Abstract
Although Chlamydia infections are widespread throughout the world, data about immunopathogenesis of genitourinary tract infections in males are very limited. In the present work we present an in vitro model of male genital tract-derived epithelial cells, more precisely prostate epithelial cells (PEC), to analyze if they are susceptible and able to respond to Chlamydia muridarum infection. Our results demonstrate that rat PEC are susceptible to C. muridarum infection and respond to this pathogen by up-regulating different proinflammatory cytokine and chemokine genes that could participate in the recruitment and local activation of immune cells, therefore influencing innate and adaptive immune responses during Chlamydia infection. Moreover, we analyzed the expression of Toll-like receptor 4 (TLR4), TLR2, and related molecules on PEC and the effect of C. muridarum infection on their expression. Our results demonstrate that PEC express significant levels of TLR4, CD14, TLR2, and the adaptor molecule MyD88 and up-regulate these proteins in response to C. muridarum infection. Indeed, TLR4, CD14, TLR2, and the adaptor MyD88 are specifically recruited to the vicinity of the bacterial inclusion, suggesting that these TLRs are actively engaged in signaling from this intracellular location in these cells. This is, to our knowledge, the first time that an in vitro model of infection with Chlamydia of male tract-derived epithelial cells has been achieved, and it provides the opportunity to determine how these cells respond and participate in modulating innate and adaptive immune response during Chlamydia infections.
Genital Chlamydia trachomatis infection is the leading cause of bacterial sexually transmitted disease in industrialized countries, particularly among young people (43). The consequences of Chlamydia infection may involve urethritis, cervicitis, epididymitis, prostatitis, pelvic inflammatory disease, neonatal conjunctivitis, neonatal pneumonitis, ectopic pregnancy, and tubal factor infertility (30). Persistent infection of mucosal sites may contribute to the development of these chronic inflammatory diseases.
In women, progression of C. trachomatis infection into the upper reproductive tract causes significant inflammation and injury to the fallopian tubes, with enormous consequences for subsequent fertility (1, 30). The pathophysiology underlying Chlamydia female infertility has been extensively studied using an experimental genital tract infection model in mice with Chlamydia muridarum, a murine pathogen closely related to C. trachomatis (3, 4, 6). However, several aspects of the pathophysiology of the infection remain unclear. It is well accepted that specific Th1 cells are involved in the immunopathogenesis of the disease (4, 7, 24, 37). Recently, a more dominant role in the pathophysiology of fallopian tube scarring (a major consequence of Chlamydia infection) has been attributed to the infected epithelial cells themselves. It has been postulated that the proinflammatory cytokines secreted by Chlamydia-infected epithelial cells and the persistence of the bacteria for long periods in the female tract could be the major responsible events for the fibrosis and scarring (44). Indeed, oviduct, endometrial, and endocervical epithelial cells respond to the infection by Chlamydia with a plethora of cytokines (21, 36, 48) such as interleukin-1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor with this putative pathogenic role (21).
On the other hand, although Chlamydia infections in males are widespread throughout the world, data on the immunopathogenesis of genitourinary tract infections in males are very limited. Among the more serious consequences of Chlamydia infections in males are urethritis and epididymitis that can very rarely result in abscess formation and infarction of the testicle (29, 46). Chronic prostatitis, another syndrome that has been related to male infertility, has also been associated with Chlamydia infections (10, 25, 47). Ascending Chlamydia infections have been thought to be an infective cause of prostatitis. Chronic prostatitis has been proved to cause scarring of the prostatic and ejaculatory ducts, resulting in low seminal volume with low fructose and alpha-glucosidase values and, consequently, severe alterations in sperm quality (9). Taking into account that the diagnostic materials (expressed prostate secretion, urine post-prostate massage, prostate biopsy specimens from the gland, and ejaculate samples) have to go through the urethra, a definitive association of an infective agent and its prostate origin is limited by factors such as urethral contamination.
The main goal of this work was to determine if prostate epithelial cells (PEC) are susceptible to Chlamydia infection and, if so, if they can respond to infection by up-regulating proinflammatory mediators that could potentially turn these cells into dominant players in the pathophysiology of the disease. We demonstrate that PEC are susceptible to C. muridarum infection and that they respond by up-regulating different proinflammatory and chemokine genes that may influence innate and adaptive immune responses during Chlamydia infections. We also show that PEC express significant levels of Toll-like receptor 4 (TLR4), CD14, and TLR2 and that they up-regulate these receptors in response to C. muridarum infection.
MATERIALS AND METHODS
Antibodies and reagents.
Mouse anti-Chlamydia spp. lipopolysaccharide (cLPS)-fluorescein isothiocyanate (FITC) monoclonal antibody was obtained from Biomerieux. Goat polyclonal anti-TLR4 antibody (L-14; recognizing an extracellular domain of mouse TLR4 and reacting with rat TLR4), rabbit polyclonal anti-CD14 (M-305; recognizing an internal region of recombinant CD14 of mouse origin and also reacting with rat CD14), goat polyclonal anti-TLR2 (D-17; recognizing a peptide near the N terminus of mouse TLR2 and reacting with rat TLR2), rabbit polyclonal anti-TLR5 antibody (H-127; recognizing a peptide near the N terminus of human TLR5 and reacting with rat TLR5), and goat polyclonal anti-MyD88 antibody (recognizing mouse MyD88 and reacting with rat MyD88) as well as mouse monoclonal anti-rat NF-κB p65 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies conjugated with FITC, Alexa 546, rhodamine, Cy-3, and corresponding isotype controls were purchased from Molecular Probes (Pitchford, OR). Griess reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture.
The MAT-LU cell line (part of the John Hopkins Special Collection) (19) is a rat adenocarcinoma cell line with characteristics of PEC obtained from American Type Culture Collection (Manassas, VA). MAT-LU cells were grown in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin, 100 units/ml streptomycin, and 250 nM dexamethasone in 5% CO2 in air at 37°C, as described previously (19).
To obtain primary PEC (PPEC), we followed the protocol described by Ilio et al. (18). Briefly, Wistar rat ventral prostates were minced in phosphate-buffered saline (PBS; pH 7.4) containing 1.0 mM dithiothreitol. After gentle stirring at 37°C for 30 min, the supernatant was discarded, and 10 ml of a dissociation solution containing enzyme type IV collagenase (200 U/ml) and trypsin (0.25%) in RPMI-1640 medium was added. After incubation at 37°C for 2 h, the tissue pieces were then passed through a tissue sieve. Cells were then washed twice in RPMI medium supplemented with 10% fetal calf serum and 5 μg/ml insulin, 0.4 μg/ml hydrocortisone, and 1 μg/ml epidermal growth factor. Cells were then cultured in 24-well plates for several days. Because murine and rat epithelial cells have properties not shared by other stromal cell types such as the expression of cytokeratins, we determined whether our PPEC express cytokeratins using a monoclonal anti-pan-cytokeratin (clone C-11, recognizing several cytokeratins [4, 5, 6, 8, 10, 13, and 18]) (catalog no. C2931; Sigma-Aldrich, St. Louis, MO) Cytokeratin 5 has been described to be a marker of basal epithelial cells and cytokeratin 8 and 18 are markers of luminal cells (45). The confluent cells were trypsinized with 0.05% trypsin-EDTA to produce single cells, after which they were seeded at 4 × 104 cells per cm2 and allowed to form subcultures.
Chlamydia strain and infection protocol. (i) Chlamydia strain.
The C. trachomatis MoPn strain (now C. muridarum MoPn) was kindly supplied by K. H. Ramsey (United States) and was propagated in LCCMK2 cells. Briefly, cultures were grown in RPMI-1640 medium supplemented with 20 μg of gentamicin/ml and 5% fetal bovine serum at 37% °C-5% CO2. Cultures infected with C. muridarum were grown for 72 h in the presence of 1 μg/ml of cycloheximide. Infected monolayers were detached by scraping and disrupted by sterile glass beads to lyse the host cells and release elementary bodies. Cell debris was removed by centrifugation at 500 × g for 15 min. Chlamydial elementary bodies were pelleted and resuspended in an isotonic sucrose-phosphate-glutamate buffer, and aliquots were stored frozen at −80°C. Infectious titers of this suspension were determined by titration on LLCMK2 cell monolayers, which were stained with an FITC-monoclonal antibody (MAb) against cLPS after 48 h of culture.
(ii) Infection protocol.
Epithelial cells were seeded into 24-well plates at a density of 3 × 105 cells/well. Monolayers were washed twice with Hanks balanced salt solution and infected with C. muridarum using a multiplicity of infection (MOI) of 0.15 to 15. The plates were spun at 1400 × g for 60 min and placed at 5% CO2 in air at 37°C for 2 h. After the incubation, the inoculum was washed twice with Hanks balanced salt solution and replaced by supplemented medium lacking gentamicin. Cultures were incubated at 37°C for up to 3 days. In some experiments, supernatants were removed at different times after infection to determine cumulative nitric oxide (NO).
Quantitation of C. muridarum infection and direct immunofluorescence.
To determine the level of infection, monolayers of MAT-LU or PPEC cells were grown in 24-well tissue culture plates on sterile coverslips and subsequently infected at different MOIs as described above. Following incubation for 48 h, cells were fixed with methanol and stained with anti-cLPS monoclonal antibody, and the presence of inclusion bodies was measured by fluorescent microscopy.
Intracellular staining of PPEC followed by flow cytometric analysis was performed on cells 48 h after the infection following the protocol described by Young et al. (49). Briefly, cells were harvested by incubation with trypsin-EDTA (Sigma) for the intracellular staining; cells were then incubated with the anti-Chlamydia LPS monoclonal antibody or an appropriate isotype control in fluorescence-activated cell sorter wash buffer (5 mM EDTA, 0.1% sodium azide, and 1% bovine serum albumin in PBS) supplemented with 0.1% saponin for 30 min at 4°C. Cells were analyzed with a Cytoron Absolute (Ortho Diagnostic System, Raritan, NJ), and the data were subsequently analyzed using WinMDI software.
Quantitation of NO in culture supernatants.
One day before infection, MAT-LU and PPEC cells were plated in 24-well plates (5 × 105 cells/well). Cells were infected at different MOIs of C. muridarum for different time periods (24 to 72 h). After C. muridarum infection, production of nitrites was measured by using the Griess reagent. Briefly, Griess reagent was prepared by mixing equal volumes of sulfanylamide (1.5% in HCl) and N-(1-naphtyl) ethylenediamide dihydrochloride (0.13% in H2O). Griess reagent (200 μl) was then mixed with 100 μl of supernatant sample and incubated for 15 min in the dark. Absorbance was measured at 540 nm, and nitrite concentration was calculated using a calibration curve. Nitrite was not detectable in cell-free medium.
RT-PCR analysis.
A standard reverse transcription-PCR (RT-PCR) assay was used in this study. Briefly, total RNA was isolated from MAT-LU cells or PPEC using Trizol reagent (Life Technologies) according to the manufacturer's protocol. Reverse transcription reactions were performed using 2 to 3 μg of total mRNA in a 25-μl mixture. Total RNA was first incubated with 0.5 μg of oligo(dT) primer (Promega, Madison, WI) for 10 min at 65°C and allowed to stand at room temperature for 2 min. Samples were then incubated with a 1.25 mM concentration of each deoxynucleoside triphosphate (Promega, Madison, WI), 10 U of RNasin Inhibitor (Boehringer Mannheim), and 16 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) for 1 h at 42°C in reverse transcriptase buffer. The cDNA obtained was subjected to PCR amplification using the following primers and PCR protocols described previously: rat β-actin (15), rat inducible nitrous oxide synthase (iNOS) (15), TNF-α (42), rat IL-1 (28), rat IL-6 (28), rat CCL2, rat CCL3, rat CCL4, rat CCL5, rat CXCL8, rat CXCL10, rat TLR4, rat CD14, rat TLR2 (15), and rat intercellular adhesion molecule (ICAM) (23). The conditions were chosen so that none of the RNAs analyzed reached a plateau at the end of the amplification protocol (in most reactions, 30 cycles of amplification were used). The PCR products were visualized in 2% agarose gels with ethidium bromide staining.
To semiquantitate and compare cDNA levels, the gels were photographed, and the intensities of the bands were analyzed using Scion Image software. The relative band intensities in each reaction were normalized to the mean intensity of the β-actin band. Results are expressed as arbitrary units corresponding to the ratio of sample intensities to the β-actin band intensity.
Confocal microscopy.
PPEC or MAT-LU cells were plated onto 12-mm circular coverslips in 24-well plates (5 × 105 cells/well) and cultured overnight. The cells were then infected and incubated for different time periods. After that period, coverslips were washed twice in PBS and then fixed with 3% formaldehyde in PBS for 10 min at room temperature. Following fixation, coverslips were washed twice in PBS and cells then were permeabilized by treatment with 0.2% Triton X-100 in PBS for 10 min, washed three times with PBS, and blocked with PBS containing 1% bovine serum albumin plus Tween-20 (0.1%, vol/vol) (blocking buffer) for 1 h at room temperature in a humid chamber. Cells were then incubated for 3 h at 4°C with blocking buffer containing antibodies against cLPS-FITC primary antibodies and antibodies against TLR4, CD14, TLR2, TLR5, and MyD88; cells were then washed with PBS-Tween 20 (0.1%, vol/vol) and incubated for 2 h at room temperature in blocking buffer with the corresponding secondary antibodies conjugated with Alexa 546, rhodamine, or Cy-3. Coverslips were washed three times, mounted with Prolong Antifade (Molecular Probes, Pitchford, OR) and visualized on an LSM 510 confocal laser scanning microscope (Zeiss, Thornwood, NY) using LSM 510 software for image analysis.
To semiquantitate the levels of activation of NF-κB, PPEC were plated onto 12-mm circular coverslips in 24-well plates (5 × 105 cells/well), cultured overnight, and then exposed to C. muridarum using an MOI of 15 for 1 h. After that period, coverslips were washed twice in PBS, fixed with 3% formaldehyde, washed, and permeabilized as described above. Cells were then incubated for 3 h at 4°C with blocking buffer containing antibodies against the p65 subunit of NF-κB. After that, PPEC were washed and then incubated with the corresponding secondary antibody and DAPI (4′,6′-diamidino-2-phenylindole). To semiquantitate the levels of activation of NF-κB, coverslips were visualized on an LSM 510 confocal laser scanning microscope; 10 fields of each condition were then analyzed, and the percentage of cells that presented nuclear p65 staining was calculated as follows: (number of cells with NF-κB p65 in the nucleus × 100)/total number of cells.
Statistical analysis.
Statistical analysis was performed using the LSD Fisher test and the InfoStat software (developed by Statistics Department, National University of Córdoba). Values of P of <0.05 were considered significant.
RESULTS
C. muridarum infects and replicates in prostate epithelial cells.
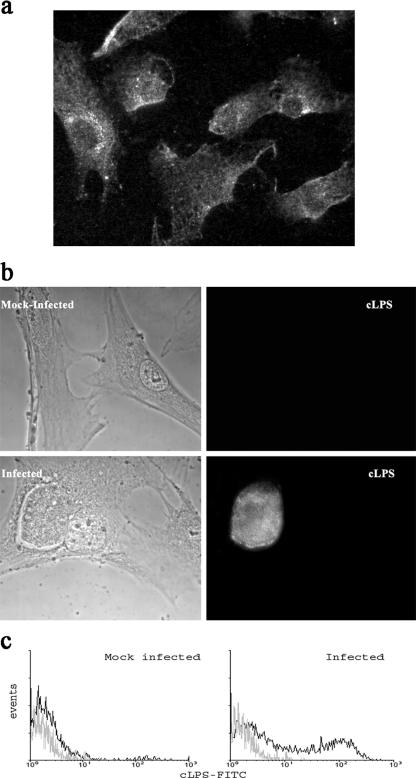
Although a significant number of reports associate chronic prostatitis with Chlamydia infection, very little is known about the capacity of C. muridarum to infect and replicate on PEC. Therefore, we analyzed if C. muridarum can also infect and replicate in a rat adenocarcinoma cell line with characteristics of prostate epithelial cells (MAT-LU) and in a PPEC line. The PPEC line, a nontransformed primary rat PEC line, was derived from rat prostate tissue in our laboratory, and as can be seen in Fig. 1a, more than 80% of the cells were positively stained for cytokeratin, retaining this marker after several passages. Infection of MAT-LU cells and PPEC resulted in the formation of inclusions, as detected by staining with an anti-Chlamydia antibody followed by fluorescence microscopy. By fluorescence, typical Chlamydia-containing inclusions were detected in MAT-LU cells (data not shown) and in PPEC (Fig. 1b). The infection rates ranged from 20 to 30% for MAT-LU cells and 30 to 40% for PPEC at MOIs of 15:1.
FIG. 1.
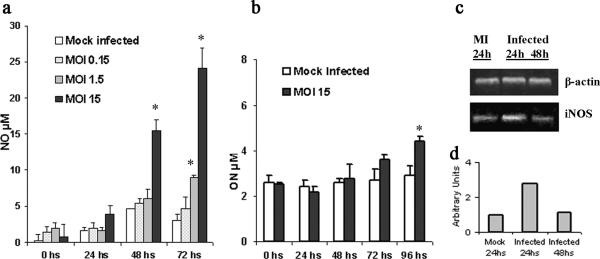
Infection of PPEC with C. muridarum. Light and fluorescence micrographs of PPEC. Cell monolayers were infected with C. muridarum (15 IFU/cell) as described in Materials and Methods and examined by fluorescence microscopy 48 h later. (a) PPEC were incubated with anti-cytokeratin antibody and Cy-3-labeled secondary antibodies. (b) PPEC stained with FITC-labeled MAb specific for cLPS. (c) Flow cytometry analysis showing the presence of intracellular cLPS in C. muridarum-infected PPEC. PPEC monolayers were infected with C. muridarum using an MOI of 15 and incubated for 48 h. Cells were treated with 0.1% saponin and incubated with FITC-labeled cLPS-specific antibodies. Figures are representative of at least five experiments performed.
We also used intracellular staining, followed by fluorescence-activated cell sorter analysis to visualize infection of cell lines with C. muridarum. As shown in Fig. 1c, when viable C. muridarum was used to infect PPEC, brightly stained populations of infected cells could be seen 48 h after infection. No staining was observed in mock-infected PPEC (Fig. 1c). Infected cells did not stain when they were fixed only, indicating that C. muridarum was intracellular (data not shown).
To determine if C. muridarum could complete its biphasic developmental cycle, supernatants from infected PPEC were collected after 48 h and used to reinfect a highly susceptible epithelial cell line such as LLCMK2. The supernatants were able to infect LLCMK2 cells, demonstrating the presence of elementary bodies that can be transformed into metabolically active reticulate bodies that replicate and form inclusions in LLCMK2 cells (data not shown).
Rat PEC respond to Chlamydia infection by up-regulating proinflammatory mediators.
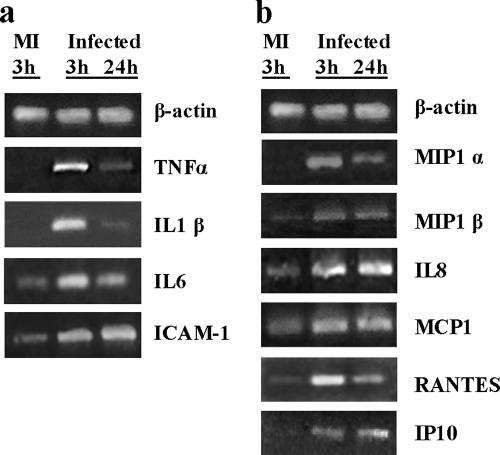
Epithelial cells have been postulated to play a dominant role in the pathophysiology of the lesion observed in infected females owing to the number of proinflammatory cytokines secreted in response to infection (36). Moreover, the plethora of inflammatory mediators produced may influence innate and adaptive immune responses during Chlamydia infections. In order to see if Chlamydia infection induces the same phenomenon in PEC, induction of chemoattractant and proinflammatory mediators was evaluated. PEC monolayers were infected with C. muridarum and cultured for up to 3 days to encompass at least one entire cycle of Chlamydia development. The initial studies focused on the production of NO in the supernatants of MAT-LU infected cells as a simple approach to begin unraveling the behavior of PEC upon C. muridarum infection. A significant NO production 48 and 72 h postinfection of MAT-LU cells was observed, with higher values for high doses of infection (Fig. 2a). PPEC also secrete NO following infection, although the levels reached by infected PPEC were considerably lower than those achieved by infected MAT-LU cells (Fig. 2b). C. muridarum infection of MAT-LU cells induced at least a threefold increase in the expression of iNOS-specific mRNA as early as 24 h poststimulation (Fig. 2c and d), suggesting that iNOS could be responsible, at least in part, for the levels of NO measured. When the expression of proinflammatory cytokines was evaluated at different times of infection, up-regulation of TNF-α-, IL-1-β-, and IL-6-specific mRNAs was observed as early as 3 h postinfection; this increase was sustained for at least 24 h (Fig. 3a). The expression of ICAM-specific mRNA was also evaluated. Some basal expression of ICAM-specific mRNAs could be observed in nontreated cells. C. muridarum infection induced a 2.5-fold increase in its expression as early as 3 h after infection (Fig. 3a), an increase that was sustained for 24 h.
FIG. 2.
Effect of Chlamydia infection on NO production. (a) Nitrite concentrations at various times postinfection from MAT-LU cells following mock infection or infection with different MOIs of C. muridarum. (b) NO concentrations at various times postinfection from PPEC cells infected with C. muridarum (MOI of 15). (c) Qualitative RT-PCR analysis of iNOS and β-actin mRNA. MAT-LU cell monolayers infected with 15 IFU/cell of C. muridarum. Total RNA was extracted at the indicated times after infection. (d) Semiquantitation of iNOS-specific mRNA in infected and mock-infected MAT-LU cells (see Materials and Methods). Errors bars represent standard deviations of triplicate assays. Significant differences are indicated with asterisks (P < 0.05). Figures are representative of at least three experiments performed. MI, mock infected.
FIG. 3.
Cytokine and chemokine mRNA induction during Chlamydia infection. PPEC monolayers in 25-cm2 flasks were infected at an MOI of 15 of C. muridarum as described in Materials and Methods. Total RNA was extracted at the indicated times after infection and analyzed by a qualitative RT-PCR to assess the expression of TNF-α, IL-1 β, IL-6, ICAM-1 (a), MIP-1α, MIP-1β, IL-8, MCP-1, RANTES, IP-10 (b), and β-actin in mock-infected and C. muridarum-infected cultures. Figures are representative of at least three experiments performed.
We then investigated if our PPEC line could up-regulate the expression of different chemokine genes. As shown in Fig. 3b, C. muridarum infection was able to induce the expression of CCL3 (macrophage inflammatory protein 1α [MIP-1α])-, CCL4 (MIP-1β)-, CCL5 (RANTES)-, and CXCL10 (inducible protein 10 [IP-10])-specific mRNAs, which were otherwise undetectable. Although some basal expression of CXCL8 (IL-8)-specific and CCL2 (monocyte chemoattractant protein 1 [MCP-1])-specific mRNAs could be observed, C. muridarum infection induced a two- to threefold increase in their expression, respectively, as early as 3 h after infection (Fig. 3b); this increase was unchanged for at least 24 h.
Taken together, these findings strongly demonstrate that PEC can respond to C. muridarum infection, secreting inflammatory mediators like NO and up-regulating the expression of proinflammatory and chemokine genes.
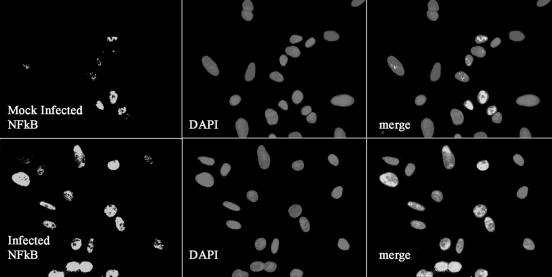
Rat PEC respond to C. muridarum infection by activating nuclear translocation of NF-κB.
The responsiveness of PPEC to C. muridarum infection shown above led us to examine the activation of the transcription factor NF-κB. PPEC were cultured on coverslips and subjected to C. muridarum infection for various time periods; indirect immunofluorescence staining was performed for the p65 subunit of NF-κB, and the nuclear localization of the transcription factor was analyzed by confocal microscopy. As shown in Fig. 4, noninfected PPEC presented basal levels of nuclear localization of NF-κB (approximately 20% of cells presented nuclear staining). In contrast, p65 nuclear translocation increased after 1 h of infection in about 50% of the cells. Thus, C. muridarum infection of PPEC induces NF-κB activation, which explains the activation of numerous proinflammatory genes.
FIG. 4.
NF-κB activation in PPEC induced by C. muridarum infection. PPEC were exposed to C. muridarum at an MOI of 15 for 2 h. Cells were subsequently stained with anti-p65 MAb and Cy-3-labeled secondary antibodies. Nuclei were counterstained with DAPI. NF-κB nuclear localization results in colocalization of red and blue signals resulting in rose fluorescence. Cells were visualized by confocal microscopy. The experiment was performed twice, and the pictures shown correspond to representative fields.
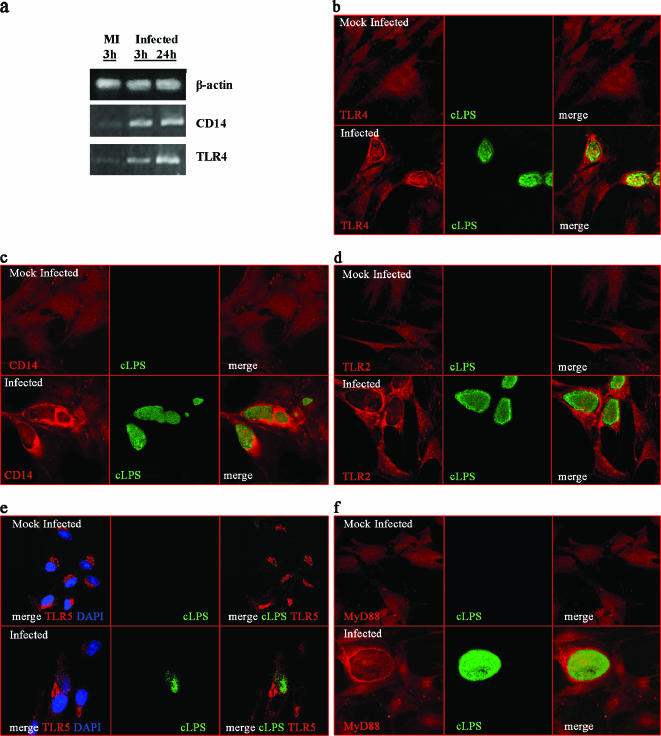
Infected PEC recruit TLR4, CD14, TLR2, and the adaptor molecule MyD88 around the inclusion.
Specific TLR recognition of cLPS does not have a consensus. Some reports have proposed that cLPS, like other forms of LPS, uses TLR4 for signaling in a CD14-dependent fashion, while others postulated TLR2 recognition (13, 34). We have recently reported that MAT-LU cells do not express TLR4, CD14, and TLR2 at the cell surface but that they do express them intracellularly and that this intracellular localization does not impede cellular activation (15). So, we wanted to investigate if PPEC express these proteins and if Chlamydia infection could change either the level of expression or the subcellular localization of these receptors. TLR4- and CD14-specific mRNAs were present in PPEC (Fig. 5a) in a constitutive way. However, up-regulation of TLR4 and CD14 mRNAs levels was seen as early as 3 h after infection and continued increasing after 24 h (Fig. 5a). To directly analyze the expression of TLR4 and CD14 by PPEC infected with C. muridarum, immunocytochemistry examination using confocal image analysis was performed. PPEC were stained for cLPS (to identify the infected cells) and for TLR4 and CD14 (Fig. 5b and c). A diffuse cytoplasmic distribution of TLR4 protein with no specific pattern was apparent in noninfected PPEC. In contrast, infected PPEC, which showed large cytoplasmic inclusions, presented an increase in the expression of TLR4, especially around the inclusion bodies (Fig. 5b). The same phenomenon was observed when MAT-LU cells were infected, showing again an increased expression of TLR4, especially around the inclusions (data not shown). When the expression of CD14 was analyzed, a similar pattern was observed, with an enhancement of its expression in infected PPEC and strong reactivity around the inclusions (Fig. 5c).
FIG. 5.
TLR4, CD14, TLR2, TLR5, and MyD88 expression in PPEC infected with C. muridarum. (a) RT-PCR analysis of mock-infected or C. muridarum-infected PEC. PPEC were infected at an MOI of 15 with C. muridarum as described in Materials and Methods. Total RNA was extracted at the indicated time after infection and analyzed by a qualitative RT-PCR. Confocal microscopy analysis of TLR4, CD14, TLR2, TLR5, and MyD88 expression on PPEC infected with C. muridarum. PPEC were grown in coverslips and infected with C. muridarum. Forty-eight hours postinfection cells were fixed, permeabilized, and stained using specific antibodies labeled with Alexa 546 or rhodamine. The appearance of chlamydia inclusions was revealed with FITC-labeled MAb specific for cLPS. Fluorescence micrographs of PPEC stained with TLR4-specific antibody (b), with CD14-specific antibody (c), with TLR2-specific antibody (d), and with TLR5-specific antibody. Nuclei were counterstained with DAPI (e) and with MyD88-specific antibody (f).
Other reports have previously shown that Chlamydia infection also involved TLR2 activation (6) and that a number of known and hypothetical lipoproteins that would be potential TLR2 ligands are present in the Chlamydia genome. When expression of TLR2 was investigated in PPEC by confocal microscopy, a comparable event was observed, with a disseminated cytoplasmic pattern of staining in mock-infected PPEC and a marked redistribution around the inclusion in infected PPEC (Fig. 5d). For the purpose of analyzing the expression of other TLRs not previously associated with Chlamydia, experiments exploring the expression of TLR5 on C. muridarum-infected PPEC were performed. The results showed a supranuclear distribution of TLR5 in mock-infected PPEC that was kept invariable upon chlamydial infection (Fig. 5e). These results strengthen the hypothesis that the most involved TLRs in chlamydial infection (TLR4 and TLR2) are the main ones recruited to the vicinity of the inclusion.
In order to evaluate if a signaling event triggered by the recognition of TLR4/TLR2 of putative components of the chlamydial inclusion (which culminates in NF-κB translocation) also starts around the inclusion, we performed staining of MyD88 in infected and noninfected cells (Fig. 5f). We could observe that the expression of MyD88 in infected cells was enriched at the periphery of the inclusion in a rim-like staining pattern indistinguishable from the intracellular staining of chlamydial inclusion membrane. Our data further demonstrate that TLR4, CD14, TLR2, and the adaptor MyD88 accumulate in the vicinity of the bacterial inclusion membrane during a productive infection with C. muridarum and suggest that TLR4 and TLR2 are actively engaged in signaling from this intracellular location in PEC.
DISCUSSION
Information currently available about the pathogenesis and immunity to C. trachomatis infection in males is scarce. Several reports regarding infection of the genital tract of female mice with C. muridarum have provided much-needed insights into the immunopathogenesis of the disease in females. Moreover, in vitro models of infection have added significant information about the contribution of particular cells to the pathogenic process (8, 21, 44). In the present work, we sought to implement similar systems that could add information to our current limited knowledge of C. trachomatis infection in males. Our results show that rat PEC are susceptible to C. muridarum infection and respond to this pathogen by up-regulating different proinflammatory cytokine and chemokine genes. To our knowledge, there are no previous reports regarding epithelial cells from the male urogenital tract and the consequences of their infection with C. trachomatis or C. muridarum. Oberley et al. (26), the only group that has attempted to approach this point, have used human PEC lines to analyze the role of surfactant protein D in the innate immunity of the prostate. These authors have shown that surfactant protein D inhibits the infection of LNCaP and P69SV40T by C. trachomatis in an in vitro infection assay; however, the investigators have not determined what the response is of the epithelial cells upon infection. No other publications regarding in vitro C. trachomatis infection of epithelial cells from epididymis, seminal vesicle, urethra, or vas deferens have been presented.
Data regarding in vivo Chlamydia male genital tract infection are not much more abundant. Pate et al. (32) demonstrated increased levels of IL-8 and specific Chlamydia antibodies in penile urethral swabs from males with PCR-confirmed C. trachomatis infection. When Wistar rats were inoculated in the vas deferens with Chlamydia psittaci, the pathogen was isolated from epididymis, testis, and prostate gland until 70 days after inoculation (20). The prostate gland of the inoculated animals presented mild interstitial edema and mild mononuclear inflammation. Similarly, S. Pal et al. (31) demonstrated that inoculation of male mice in the meatus urethra with C. muridarum resulted in an infection of the genitourinary tract and a specific humoral and cellular response with gamma interferon (IFN-γ). It appears that the type of inflammatory infiltration found in urethra and urinary bladder depends on the kind of chemokines and cytokines up-regulated by infected epithelial cells of the male genital tract.
Existent data regarding female tract epithelial cell cytokine and chemokine responses upon infection with C. trachomatis are contradictory. Indeed, the pattern of cytokines secreted seems to vary depending on whether epithelial tumor or nontumoral cell lines were used (8, 17, 21, 36). This fact suggested that in vitro models based on epithelial tumor cell lines might not fully mimic the immunobiology of Chlamydia-infected epithelium. For this reason, in the present work we have used MAT-LU cells (a rat prostate epithelial tumor cell line) and rat primary cultured epithelial cells and found that both types of cells are susceptible to C. muridarum infection and respond in a similar way. MAT-LU cells respond to C. muridarum infection by secreting NO and up-regulating iNOS mRNA, findings that are in agreement with those reported in female tract epithelial cells in which NO production was involved in the mucosal defense against Chlamydia (38). Infected PPEC also up-regulated proinflammatory cytokine and chemokine genes that have consequences on the recruitment of neutrophils, macrophages, monocytes, dendritic cells, natural killer cells, and T lymphocytes such as IL-8, MCP-1, MIP-1α, RANTES, and IP-10 (11, 12). Moreover, it is an accepted fact that the host response to urogenital infections by C. trachomatis is dominated by IFN-γ, both in animal models and human infections (4, 7, 24, 37). Our data suggest that Chlamydia-infected PEC could facilitate this IFN-γ production through the up-regulation of chemokines that preferentially recruit Th1 over Th2 lymphocytes, such as IP-10 (39).
The limited data about in vivo or in vitro male genital tract infections with Chlamydia do not allow us to determine whether our in vitro results are in agreement with what it is happening in Chlamydia-infected men. The major problem in studying Chlamydia infections in human individuals is the fact that most of them are asymptomatic, and for that reason, it is difficult to know if a patient is in the course of a primary infection, a reinfection, or a chronic one. This difficulty generates heterogeneous groups of patients enrolled for only positive Chlamydia detection in particular studies.
Data regarding the expression of TLRs on epithelial cells from the genital tract are mainly related to the female genitourinary tract (33, 40). It has been shown that primary and immortalized epithelial cells derived from the ectocervix and endocervix express TLRs 1 to 6 but lack TLR4 (33), while a transformed human uterine epithelial cell line and primary uterine epithelial cells express TLRs 1 to 9, including TLR4 (14). Similar results were shown for murine oviduct epithelial cell lines that also lack TLR4 (8).
In the present report, we demonstrate that rat primary PEC constitutively express TLR4, CD14, TLR2, TLR5, and the adaptor molecule MyD88, making these cells able to respond to numerous known and hypothetical ligands present in C. muridarum. Indeed, after C. muridarum infection, TLR4- and CD14-specific mRNA up-regulation was seen. Moreover, infected PEC recruit TLR4, CD14, TLR2, and the adaptor molecule MyD88 around the inclusion bodies, suggesting that TLR4 and TLR2 are actively engaged in signaling from this intracellular location in prostate epithelial cells.
It is well known that Chlamydia is an intracellular bacterium which differs from other intracellular pathogens in its unique ability to form inclusions (41). The subcellular localization of the chlamydial inclusion in the Golgi region was thought to provide an immunologically hidden site for the bacteria to evade immune detection. However, the active recruitment of TLR4, CD14, TLR2, and MyD88 around the intracellular chlamydial inclusion suggests that, in fact, this is not the case. Our results are in accordance with those reported by O'Connell et al. (27) using TLR4- and TLR2-transfected human cervical epithelial cell lines. They suggest that intracellular TLR2 could be responsible for the initiation of signal transduction events during infection with C. trachomatis (27).
Chlamydia expresses a variety of antigens that could serve as potential TLR ligands. For example, cLPS has been extensively studied (35) and has been found to have low endotoxic activity (16); it is currently under discussion whether LPS signals through TLR4 or TLR2 (13, 34). A second important Chlamydia antigen is the heat shock protein. Indeed, purified HSP60 preparations were able to activate the transcriptional factor NF-κB (22), although it is still not clear if Chlamydia HSP60 could activate TLR2 and/or TLR4 (2, 5). The Chlamydia genome has also been shown to contain a number of known and hypothetical lipoproteins that would be potential TLR2 ligands. While a variety of Chlamydia antigens might be recognized by individual TLRs, the role of specific TLRs during a productive infection with live bacteria remains unclear. Remarkably, using TLR2 knockout (KO) and TLR4 KO mice in in vivo models of C. trachomatis female genital tract infection, Darville et al. (6) showed that TLR2 KO mice exhibited a significant reduction in oviduct and mesosalpinx pathology at late time points, while TLR4 KO mice responded to in vivo infection similarly to wild-type controls and developed similar pathology.
The lack of an experimental model and the limited access to specimens from the human genitourinary tract have hindered our understanding of the pathogenesis of C. trachomatis infections in males. The implementation and extensive analysis of in vitro and in vivo models of male genital infection will allow the characterization of the main features of acute and chronic stages of Chlamydia infection in males and the possible immune pathogenic mechanisms implicated in it. This is, to our knowledge, the first time that an in vitro model of infection of male tract-derived epithelial cells was achieved, and it should provide an opportunity to determine how epithelial cells from the male genitourinary tract respond and how they participate in influencing the innate and adaptive immune response of the host.
Acknowledgments
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) PICT 2003 05-13624, Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET) PIP number 6437, and Fundación Antorchas 2003 (to V.R.).
We thank Sirard for providing us some antibodies and Paula Icely for technical assistance.
J.P.M.-O. is a Ph.D. fellow of CONICET. M.M. and V.E.R. are members of the Researcher Career of CONICET.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Andersen, B., L. Ostergaard, E. Puho, M. V. Skriver, and H. Schonheyder. 2005. Ectopic pregnancies and reproductive capacity after Chlamydia trachomatis positive and negative test results: a historical follow-up study. Sex. Transm. Dis. 32:377-381. [DOI] [PubMed] [Google Scholar]
- 2.Ausiello, C. M., G. Fedele, R. Palazzo, F. Spensieri, A. Ciervo, and A. Cassone. 2006. 60-kDa heat shock protein of Chlamydia pneumoniae promotes a T helper type 1 immune response through IL-12/IL-23 production in monocyte-derived dendritic cells. Microbes Infect. 8:714-720.. [DOI] [PubMed] [Google Scholar]
- 3.Belay, T., F. O. Eko, G. A. Ananaba, S. Bowers, T. Moore, D. Lyn, and J. U. Igietseme. 2002. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 70:844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 5.Costa, C. P., C. J. Kirschning, D. Busch, S. Durr, L. Jennen, U. Heinzmann, S. Prebeck, H. Wagner, and T. Miethke. 2002. Role of chlamydia heat shock protein 60 in the stimulation of innate immune cells by Chlamydia pneumoniae. Eur. J. Immunol. 32:2460-2470. [DOI] [PubMed] [Google Scholar]
- 6.Darville, T., J. M. O'Neill, C. W. Andrews. Jr., U. M. Nagarajan, L. Stahl, and D. M. Ojcius. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydia genital tract infection. J. Immunol. 171:6187-6197. [DOI] [PubMed] [Google Scholar]
- 7.Debattista, J., P. Timms, and J. Allan. 2003. Immunopathogenesis of Chlamydia trachomatis infections in women. Fertil. Steril. 79:1273-1287. [DOI] [PubMed] [Google Scholar]
- 8.Derbigny, W. A., M. S. Kerr, and R. M. Johnson. 2005. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J. Immunol. 175:6065-6075. [DOI] [PubMed] [Google Scholar]
- 9.Dohle, G. R. 2003. Inflammatory-associated obstructions of the male reproductive tract. Andrologia 35:321-324. [PubMed] [Google Scholar]
- 10.Domingue, G. J., Sr., and W. J. Hellstrom. 1998. Prostatitis. Clin. Microbiol. Rev. 11:604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 12.el-Sawy, T., N. M. Fahmy, and R. L. Fairchild. 2002. Chemokines: directing leukocyte infiltration into allografts. Curr. Opin. Immunol. 14:562-568. [DOI] [PubMed] [Google Scholar]
- 13.Erridge, C., A. Pridmore, A. Eley, J. Stewart, and I. R. Poxton. 2004. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via Toll-like receptor 2. J. Med. Microbiol. 53:735-740. [DOI] [PubMed] [Google Scholar]
- 14.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 15.Gatti, G., V. Rivero, R. D. Motrich, and M. Maccioni. 2006. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J. Leukoc. Biol. 79:989-998. [DOI] [PubMed] [Google Scholar]
- 16.Heine, H., S. Muller-Loennies, L. Brade, B. Lindner, and H. Brade. 2003. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur. J. Biochem. 270:440-450. [DOI] [PubMed] [Google Scholar]
- 17.Hess, S., C. Rheinheimer, F. Tidow, G. Bartling, C. Kaps, J. Lauber, J. Buer, and A. Klos. 2001. The reprogrammed host: Chlamydia trachomatis-induced up-regulation of glycoprotein 130 cytokines, transcription factors, and antiapoptotic genes. Arthritis Rheum. 44:2392-2401. [DOI] [PubMed] [Google Scholar]
- 18.Ilio, K. Y., J. A. Nemeth, S. Lang, and C. Lee. 1998. The primary culture of rat prostate basal cells. J. Androl. 19:718-724. [PubMed] [Google Scholar]
- 19.Isaacs, J. T., W. B. Isaacs, W. F. Feitz, and J. Scheres. 1986. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 9:261-281. [DOI] [PubMed] [Google Scholar]
- 20.Jantos, C., W. Baumgartner, B. Durchfeld, and H. G. Schiefer. 1992. Experimental epididymitis due to Chlamydia trachomatis in rats. Infect. Immun. 60:2324-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, R. M. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect. Immun. 72:3951-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164:13-17. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y. Y., X. L. Li, C. X. Yang, H. Zhong, H. Yao, and L. Zhu. 2003. Effects of tetrandrine and QYT on ICAM-1 and SOD gene expression in pancreas and liver of rats with acute pancreatitis. World J. Gastroenterol. 9:155-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motrich, R. D., C. Cuffini, J. P. Mackern Oberti, M. Maccioni, and V. E. Rivero. 2005. Chlamydia trachomatis occurrence and its impact on sperm quality in chronic prostatitis patients. J. Infect. 53:175-183. [DOI] [PubMed] [Google Scholar]
- 26.Oberley, R. E., K. L. Goss, L. Dahmoush, K. A. Ault, E. C. Crouch, and J. M. Snyder. 2005. A role for surfactant protein D in innate immunity of the human prostate. Prostate 65:241-251. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell, C. M., I. A. Ionova, A. J. Quayle, A. Visintin, and R. R. Ingalls. 2006. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J. Biol. Chem. 281:1652-1659. [DOI] [PubMed] [Google Scholar]
- 28.Okada, K., K. Matsunaga, T. Yuhi, E. Kuroda, U. Yamashita, and S. Tsuji. 2002. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 957:37-41. [DOI] [PubMed] [Google Scholar]
- 29.Ostaszewska, I., B. Zdrodowska-Stefanow, B. Darewicz, J. Darewicz, J. Badyda, K. Pucilo, V. Bulhak, and M. Szczurzewski. 2000. Role of Chlamydia trachomatis in epididymitis. Part II: Clinical diagnosis. Med. Sci. Monit. 6:1119-1121. [PubMed] [Google Scholar]
- 30.Paavonen, J., and W. Eggert-Kruse. 1999. Chlamydia trachomatis: impact on human reproduction. Hum. Reprod. Update 5:433-447. [DOI] [PubMed] [Google Scholar]
- 31.Pal, S., E. M. Peterson, and L. de la Maza. 2004. New murine model for the study of Chlamydia trachomatis genitourinary tract infections in males. Infect. Immun. 72:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pate, M. S., S. R. Hedges, D. A. Sibley, M. W. Russell, E. W. Hook III, and J. Mestecky. 2001. Urethral cytokine and immune responses in Chlamydia trachomatis-infected males. Infect. Immun. 69:7178-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pioli, P. A., E. Amiel, T. M. Schaefer, J. E. Connolly, C. R. Wira, and P. M. Guyre. 2004. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect. Immun. 72:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prebeck, S., H. Brade, C. J. Kirschning, C. P. da Costa, S. Durr, H. Wagner, and T. Miethke. 2003. The gram-negative bacterium Chlamydia trachomatis L2 stimulates tumor necrosis factor secretion by innate immune cells independently of its endotoxin. Microbes Infect. 5:463-470. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi, N., I. Kaltashov, K. Walker, V. Doroshenko, R. J. Cotter, K. Takayama, T. R. Sievert, P. A. Rice, J. S. Lin, and D. T. Golenbock. 1997. Structure of the monophosphoryl lipid A moiety obtained from the lipopolysaccharide of Chlamydia trachomatis. J. Biol. Chem. 272:10594-10600. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, L., Y. Zhang, J. A. Anderson, J. A., J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy, B. S., S. Rastogi, B. Das, S. Salhan, S. Verma, and A. Mittal. 2004. Cytokine expression pattern in the genital tract of Chlamydia trachomatis-positive infertile women implication for T-cell responses. Clin. Exp. Immunol. 137:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roshick, C., H. Wood, H. D. Caldwell, and G. McClarty. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 74:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer, T. M., K. Desouza, J. V. Fahey, K. W. Beagley, and C. R. Wira. 2004. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scidmore, M. A., D. D. Rockey, E. R. Fischer, R. A. Heinzen, T. Hackstadt. 1996. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect. Immun. 64:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorimachi, K., K. Akimoto, Y. Hattori, T. Ieiri, and A. Niwa. 1999. Secretion of TNF-α, IL-8 and nitric oxide by macrophages activated with polyanions, and involvement of interferon-γ in the regulation of cytokine secretion. Cytokine 11:571-578. [DOI] [PubMed] [Google Scholar]
- 43.Spiliopoulou, A., V. Lakiotis, A. Vittoraki, D. Zavou, and D. Mauri. 2005. Chlamydia trachomatis: time for screening? Clin. Microbiol. Infect. 11:687-689. [DOI] [PubMed] [Google Scholar]
- 44.Stephens, R. S. 2003. The cellular paradigm of chlamydia pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 45.Verhagen, A. P., F. C. Ramaekers, T. W. Aalders, H. E. Schaafsma, F. M. Debruyne, and J. A. Schalken. 1992. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 52:6182-6187. [PubMed] [Google Scholar]
- 46.Wagenlehner, F. M., W. Weidner, and K. G. Naber. 2006. Chlamydia infections in urology. World J. Urol. 24:4-12. [DOI] [PubMed] [Google Scholar]
- 47.Weidner, W., T. Diemer, P. Huwe, H. Rainer, and M. Ludwig. 2002. The role of Chlamydia trachomatis in prostatitis. Int. J. Antimicrob Agents 19:466-470. [DOI] [PubMed] [Google Scholar]
- 48.Xia, M., R. E. Bumgarner, M. F. Lampe, and W. E. Stamm. 2003. Chlamydia trachomatis infection alters host cell transcription in diverse cellular pathways. J. Infect. Dis. 187:424-434. [DOI] [PubMed] [Google Scholar]
- 49.Young, J. L., L. Smith, M. K. Matyszak, and J. S. Gaston. 2001. HLA-B27 expression does not modulate intracellular Chlamydia trachomatis infection of cell lines. Infect. Immun. 69:6670-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]