Abstract
Vibrio cholerae is an aquatic bacterium that causes the severe diarrheal disease cholera. V. cholerae strains of the O1 serogroup exist as two biotypes, classical and El Tor. Toxigenic strains of the El Tor biotype emerged to cause the seventh pandemic of cholera in 1961 and subsequently displaced strains of the classical biotype both in the environment and as a cause of cholera within a decade. The factors that drove emergence of the El Tor biotype and the displacement of the classical biotype are unknown. Here, we show a unique difference in carbohydrate metabolism between these two biotypes. When grown with added carbohydrates, classical biotype strains generated a sharp decrease in medium pH, resulting in loss of viability. However, growth of El Tor biotype strain N16961 was enhanced due to its ability to produce 2,3-butanediol, a neutral fermentation end product, and suppress the accumulation of organic acids. An N16961 mutant (SSY01) defective in 2,3-butanediol synthesis showed the same defect in growth that classical biotype strains show in media rich in carbohydrates. Importantly, the SSY01 mutant was attenuated in its ability to colonize the intestines of infant mice, suggesting that host carbohydrates may be available to V. cholerae within the intestinal environment. Similarly, the SSY01 mutant failed to develop biofilms when utilizing N-acetyl-d-glucosamine as a carbon source. Because growth on N-acetyl-d-glucosamine likely reflects the ability of a strain to grow on chitin in certain aquatic environments, we conclude that the strains of classical biotype are likely defective compared to those of El Tor in growth in any environmental niche that is rich in chitin and/or other metabolizable carbohydrates. We propose that the ability to metabolize sugars without production of acid by-products might account for the improved evolutionary fitness of the V. cholerae El Tor biotype compared to that of the classical biotype both as a global cause of cholera and as an environmental organism.
Toxigenic strains of Vibrio cholerae can cause cholera. Strains that display two serogroups, O1 and O139, typically cause epidemic and endemic disease. O1 strains can be further classified into two biotypes, termed classical and El Tor. The first six pandemics of cholera were caused by classical biotype strains, and the current, seventh pandemic is caused by a closely related group of the El Tor biotype. The seventh-pandemic El Tor O1 “clone” emerged in 1961 in Indonesia. However, within a decade this highly successful clone displaced all classical strains as a cause of cholera throughout areas of the world where cholera is endemic. The seventh-pandemic El Tor clone is also remarkable in that it has established endemicity in virtually every area of the world where it has been introduced, including Africa and Latin America. Most recently, strains of the O139 serogroup have emerged to also cause cholera in southern Asia (4, 51). These strains are clearly derived from the seventh-pandemic El Tor O1 clone and now coexist with O1 El Tor strains in locations of cholera endemicity, such as India and Bangladesh (44).
Epidemics caused by strains of the El Tor biotype produce a lower case fatality rate and a higher incidence of asymptomatic infections than those caused by strains of the classical biotype (26). To date, several biotype-specific biochemical features, including (i) hemolysis (1, 41), (ii) hemagglutination of chicken erythrocytes (19), (iii) resistance to polymyxin B (37), (iv) bacteriophage-mediated lysis (30, 49), and (v) acetoin synthesis (21, 27), have been used to distinguish strains of the El Tor biotype from those of the classical biotype. Among these, acetoin biosynthesis has been used most widely for biotyping. Seventh-pandemic O1 El Tor strains and all strains of the O139 serogroup are positive in this assay, while classical strains are not. The molecular basis, however, that accounts for the negative test result for classical O1 V. cholerae is not understood.
In addition to the aforementioned differences in biochemical traits, comparative DNA analyses revealed differences in the gene contents (8) and polymorphic sequence variations in several regions of the genome, including loci responsible for the production of virulence factors in these two biotypes. Biotype-specific DNA sequence differences in the gene encoding TcpA, a structural component of toxin-coregulated pilus, have been used to differentiate El Tor strains and classical strains by simply comparing the sizes of PCR products (23, 50). Single-base-pair differences in the tcpPH promoter region were also found and proposed to be responsible for the differential regulation of virulence gene expression in these two biotype strains (28, 36). Recently, two genes in the tRNA operon 1 were reported to be missing in the classical biotype compared to El Tor (11). Each strain also requires different culture conditions to produce cholera toxin (CT), the major virulence determinant of V. cholerae. CT can be produced in classical strains growing under mildly acidic conditions Luria-Bertani (LB), 30°C, pH 6.5). In contrast, CT production from El Tor strains is efficient only under unusual biphasic growth conditions typically referred to as AKI conditions (7, 15). Based on the aforementioned studies, it was suggested that classical and El Tor biotypes may have evolved independently from different lineages (11, 22).
In this study, we identified a dramatic difference in carbohydrate metabolism between strains of the classical and El Tor biotypes. This difference leads classical biotype strains to produce organic acids when metabolizing carbohydrates, with a concomitant drop in pH of the growth media. Because V. cholerae is a profoundly acid-sensitive organism, this drop in pH rapidly inhibits further growth of strains of classical biotype on carbohydrate-rich media. El Tor strains do not accumulate such acids but instead produce 2,3-butanediol as their fermentation end product and thus grow to much higher densities in media containing carbohydrates. A mutant of an El Tor biotype strain deficient in the 2,3-butanediol fermentation showed the same growth defect displayed by classical biotype strains under carbohydrate-rich conditions. Thus, El Tor biotype strains which produce 2,3-butanediol are predicted to have a profound selective advantage over classical biotype strains in any environment that is rich in metabolizable carbohydrates. Such a metabolic selection can explain the dramatically improved fitness of toxigenic El Tor compared to the earlier classical biotype strains. We provide evidence here that this metabolic selection might have occurred both within the human host and in carbohydrate-rich aquatic environments.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains and plasmids used in this study are listed in Table 1. Bacterial cultures were grown at 37°C in LB (10 g tryptone, 5 g NaCl, and 5 g yeast extract per liter) with shaking unless otherwise noted. The antibiotics streptomycin (200 μg/ml for V. cholerae), kanamycin (100 μg/ml for V. cholerae and 50 μg/ml for Escherichia coli), ampicillin (100 μg/ml for E. coli), and chloramphenicol (10 μg/ml for E. coli) were used as required. In-frame deletion mutants of ald (VC1589) and VC1591 were constructed following a gene replacement procedure involving sucrose counterselection (46). Phenol red (20 mg/liter; Sigma Chemical Co., St. Louis, MO) was added in pH indicator plates.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Serogroup/biotype | Relevant genotype or description | Reference or source |
|---|---|---|---|
| Strains | |||
| V. cholerae | |||
| N16961 | O1/El Tor | SmrlacZ | 8 |
| O395 | O1/classical | SmrlacZ | 8 |
| CA401 | O1/classical | 6 | |
| 569B | O1/classical | Smr | 6 |
| MO10 | O139 | Smr | 51 |
| AM19226 | Non-O1, non-O139 | Smr | 9 |
| V52 | Non-O1, non-O139 | Smr | 39 |
| SSY01 | N16961, als::TnKGL3 | This study | |
| SSY02 | N16961, Δald | This study | |
| SSY03 | N16961, ΔVC1591 | This study | |
| SSY04 | O395, pgi::TnKGL3 | This study | |
| E. coli | |||
| DH5α | supE44 ΔlacU169 (φ80dlacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 13 | |
| SM10λpir | Kmrthi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu pir+ | 5 | |
| BW20767 | Km::Tn7 leu-63::IS10 recA1 creC510 hsdR17 endA1 zbf-5 uidA(ΔMlu1)::pir+thi | 34 | |
| P. aeruginosa | |||
| PAO1 | 14 | ||
| Plasmids | |||
| pWM91 | sacB suicide vector, Apr | 34 | |
| pTnKGL3 | Suicide vector bearing TnKGL3, Cmr Kmr | This study | |
| pVIK112 | Suicide vector for lacZ reporter fusion, Kmr | 20 |
Transposon mutagenesis and identification of genes interrupted by transposon insertions.
A library of random transposon insertion mutants was constructed by introducing TnKGL3, a mariner-based transposon, into V. cholerae recipient strains through conjugation. TnKGL3 was derived from TnTGL3 (42) by replacing the Tetr cassette with a Kmr cassette. Kmr transconjugants were obtained by biparental matings (5) and screened for a mutant that acidified the culture medium in the presence of glucose. An arbitrary PCR was performed to determine the location of the TnKGL3 insertion site in each mutant. It was first performed using a transposon-specific primer, Mar1 (5′-GGGAATCATTTGAAGGTTGGT-3′), and two random primers, Arb1 (5′-GGCCACGCGTCGACTAGTACNNNNNNNNNNGATAT-3′) and Arb6 (5′-GGCCACGCGTCGACTAGTACNNNNNNNNNNACGCC-3′), and was followed by a second-round PCR using the first-round PCR product as the template and Mar2 (5′-TAGCGACGCCATCTATGTGTC-3′) and Arb2 (5′-GGCCACGCGTCGACTAGTAC-3′) as the primers. PCR products were sequenced using the primer Mar2, which allows sequencing outward across the insertion junction of TnKGL3. A BLAST search of DNA sequences was done against the whole genome of N16961 available in public databases.
Construction of lacZ transcriptional fusions and LacZ assay.
To construct single-copy lacZ transcriptional fusions, DNA fragments containing the promoter region of ald (representing the first gene of the cluster, VC1589) or als (representing the second gene, VC1590) were flanked with EcoRI and KpnI restriction sites and ligated with an EcoRI-KpnI digest of pVIK112 (20) containing the promoterless lacZ structural gene with its own ribosomal binding site. E. coli BW20767, harboring each transcriptional lacZ fusion, was used as a donor strain in biparental matings. β-Galactosidase activity was measured as described previously (35).
Quantification of glycolysis metabolites in the culture supernatants.
The glucose level remaining in the media was measured using a QuantiChrom glucose assay kit (Bioassay Systems, Hayward, CA). The acetoin level was quantitatively measured as described previously (43). Levels of two organic acids (lactic acid and acetic acid) were determined using an assay kit (Megazyme International Ireland Ltd., Wicklow, Ireland) by following the instructions provided by the manufacturer.
Infant mouse colonization assay.
For the infant mouse colonization assay, ∼105 to 106 total CFU was inoculated intragastrically into an infant mouse (∼5 to 6 days old; Charles River Breeding Laboratories) as described previously (52). After a period of colonization, intestinal homogenates were collected and viable cells were enumerated by spreading serial diluents on LB agar containing streptomycin (for total bacterial count) or streptomycin-kanamycin (for SSY01 count). Strains to be inoculated were grown in LB to an optical density at 600 nm (OD600) of ∼0.5 and diluted 500-fold in LB or LB plus 2.5% glucose (LBG) before inoculation. Competitive indices were calculated by dividing the output CFU of SSY01 by the output CFU of N16961.
Microscopic examination of biofilms.
For examination of biofilm architecture and cell viability, an eight-chambered coverslip system (Lab-Tek Inc., Campbell, CA) was used. LB (0.4 ml) was inoculated with 4 μl of overnight-grown LB starter culture. After 24 h of static growth at 37°C, biofilms were washed two times with modified artificial seawater (MASW) (234 mM NaCl, 27.5 mM MgSO4, 1.5 mM NaHCO3, 4.95 mM CaCl2, 5.15 mM KCl, 18.7 mM NH4Cl, pH 7.2) and treated with MASW containing 0% or 1% N-acetyl-d-glucosamine (NAG) for 24 h. Biofilms were stained with 0.2 ml of a LIVE/DEAD BacLight (Molecular Probes, Inc., Eugene, OR) bacterial viability stain for 15 min. Images were acquired on a Nikon TE200 inverted microscope with a C1 laser scanning confocal unit attached (Nikon Instruments, Inc., Melville, NY). For two-color images, samples were scanned sequentially at 488 and 546 nm. Syto-9 (green fluorescence) was detected through a 505- to 530-nm band-pass filter, and propidium iodide (red fluorescence) was detected through a 560-nm long-pass filter; these were presented in two channels of a 512- by 512-pixel, 8-bit image. MetaMorph (Molecular Device Corp., Sunnyvale, CA) imaging software was used to condense 25 serial stacks of confocal images.
RESULTS
Classical biotype V. cholerae strain O395 loses viability during in vitro culture in the presence of glucose.
To examine the effect of glucose on growth of two biotypes of O1 V. cholerae, the El Tor strain N16961 and the classical strain O395 were grown in LB containing 0% or 1% glucose. We chose N16961 because its genomic sequence has been determined and thus it is a well-characterized seventh-pandemic El Tor strain and O395 because it has been investigated extensively in previous studies. As shown in Fig. 1A, N16961 grew better in a medium supplemented with glucose (OD600 of ∼8.0 versus ∼6.0). Consistently, ∼10-fold more viable cells were detected in the glucose-supplemented culture than in the control, unsupplemented medium (Fig. 1C). In contrast, O395 showed a significant growth defect under the same culture conditions. In the presence of 1% glucose, bacterial growth virtually ceased after 4 h and reached an OD600 of only ∼1.8 after 8 h of culture (Fig. 1B). Importantly, no viable bacteria were recovered after 15 h of culture with 1% glucose (Fig. 1C). The loss of viability was also observed in the culture medium containing glucose levels as low as 0.3% or other medium carbohydrates, such as sucrose (data not shown). Since V. cholerae is highly susceptible to acidic pH (33, 52) and glucose fermentation leads to the production of organic acids, such as lactic acid and acetic acid, we monitored pH change in culture media. After a small drop to ∼6.0, medium pH rose to >8.0 with time in the glucose-supplemented culture of N16961. O395, however, acidified culture media persistently, and after 8 h, the pH dropped to about ∼5.0 (Fig. 1D). When O395 was grown in pH-buffered media (pH 8.0) supplemented with 1% glucose, the pH drop was minimized and the loss of viability was not detected (data not shown). Thus, metabolism of glucose by O395 resulted in the acidification of the growth medium and killing of cells at low pH in glucose-supplemented but not unsupplemented medium. Collectively, these data suggest that glucose exerts a differential effect on the growth and viability of strains of V. cholerae depending on biotype.
FIG. 1.
Killing of V. cholerae O1 classical biotype by glucose-induced pH drop. (A and B) Aerobic flask culture of El Tor biotype strain N16961 and classical biotype strain O395. Bacteria were cultured in LB containing 0% (LB) or 1% (LBG) glucose, and growth was monitored by measuring OD600. (C) Loss of viability in the glucose-supplemented culture of O395. To enumerate viable cells, 10 μl of serial dilutions (∼10−3 to 10−7) was spotted on LB agar plates and incubated at 37°C overnight. The left and right sides of each plate represent bacteria grown in LB and LBG, respectively, for 15 h. (D) pH change of the culture supernatants in the culture of N16961 or O395. Bacteria were grown in LB plus 1% glucose.
O139 and non-O1/non-O139 strains revealed a glucose growth phenotype identical to that of N16961.
As shown in Fig. 2, two other classical strains, CA401 and 569B, also exhibited a glucose growth phenotype identical to that of O395, suggesting that the glucose-dependent pH drop may occur universally among classical biotype strains. In contrast, growth of strain MO10 (O139 serogroup) in the presence of glucose was enhanced, further confirming previous reports that pathogenic O139 strains are closely related to pandemic O1 El Tor biotype strains (2, 4, 18, 22). Two non-O1/non-O139 strains, AM19226 (8) and V52 (39), were also identical to N16961 or MO10 as far as glucose growth phenotype (Fig. 2). In addition, two other gram-negative bacteria, Pseudomonas aeruginosa PAO1 and Escherichia coli DH5α, grew better or equally well in the presence versus absence of glucose, respectively. These results indicate that glucose-induced loss of viability occurs uniquely in V. cholerae O1 classical biotype strains.
FIG. 2.
Glucose growth phenotypes of various V. cholerae strains. Seven V. cholerae strains and two control gram-negative strains were grown in LB or LBG. The OD600 of each culture at 15 h is shown. *, no viable cells were detected in these cultures; **, the increase in growth in the presence versus absence of glucose is statistically significant (P < 0.05).
The 2,3-butanediol fermentation pathway is essential for maintaining viability in glucose-containing media.
To identify the molecular basis accounting for such dramatic differences in glucose metabolism, a random transposon insertion mutant library of N16961 was constructed to screen for mutants that exhibit glucose growth phenotypes identical to that of O395. Because these glucose-dependent growth phenotypes were related to acid production, we took advantage of phenol red, a pH-dependent color indicator, which is pink or yellow under basic and acidic conditions, respectively. Consistent with the results in Fig. 1D, strains N16961 and O395 growing on such phenol red pH indicator plates substituted with glucose were pink and yellow in color, respectively (Fig. 3A). We isolated an N16961 transposon mutant (strain SSY01) that acidified the culture media and was yellow on the same pH indicator plate (Fig. 3A). As expected, this mutant also lost viability due to the glucose-induced pH drop, as observed for the classical biotype strain O395 (data not shown). The gene disrupted by the transposon insertion carried by SSY01 was determined to be als (VC1590), encoding acetolactate synthase (Als). Als is involved in the 2,3-butanediol fermentation pathway, catalyzing the first step in the pathway from pyruvate to 2,3-butanediol (Fig. 3B). To support the conclusion that synthesis of 2,3-butanediol could block production of acidic metabolites in the presence of glucose, we constructed clean deletion mutants of VC1589 and VC1591, which are deficient in acetolactate decarboxylase (Ald) and oxidoreductase, respectively. The strain carrying the in-frame deletion of ald (SSY02) showed a phenotype identical to that of SSY01 in growth and pH change (Fig. 3C), further supporting an essential role for the 2,3-butanediol pathway in maintaining viability in media containing glucose. Interestingly, the VC1591 mutant SSY03, which is not able to convert acetoin to 2,3-butanediol, still acidified culture media but not to the same extent as the other two mutants (SSY01 and SSY02) (Fig. 3C). This suggests that (i) all participating enzymes should be functional to prevent the pH drop and (ii) the first two steps play a more important role in reducing the availability of pyruvate towards the mixed acid fermentation pathway. The SSY03 mutant maintained viability in the culture with 1% glucose, while SSY01 and SSY02 lost their viabilities (data not shown). We then asked whether a mutant of O395 defective in glycolysis could survive the glucose-supplemented culture. A transposon insertion mutant of strain O395 (SSY04) in the gene pgi, encoding phosphoglucose isomerase (VC0374), which mediates the second step of glycolysis, indeed could not acidify the culture media containing glucose (Fig. 3A) and thus maintained viability in the presence of glucose (data not shown). These data confirm that glucose metabolism through the glycolytic pathway causes classical biotype strains, such as O395, to acidify growth media containing glucose. These results also suggest that the classical biotype of V. cholerae O1 may possess an inherent defect in the 2,3-butanediol fermentation pathway.
FIG. 3.
Role of the 2,3-butanediol fermentation pathway in maintaining viability and effect of each mutation on pH drop and growth in the presence of glucose. (A) Identification of an N16961 mutant that acidified culture medium in the presence of glucose. Color changes of overnight growth of N16961, O395, SSY01, and SSY04 on a pH indicator plate (see Materials and Methods). (B) Biochemical pathway of 2,3-butanediol fermentation. Pyruvate, a glycolysis product, is fermented to 2,3-butanediol with intermediates that include acetolactate and acetoin. Enzymes involved in each step are Als, Ald, and oxidoreductase. Mixed acid fermentation leading to the production of organic acids is also shown on the right. Mutants of VC1590, VC1589, and VC1591 are named SSY01, SSY02, and SSY03, respectively. EtOH, ethanol. (C) Bacterial growth and pH change profiles. Experimental conditions were identical to those described in the legend for Fig. 1. Bacteria were grown in LBG for 8 h.
Glucose derepresses transcription of genes involved in the 2,3-butanediol pathway in El Tor strain N16961 but not in classical strain O395.
Examination of the coding sequences of ald and als in strains N16961 and O395 revealed no obvious defects in these two structural genes in strain O395. Accordingly, we asked whether the lack of a functional 2,3-butanediol fermentation pathway in strain O395 was caused by a biotype-specific difference in transcriptional expression of genes encoding enzymes in the pathway. To test this, we monitored the promoter activities of ald and als by constructing lacZ reporter gene fusions to these two genes. The constructs were integrated onto the chromosomes of lacZ-defective derivatives of N16961 and O395. β-Galactosidase activity was monitored in media supplemented with or without glucose (Fig. 4). In strain O395, extremely low levels of LacZ were detected for both reporter strains, irrespective of the presence or absence of added medium glucose (Fig. 4), suggesting that transcription of these genes was very low in O395. In contrast, ald promoter activity was increased ∼50-fold in the presence versus absence of glucose for N16961 (Fig. 4). This suggests that the 2,3-butanediol fermentation pathway is highly induced by the presence of excess glucose in this El Tor biotype strain. Curiously, we observed that glucose caused only an ∼2.5-fold increase of als promoter activity in N16961, suggesting that regulation of the second gene (ald) in the pathway from pyruvate to 2,3-butanediol accounts for the metabolic differences between N16961 and O395.
FIG. 4.
Transcriptional regulation of genes involved in the 2,3-butanediol pathway in classical and El Tor biotypes. Shown are the organization of genes encoding enzymes in this pathway and the promoter activities of two potential promoters present in this cluster. Bacterial strains harboring single-copy ald-lacZ or als-lacZ fusions were assayed for β-galactosidase activity in triplicate, and means ± standard errors of the means are presented.
Strain N16961 maintains viability in the glucose-supplemented cultures by producing a high level of acetoin, a neutral fermentation product, and by suppressing the accumulation of organic acids.
To better understand the discrepancy between glucose metabolism in classical versus El Tor biotypes, we quantitatively monitored glucose levels and three different end products (i.e., acetoin, lactic acid, and acetic acid) of the glucose fermentation in the culture media (Fig. 3B). As shown in Fig. 5A, strain N16961 consumed glucose completely in 6 h. In contrast, only ∼15% or ∼30% of the initial glucose was consumed by strain O395 or SSY01, respectively, in 8 h. This result, in part, accounts for our initial observation that N16961 growth was enhanced in the presence of glucose (Fig. 1A). The ability to produce acetoin, one of two equilibrium end products of the 2,3-butanediol fermentation pathway, has been one of the major parameters for biotyping of classical and El Tor strains (21). As expected, high levels of acetoin were detected in cultures of N16961 supplemented with glucose, whereas no acetoin was produced in strain O395 or SSY01 (Fig. 5B). However, we were astonished to find that after 8 h of growth on glucose, N16961 produced ∼22.5 mM of acetoin, a level that is ∼40% of the initial glucose concentration (1%, 55.6 mM). In contrast, lactic and acetic acid concentrations continued to rise in cultures of strain O395 or the SSY01 mutant (Fig. 5C and D) during the same period of acetoin production by N16961. Lactic acid levels, in particular, were dramatically increased in the culture media of the SSY01 mutant compared to those of its parental strain and O395 (Fig. 5C). These data are similar to the observation that higher glucose levels were consumed in SSY01 than in strain O395, suggesting that glucose consumption drives lactic acid accumulation in both of these strains (Fig. 5A and C). Acetic acid levels were significantly higher than those of lactic acid during the entire growth period (Fig. 5C and D), suggesting that the pH drop is mainly due to acetic acid. Importantly, strain N16961 was capable of minimizing the accumulation of both lactic and acetic acids. Acetic acid and lactic acid were produced in the early logarithmic phase of growth, while levels decreased to baseline at ∼6 h (Fig. 5C and D). Taken together, these results suggest that El Tor strain N16961 possesses the metabolic potential to turn glucose into acetoin and 2,3-butanediol and keep production of two harmful organic acids to a minimum. This metabolic strategy results in the full utilization of available glucose, compared to classical biotype strains, which do not have this capacity.
FIG. 5.
Time profiles of metabolites in the glucose-supplemented culture. Three bacterial strains, N16961, O395, and SSY01, were grown in LB containing 1% glucose for 8 h. Levels of glucose, acetoin, lactic acid, and acetic acid in culture supernatants were measured as described in Materials and Methods and plotted with respect to time. In panel B, lines for O395 and SSY01 are superimposed.
The N16961 als mutant is defective in colonizing infant mouse intestine.
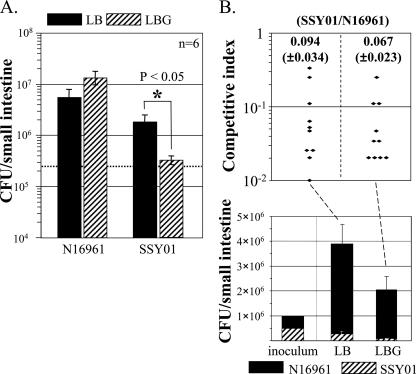
We have observed previously that classical biotype strains are less able to colonize the intestines of infant mice than strains of the El Tor biotype (unpublished observations). The molecular basis for this was not understood. We hypothesized that differences in sensitivity to glucose between classical and El Tor biotypes might account for this difference in in vivo colonization. The average luminal glucose concentration in the small intestine was previously reported to range between 0.2 and 48 mM in normally feeding animals, with the higher concentrations found in the proximal small intestine (10). Because SSY01 showed a sensitivity to glucose that was comparable to that of classical strain O395, we tested whether this mutant strain would have a colonization defect in the intestines of infant mice. To better evaluate the effect of glucose on in vivo colonization, bacteria were also inoculated into animals with extra glucose. After 24 h of intestinal growth, the total CFU of strain N16961 and its isogenic als mutant increased ∼20-fold and ∼8-fold, respectively (Fig. 6A). When inoculated with additional glucose, ∼2-fold more CFU of wild-type N16961 were recovered. In contrast, the isogenic N16961 als mutant SSY01 showed a statistically significant colonization defect when inoculated with glucose compared to results when inoculated without glucose (Fig. 6A). The level of colonization by SSY01 was ∼3-fold (without glucose in the inoculum) or ∼42-fold (with glucose in the inoculum) less that of N16961 (Fig. 6A). These data suggest that glucose-mediated toxicity may still occur in vivo and is enhanced by addition of glucose to the inoculum.
FIG. 6.
Effect of glucose on in vivo colonization of SSY01. (A) Infant mice were individually infected with 2.5 × 105 CFU (dashed line) of wild-type N16961 or SSY01. In 24 h, bacteria were recovered from mice and plated on appropriate media for enumeration. Prior to inoculation, bacterial cells, which were grown in LB to an OD600 of ∼0.5, were diluted 500-fold in fresh LB containing 0% (LB) or 2.5% (LBG) glucose. Six mice were used for each group, and means ± standard errors of the means are presented on a logarithmic scale. For SSY01, the decrease in CFU upon coinoculation with glucose was statistically significant (*, P < 0.05). (B) Infant mice were coinfected with 5 × 105 CFU of each strain. Again, bacterial cells were diluted in LB or LB containing 2.5% glucose before inoculation. After 24 h of incubation, CFU of each strain was enumerated and plotted in a linear scale. The competitive index represents the ratio of SSY01 to N16961 recovered after incubation. Ten mice were used for each infection, and means ± standard errors of the means are presented.
Based upon these data, we next determined the competitive index of the SSY01 mutant compared to that of its parental strain. Even without additional glucose in the inoculum, SSY01 was only ∼9.4% as competitive as wild-type N16961 (Fig. 6B, top). When a 1:1 mixture of bacteria was inoculated together with glucose, the competitive index did not change significantly (∼6.7%, P = 0.167 versus glucose-free infection). This was surprising because N16961 colonized better with extra glucose whereas colonization of SSY01 was decreased, as shown in Fig. 6A. Interestingly, the total number of viable organisms of both strains decreased ∼2-fold compared with that recovered from glucose-free infected mice (Fig. 6B, bottom). These results suggest that the glucose-mediated toxic effect caused by the mutant may alter the colonization properties of the wild-type strain in vivo. This would be expected if both strains form mixed microcolonies or biofilms on the mucosal surface that then become acidic as a result of in vivo carbohydrate utilization by SSY01. Such clumping of V. cholerae on the mucosal surface has previously been observed with the infant mouse model (25).
NAG, a monomeric unit of chitin, inhibits the biofilm development of classical biotype strain O395.
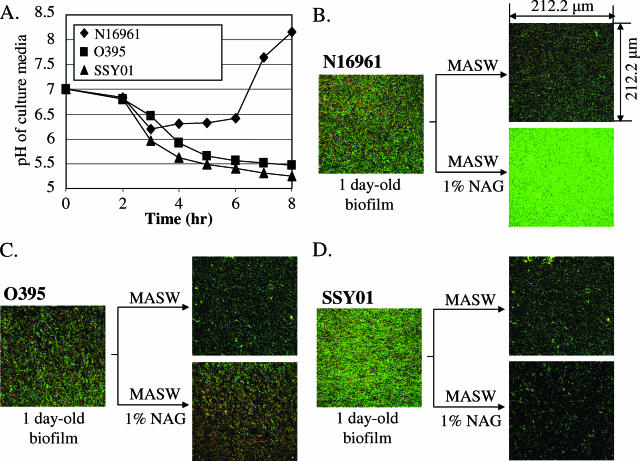
V. cholerae inhabits aquatic environments and is capable of forming multicellular communities known as biofilms on the surface of chitin, one of the most predominant carbohydrate polymers in nature (24, 31, 32). V. cholerae expresses chitinases, and the product of the action of these enzymes is NAG. We asked whether NAG was similar to glucose in terms of its differential effect on the growth of El Tor versus classical biotypes of V. cholerae. We found that cultures of strain O395 or SSY01, but not cultures of N16961, produced acid when grown in LB supplemented with 1% NAG but not in its absence (Fig. 7A). A similar drop in pH was also observed when each strain was grown with the hexameric unit of chitin, hexa-N-acetylchitohexaose (data not shown). These results led us to hypothesize that El Tor biotype strains of V. cholerae might also have a survival advantage over strains of classical biotype in the environment, where bacteria grow as biofilms in association with chitinous surfaces. To test this hypothesis, we treated routine LB-grown, 1-day-old biofilms with MASW with or without 1% NAG to assess whether these treatments would affect biofilm density and/or cell viability. When strains were grown statically to form biofilms in regular LB media for 1 day, the classical strain O395 developed a somewhat less dense biofilm than N16961 or SSY01 (Fig. 7B, C, and D, left panels). In each case, biofilm cell density was invariably decreased when treated with only MASW (Fig. 7B, C, and D, top right panels), suggesting that bacteria in a biofilm still require a carbon source to maintain biofilm structure. Upon treatment with 1% NAG, biofilm cell density was robustly increased for strain N16961 (Fig. 7B, bottom right panel). In contrast, there were significantly increased numbers of dead bacteria in the biofilms of O395 after the same treatment (Fig. 7C, bottom right panel). Similarly, a significant reduction of biofilm cell density was observed for the biofilm of SSY01, which also acidified the culture media in the presence of NAG (Fig. 7D, bottom right panel). The decreased cell density in these biofilms was likely due to the detachment of dead bacteria from the biofilm as a result of glucose-induced acidification of the biofilm. Collectively, these results suggest that El Tor biotype strains are more capable than classical biotype strains of propagating and maintaining themselves in aquatic environments through utilization of chitin as a carbon and energy source.
FIG. 7.
Effect of NAG on biofilm viability. (A) pH change of culture supernatants in the NAG-supplemented culture. Culture conditions were identical to those described in the legend for Fig. 1D, except for the use of 1% NAG in the media. (B, C, and D) Confocal laser microscopic analysis of biofilms of N16961, O395, and SSY01. To acquire images, live cells were stained with Syto-9 (green) and dead cells were stained with propidium iodide (red). Top (x-y plane) views were projected from a stack of 25 images taken at 0.5-μm intervals for a total of 12 μm. Before staining, 1-day-old biofilms were treated with MASW (see Materials and Methods) containing 0% (top right panel of each set) or 1% (bottom right panel of each set) NAG for 1 day.
DISCUSSION
One of the most long-standing mysteries in cholera epidemiology is the mechanism(s) by which the classical biotype of V. cholerae was replaced by the seventh-pandemic El Tor biotype as the cause of epidemic and endemic cholera worldwide. This process began in 1961 and was virtually complete a decade later. While there were reports of the occasional isolation of the classical strain in parts of rural Bangladesh as late as the mid-1980s, current information suggests that even these strains are actually “hybrid” strains with characteristics of both the seventh-pandemic El Tor clone and classical strains. Thus, it is quite possible that the emergence of the seventh-pandemic El Tor clone has resulted in the extinction of strains of the classical biotype on a global scale (37). The molecular and ecological events that led to this change in the epidemiology of cholera remain a mystery despite the accumulation of many new facts about the molecular differences between these biotypes. For example, a recent comparative genomic study revealed that classical biotype strains are missing ∼22 genes compared to seventh-pandemic El Tor strains, with most of these genes located on two chromosomal islands (8). Among the phenotypic and genetic factors that have been proposed in the epidemiological shift of biotypes are differences in environmental fitness (38), susceptibility to lytic vibriophages (30, 49), regulation of virulence gene expression (7, 28, 36), virulence gene content (8), and primary sequence of virulence gene products (23, 50). In this study, we identified a unique difference in carbohydrate metabolism between these two biotypes of V. cholerae and propose that this difference could plausibly account for the improved evolutionary fitness of the seventh-pandemic clone versus strains of the classical biotype in both the host and the aquatic environment.
We have shown that classical biotype O1 strains grow poorly in media containing high levels of carbohydrates and lose viability in such media due to the glycolysis-dependent production of organic acids and the resultant acidification of the medium. In contrast, O1 strains of the El Tor biotype grow better in the presence of excess carbohydrates and avoid acidification of their growth medium by production of the neutral fermentation end products acetoin and 2,3-butanediol (Fig. 1 to 3). Furthermore, other V. cholerae strains of recent clinical or environmental importance (i.e., O139 and non-O1/non-O139 serogroups) show the same phenotype as the El Tor biotype of O1 V. cholerae in terms of carbohydrate metabolism.
A molecular basis responsible for this dramatic difference was identified by a simple genetic screen (Fig. 3). 2,3-Butanediol fermentation is a highly conserved fermentation pathway present in many bacterial species and has recently been shown to be important for maintaining viability under carbohydrate-rich growth conditions in V. cholerae (27). Early physiological and biochemical characterization of this pathway in Enterobacter aerogenes and Klebsiella terrigena revealed that the production of 2,3-butanediol is enhanced under acidic and/or anaerobic growth conditions (3, 17). Consistent with these findings, an acetoin level was not detected until the medium pH dropped to ∼6.0 in the glucose-supplemented culture of N16961 (Fig. 1D and 5B). Most importantly, no further pH drop was observed until the exogenously added glucose was completely consumed (Fig. 5A), indicating that 2,3-butanediol synthesis is indispensable to prevent hyperacidification under such carbohydrate-rich growth conditions. Our data also showed that pH homeostasis in El Tor biotype strains was achieved by active degradation of acetate and lactate (Fig. 5C and D).
In V. cholerae, production of virulence factors is strongly influenced by environmental signals, such as pH and temperature. It is not clearly defined, however, why virulence gene expression, especially in strains of the classical biotype, is increased at pH 6.5 and 30°C (48). Recently, an intriguing link between virulence and energy metabolism in V. cholerae has been proposed by Kovacikova and colleagues (27). In this work, AphA, a transcriptional activator for the tcpPH promoter, was reported to repress the expression of genes for 2,3-butanediol synthesis by directly binding to the transcriptional start site of alsD (VC1589), the first gene in the cluster (27). In the same study, it was also shown that expression of biosynthetic genes was derepressed by AlsR (VC1588), which becomes activated upon acidification. This result might (i) suggest that AphA, coupled with AlsR, can reciprocally regulate virulence gene expression and 2,3-butanediol synthesis in response to pH change and (ii) provide a possible explanation for why the virulence gene expression is elevated at pH 6.5 (29, 48). Interestingly, when mutation of the aphA gene was introduced, expression of the tcpP gene was significantly decreased (>10-fold) in O395, whereas only a twofold decrease was observed to occur in C6706, another El Tor biotype strain (29). This suggests that AphA-dependent gene regulation may occur more actively in strains of classical biotype and, thus, gene expression for 2,3-butanediol synthesis is suppressed to a higher extent in classical biotype strains than in El Tor. Additional experiments are necessary in order to understand the different gene regulation by AphA in the two biotypes.
We also obtained evidence that 2,3-butandediol synthesis confers El Tor biotypes a survival advantage in vivo during infection. The N16961 als mutant (SSY01), which showed an in vitro growth phenotype in control media identical to that of its parental strain, was defective in colonizing the intestines of infant mice (Fig. 6). Because the colonization defect of this mutant became more severe when the inoculum was “spiked” with added glucose, we postulate that a glucose-mediated toxic effect in classical biotypes still occurs in vivo and that the functional 2,3-butanediol pathway is required for better colonization in the host intestine.
It is interesting to note that oral rehydration solution (ORS) containing 20 g/liter glucose (12, 40) is frequently used to treat the dehydration caused by V. cholerae infection (45, 47). ORS has been used since the early 1960s, and this period happens to correspond to the same decade in which the seventh-pandemic clone emerged and replaced the classical biotype. It is tempting to speculate that the glucose present in ORS may have contributed to an in vivo selection of El Tor strains, at least in those patients that were not simultaneously treated with antibiotics. Thus, ORS may have been antibacterial for classical biotype strains that would be sensitive to added glucose but stimulatory to El Tor biotype strains that actually could utilize this sugar with no deleterious effects.
Biofilms represent the major V. cholerae growth mode in aquatic environments (52). Thus, we also examined the role of 2,3-butanediol synthesis in pH homeostasis in planktonic cultures and biofilms growing in the presence of NAG, the end product of chitin degradation. In planktonic cultures, medium acidification and resultant cell death were observed for classical strain O395 and the N16961 als mutant SSY01 in the presence of NAG (Fig. 7A). Thus, both biotypes can metabolize NAG but 2,3-butanediol synthesis is required to avoid acid production and killing in planktonic cultures. Upon treatment with NAG, N16961 biofilms show a dramatic increase in biofilm cell density (Fig. 7B). In contrast, a decrease in cell viability and/or cell density was detected in biofilms of two sensitive strains, O395 and SSY01 (Fig. 7C and D). These data suggest that (i) El Tor strains growing as a biofilm can utilize NAG as a carbon and energy source and (ii) the utilization of chitin, a ubiquitous carbohydrate in the aquatic milieu, may exert a harmful effect on the survival of classical biotype strains when they grow in biofilms. Recently, it has been reported that V. cholerae becomes competent for DNA uptake and transformation when exposed to chitin (31), suggesting that growth on chitinous surfaces may be a common adaptation of this species and tied intimately to its genetic variability and evolution. Accordingly, it is interesting to note that classical strains are far less genetically diverse than non-O1, non-O139 strains (9). As noted earlier, non-O1 and non-O139 strains are similar to El Tor O1 strains in terms of carbohydrate metabolism (i.e., they produce 2,3-butanediol) and these strains are also quite unique in that they have acquired novel virulence loci, such as type III secretion, by horizontal transfer (9). Resistance to chitin-induced acidification of biofilms may be responsible for the higher levels of diversity seen in these strains driven by chitin-induced DNA transformation and recombination.
It has also been suggested that the displacement of the classical biotype by El Tor may be due to the ability of El Tor biotype strains to grow better in foods, such as cooked rice (26). Because the infectious dose of V. cholerae is known to be quite high for humans with normally acidic stomach contents, transmission via contaminated food may be more important than transmission through contaminated water alone. Recently, the results of mathematical modeling of phage-mediated modulation of cholera epidemics have provided compelling evidence that amplification of V. cholerae in food sources may an important rate-limiting step in cholera epidemics (16). Our results suggest that the El Tor biotype might replicate in carbohydrate-rich foodstuffs (e.g., rice) better than the classical biotype and that this too could have contributed to the demise of the classical biotype if food-mediated amplification of V. cholerae is a necessary step in the generation and propagation of cholera epidemics.
Acknowledgments
We thank David Raskin, Stefan Pukatzki, and Drew Revel for critical reading of the manuscript.
This work was supported by National Institutes of Health grant AI-18045 (to J.J.M.).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Barrett, T. J., and P. A. Blake. 1981. Epidemiological usefulness of changes in hemolytic activity of Vibrio cholerae biotype El Tor during the seventh pandemic. J. Clin. Microbiol. 13:126-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berche, P., C. Poyart, E. Abachin, H. Lelievre, J. Vandepitte, A. Dodin, and J. M. Fournier. 1994. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J. Infect. Dis. 170:701-704. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist, K., M. Nikkola, P. Lehtovaara, M. L. Suihko, U. Airaksinen, K. B. Straby, J. K. Knowles, and M. E. Penttila. 1993. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J. Bacteriol. 175:1392-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calia, K. E., M. Murtagh, M. J. Ferraro, and S. B. Calderwood. 1994. Comparison of Vibrio cholerae O139 with V. cholerae O1 classical and El Tor biotypes. Infect. Immun. 62:1504-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang, S. L., and J. J. Mekalanos. 2000. Construction of a Vibrio cholerae vaccine candidate using transposon delivery and FLP recombinase-mediated excision. Infect. Immun. 68:6391-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, B. M., K. E. Moyer, E. F. Boyd, and M. K. Waldor. 2000. CTX prophages in classical biotype Vibrio cholerae: functional phage genes but dysfunctional phage genomes. J. Bacteriol. 182:6992-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiRita, V. J., M. Neely, R. K. Taylor, and P. M. Bruss. 1996. Differential expression of the ToxR regulon in classical and E1 Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc. Natl. Acad. Sci. USA 93:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziejman, M., D. Serruto, V. C. Tam, D. Sturtevant, P. Diraphat, S. M. Faruque, M. H. Rahman, J. F. Heidelberg, J. Decker, L. Li, K. T. Montgomery, G. Grills, R. Kucherlapati, and J. J. Mekalanos. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. USA 102:3465-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraris, R. P., S. Yasharpour, K. C. Lloyd, R. Mirzayan, and J. M. Diamond. 1990. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. 259:G822-G837. [DOI] [PubMed] [Google Scholar]
- 11.Ghatak, A., A. Majumdar, and R. K. Ghosh. 2005. Structural organization of the transfer RNA operon I of Vibrio cholerae: differences between classical and El Tor strains. J. Biosci. 30:469-474. [DOI] [PubMed] [Google Scholar]
- 12.Guerrant, R. L., B. A. Carneiro-Filho, and R. A. Dillingham. 2003. Cholera, diarrhea, and oral rehydration therapy: triumph and indictment. Clin. Infect. Dis. 37:398-405. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 14.Holloway, B. W. 1969. Genetics of Pseudomonas. Bacteriol. Rev. 33:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 16.Jensen, M. A., S. M. Faruque, J. J. Mekalanos, and B. R. Levin. 2006. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc. Natl. Acad. Sci. USA 103:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen, L., K. Bryn, and F. C. Størmer. 1975. Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J. Bacteriol. 123:1124-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, J. A., C. A. Salles, P. Panigrahi, M. J. Albert, A. C. Wright, R. J. Johnson, and J. G. Morris, Jr. 1994. Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect. Immun. 62:2108-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonson, G., J. Sanchez, and A. M. Svennerholm. 1989. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J. Gen. Microbiol. 135:111-120. [DOI] [PubMed] [Google Scholar]
- 20.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 21.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaolis, D. K., R. Lan, and P. R. Reeves. 1995. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J. Bacteriol. 177:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keasler, S. P., and R. H. Hall. 1993. Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet 341:1661. [DOI] [PubMed] [Google Scholar]
- 24.Kirn, T. J., B. A. Jude, and R. K. Taylor. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438:863-866. [DOI] [PubMed] [Google Scholar]
- 25.Kirn, T. J., M. J. Lafferty, C. M. Sandoe, and R. K. Taylor. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35:896-910. [DOI] [PubMed] [Google Scholar]
- 26.Kolvin, J. L., and D. Roberts. 1982. Studies on the growth of Vibrio cholerae biotype eltor and biotype classical in foods. J. Hyg. (London) 89:243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova, G., W. Lin, and K. Skorupski. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57:420-433. [DOI] [PubMed] [Google Scholar]
- 28.Kovacikova, G., and K. Skorupski. 2000. Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae. J. Bacteriol. 182:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuyyakanond, T., S. Nakamura, W. Manmontri, and M. Iwanaga. 1990. Vibrio cholerae serogroup O1 in northeast Thailand. J. Clin. Microbiol. 28:872-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meibom, K. L., M. Blokesch, N. A. Dolganov, C. Y. Wu, and G. K. Schoolnik. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824-1827. [DOI] [PubMed] [Google Scholar]
- 32.Meibom, K. L., X. B. Li, A. T. Nielsen, C. Y. Wu, S. Roseman, and G. K. Schoolnik. 2004. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA 101:2524-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 34.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 35.Miller, J. H. (ed.). 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Murley, Y. M., J. Behari, R. Griffin, and S. B. Calderwood. 2000. Classical and El Tor biotypes of Vibrio cholerae differ in timing of transcription of tcpPH during growth in inducing conditions. Infect. Immun. 68:3010-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair, G. B., S. M. Faruque, N. A. Bhuiyan, M. Kamruzzaman, A. K. Siddique, and D. A. Sack. 2002. New variants of Vibrio cholerae O1 biotype El Tor with attributes of the classical biotype from hospitalized patients with acute diarrhea in Bangladesh. J. Clin. Microbiol. 40:3296-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neogy, K. N. 1965. Viability of V. cholerae and V. El Tor in food and water. Bull. Calcutta Sch. Trop. Med. 13:10-11. [PubMed] [Google Scholar]
- 39.Pukatzki, S., A. T. Ma, D. Sturtevant, B. Krastins, D. Sarracino, W. C. Nelson, J. F. Heidelberg, and J. J. Mekalanos. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 103:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishna, B. S., S. Venkataraman, P. Srinivasan, P. Dash, G. P. Young, and H. J. Binder. 2000. Amylase-resistant starch plus oral rehydration solution for cholera. N. Engl. J. Med. 342:308-313. [DOI] [PubMed] [Google Scholar]
- 41.Richardson, K., J. Michalski, and J. B. Kaper. 1986. Hemolysin production and cloning of two hemolysin determinants from classical Vibrio cholerae. Infect. Immun. 54:415-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rietsch, A., M. C. Wolfgang, and J. J. Mekalanos. 2004. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect. Immun. 72:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romick, T. L., and H. P. Fleming. 1998. Acetoin production as an indicator of growth and metabolic inhibition of Listeria monocytogenes. J. Appl. Microbiol. 84:18-24. [DOI] [PubMed] [Google Scholar]
- 44.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-233. [DOI] [PubMed] [Google Scholar]
- 45.Schultz, S. G., and P. F. Curran. 1970. Stimulation of intestinal sodium absorption by sugars. Am. J. Clin. Nutr. 23:437-440. [DOI] [PubMed] [Google Scholar]
- 46.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 47.Serebro, H. A., T. M. Bayless, T. R. Hendrix, F. L. Iber, and T. McGonagle. 1968. Absorption of d-glucose by the rabbit jejunum during cholera toxin-induced diarrhoea. Nature 217:1272-1273. [DOI] [PubMed] [Google Scholar]
- 48.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 49.Takeya, K., T. Otohuji, and H. Tokiwa. 1981. FK phage for differentiating the classical and El Tor groups of Vibrio cholerae. J. Clin. Microbiol. 14:222-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, R., C. Shaw, K. Peterson, P. Spears, and J. Mekalanos. 1988. Safe, live Vibrio cholerae vaccines? Vaccine 6:151-154. [DOI] [PubMed] [Google Scholar]
- 51.Waldor, M. K., and J. J. Mekalanos. 1994. Vibrio cholerae O139 specific gene sequences. Lancet 343:1366. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]