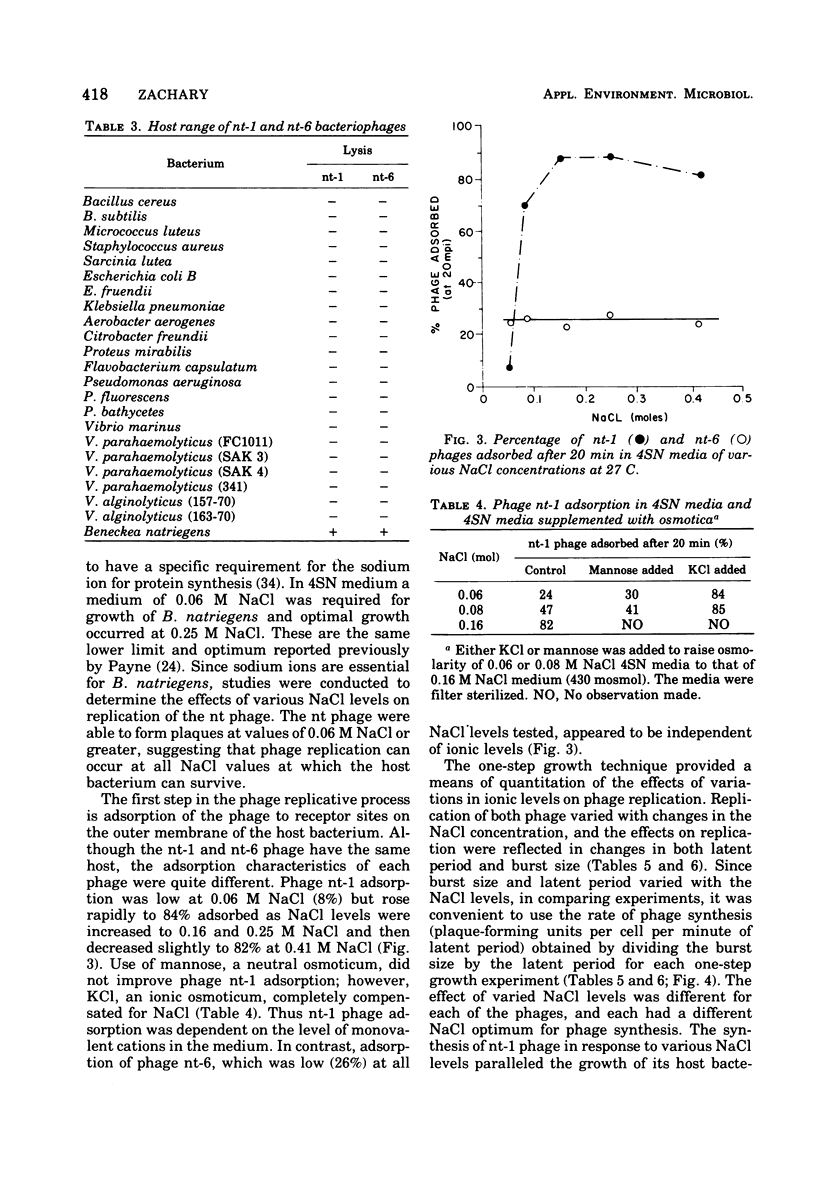
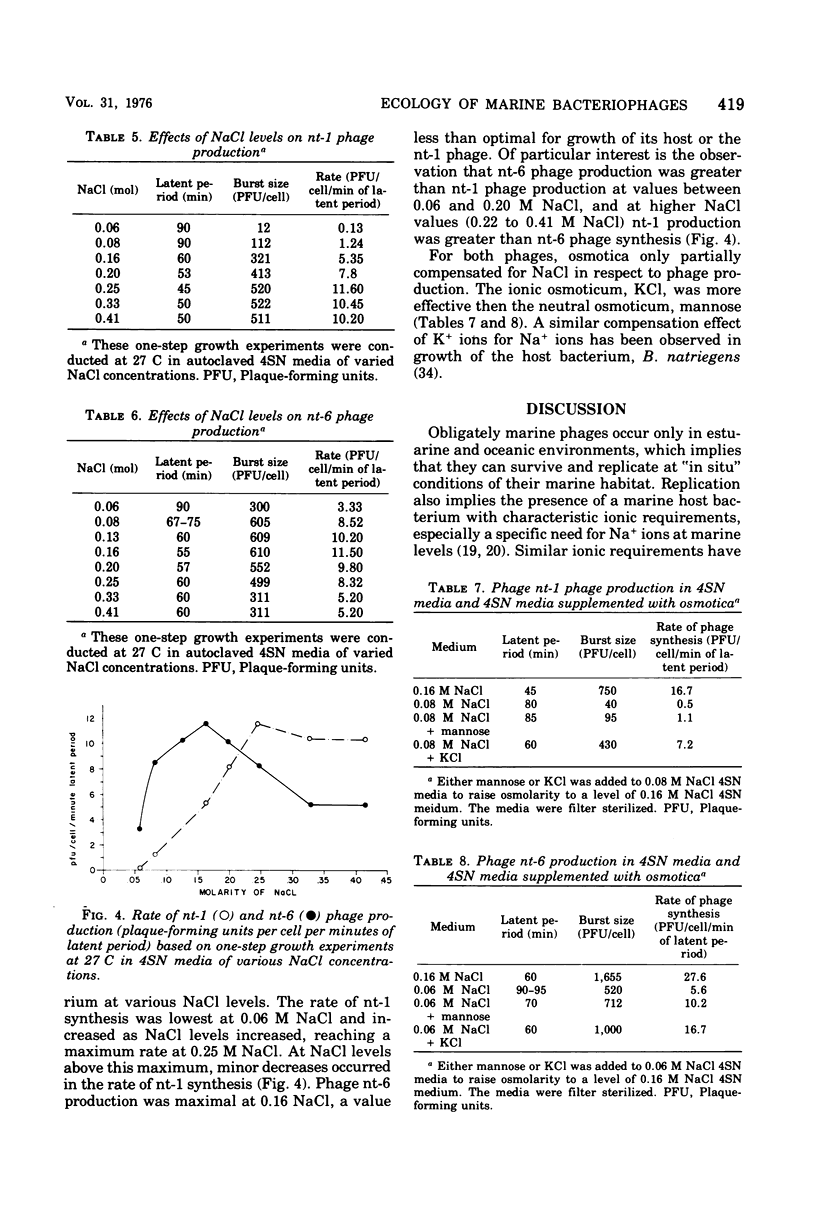



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W., Eisenstark A. The present state of phage taxonomy. Intervirology. 1974;3(4):201–219. doi: 10.1159/000149758. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971 Jul;107(1):268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. W., Eagon R. G. Factors affecting the pathways of glucose catabolism and the tricarboxylic acid cycle in Pseudomonas natriegens. J Bacteriol. 1967 Mar;93(3):866–873. doi: 10.1128/jb.93.3.866-873.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., CHO H. W. MAJOR PRODUCTS OF GLUCOSE DISSIMILATION BY PSEUDOMONAS NATRIEGENS. J Bacteriol. 1965 May;89:1209–1211. doi: 10.1128/jb.89.5.1209-1211.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J Bacteriol. 1962 Apr;83:736–737. doi: 10.1128/jb.83.4.736-737.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., WANG C. H. Dissimilation of glucose and gluconic acid by Pseudomonas natriegens. J Bacteriol. 1962 Apr;83:879–886. doi: 10.1128/jb.83.4.879-886.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagon R. G. PYRIDINE NUCLEOTIDE-LINKED REACTIONS OF PSEUDOMONAS NATRIEGENS. J Bacteriol. 1962 Oct;84(4):819–821. doi: 10.1128/jb.84.4.819-821.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., Warren R. A. Isolation and properties of a bacteriophage lytic for a wide range of pseudomonads. Can J Microbiol. 1971 May;17(5):677–682. doi: 10.1139/m71-109. [DOI] [PubMed] [Google Scholar]
- Keynan A., Nealson K., Sideropoulos H., Hastings J. W. Marine transducing bacteriophage attacking a luminous bacterium. J Virol. 1974 Aug;14(2):333–340. doi: 10.1128/jvi.14.2.333-340.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles C. J., Calcott P. H., MacLeod R. A. Periplasmic CO-binding c-type cytochrome in a marine bacterium. FEBS Lett. 1974 Dec 1;49(1):78–83. doi: 10.1016/0014-5793(74)80636-8. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., EAGON R. G., WILLIAMS A. K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek. 1961;27:121–128. doi: 10.1007/BF02538432. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J. Effects of sodium and potassium ions on growth and substrate penetration of a marine pseudomonad. J Bacteriol. 1960 Nov;80:696–700. doi: 10.1128/jb.80.5.696-700.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J. Studies on bacterial utilization of uronic acids. III. Induction of oxidative enzymes in a marine isolate. J Bacteriol. 1958 Sep;76(3):301–307. doi: 10.1128/jb.76.3.301-307.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES M. E., PAYNE W. J. Further observations on effects of cations on enzyme induction in marine bacteria. Antonie Van Leeuwenhoek. 1962;28:302–314. doi: 10.1007/BF02538743. [DOI] [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P. Effect of sodium chloride on growth of heterotrophic marine bacteria. Arch Microbiol. 1974 May 20;97(4):329–345. doi: 10.1007/BF00403071. [DOI] [PubMed] [Google Scholar]
- Rhodes M. E., Payne W. J. Influence of Na+ on synthesis of a substrate entry mechanism in a marine bacterium. Proc Soc Exp Biol Med. 1967 Mar;124(3):953–955. doi: 10.3181/00379727-124-31894. [DOI] [PubMed] [Google Scholar]
- Rhodes M. E., Payne W. J. Influence of cations on spheroplasts of marine bacteria functioning as osmometers. Appl Microbiol. 1967 May;15(3):537–542. doi: 10.1128/am.15.3.537-542.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. E., Payne W. J. Substrate binding by crude membranes and solubilized membrane extracts from Pseudomonas natriegens. Antonie Van Leeuwenhoek. 1968;34(3):298–312. doi: 10.1007/BF02046451. [DOI] [PubMed] [Google Scholar]
- SMITH L. S., KRUEGER A. P. Thermal inactivation of a new vibrio phage. Proc Soc Exp Biol Med. 1952 Nov;81(2):371–374. doi: 10.3181/00379727-81-19879. [DOI] [PubMed] [Google Scholar]
- Webb C. D., Payne W. J. Influence of Na+ on synthesis of macromolecules by a marine bacterium. Appl Microbiol. 1971 Jun;21(6):1080–1088. doi: 10.1128/am.21.6.1080-1088.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston J. A., Collins P. A., Knowles C. J. The respiratory system of the marine bacterium Beneckea natriegens. II. Terminal branching of respiration to oxygen and resistance to inhibition by cyanide. Biochim Biophys Acta. 1974 Nov 19;368(2):148–157. doi: 10.1016/0005-2728(74)90145-5. [DOI] [PubMed] [Google Scholar]
- Weston J. A., Knowles C. J. A soluble CO-binding c-type cytochrome from the marine bacterium Beneckea natriegens. Biochim Biophys Acta. 1973 Apr 27;305(1):11–18. doi: 10.1016/0005-2728(73)90226-0. [DOI] [PubMed] [Google Scholar]
- Weston J. A., Knowles C. J. The respiratory system of the marine bacterium Beneckea natriegens. Oxidation--reduction potentials of the cytochromes. FEBS Lett. 1974 Jul 15;43(2):235–238. doi: 10.1016/0014-5793(74)81008-2. [DOI] [PubMed] [Google Scholar]
- Zachary A. Isolation of bacteriophages of the marine bacterium Beneckea natriegens from coastal salt marshes. Appl Microbiol. 1974 May;27(5):980–982. doi: 10.1128/am.27.5.980-982.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




