Abstract
Relapsing fever Borrelia spp. undergo antigenic variation, achieve high levels in blood, and require rapid production of immunoglobulin M (IgM) for clearance. MyD88-deficient mice display defective clearance of many pathogens; however, the IgM response to persistent infection is essentially normal. Therefore, MyD88−/− mice provided a unique opportunity to study the effect of nonantibody, innate host defenses to relapsing fever Borrelia. Infected MyD88−/− mice harbored extremely high levels of B. hermsii in the blood compared to wild-type littermates. In the comparison of MyD88−/− mice and B- and T-cell-deficient scid mice, two features stood out: (i) bacterial numbers in blood were at least 10-fold greater in MyD88−/− mice than scid mice, even though the production of IgM still occurred in MyD88−/− mice; and (ii) many of the MyD88−/− mice were able to exert partial clearance, although with delayed kinetics relative to wild-type mice, a feature not seen in scid mice. Further analysis revealed a delay in the IgM response to lipoproteins expressed by the original inoculum; however, by 6 days of infection antibodies were produced in MyD88−/− mice that could clear spirochetemia in scid mice. While these results indicated that the production of IgM was delayed in MyD88−/− mice, they also point to a second, antibody-independent role for MyD88 signaling in host defense to relapsing fever Borrelia. This second defect was apparent only when antibody levels were limiting.
Relapsing fever is a vector-borne disease that can be caused by multiple species of Borrelia, including B. hermsii, B. turicatae, and B. parkeri. While relapsing fever Borrelia spp. can invade multiple tissues, including the heart (27, 75), brain (16, 26, 27), and joints (18, 37, 56), the most notable niche is the blood, where the bacteria can reach extremely high densities (106 to 108 per ml). Although rapidly produced antibodies initially clear the organism from the blood, the infection is characterized by recurring episodes of bacteremia. The expression of antigenically distinct variable-surface proteins allows relapsing fever Borrelia to evade host defenses and repopulate the bloodstream (63, 69). This antigenic variation results from alterations in expression of the variable major surface lipoproteins (Vmps) through gene conversion from silent cassettes into an expression locus (9, 23, 32, 47, 57).
Immunoglobulin M (IgM) antibody plays a critical role in the host defense to relapsing fever Borrelia, as indicated by studies using scid, RAG−/−, B-cell-deficient, and surface IgM−/− mice (4, 18, 21). IgM antibodies that react with relapsing fever Borrelia can be directly bactericidal in the absence of host complement and can neutralize growth of Borrelia both in vitro and in vivo (10, 11, 21, 22, 69). The production of antibodies and clearance of relapsing fever Borrelia notably occurs in the absence of T cells, as demonstrated in thymectomized mice and T-cell receptor−/− mice (4, 53). This supports a role for cells that respond to T-independent antigens such as B1 B cells and marginal-zone B cells. Recent studies by Alugupalli et al. strongly support a role for B1b B cells, which are primarily found in the peritoneum, in IgM production and host defense toward B. hermsii (4, 5). Additionally, the finding that splenectomized mice show a deficiency in the ability to control the first episode of bacteremia when infected with low-passage B. hermsii provides evidence for involvement of splenic B cells in this response (4). Recent work by Belperron et al. further implicated marginal-zone B cells of the spleen in the rapid IgM production important for early control of B. hermsii (13).
While antibody is clearly central to the defense against relapsing fever Borrelia, other factors of the innate defense may also contribute to host control of the pathogen. Complement was not found to play an essential role in clearance of relapsing fever Borrelia in studies using C1q-, C3-, or C5-deficient mice (21, 22, 52). This may be due to the expression of a factor H-binding protein by B. hermsii, which would provide a mechanism for complement evasion by this organism (36). Guyard et al. have recently identified a serine protease in relapsing fever Borrelia that increases spirochete resistance to oxidative stress and polymorphonuclear leukocyte (PMN) killing (29). Platelets bind to B. hermsii during infection and are thought to play an important role in defense and clearance of relapsing fever Borrelia (6, 7). Additionally, the spleen is a major filtering organ of the blood that may contribute to efficient removal of high levels of bacteria from the blood (4).
Toll-like receptors (TLRs) are receptors of the innate immune response that are involved in detection and response to pathogen-associated molecules such as lipopolysaccharide (20, 35, 41, 58, 59), bacterial lipoproteins (3, 33, 42, 71), bacterial flagellin (2, 30), and unmethylated CpG DNA (31). In previous studies, Toll-like receptors were found to be critical in the host response to the related tissue-associated pathogen B. burgdorferi (2, 12, 14, 43, 77) and in response to blood-borne pathogens such as group B streptococcus (46). Others have predicted that recognition and signaling by the variable-surface lipoproteins expressed by relapsing fever Borrelia is involved in the inflammatory and febrile responses during bacteremic episodes (18, 73). TLR signaling also has a significant role in host defense to numerous pathogens (24, 25, 60, 67, 70). The experiments presented in this report reveal that TLR signaling is important in two aspects of host response to B. hermsii: the rapid antibody production against B. hermsii lipoproteins and an antibody-independent entity required for clearance of blood-borne B. hermsii.
MATERIALS AND METHODS
Mice.
MyD88-deficient mice (1) were maintained through breeding of heterozygous mice at the sixth-generation backcross on the C57BL/6 background. Mice were kept on sulfamethoxazole-trimethoprim antibiotics prior to infection. MyD88-deficient mice and littermates were genotyped using PCR. Two independent sets of primers were used to confirm the genotype of each mouse: MyD88.F2 (5′-GGT CCA TTG CCA GCG AGC-3′) and MyD88.R2 (5′-GCC AGT CAT CAT TGA ACA CG-3′); MyD88.F1 (5′-TGG CAT GCC TCC ATC ATA GTT AAC C-3′) (38), MyD88.R4 (5′-GAA TCA GTC GCT TCT GTT GGA CAC-3′), and neo.R (5′-ATC GCC TTC TAT CGC CTT CTT GAC G-3′) (38). TLR2-deficient mice were provided by Tularik, Inc. (San Francisco, CA) and were generated by Deltagen, Inc. (Redwood City, CA) (76). TLR2−/− mice were on the 10th-generation backcross to C57BL/6. TLR2−/−/scid double mutant mice on a C57BL/6 background were generated and maintained as described previously (74). C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD), and B6.CB17-Prkdcscid mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in the Animal Resource Center at the University of Utah Medical Center (Salt Lake City) according to the National Institutes of Health guidelines for care and use of laboratory animals.
B. hermsii culture and infection.
Infections were with the DAH isolate of B. hermsii, at passage 3 from the mouse (62). Spirochetes were cultured in Barbour-Stoenner-Kelly II (BSK-II) medium containing 12% rabbit serum (Sigma, St. Louis, MO) for 3 days prior to infection. Mice between the ages of 10 and 16 weeks were infected intraperitoneally with 1 × 106 B. hermsii cells, a dose shown to result in rapid appearance of spirochetemia (62). Mice were monitored daily for spirochete levels in the blood.
DNA isolation.
Mice were bled from the lateral tail vein, and DNA was isolated from a 10-μl volume of blood. Each blood sample was spiked with 0.7 μg B. burgdorferi DNA prior to extraction to account for any loss of DNA during extraction methods. DNA was isolated from blood samples that were incubated at 60°C for 8 h in 50 mM NaCl, 50 mM Tris-HCl (pH 7.5), 10 mM EDTA, 1% (vol/vol) Triton X-100 lysis buffer containing 0.2 mg/ml Proteinase K (66). Samples were treated with cetyltrimethylammonium bromide, and DNA was recovered by phenol-chloroform extraction and ethanol precipitation (8). A fivefold dilution of the recovered DNA was prepared for PCR quantification of both B. hermsii and B. burgdorferi DNA.
Quantification of B. hermsii DNA in mouse blood.
Continuous fluorescent monitoring PCR was performed using the LightCycler (Roche, Indianapolis, IN) to assess B. hermsii levels in blood of infected animals. The copy number of the B. hermsii glpT gene (61) was calculated using the cycle threshold of amplification, determined by Roche LightCycler software. The copy number of the B. burgdorferi recA gene was also calculated (51), and the percentage of recA recovered from the B. burgdorferi DNA that was spiked in with the blood sample was determined. B. hermsii glpT copy numbers were then normalized to the percentage of recovered B. burgdorferi recA. Values for the peak levels of B. hermsii in the blood of wild-type mice were similar to those previously obtained by microscopic counting (4). Oligonucleotide primers used for quantification of B. hermsii glpT were glpT.F289 (5-CGA AGT AAT CCC AGG TAT TTC TTA GC-3′) and glpT.R515 (5′-GCA ACA GTT AGT CCT CTT TCT TTT CTT G-3′), derived from GenBank accession number AF506981 (61). Oligonucleotide primers used for the quantification of the recA gene of B. burgdorferi were ntm17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and ntm17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′) (51) and do not amplify the B. hermsii recA gene.
Reagents.
B. hermsii antigens were prepared by sonication of a 10-day washed culture of the DAH strain of B. hermsii. Protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Escherichia coli K12, D31m4 (Re) lipopolysaccharide (LPS) (List Biological Laboratories, Campbell, CA) was repurified by phenol extraction as previously described (34). Antibodies and standards for cytokine enzyme-linked immunosorbent assay (ELISA) were obtained from PharMingen (San Diego, CA). IgG, IgM, and IgA for Ig ELISA were from Zymed (San Francisco, CA), and μ-chain-specific detection antibody was from Invitrogen (Carlsbad, CA).
Western blot analysis.
Western blot analysis of mouse sera was conducted as previously described (77) using a sonicated preparation of B. hermsii or the n-butanol extract of B. hermsii that was prepared as described for B. burgdorferi (45). Briefly, 120 μg of B. hermsii sonicate or 12 μg protein from the aqueous phase of an n-butanol extraction of B. hermsii was separated on a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gel and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). Blots were incubated with a 1/50 dilution of infected or control mouse sera. Bands were detected using alkaline phosphatase (AP)-conjugated goat anti-mouse IgM or AP-conjugated goat anti-mouse IgG antibodies (Invitrogen). GlpQ and Vsp33 were identified with rabbit antisera (65) or mouse monoclonal antibody H4825 (55), respectively.
Passive transfer of sera.
Sera were collected from naïve C57BL/6 mice, C57BL/6 mice infected with B. hermsii for 6 days, C57BL/6 mice infected with B. hermsii for 9 weeks, or MyD88-deficient mice infected with B. hermsii for 6 days. Undiluted pooled sera (300 μl) were filter sterilized to remove B. hermsii and transferred via intraperitoneal injection into recipient animals 72 h following infection with B. hermsii. Spirochete levels in the blood were monitored daily using quantitative PCR.
Fluorescent-activated cell sorter (FACS) analysis of B1b cells.
Peritoneal cells were isolated from individual mice and stained with CD16/32 (Fc block; clone 93) followed by IgM (clone 1B4B1), IgD (clone 11-26c), CD11b (clone M1/70), or CD5 (clone 53-7.3) antibody (eBioscience, San Diego, CA) for determining the frequency of B-cell populations. Samples were analyzed using a FACScan flow cytometer and Cell Quest software (Becton Dickinson, San Jose, CA).
In vitro IgM antibody production.
Peritoneal cells from uninfected MyD88+/− and MyD88−/− mice were collected and plated at 2 × 105 cells per well in a 24-well dish. Cells were treated with media, repurified lipopolysaccharide (200 ng/ml) (34), B. hermsii sonicate (50 μg/ml), or 2.4 × 107 live B. hermsii cells for 5 days at 37°C. IgM levels in cell-free supernatants were determined by ELISA.
In vitro macrophage killing assay.
Bone marrow-derived macrophages were isolated from MyD88-deficient mice and littermate control animals as described previously (15). Adherent macrophages were collected after 7 days and plated at a density of 3 × 105 cells per well in a 24-well dish, as were mouse L cells. After overnight incubation, bone marrow-derived macrophages were washed and 3 × 106 B. hermsii cells were added in BSK-II media containing 6% rabbit serum. Viable spirochetes were counted after 24 h, 48 h, and 72 h by dark-field microscopy using a Petroff-Hausser counting chamber.
Statistics.
Data sets were analyzed using Microsoft Excel. Significant differences among groups were determined using a two-tailed, two-sample unequal-variance Student's t test. Statistical significance was defined as P < 0.05. For survival analysis, Kaplan-Meier plots were constructed and analyzed by the Mantel-Haenszel logrank test using Prism version 4.01 for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
TLR signaling plays an important role in the host defense against B. hermsii.
A role for Toll-like receptor signaling in the response to the relapsing fever agent B. hermsii was examined using mice that lack MyD88, a common adapter molecule in the TLR signaling pathway. MyD88-deficient mice displayed significant mortality starting at day 8 of infection with B. hermsii, with most MyD88−/− mice succumbing by day 16 of infection (Fig. 1A). Infected MyD88+/+ and MyD88+/− littermate mice survived throughout the 16 days of experimental infection and were indistinguishable in all characteristics of infection. Histological examination of B. hermsii-infected MyD88−/− mouse lungs revealed intra-alveolar hemorrhaging, cellular infiltration, and thickened septae. Further, infected MyD88−/− mice exhibited grossly enlarged spleens, with disrupted splenic architecture and large numbers of activated lymphoid cells (data not shown). Although the cause of death was not determined for the MyD88−/− mice, the abnormalities of lung and spleen were not seen in wild-type mice and could have been contributing factors.
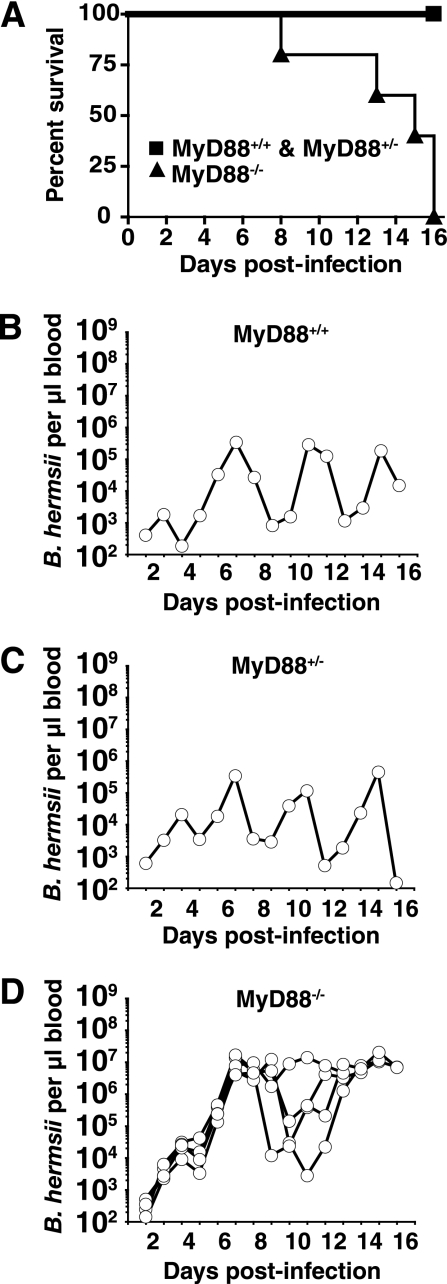
FIG. 1.
TLR signaling plays a crucial role in the control of B. hermsii levels in the blood. Mice were infected intraperitoneally with 1 × 106 B. hermsii cells (DAH isolate) and monitored daily for survival and bacterial levels in blood. DNA was isolated from 10 μl of blood, and B. hermsii levels were determined by quantitative PCR as described in Materials and Methods. Numbers reflect copies of the B. hermsii gene glpT normalized to the volume of blood. Kaplan-Meier survival plot of MyD88+/+, MyD88+/−, and MyD88−/− mice infected with B. hermsii (A) displaying significantly greater survival in MyD88+/+ and MyD88+/− mice than MyD88−/− mice infected with B. hermsii (P < 0.5). Also shown are B. hermsii levels in blood of one MyD88+/+ mouse, representative of three mice (B), B. hermsii levels in blood of one MyD88+/− mouse, representative of four mice (C), and B. hermsii levels in blood of five MyD88−/− mice (D).
Quantitative PCR was employed to determine spirochete levels in blood collected daily from infected mice. The initial expansion of B. hermsii observed in blood (days 1 to 3) was similar among MyD88-deficient mice and littermate wild-type and heterozygous animals; however, the peak level of B. hermsii in the blood of MyD88−/− mice was consistently elevated over several days (Fig. 1B to D). At the peak of bacteremia, MyD88-deficient mice harbored 1 × 107 to 2 × 107 B. hermsii cells per μl of blood, at least 30-fold higher than the 6 × 105-cells/μl peak level of B. hermsii attained in control animals. During infection, some of the MyD88-deficient mice were able to markedly reduce the level of B. hermsii in blood, suggesting that there was residual host defense in the absence of MyD88-dependent TLR signaling; however, the kinetics of B. hermsii clearance in these animals was generally delayed to day 8 of infection compared with littermate controls, where the initial clearance was observed at 3 to 4 days of infection (Fig. 1). This trend of delayed clearance in MyD88−/− mice was reproduced in several experiments, although some variability was observed in the time of onset of B. hermsii clearance in MyD88−/− mice as shown in later figures. Furthermore, clearance that occurred in MyD88-deficient mice was less complete than seen with wild-type mice.
TLR signaling is not required for the IgM response to B. hermsii infection.
Antibodies play an important role in the clearance of B. hermsii from the bloodstream, and IgM antibodies specific for the variable-surface lipoproteins are particularly important in clearing individual serotypes of B. hermsii (4, 21, 69). The antibody response of infected MyD88−/− mice was analyzed to determine if deficient antibody production was responsible for the elevated levels of B. hermsii observed in MyD88−/− mice. Circulating levels of IgM in uninfected MyD88−/− mice were similar to those of wild-type littermates, and infection with B. hermsii resulted in a greater than sevenfold increase in total serum IgM in both mouse genotypes, suggestive of a mitogen response to the pathogen (Table 1). Additionally, B. hermsii-infected MyD88−/− mice and wild-type mice produced similar amounts of B. hermsii-specific IgM as determined by ELISA. This finding is consistent with previous findings of normal or even elevated IgM production in MyD88−/− mice infected with B. burgdorferi (14, 43).
TABLE 1.
IgM response of MyD88-deficient mice infected with B. hermsii
| Genotype | Total serum IgM in uninfected micea (mg/ml) | Serum IgM in 6-day-postinfection miceb
|
|
|---|---|---|---|
| Total (mg/ml) | B. hermsii specific (μg/ml) | ||
| Wild type | 0.16 ± 0.07 | 1.18 ± 0.31 | 7.31 ± 2.29 |
| MyD88−/− | 0.09 ± 0.02 | 1.42 ± 0.54 | 10.1 ± 4.56 |
Uninfected groups consisted of 12 wild-type mice and 27 MyD88-deficient mice.
Groups infected with B. hermsii consisted of 20 wild-type mice and 22 MyD88-deficient mice.
Defect in the in vitro polyclonal response of B1b B cells from MyD88−/− mice.
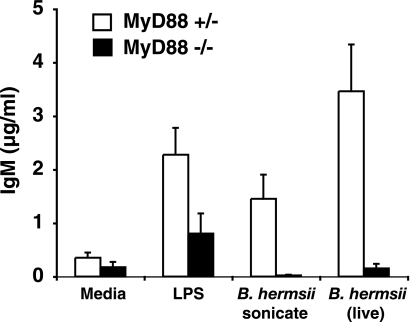
Cells from the peritoneum, a rich source of B1 B cells, were collected from uninfected wild-type and MyD88−/− mice, and IgM production was determined in response to cultured, living B. hermsii. Cultured peritoneal cells from naïve MyD88−/− mice failed to produce IgM when treated with B. hermsii, whereas peritoneal cells from heterozygous littermates responded vigorously (Fig. 2). The lack of an antibody response by MyD88−/− cells in vitro supported a deficiency in the ability to properly activate B1 B cells, which could translate into a failure to rapidly produce IgM antibody. IgM production from cultured MyD88-deficient peritoneal cells treated with LPS indicated that B cells were still present in the peritoneum of MyD88−/− mice and that these B cells were capable of IgM production when given a stimulus that can signal via the TRIF adapter molecule in the absence of MyD88. To directly determine if B1 B cells were present in the peritoneal cavity of MyD88−/− mice, cells isolated from peritoneal washes were analyzed for IgM, IgD, CD11b (Mac1), and CD5 surface expression by flow cytometry. Similar numbers of total cells were recovered from peritoneal washes of wild-type and MyD88−/− mice, and flow cytometry revealed similar levels of B1 B cells, including both B1a (IgM+ IgD+ CD11blo CD5+) and B1b (IgM+ IgD+ CD11blo CD5−) B-cell subsets (Table 2). These results indicated that B1b B cells are present in the peritoneal cavity of MyD88−/− mice and suggest that the absence of IgM production by cultured cells might reflect the requirement for MyD88 in an accessory cell. As an attempt to correct the defect in IgM production by MyD88−/− peritoneal B cells, wild-type bone marrow-derived macrophages, which respond to B. hermsii by producing cytokines, were cocultured with peritoneal cells from MyD88−/− mice. This did not promote increased IgM production by MyD88−/− B cells (data not shown). This distinction between in vitro and in vivo IgM response suggested a more subtle defect in antibody production and prompted us to further characterize the production of antibody directed against B. hermsii during infection of MyD88−/− mice.
FIG. 2.
MyD88-deficient peritoneal cells fail to produce IgM in response to B. hermsii in vitro. Peritoneal cells were treated with LPS (200 ng/ml), B. hermsii sonicate (50 μg/ml), or live B. hermsii (2.4 × 107) for 5 days at 37°C. Supernatants were collected, and total IgM production was measured by ELISA. Data bars represent the average IgM levels from five MyD88+/− mice and four MyD88−/− mice.
TABLE 2.
Frequency of B1b B-cell population in naïve MyD88-deficient micee
| Genotype | Total no. of cells recovereda (106) | %B1a + B1b B cellsb | %B1a B cellsc | %B1b B cellsd |
|---|---|---|---|---|
| Wild type | 4.0 ± 1.9 | 10.9 ± 1.6 | 6.5 ± 1.3 | 4.4 ± 0.8 |
| MyD88−/− | 5.7 ± 2.9 | 11.2 ± 1.6 | 7.6 ± 1.1 | 3.6 ± 0.7 |
B-cell populations in the peritoneal wash were quantified by FACS analysis as described in Materials and Methods. Values represent the average frequency of cell populations from nine wild-type mice and five MyD88-deficient mice. At least 20,000 events were analyzed to determine B-cell frequency in individual mice.
Total B1 B-cell populations were characterized as IgM+ IgD+ CD11blo.
B1a B cells were characterized as IgM+ IgD+ CD11blo CD5+.
B1b B-cell populations (IgM+ IgD+ CD11blo CD5−) were calculated by subtracting the frequency of B1a B cells from the total IgM+ IgD+ CD11blo B-cell population in individual mice and are reported as the means ± standard deviations for each group.
Significantly different values for wild-type and MyD88−/− mice were not achieved in any category by Student's t test.
Complexity and kinetics of antibody response in MyD88−/− mice.
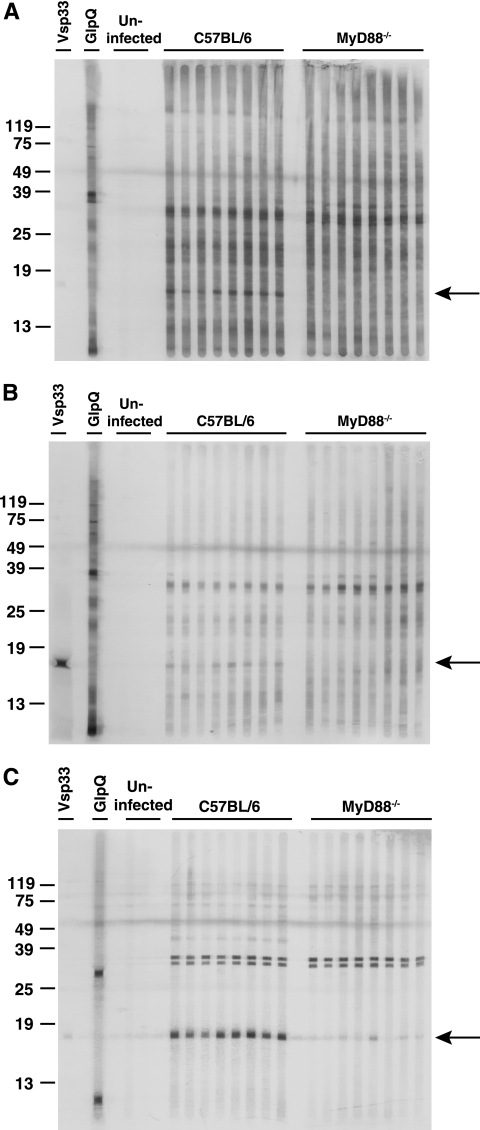
Sera collected from wild-type and MyD88−/− mice infected for 6 days with B. hermsii were assessed for reactivity with B. hermsii antigens. Western blot analysis using a preparation of sonicated bacteria revealed IgM and IgG reactivity with several antigens and selective lack of reactivity in MyD88−/− mouse sera toward a B. hermsii antigen of approximately 17 kDa (Fig. 3A and B). The Vsp33 monoclonal antibody H4825 (IgG) gave strong reactivity with the band at 17 kDa, demonstrating it contained Vsp33 (Fig. 3B). Most of the spirochetes in the passage-3 DAH culture stained positively for Vsp33, with a small proportion staining for serotype 7 (data not shown). Taken together, these findings suggest that an early antibody response to Vsp33 is responsible for the reactivity to the 17-kDa band. Reactivity with several bands was enhanced when the Western blot was performed with the lipoprotein-enriched n-butanol extract of B. hermsii, indicating these antigens were likely to be lipoproteins. This included reactivity of wild-type sera to the 17-kDa lipoprotein, likely Vsp33, with diminished reactivity in sera from MyD88−/− mice (Fig. 3C).
FIG. 3.
Reactivity of sera from infected MyD88-deficient mice to B. hermsii. IgM antibodies (A and C) and IgG antibodies (B) against B. hermsii were detected in sera from mice sacrificed at 6 days of infection by Western blot analysis with sonicated B. hermsii (A and B) or with the lipoprotein-enriched n-butanol extract of B. hermsii, as described in Material and Methods. Uninfected control sera were from C57BL/6 and MyD88−/− mice. GlpQ was detected with rabbit antisera (IgG). Arrows denote reactivity to an ∼17-kDa lipoprotein.
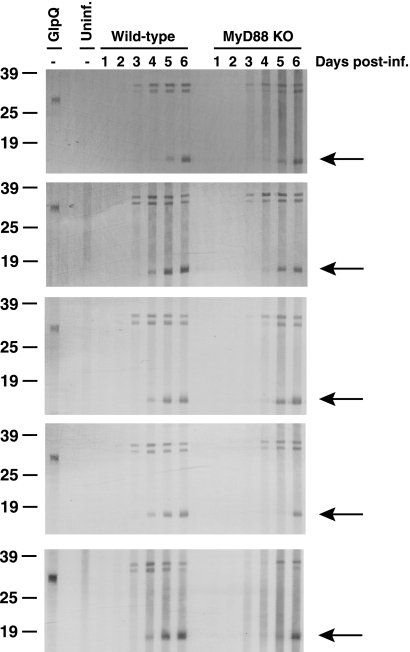
To assess the kinetics of antibody production to B. hermsii lipoproteins, sera from B. hermsii-infected wild-type and MyD88−/− mice were collected daily and analyzed for IgM reactivity to B. hermsii lipoprotein antigens (Fig. 4). Western blot analysis using n-butanol extracts of B. hermsii demonstrated that MyD88−/− mice were able to produce antibody to B. hermsii lipoproteins; however, sera from all five MyD88−/− mice showed a 1- to 2-day delay in the response to several B. hermsii lipoprotein antigens relative to wild-type littermates, including Vsp33 at 17 kDa. This finding is interesting in light of the limited and delayed clearance of B. hermsii from the blood in MyD88−/− mice (Fig. 1).
FIG. 4.
MyD88-deficient mice have a delayed IgM response to B. hermsii lipoproteins. Antibodies against lipoprotein-enriched n-butanol extract of B. hermsii were analyzed each day of infection, day 1 through day 6. Each panel shows reactivity of sera collected from one wild-type and one MyD88−/− mouse, for a total of five mice for each genotype. Arrows denote reactivity to an ∼17-kDa lipoprotein. Uninf., uninfected; KO, knockout; post-inf., postinfection.
TLR signaling contributes to both antibody production against B. hermsii and additional responses involved in host defense.
Although there was a delay in the production of antibodies to B. hermsii lipoproteins, the blots of Fig. 4 indicated IgM reactivity eventually appeared. Some experiment-to-experiment variation does occur in the development of antibody to the 17-kDa antigen, as seen by comparison of the 6-day samples in Fig. 3 and 4 from different experiments. Because the day-6 sera shown in Fig. 3 contained equivalent levels of reactivity by ELISA but reduced activity against the 17-kDa band by Western blotting, we hypothesized that the reduced recognition of this band might correlate with lack of bactericidal or other protective capacity. Pooled 6-day sera from samples shown in Fig. 3 were transferred from wild-type or MyD88−/− mice into scid mice 72 h following inoculation with B. hermsii (Fig. 5). scid mice were expected to have normal MyD88-dependent responses in cell types other than lymphocytes. The maximal level of B. hermsii in blood of scid mice receiving nonimmune sera was 10-fold higher than peak B. hermsii levels in wild-type mice (Fig. 5A and C), and scid mice failed to display recurring episodes of bacterial clearance, similar to previous reports (4, 18). MyD88−/− mice harbored approximately eightfold greater levels of B. hermsii than scid mice at the peak of infection (Fig. 5B and C). Sera from either wild-type or MyD88−/− mice led to the transient clearance of B. hermsii from the blood of recipient scid animals within 12 h after the transfer (Fig. 5D and E). These findings support our observation that MyD88−/− mice were capable of producing B. hermsii-specific antibodies and, in addition, demonstrate that these antibodies can efficiently clear B. hermsii from the blood. The two observations of (i) a greater defect in control of the spirochetes in MyD88−/− mice compared to scid mice and (ii) the ability of antibody in sera from MyD88−/− mice to clear B. hermsii upon transfer to scid mice strongly argue that the defect in control of B. hermsii in MyD88−/− mice is not solely due to a B-cell defect or deficiency of antibody production.
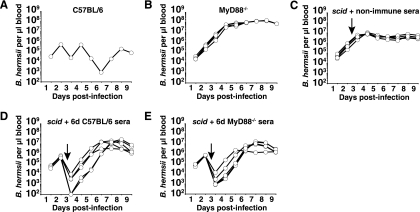
FIG. 5.
Sera from B. hermsii-challenged MyD88-deficient mice can clear persistent spirochetemia in recipient scid mice. Pooled sera (300 μl) from naïve C57BL/6 mice (non-immune sera), C57BL/6 mice infected with B. hermsii for 6 days (6d C57BL/6 sera), or MyD88-deficient mice infected with B. hermsii for 6 days (6d MyD88−/− sera) were transferred to B. hermsii-infected scid mice 72 h after infection. B. hermsii levels in the blood were monitored as described in the legend to Fig. 1. Shown are B. hermsii levels in blood of one C57BL/6 mouse, representative of three mice (A), B. hermsii levels in blood of four MyD88-deficient mice (B), five scid mice receiving nonimmune sera from C57BL/6 mice (C), five scid mice receiving sera from B. hermsii-challenged MyD88-deficient mice (D), and five scid mice receiving sera from B. hermsii-challenged C57BL/6 mice (E). Two samples shown in panel D that were PCR negative for B. hermsii glpT DNA are plotted as 102 B. hermsii per μl of blood based on sensitivity of the assay.
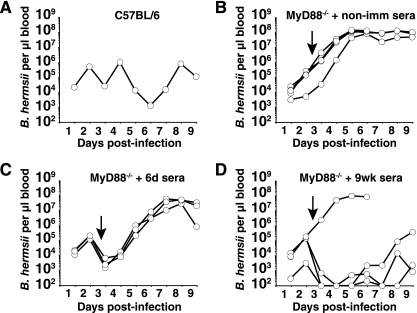
The impact of the delayed early antibody response on the failure of MyD88−/− mice to efficiently control spirochete numbers in the blood was investigated by transferring sera from wild-type mice that had been infected with B. hermsii for either 6 days or 9 weeks (Fig. 6). MyD88−/− mice receiving nonimmune sera at 72 h of infection failed to clear B. hermsii from the blood by 9 days (Fig. 6B). At 72 h postinfection, MyD88−/− mice that received sera from C57BL/6 mice infected for 6 days or 9 weeks displayed rapid clearance of B. hermsii from the blood, lowering the B. hermsii burden to levels similar to wild-type mice during clearance phases of infection (Fig. 6). All five MyD88−/− mice receiving 6-day sera and four out of five MyD88−/− mice receiving 9-week sera exhibited rapid clearance of B. hermsii from the bloodstream (Fig. 6C and D). One MyD88−/− mouse receiving 9-week immune sera failed to reduce B. hermsii levels following transfer. It is most likely that in this mouse, B. hermsii underwent a rapid switch in variable antigen expression to a serotype not recognized by antibodies in the 9-week immune sera, allowing the bacteria to persist in this animal.
FIG. 6.
Sera from B. hermsii-challenged C57BL/6 mice can clear high levels of spirochetes in recipient MyD88-deficient mice. Pooled sera (300 μl) from naïve C57BL/6 mice (non-imm sera), C57BL/6 mice infected with B. hermsii for 6 days (6d sera), or C57BL/6 mice infected with B. hermsii for 9 weeks (9wk sera) were transferred to B. hermsii-infected MyD88-deficient mice 72 h after infection. Survival and B. hermsii levels in the blood were monitored as described in the legend to Fig. 1. Shown are B. hermsii levels in blood of one C57BL/6 mouse, representative of three mice (A), B. hermsii levels in blood of five MyD88-deficient mice receiving nonimmune sera from C57BL/6 mice (B), three MyD88-deficient mice receiving sera from 6d B. hermsii-challenged C57BL/6 mice (C), and four MyD88-deficient mice receiving sera from 9wk B. hermsii-challenged C57BL/6 mice (D). Results shown are representative of two separate experiments. Samples that were PCR negative for B. hermsii DNA in panel D are plotted as 102 B. hermsii per μl of blood based on sensitivity of the assay.
Contribution of TLR2 to host defense against B. hermsii.
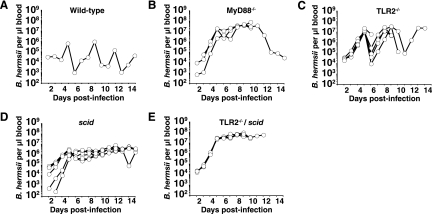
Previous studies have demonstrated that TLR2, acting in a heterodimeric complex with TLR1, signals in response to Borrelia lipoproteins (2, 72). To determine the involvement of TLR2 in the host defense against B. hermsii and to more fully characterize the nature of the defect in host defense against B. hermsii, we compared survival and spirochete burden in blood of MyD88−/−, TLR2−/−, scid, and TLR2−/−/scid double mutant mice (Fig. 7). MyD88−/−, TLR2−/−, and TLR2−/−/scid mice succumbed to infection beginning at 7 days of infection, as shown previously with MyD88−/− mice (Fig. 1A). Levels of B. hermsii in the blood of TLR2−/− mice were very high, approaching those seen in infected MyD88−/− mice (Fig. 7A to C). However, TLR2−/− mice underwent clearance of bacteremia at a frequency similar to that seen with wild-type animals, suggesting that other TLRs are also involved in host defense against B. hermsii (Fig. 7A and C). In comparison, TLR2−/−/scid double mutant mice harbored higher levels of B. hermsii than either TLR2−/− mice or scid mice and similar to levels for MyD88−/− mice (Fig 7B to E). This observation further supports the influence of a second, antibody-independent requirement for TLR signaling in host defense to B. hermsii. It is also possible that TLR signaling influences tissue invasion, altering the balance between blood and tissue infection.
FIG. 7.
TLR2−/−/scid double mutant mice have a greater defect in host defense against B. hermsii than either individual mutant alone. Mice of the indicated genotypes were infected with B. hermsii and monitored for survival and B. hermsii levels in the blood as described in the legend to Fig. 1. Shown are B. hermsii levels in blood of one C57BL/6 mouse, representative of six mice (A), and B. hermsii levels in blood of four MyD88−/− mice (B), five TLR2−/− mice (C), six scid mice (D), and four TLR2−/−/scid mice (E).
Killing of B. hermsii by macrophages from MyD88−/− and wild-type mice.
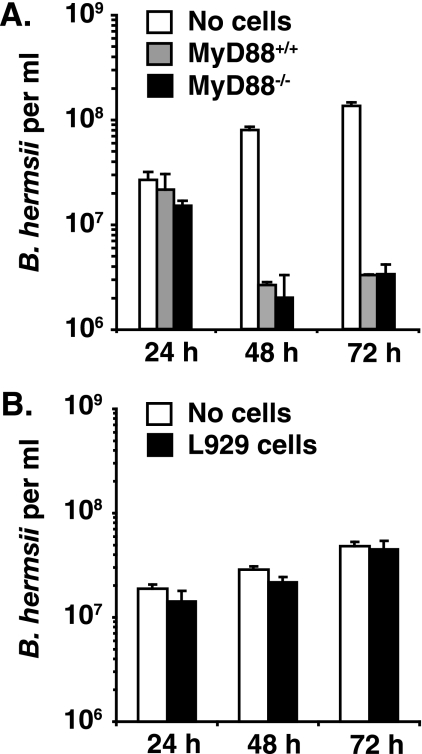
The possibility that the antibody-independent defect in MyD88−/− mice was related to macrophage function was tested with bone marrow-derived macrophages, which had previously been found to effectively kill B. burgdorferi in vitro (43). Efficient killing of B. hermsii was observed in 48-h and 72-h cocultures with bone marrow-derived macrophages from wild-type mice or MyD88−/− mice but not observed in cocultures with murine fibroblasts (Fig. 8). Importantly, the slow kinetics of this assay argue that it is not mediated by classical phagocytosis but possibly by a mechanism of extracellular killing, as previously described for killing of unopsonized Borrelia by human PMNs (44). Interestingly, antibody to B. burgdorferi was previously found to increase B. burgdorferi killing in vitro (43), consistent with our finding that serum transfer into MyD88−/− mice helps to resolve relapsing fever spirochetemia (Fig. 6).
FIG. 8.
Bone marrow-derived macrophages from MyD88-deficient mice and littermate mice control in vitro growth of B. hermsii with similar efficiency. Bone marrow-derived macrophages or mouse fibroblast cell line cells (L929 cells) were plated at a density of 3 × 105 in a 24-well dish. After overnight incubation, 3 × 106 B. hermsii cells were added to the macrophages (A) or L929 cells (B) for a 10:1 starting ratio. After 24 h, 48 h, and 72 h, supernatants were collected and B. hermsii levels were quantified by dark-field microscopy. Averages of duplicate (A) or triplicate (B) samples are shown.
MyD88-independent inflammatory responses to B. hermsii infection.
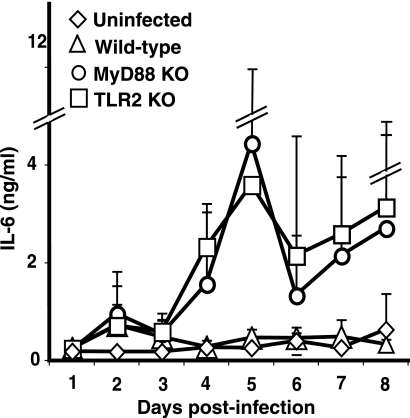
While a defect in control of B. hermsii was clearly evident in MyD88-deficient mice, the production of antibody to B. hermsii and the partial clearance of spirochetes from blood in some MyD88−/− mice indicated residual responses to B. hermsii independent of TLR signaling. In vitro inflammatory cytokine production in response to Borrelia is dependent on TLR2 signaling, therefore, it was surprising to find that infected MyD88−/− mice and TLR2−/− mice expressed higher levels of interleukin-6 (IL-6) in their sera than wild-type mice (Fig. 9). Extremely high levels of IL-6 were found in the sera of several of the MyD88−/− and TLR2−/− mice, generating large standard deviations. However, close inspection of the individual samples revealed that each of the infected MyD88−/− and TLR2−/− mice displayed at least fivefold higher levels of IL-6 than wild-type mice on two consecutive days of infection, most prominently days 4 and 5 (Fig. 9). Although there was not a direct correlation with spirochete levels in the blood at the time the sample was taken, there did appear to be a periodicity to the increased levels of serum IL-6 that could reflect episodes of spirochetemia. Levels of IL-6 in uninfected wild-type, MyD88−/−, or TLR2−/− mice were similar, ranging from 70 to 150 pg/ml and as shown for TLR2−/− mice in Fig. 9.
FIG. 9.
Serum IL-6 cytokine levels during B. hermsii infection. Serum IL-6 levels were measured each day for 8 days following B. hermsii infection. Each plot shows average IL-6 levels, as determined by ELISA, for five mice of the indicated genotype. The uninfected group consisted of TLR2−/− mice. KO, knockout.
DISCUSSION
A notable feature shared by the vector-borne spirochetes of the Borrelia species is the ability of these extracellular pathogens to establish persistent infections. This ability to persist requires that the bacteria possess mechanisms allowing evasion of both acquired and innate host defenses. Antigenic variation plays a major role in immune evasion by both the Lyme disease spirochete B. burgdorferi and the relapsing fever spirochetes, including B. hermsii (9, 23, 78). While antigenic variation, resulting from gene conversion events, is the foundation for the ability of Borrelia to evade antibody-mediated clearance and persist in mammalian hosts, alteration in lipoprotein expression also plays an important role in the natural life cycle of the bacterium (19, 28, 39, 40, 54, 64, 68).
Innate host defenses also play a major role in the host defense to B. burgdorferi. We and others have previously studied the involvement of MyD88 in host defense to B. burgdorferi and found that mice missing this important TLR adapter molecule harbor extremely high levels of spirochetes in several tissues, averaging 70-fold greater spirochete levels in ankle tissue than wild-type littermates (12, 14, 43), higher than levels of B. burgdorferi in antibody-deficient scid mice (53). Interestingly, even in the absence of MyD88-dependent signaling pathways, a robust antibody response to B. burgdorferi develops, with high levels of IgM and a strong IgG response (14, 43). In fact, transfer of sera from B. burgdorferi-infected MyD88-deficient mice is sufficient to protect naïve recipients from challenge with B. burgdorferi (14), indicating that MyD88-dependent factors independent of the production of antibody are also important for controlling B. burgdorferi in tissues.
In contrast to B. burgdorferi, the relapsing fever Borrelia achieves very high levels in blood during infection of mice. This suggested that the relapsing fever B. hermsii spirochetes might provide a better experimental model than B. burgdorferi for assessment of host defense strategies against Borrelia. The critical involvement of IgM in resolution of spirochetemic episodes of relapsing fever Borrelia has been well documented, as has the emergence of bacterial clones expressing novel variable-surface lipoproteins (4, 9, 18, 21). Two recent studies pointed to B1b B cells and marginal-zone B cells as important sources of IgM in control of B. hermsii (5, 13). Therefore, we focused on the role of MyD88 in two aspects of host response to B. hermsii: the rapid generation of Borrelia-specific IgM and the ability of MyD88-deficient mice to clear episodes of spirochetemia.
MyD88-deficient mice were severely compromised in the ability to effectively clear B. hermsii from the blood, averaging 30-fold more spirochetes than wild-type littermates (Fig. 1 and 5 to 7). We initially suspected that inadequate antibody response in MyD88-deficient mice was responsible for the impaired clearance of B. hermsii and assessed IgM production in these mice. However, MyD88-deficient mice generated an active IgM response, as determined by ELISA, achieving similar or higher levels than infected wild-type mice at 6 days of infection (Table 1). Western blot analysis revealed IgM recognition of multiple B. hermsii antigens by both MyD88-deficient mice and wild-type mice with one notable exception: reduced reactivity to a 17-kDa band that contained Vsp33, the lipoprotein expressed by cultured and tick-associated bacteria (Fig. 3B and C). These results suggested that although MyD88-deficient mice could recognize B. hermsii, there might be an impaired ability to rapidly respond to new antigenic variants. Consistent with this hypothesis, the kinetics of the appearance of antibody to several B. hermsii lipoproteins was delayed (Fig. 4). Definitive assessment of the kinetics of appearance of new serotypes will require low-dose infection of a single serotype.
Even more surprising was the observation that the defect in host defense in MyD88-deficient mice was greater than that in scid mice that lack B cells, T cells, and antibody production: MyD88−/− mice harbored 10-fold more B. hermsii than scid mice (Fig. 5 and 7). This was a strong indicator that the defect in MyD88−/− mice was not entirely due to inadequacy in the antibody response. This conclusion was further supported by antibody transfer experiments: sera from MyD88−/− mice could clear B. hermsii from infected scid mice, indicating that anti-B. hermsii antibody was produced in MyD88−/− mice but was not fully effective in the absence of MyD88-expressing effector cells (Fig. 5). However, when high levels of antibody from wild-type mice were provided to MyD88−/− mice, they could clear B. hermsii from the blood (Fig. 6). This argues that two defects occur in the absence of MyD88−/−: (i) delayed appearance of IgM, and (ii) a second defect distinct from antibody and needed for the most efficient clearance of B. hermsii. That this second effector is more than a facilitator of clearance of antibody-coated cells is supported by the ability of scid mice to better control infection than MyD88−/− mice, although the requirement for the second effector can be overcome by transfer of high levels of antibody-containing serum.
TLR2−/− mice were able to partially clear B. hermsii infection, with peak levels in the blood higher than those found with wild-type or scid mice (Fig. 7). TLR2−/− mice also displayed greater mortality than either wild-type or scid mice. Interestingly, a recent study using a different species of relapsing fever, B. turicatae, found clearance in TLR2−/− mice and incidence of vestibular disease to be similar to that seen with infected scid mice (17). This difference in TLR2 requirement in host defense could reflect differences in target tissues of these two distinct but related organisms.
Macrophages were selected to assess MyD88-dependent activity of a cell important to the host defense that could be facilitating clearance of B. burgdorferi in vivo. Several studies have been performed with human macrophages and PMNs, establishing that macrophages can phagocytize unopsonized B. burgdorferi, whereas PMN-mediated killing in the absence of antibody is primarily extracellular (48). Murine macrophages from MyD88−/− mice were assessed for the ability to kill B. hermsii, as these cells respond strongly to Borrelia antigens in a MyD88-dependent manner in vitro and have been shown to kill B. burgdorferi in vitro (14, 43, 49, 50). The coculture assay demonstrated reduction in viability of B. hermsii cocultured with murine macrophages but not with fibroblasts and was compatible with both intracellular and extracellular killing of the bacteria. This assay failed to reveal a defect in killing of B. hermsii by MyD88-deficient macrophages (Fig. 8). Liu et al. recently demonstrated that TLR signaling was involved in the degradation of B. burgdorferi following phagocytosis, suggesting that MyD88-dependent signaling in response to Borrelia could affect phagosome maturation and kinetics of killing (43); however, with time MyD88−/− macrophages were fully capable of killing B. burgdorferi, similar to our findings with B. hermsii. Together, these results imply the presence of an additional, nonmacrophage, non-B-cell defect in MyD88−/− mice crucial to host defense against B. hermsii. B. hermsii is also highly resistant to complement-mediated killing and to PMN-mediated oxidative killing (21, 29). Thus, the MyD88-dependent host defense complementary to antibody-mediated clearance has yet to be identified and will require further studies.
Flow cytometry confirmed the presence of cells bearing surface markers for B1b B cells in the peritoneal cavity of MyD88−/− mice at a frequency similar to that observed in wild-type mice (Table 2). Furthermore, these cells could be activated by LPS through a MyD88−/−-independent pathway but failed to respond to the MyD88-dependent ligands of B. hermsii or B. burgdorferi. The dichotomy between in vitro failure of B cells to produce IgM in response to the mitogenic activities of B. hermsii and the strong IgM response found in B. hermsii-infected mice indicates an additional cell type is required that was not included in these cultures and could not be replaced with bone marrow-derived macrophages. One likely candidate is the marginal-zone B cell, whose contribution in vivo was demonstrated by Belperron et al. (13).
The cellular source of these additional factors remains to be determined. In fact, there is clearly a source of proinflammatory cytokines in response to B. hermsii in infected TLR2-deficient and MyD88-deficient mice, as levels of IL-6 in serum were extremely high, much greater than in wild-type mice (Fig. 9). This is quite surprising, as the IL-6 response to lipoproteins from Borrelia spp. and other bacterial species by macrophages is dependent on TLR2 and MyD88 signaling (14, 77). Responses to the lipoproteins have been proposed to drive the Jarisch-Herxheimer reaction of relapsing fever, and this elevation in cytokines was thought to be dependent on MyD88 signaling (73). These results clearly point to alternative pathways allowing cytokine production in the absence of TLR signaling. We hypothesize that the cellular source of IL-6 in vivo could also be the source of the host defense molecule(s) or pathways responsible for efficient clearance of B. hermsii during infection. Therefore, further characterization of these pathways could allow identification of the components of host defense that act in concert with IgM in clearing B. hermsii and could also shed light on the host defense against B. burgdorferi. Identification of these components could also provide tools for development of novel targets for these interesting, and persistent, pathogens.
Acknowledgments
This work was supported by U.S. Public Health Service grants AI-32223 and AI-43521 (to J.J.W.), AI-24158 (to J.H.W.), and 5P30-CA-42014 to the University of Utah, funds from Associated Regional University Pathologists, National Institutes of Health Training grant GM07464 (to D.D.B.), and the Division of Intramural Research, NIAID, NIH (T.G.S.).
We thank Abbas Zoufer and Xiaohui Wang for maintaining the MyD88−/− and TLR2−/−/scid mouse colonies, Wayne Green for assistance with the University of Utah Flow Cytometry Core Facility, Joe Holden for examining histology slides, and Lynn Sonderegger and Hillary Crandall for helpful discussion.
Editor: F. C. Fang
Footnotes
Published ahead of print on 9 October 2006.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 4.Alugupalli, K. R., R. M. Gerstein, J. Chen, E. Szomolanyi-Tsuda, R. T. Woodland, and J. M. Leong. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819-3827. [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. [DOI] [PubMed] [Google Scholar]
- 6.Alugupalli, K. R., A. D. Michelson, M. R. Barnard, D. Robbins, J. Coburn, E. K. Baker, M. H. Ginsberg, T. G. Schwan, and J. M. Leong. 2001. Platelet activation by a relapsing fever spirochaete results in enhanced bacterium-platelet interaction via integrin alphaIIbbeta3 activation. Mol. Microbiol. 39:330-340. [DOI] [PubMed] [Google Scholar]
- 7.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843-2850. [DOI] [PubMed] [Google Scholar]
- 8.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y.
- 9.Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. [DOI] [PubMed] [Google Scholar]
- 10.Barbour, A. G., and V. Bundoc. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbour, A. G., S. L. Tessier, and H. G. Stoenner. 1982. Variable major proteins of Borrelia hermsii. J. Exp. Med. 156:1312-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 74:1462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 14.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 15.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadavid, D., V. Bundoc, and A. G. Barbour. 1993. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J. Infect. Dis. 168:143-151. [DOI] [PubMed] [Google Scholar]
- 17.Cadavid, D., M. Sondey, E. Garcia, and C. L. Lawson. 2006. Residual brain infection in relapsing-fever borreliosis. J. Infect. Dis. 193:1451-1458. [DOI] [PubMed] [Google Scholar]
- 18.Cadavid, D., D. D. Thomas, R. Crawley, and A. G. Barbour. 1994. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J. Exp. Med. 179:631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 21.Connolly, S. E., and J. L. Benach. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 167:3029-3032. [DOI] [PubMed] [Google Scholar]
- 22.Connolly, S. E., D. G. Thanassi, and J. L. Benach. 2004. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J. Immunol. 172:1191-1197. [DOI] [PubMed] [Google Scholar]
- 23.Dai, Q., B. I. Restrepo, S. F. Porcella, S. J. Raffel, T. G. Schwan, and A. G. Barbour. 2006. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol. 60:1329-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 25.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758-4764. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Monco, J. C., N. S. Miller, P. B. Backenson, P. Anda, and J. L. Benach. 1997. A mouse model of Borrelia meningitis after intradermal injection. J. Infect. Dis. 175:1243-1245. [DOI] [PubMed] [Google Scholar]
- 27.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Investig. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyard, C., J. M. Battisti, S. J. Raffel, M. E. Schrumpf, A. R. Whitney, J. G. Krum, S. F. Porcella, P. A. Rosa, F. R. Deleo, and T. G. Schwan. 2006. Relapsing fever spirochaetes produce a serine protease that provides resistance to oxidative stress and killing by neutrophils. Mol. Microbiol. 60:710-722. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 31.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 32.Hinnebusch, B. J., A. G. Barbour, B. I. Restrepo, and T. G. Schwan. 1998. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect. Immun. 66:432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 34.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 36.Hovis, K. M., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange, W. R., T. G. Schwan, and J. D. Frame. 1991. Can protracted relapsing fever resemble Lyme disease? Med. Hypotheses 35:77-79. [DOI] [PubMed] [Google Scholar]
- 38.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- 39.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 43.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 45.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancuso, G., A. Midiri, C. Beninati, C. Biondo, R. Galbo, S. Akira, P. Henneke, D. Golenbock, and G. Teti. 2004. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J. Immunol. 172:6324-6329. [DOI] [PubMed] [Google Scholar]
- 47.Meier, J. T., M. I. Simon, and A. G. Barbour. 1985. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell 41:403-409. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 49.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1993. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. J. Immunol. 150:909-915. [PubMed] [Google Scholar]
- 51.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newman, K., Jr., and R. C. Johnson. 1981. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect. Immun. 31:465-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman, K., Jr., and R. C. Johnson. 1984. T-cell-independent elimination of Borrelia turicatae. Infect. Immun. 45:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park, H. K., B. E. Jones, and A. G. Barbour. 1986. Erythema chronicum migrans of Lyme disease: diagnosis by monoclonal antibodies. J. Am. Acad. Dermatol. 15:406-410. [DOI] [PubMed] [Google Scholar]
- 56.Pennington, P. M., C. D. Allred, C. S. West, R. Alvarez, and A. G. Barbour. 1997. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect. Immun. 65:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plasterk, R. H., M. I. Simon, and A. G. Barbour. 1985. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature 318:257-263. [DOI] [PubMed] [Google Scholar]
- 58.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 59.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 61.Schwan, T. G., J. M. Battisti, S. F. Porcella, S. J. Raffel, M. E. Schrumpf, E. R. Fischer, J. A. Carroll, P. E. Stewart, P. Rosa, and G. A. Somerville. 2003. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J. Bacteriol. 185:1346-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280:1938-1940. [DOI] [PubMed] [Google Scholar]
- 63.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwan, T. G., M. E. Schrumpf, B. J. Hinnebusch, D. E. Anderson, Jr., and M. E. Konkel. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 34:2483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scopel, K. K., C. J. Fontes, A. C. Nunes, M. F. Horta, and E. M. Braga. 2004. Low sensitivity of nested PCR using Plasmodium DNA extracted from stained thick blood smears: an epidemiological retrospective study among subjects with low parasitaemia in an endemic area of the Brazilian Amazon region. Malar. J. 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 68.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 73.Vidal, V., I. G. Scragg, S. J. Cutler, K. A. Rockett, D. Fekade, D. A. Warrell, D. J. Wright, and D. Kwiatkowski. 1998. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat. Med. 4:1416-1420. [DOI] [PubMed] [Google Scholar]
- 74.Wang, X., Y. Ma, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2005. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect. Immun. 73:657-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wengrower, D., H. Knobler, S. Gillis, and T. Chajek-Shaul. 1984. Myocarditis in tick-borne relapsing fever. J. Infect. Dis. 149:1033. [DOI] [PubMed] [Google Scholar]
- 76.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 77.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 78.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]