Abstract
Abnormal nitric oxide (NO) synthesis has been implicated in the pathogenesis of both periodontal disease and diabetes mellitus. In diabetic patients, increased inducible NO synthase in inflamed gingiva correlated with NO in gingival crevicular fluid. Although increased NO reflected more-severe inflammation, it was associated with reductions in CFU of Prevotella intermedia, a major periodontopathogen, highlighting dual roles for NO.
Nitric oxide (NO), a toxic free radical (25) with multiple biological functions, including inhibition of neutrophil chemotaxis (14), adhesion to endothelium (17), and upregulation of tumor necrosis factor alpha (29), is generated by oxidative deamination of l-arginine by nitric oxide synthase (NOS). The inducible form of NOS (iNOS) is rapidly and durably expressed by inflammatory cells in response to bacteria or their products, such as lipopolysaccharide (LPS) (32). Small amounts of NO induced by constitutive NOS are considered beneficial, whereas excess iNOS-induced NO can mediate cell and tissue injury. Periodontal diseases are chronic inflammatory infections associated with gram-negative bacteria, including Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans (26), which stimulate macrophages to generate NO (1, 7, 16). Moreover, NO is increased in inflamed gingival tissue (11, 15), and mercaptoethylguanidine, a selective iNOS inhibitor, prevents bone destruction in ligature-induced rodent periodontitis (21).
Periodontal disease is often a chronic complication of diabetes mellitus (20), with evidence of increased gingival inflammation, deeper periodontal pockets, and greater clinical attachment and bone loss (22). Hyperglycemia stimulates the production of advanced glycolysated end products, enhances the polyol pathway, and activates protein kinase C, which may lead to increased oxidative stress (12). Increased NO concentrations were demonstrated in sera of patients with type I diabetes and persistent microalbuminuria (2).
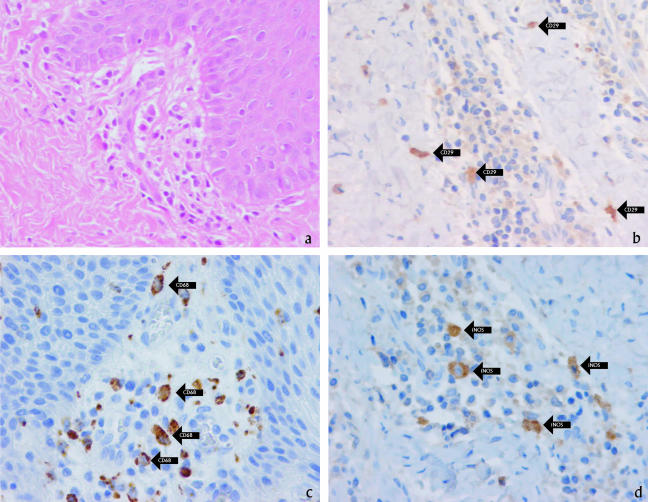
The aim of our study was to evaluate expression of NO in gingivae of type I diabetic patients presenting with periodontal disease and to correlate the level of NO with P. intermedia infection. Gingival tissues were obtained during modified Widman flap surgery from diabetic patients (three males and two females; mean age [±standard deviation], 48.2 ± 6.9 years) diagnosed with moderate (probing depth of ≤5 mm) or advanced (probing depth of >5 mm) periodontitis. Noninflamed gingival tissue was obtained during the crown-lengthening procedure of diabetic patients (two females, aged 44 and 51 years) (protocol approved by National Medical Ethics Committee of Slovenia; patients signed informed consent). Fixed and embedded tissue sections were stained with hematoxylin-eosin (H&E) or antibodies against iNOS (monoclonal antibody 9502, 1:100 in 1% bovine serum albumin; R&D Systems, Minneapolis, MN), CD29 (monoclonal antibody 1778, 1:100; R&D Systems, Minneapolis, MN), and CD68 (M0876, 1:50; DAKO Corporation, Carpinteria, CA) by use of an indirect biotin streptavidin system for detection (basic 3,3′-diaminobenzidine tetrahydrochloride detection kit 760-001; Ventana Medical Systems, Tucson, AZ) (18). An intense inflammatory infiltrate composed predominantly of mononuclear cells, including lymphocytes and macrophages, was observed in H&E-stained gingival tissues from periodontally involved type 1 diabetic patients (Fig. 1a). Immunostaining confirmed the presence of CD68-positive macrophages (Fig. 1c) within the inflammatory site as well as CD29-positive fibroblasts (Fig. 1b) along the margins of the infiltrate. Importantly, iNOS-positive cells were identified within the lesion (Fig. 1d). Similarly to the results of Hirose et al. (11), who did not find iNOS expression in noninflamed gingival tissue of nondiabetic patients, we did not demonstrate iNOS expression in noninflamed gingival tissue of our diabetic patients (data not shown).
FIG. 1.
Mononuclear cell infiltration in gingival tissue of diabetic patient with severe periodontitis. Gingival tissue sections were stained with H&E (a), anti-CD29 (fibroblasts) (b), anti-CD68 (macrophages) (c), or anti-human iNOS (d). Magnification, ×576.
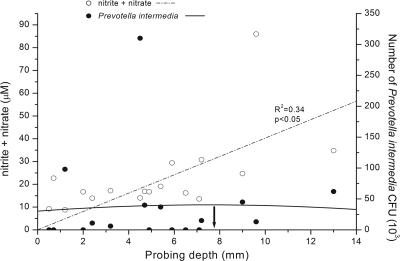
Based on the elevated iNOS expression in inflamed gingival tissue, gingival fluid samples were obtained from diabetic patients (13 males and 5 females; mean age, 38.8 years; range, 24 to 58 years; mean duration of diabetes, 16.1 years; range, 5 to 35 years) by use of 2-μl microcapillary tubes (Drummond Co., Pennsylvania). Fluid was diluted into 50 μl phosphate-buffered saline containing gentamicin (10 μg/ml), filtered (Ultrafree microcentrifuge filter, 10,000 molecular weight), treated with nitrate reductase to convert nitrate to nitrite, and reacted with 2,3-diaminonaphthalene (23). Fluorescence was measured at a wavelength of 365/450 (excitation/emission) by use of a fluorescence plate reader (Idexx Laboratories, Westbrook, ME) and based on a standard curve, with data reported as μM nitrite plus nitrate. Patients were assessed for degree of periodontitis by plaque index (PI), gingival index (GI) (19), probing depth, and clinical attachment loss (mm) by electronic periodontal probe (Peri-probe; Vivadent, Liechtenstein) and Williams periodontal probe. Consistent with iNOS detection in tissues, NO was quantified in all fluid samples, ranging from 10.7 to 86.0 μM (mean, 22.98 ± 4.32 μM). Samples from tooth sites with small or moderate plaque (PI of 1 or 2) contained increased NO, although the difference was not significant compared to sites with no plaque (PI of 0) (P > 0.05, Student's t test). Based on gingival inflammation, sites with a GI of 1 or 2 contained significantly higher NO than those with no inflammation (GI of 0) (P = 0.012). Likewise, significantly increased NO in gingival exudates was associated with sites of increased probing depth (r2 = 0.34, P < 0.05, correlation and polynomial regression analysis) (Fig. 2), all consistent with higher NO in the inflamed sites.
FIG. 2.
Correlation between periodontal pocket depth and NO production and number of P. intermedia CFU. Gingival fluid and subgingival plaque samples from periodontal pockets of increasing depth were analyzed for NOx and number of bacteria, respectively. Increased NO production was associated with increased periodontal pocket depth. In contrast, the numbers of P. intermedia CFU in deeper (>6 mm) periodontal pockets decreased considerably from the numbers found in moderate pockets.
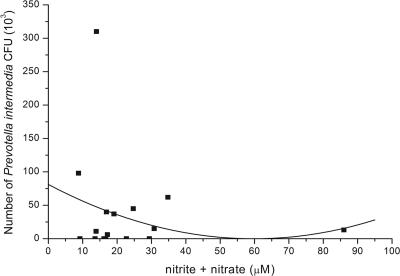
Because Prevotella intermedia is one of the causative pathogens of periodontal disease and P. intermedia LPS induces iNOS and release of NO in murine macrophages (12), we analyzed DNA from subgingival plaque samples (isolated from the same sites where the gingival crevicular fluid was collected) of 18 diabetic patients for P. intermedia. Loosely adherent supragingival plaque in the intercanine sector was gently removed by cotton gauze. A sterile paper point was inserted into the apical extent of periodontal pocket sulcus for 10 s, and after elution and denaturation, samples were analyzed by slot blot analysis for bacterial species, including P. intermedia (Omnigene Laboratory, Cambridge, MA), as described by French et al. (6). Questions regarding the sensitivity and specificity of the Omnigene probe for P. intermedia have been raised by van Steenbergen et al. (30), who claimed that the Omnigene probe did not distinguish between P. intermedia and Prevotella nigrescens. Quantitative differentiation between P. intermedia and P. nigrescens by oligonucleotide probes to specific rRNA sequences, as described by Gmür and Thurnheer (8), was not performed in our study. However, Moore et al. (24) detected five times as many P. intermedia CFU as P. nigrescens CFU in periodontal pockets but found seven times more P. nigrescens CFU than P. intermedia CFU at healthy sites. In addition, Dahlen et al. (4) identified two-thirds of their P. intermedia-like isolates from “destructive periodontal disease” as P. intermedia isolates, whereas 75% of the isolates from healthy control subjects were P. nigrescens isolates. According to these results and the fact that our subgingival plaque samples were collected predominantly from moderate and deep periodontal pockets, we assume that P. intermedia and not P. nigrescens was the main detected species in our samples. P. intermedia isolates were detected in our plaque samples, ranging from 0 to 310 × 103 bacteria. When P. intermedia levels were compared by polynomial regression analysis, based on the depth of the periodontal pocket source, we found a modest increase in P. intermedia DNA in moderate depth pockets (<6 mm) but decreased numbers in pockets deeper than 8 mm (Fig. 2). Importantly, polynomial regression analysis of NO and P. intermedia in periodontal pockets confirmed the reduction in P. intermedia DNA in pockets where NO levels were increased (Fig. 3) (r2 = 0.34, P < 0.05).
FIG. 3.
Correlation between NO levels and numbers of P. intermedia CFU in periodontal pockets from diabetic patients. The concentrations of NOx in gingival crevicular fluid and the numbers of P. intermedia CFU in subgingival plaque samples were compared in diabetic patients with various degrees of periodontal disease. Increased concentrations of NOx in gingival crevicular fluid were associated with reduced numbers of P. intermedia CFU.
The correlation between increased NO in gingival fluid and decreased CFU of the periodontal pathogen P. intermedia in deep periodontal pockets (>6 mm) suggested a possible microbicidal effect. Nitric oxide induced by iNOS has been shown to possess immunomodulatory, cytotoxic, and antibacterial effects (33), consistent with a role for reactive oxygen and nitrogen species in periodontal tissue damage as well as in microbial killing. Recent data indicate that modulation of superoxide levels by NO influences phagocytic functions of neutrophils and macrophages and that NO is an important element of host defense against P. gingivalis (9). Since P. gingivalis LPS can also induce macrophages to produce NO in an l-arginine- and gamma interferon-dependent mechanism (27), this NO may potentially be an important mediator of bone resorption. In this regard, iNOS null mice demonstrate a significantly reduced osteoclast response (13). In addition, iNOS influences both osteoblast and osteocyte function in bone remodeling (31).
The assessment of NO stable end products, nitrite and nitrate (NOx), is commonly used as a measure of NO production in biological fluids. Both NOx and vascular endothelial growth factor concentrations are increased, although not related, in the vitreous fluid of diabetic patients with proliferative diabetic retinopathy (10), and NO has been implicated in angiogenesis (3). Rapid serum diffusion of NO could contribute to increased aqueous NOx (10), implicating NO in the pathophysiology and progression of diabetic retinopathy (28) as well as in periodontal disease. These molecules may serve as therapeutic targets for the treatment and/or prevention of systemic and ocular microvascular complications in diabetes (5). Similarly, subgingival local delivery of NO inhibitors might be useful in the treatment of periodontal inflammation, particularly as systemic delivery of an NO inhibitor was shown to reduce bone resorption in an animal model of experimental periodontitis (21).
Acknowledgments
This study was supported by the Ministry of Education and Science of Republic Slovenia (periodontal medicine research grant no. K71/P3-0293) and in part by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research.
Editor: F. C. Fang
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Blix, I. J., and K. Helgeland. 1998. LPS from Actinobacillus actinomycetemcomitans and production of nitric oxide in murine macrophages J774. Eur. J. Oral Sci. 106:576-581. [DOI] [PubMed] [Google Scholar]
- 2.Chiarelli, F., F. Cipollone, F. Romano, S. Tumini, F. Costantini, L. di Ricco, M. Pomilio, S. D. Pierdomenico, M. Marini, F. Cuccurullo, and A. Mezzetti. 2000. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes 49:1258-1263. [DOI] [PubMed] [Google Scholar]
- 3.Cooke, J. P. 2003. NO and angiogenesis. Atheroscler. Suppl. 4:53-60. [DOI] [PubMed] [Google Scholar]
- 4.Dahlén, G., M. Wikström, S. Renvert, R. Gmür, and B. Guggenheim. 1990. Biochemical and serological characterization of Bacteroides intermedius strains isolated from the deep periodontal pocket. J. Clin. Microbiol. 28:2269-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doganay, S., C. Evereklioglu, H. Er, Y. Turkoz, A. Sevinc, N. Mehmet, and H. Savli. 2002. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye 16:163-170. [DOI] [PubMed] [Google Scholar]
- 6.French, C. K., E. D. Savitt, S. L. Simon, S. M. Eklund, M. C. Chen, L. C. Klotz, and K. K. Vaccaro. 1986. DNA probe detection of periodontal pathogens. Oral Microbiol. Immunol. 1:58-62. [DOI] [PubMed] [Google Scholar]
- 7.Frolov, I., Y. Houri-Hadad, A. Soskolne, and L. Shapira. 1998. In vivo exposure to Porphyromonas gingivalis up-regulates nitric oxide but suppresses tumour necrosis factor-alpha production by cultured macrophages. Immunology 93:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gmür, R., and T. Thurnheer. 2002. Direct quantitative differentiation between Prevotella intermedia and Prevotella nigrescens in clinical specimens. Microbiology 148:1379-1387. [DOI] [PubMed] [Google Scholar]
- 9.Gyurko, R., G. Boustany, P. L. Huang, A. Kantarci, T. E. Van Dyke, C. A. Genco, and F. C. Gibson III. 2003. Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect. Immun. 71:4917-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez, C., A. Lecube, R. M. Segura, L. Sararols, and R. Simo. 2002. Nitric oxide and vascular endothelial growth factor concentrations are increased but not related in vitreous fluid of patients with proliferative diabetic retinopathy. Diabet. Med. 19:655-660. [DOI] [PubMed] [Google Scholar]
- 11.Hirose, M., K. Ishihara, A. Saito, T. Nakagawa, S. Yamada, and K. Okuda. 2001. Expression of cytokines and inducible nitric oxide synthase in inflamed gingival tissue. J. Periodontol. 72:590-597. [DOI] [PubMed] [Google Scholar]
- 12.Honing, M. L., P. J. Morrison, J. D. Banga, E. S. Stroes, and T. J. Rabelink. 1998. Nitric oxide availability in diabetes mellitus. Diabetes Metab. Rev. 14:241-249. [DOI] [PubMed] [Google Scholar]
- 13.Jung, J. Y., A. C. Lin, L. M. Ramos, B. T. Faddis, and R. A. Chole. 2003. Nitric oxide synthase I mediates osteoclast activity in vitro and in vivo. J. Cell. Biochem. 89:613-621. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, S. S., T. Billiar, R. D. Curran, U. E. Zdziarski, R. L. Simmons, and R. E. Basford. 1989. Inhibition of chemotaxis Ng-monomethyl-L-arginine: a role for cyclic GMP. Blood 74:1885-1887. [PubMed] [Google Scholar]
- 15.Kendall, H. K., H. R. Haase, H. Li, Y. Xiao, and P. M. Bartold. 2000. Nitric oxide synthase type-II is synthesized by human gingival tissue and cultured human gingival fibroblasts. J. Periodontal Res. 35:194-200. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S. J., M. S. Ha, E. Y. Choi, J. I. Choi, and I. S. Choi. 2004. Prevotella intermedia lipopolysaccharide stimulates release of nitric oxide by inducing expression of inducible nitric oxide synthase. J. Periodontal Res. 39:424-431. [DOI] [PubMed] [Google Scholar]
- 17.Kubes, P., M. Suzuki, and D. N. Granger. 1991. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 88:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd, R. V., K. Schmidt, L. Blaivas, J. P. McCoy, and B. S. Wilson. 1985. A rapid immunostaining method utilizing preformed antibody-avidin-biotin-peroxidase complexes. Am. J. Clin. Pathol. 83:636-639. [DOI] [PubMed] [Google Scholar]
- 19.Loe, H. 1967. The gingival index, the plaque index and the retention index systems. J. Periodontol. 38(Suppl.):610-616. [DOI] [PubMed] [Google Scholar]
- 20.Loe, H. 1993. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16:329-334. [PubMed] [Google Scholar]
- 21.Lohinai, Z., P. Benedek, E. Feher, A. Gyorfi, L. Rosivall, A. Fazekas, A. L. Salzman, and C. Szabo. 1998. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br. J. Pharmacol. 123:353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mealey, B. 1999. Diabetes and periodontal diseases. J. Periodontol. 70:935-949. [DOI] [PubMed] [Google Scholar]
- 23.Misko, T. P., R. J. Schilling, D. Salvemini, W. M. Moore, and M. G. Currie. 1993. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 214:11-16. [DOI] [PubMed] [Google Scholar]
- 24.Moore, L. V., W. E. Moore, E. P. Cato, R. M. Smibert, J. A. Burmeister, A. M. Best, and R. R. Ranney. 1987. Bacteriology of human gingivitis. J. Dent. Res. 66:989-995. [DOI] [PubMed] [Google Scholar]
- 25.Palmer, R. M., A. G. Ferrige, and S. Moncada. 1987. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524-526. [DOI] [PubMed] [Google Scholar]
- 26.Socransky, S. S., and A. D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63:322-331. [DOI] [PubMed] [Google Scholar]
- 27.Sosroseno, W. 2000. Nitric oxide production by murine spleen cells stimulated with Porphyromonas gingivalis-derived lipopolysaccharide. Asian Pac. J. Allergy Immunol. 18:209-214. [PubMed] [Google Scholar]
- 28.Tsai, D. C., S. H. Chiou, F. L. Lee, C. K. Chou, S. J. Chen, C. H. Peng, Y. H. Kuo, C. F. Chen, L. L. Ho, and W. M. Hsu. 2003. Possible involvement of nitric oxide in the progression of diabetic retinopathy. Ophthalmologica 217:342-346. [DOI] [PubMed] [Google Scholar]
- 29.Van Dervort, A. L., L. Yan, P. J. Madara, J. P. Cobb, R. A. Wesley, C. C. Corriveau, M. M. Tropea, and R. L. Danner. 1994. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. J. Immunol. 152:4102-4109. [PubMed] [Google Scholar]
- 30.van Steenbergen, T. J., M. F. Timmerman, F. H. Mikx, G. de Quincey, G. A. van der Weijden, U. van der Velden, and J. de Graaff. 1996. Discrepancy between culture and DNA probe analysis for the detection of periodontal bacteria. J. Clin. Periodontol. 23:955-959. [DOI] [PubMed] [Google Scholar]
- 31.van't Hof, R. J., and S. H. Ralston. 2001. Nitric oxide and bone. Immunology 103:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong, J. M., and T. R. Billiar. 1995. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv. Pharmacol. 34:155-170. [DOI] [PubMed] [Google Scholar]
- 33.Zamora, R., Y. Vodovotz, and T. R. Billiar. 2000. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 6:347-373. [PMC free article] [PubMed] [Google Scholar]