Abstract
There is no licensed vaccine available against Chlamydia trachomatis, the leading cause of bacterial sexually transmitted disease. We have found that intranasal immunization with recombinant chlamydial protease-like activity factor (CPAF) induces CD4+ T-cell- and gamma interferon (IFN-γ)-dependent protective immunity against murine genital chlamydial infection, thus making CPAF a viable vaccine candidate for further characterization. HLA-DR4 is the predominant allele involved in chlamydial antigen presentation to CD4+ T cells in humans. We used engineered mice that lack endogenous major histocompatibility complex class II (MHC-II) alleles but express a human HLA allele (HLA-DR4 transgenic [tg] mice) to examine primary immune and CPAF-mediated responses against genital Chlamydia muridarum challenge. Upon primary bacterial exposure, HLA-DR4 tg mice developed Chlamydia-specific IFN-γ and antibody production and resolved the infection within 30 days, similar to challenged conventional C57BL/6 animals. Moreover, C. muridarum-challenged HLA-DR4 tg mice exhibited CPAF-specific antibody and IFN-γ production. Upon CPAF-plus-interleukin-12 (IL-12) vaccination, HLA-DR4 tg animals exhibited robust CPAF-specific IFN-γ production and elevated titers of anti-CPAF total antibody and immunoglobulin G2a (IgG2a) and lower titers of IgG2b and IgG1 antibodies. HLA-DR4 tg and C57BL/6 mice vaccinated with CPAF plus IL-12 resolved the primary genital chlamydial infection significantly earlier than mock-immunized animals, whereas similarly vaccinated MHC class II-deficient mice displayed minimal antigen-specific immune responses and failed to resolve the infection even at 30 days postchallenge. Together, these results demonstrate the importance of human HLA-DR4 molecules in the recognition and presentation of CPAF epitopes, leading to the generation of protective antichlamydial immunity and making these mice a valuable model for mapping HLA-DR4-restricted chlamydial epitopes.
Chlamydia trachomatis is the leading cause of sexually transmitted bacterial disease, with an estimated 90 million cases occurring worldwide each year (2). Recurrent chlamydial infections are common (11) and lead to chronic inflammatory complications, including pelvic inflammatory disease, ectopic pregnancy, and infertility (2, 11, 25, 26). The incidence rate of this infection has continued to increase over the last decade despite the availability of potent antimicrobial regimens against chlamydiae (18), underscoring an urgent need to develop an effective antichlamydial vaccine (2, 14).
Chlamydia has evolved several strategies to evade host immune responses. A secreted chlamydial protease-like activity factor (CPAF) has been reported by Zhong and colleagues to cleave host major histocompatibility complex (MHC) transcription factors (28) and keratin-8 (4), potentially allowing chlamydial survival and expansion within infected cells, respectively. High titers of anti-CPAF antibodies in Chlamydia-seropositive humans have also been observed (21). Additionally, these antibodies have been demonstrated to neutralize the protease activity of CPAF (22). We recently demonstrated the efficacy of intranasal CPAF vaccination in inducing robust gamma interferon (IFN-γ)-dependent protective immunity against Chlamydia muridarum challenge (A. K. Murthy and B. P. Arulanandam, unpublished data) in a murine model of genital chlamydial infection (14). These results suggest an important role for CPAF in antichlamydial immunity and the relevance of additional characterization of this protein as a potential vaccine candidate for humans.
Chlamydial infection in mice induces a robust CD4+ T-cell-mediated protective cellular immune response (14-16, 23, 24), indicating that critical epitopes of chlamydial antigens are presented via the MHC class II pathway. The mapping of T-cell epitopes by using conventional mice is problematic because of the differences in MHC binding properties between murine and human MHCs (6). These constraints can be overcome by using mice that lack endogenous murine MHC class II molecules but express a human MHC class II (HLA) allele (7). Through exon shuffling, these mice are engineered to express the extracellular human α1 and β1 domains of HLA-DRA and HLA-DRB1*0401, which jointly form the peptide binding groove that defines peptide binding specificity, in conjunction with the murine membrane proximal α2 and β2 domains, which form the binding site for the (murine) CD4 molecule (10). HLA-DR4 has been shown to be the predominant MHC allele involved in the presentation of chlamydial antigens to T cells (3, 5, 19, 20, 27), and the frequency of the HLA-DR allele in humans is 29% in Caucasian individuals, 10% in African Americans, and 34% in others (13), indicating the translational value of the epitopes identified in these mice for humans.
In this study, we have established a genital C. trachomatis mouse pneumonitis (recently designated the separate species C. muridarum) infection model using HLA-DR4 transgenic (tg) mice. HLA-DR4 tg animals infected intravaginally (i.vag.) with C. muridarum were found to exhibit an immune response and resolution kinetics comparable to those of conventional C57BL/6 animals. HLA-DR4 tg mice mounted both antibody and cellular immune responses to CPAF after i.vag. C. muridarum challenge. In addition, intranasal vaccination with CPAF plus interleukin-12 (IL-12) induced robust Th1-type anti-CPAF cellular and humoral responses and significantly accelerated the resolution of genital C. muridarum infection, similar to what was observed for vaccinated conventional C57BL/6 animals. Collectively, these results indicate that CPAF contains human HLA-DR4 determinants that are capable of inducing protective antichlamydial immunity. Thus, the HLA-DR4 tg mice may be useful for mapping CPAF determinants that have translational value for humans.
MATERIALS AND METHODS
Bacteria.
Chlamydia muridarum was grown on confluent HeLa cell monolayers. The cells were lysed using a sonicator (Fisher, Pittsburgh, PA), and the elementary bodies were purified on Renografin gradients as described previously (17, 29). Aliquots of bacteria were stored at −70°C in sucrose-phosphate-glutamine (SPG) buffer. Chlamydia genus-specific murine monoclonal antibody was used to confirm the identity of the purified bacterium (17).
Mice.
HLA-DRA-IEα/HLA-DRB1*0401-IEβ tg mice were generated and backcrossed to MHC class II-deficient mice (MHC-IIΔ/Δ mice) to eliminate any effect of endogenous MHC class II proteins as described previously (10). The MHC-IIΔ/Δ mice were generated by complete deletion of the H2-Aa, H2-Eb1, and H2-Eb2 genes and bred to C57BL/6 mice as described previously (12). The HLA-DR4 tg mice, MHC-IIΔ/Δ mice (Jackson Laboratories, Bar Harbor, ME), and C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were housed and bred at the University of Texas at San Antonio Animal Care Facility and provided food and water ad libitum. Animal care and experimental procedures were performed in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines. Four- to 6-week-old female mice were used for all experiments.
Intravaginal infection.
Mice were anesthetized intranasally (i.n.) using 3% isofluorane in a rodent anesthesia system (Harvard Apparatus, Holliston, MA) and immediately inoculated i.vag. with 1,500 inclusion-forming units (IFU) of C. muridarum in 5 μl of sterile SPG buffer (17). Vaginal vaults of challenged mice were swabbed at 3-day intervals, and swabs were collected into Eppendorf tubes containing 4-mm glass beads (Kimble, Vineland, NJ) and 500 μl of sterile SPG buffer. The tubes were vortexed for 1 min, and swab material was plated and incubated for 28 h with HeLa cells grown on coverslips in 24-well plates. The infected HeLa cells were fixed with 2% paraformaldehyde and permeabilized with 2% saponin. Cells were washed using phosphate-buffered saline (PBS) and incubated with Dulbecco's modified Eagle's medium containing 10% fetal bovine serum for 1 h to block nonspecific binding. Thereafter, cells were washed and incubated with polyclonal rabbit anti-Chlamydia antibody for 1 h and then incubated for an additional 2 h with goat anti-rabbit immunoglobulin (Ig) conjugated to fluorescein isothiocyanate (Sigma, St. Louis, MO) plus Hoechst nuclear staining. The treated coverslip cultures were then washed and mounted onto Superfrost microscope slides (Fisher) by using Fluorsave reagent (Calbiochem, La Jolla, CA). Slides were visualized using a Zeiss Axioskop 2 Plus research microscope (Zeiss, Thornwood, NY). The bacterial shedding was calculated and expressed as the number of inclusion-forming units per animal.
Determination of cytokine responses by enzyme-linked immunosorbent assay (ELISA).
Spleens from HLA-DR4 tg mice were removed on day 14 after i.vag. C. muridarum challenge or i.n. CPAF (15 μg)-plus-IL-12 (0.5 μg) immunization, and single-cell suspensions were made. Splenocytes (106 cells/well) were plated on 96-well culture plates, stimulated with 0.5 μg/ml of recombinant CPAF (rCPAF) or hen egg lysozyme (HEL), and incubated for 72 h. The culture supernatants were then assayed for IFN-γ and IL-4 by using BD optELISA kits (BD Pharmingen, New Jersey) according to the manufacturer's instructions. Results were calculated and expressed as pg/ml of IFN-γ or IL-4.
Detection of antibody levels by ELISA.
Microtiter plates (96 well) were coated overnight with UV-inactivated C. muridarum (105 IFU/well) or 5 μg/well of CPAF in sodium bicarbonate buffer (pH 9.5), washed with PBS containing 0.3% Brij-35 (Sigma), and blocked for 1 h at room temperature with PBS containing 2% bovine serum albumin (EM Science, Gibbstown, NJ). Serial dilutions of sera were added to the wells and incubated at room temperature for 2 h. The plates were then washed and incubated for an additional 1 h with goat anti-mouse total Ig conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). After incubation for 1 h, the plates were washed and p-nitrophenyl phosphate substrate was added for color development. Absorbance at 405 nm was measured using an ELISA microplate reader (Biotek Instruments, Winooski, VT). No binding of immune sera was observed when the plates were coated with the unrelated antigen HEL.
Cloning and expressing CPAF.
The open reading frames coding for CPAF from the C. trachomatis L2 genome were cloned into pBAD vectors and expressed as fusion proteins with a six-His tag at the N terminus. The amino acid sequences of CPAF from serovar L2 and C. muridarum share significant identity (82%). Expression of the fusion protein designated CPAF (CPAF with a six-His tag) was induced with l-arabinose (Sigma), and the fusion proteins were extracted by lysing the bacteria via sonication in Triton X-100 lysis buffer (1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 75 U of aprotinin/ml, 20 μM leupeptin, and 1.6 μM pepstatin). After high-speed centrifugation to remove debris, the fusion protein-containing supernatants were purified further with Ni-nitrilotriacetic acid agarose beads (QIAGEN, Valencia, CA) for six-His tag proteins.
i.n. immunization procedures.
Intranasal immunization was performed as described previously (1). Briefly, mice were anesthetized i.n. with 3% isofluorane by using a rodent anesthesia system (Harvard Apparatus, Holliston, MA). Mice were immunized i.n. on day 0 with 15 μg rCPAF dissolved in 25 μl sterile PBS and on days −1, 0, and +1 with or without 0.5 μg of recombinant murine IL-12 (Wyeth, Cambridge, MA) in PBS containing 1% normal mouse serum. Mice were boosted i.n. with 15 μg rCPAF with or without IL-12 (0.5 μg) on days 14 and 28. Some mice received only PBS-normal mouse serum (no rCPAF vaccine). As previously described, no toxicity was observed with the IL-12 treatment regimen (9). The dose of rCPAF that provided optimal protection against genital C. trachomatis challenge in BALB/c mice was used (Murthy and Arulanandam, unpublished).
Statistical analyses.
Sigma Stat (Chicago, IL) was used to perform all tests of significance. The Kruskal-Wallis test was used to determine differences in vaginal chlamydial shedding between experimental groups. The infection resolution times between groups were compared using the Kaplan-Meier test. Differences were considered statistically significant if P values were <0.05. All data shown are representative of at least two independent experiments and have been expressed as means ± standard deviations (SD).
RESULTS
HLA-DR4 tg mice resolve primary genital C. muridarum infection similar to conventional C57BL/6 mice.
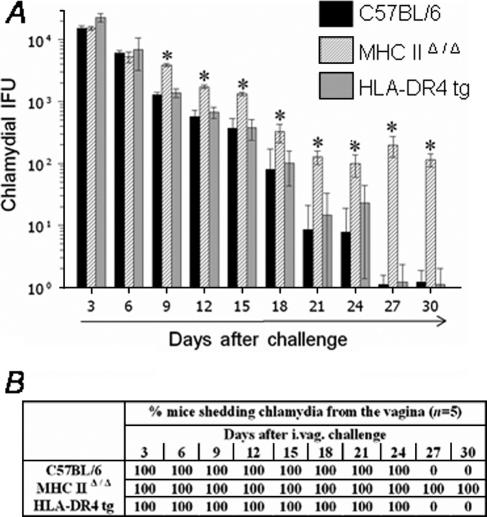
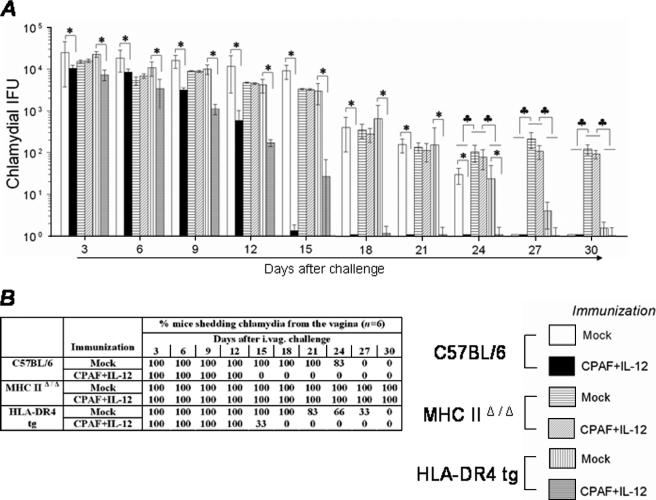
The resolution of primary genital C. muridarum infection following challenge with 1,500 IFU was analyzed by comparing vaginal chlamydial shedding results at timed intervals in conventional C57BL/6, MHC-IIΔ/Δ, and HLA-DR4 tg mice. As shown in Fig. 1A and B, challenged HLA-DR4 tg mice displayed resolution kinetics comparable to those of conventional C57BL/6 animals. The chlamydial shedding progressively reduced (Fig. 1A) such that 100% of HLA-DR4 tg and C57BL/6 animals resolved the infection by day 27 after challenge (Fig. 1B). In contrast, challenged MHC-IIΔ/Δ animals shed significantly greater numbers of Chlamydia (≥0.5 log) than similarly treated HLA-DR4 tg and conventional C57BL/6 animals at day 9 after challenge and at all subsequent time points examined (Fig. 1A). All of the challenged MHC-IIΔ/Δ animals were still shedding considerable numbers of Chlamydia (114 ± 12) even at day 30 after challenge (Fig. 1B). These results indicate that (i) MHC class II molecules are important in chlamydial clearance, (ii) chlamydial antigenic epitopes presented on human HLA-DR4 molecules elicit protective immunity, and (iii) epitopes presented on HLA-DR4 molecules induce protective immunity comparable to that of epitopes presented on murine MHC class II molecules, suggesting that HLA-DR4 tg mice are a useful model for studying immunity against genital chlamydial infections.
FIG. 1.
Resolution of genital infection in C. muridarum-challenged HLA-DR4 tg mice. HLA-DR4 tg, MHC-IIΔ/Δ, and conventional C57BL/6 animals (five mice/group) were challenged i.vag with 1,500 IFU of C. muridarum, and vaginal chlamydial shedding was monitored at 3-day intervals. (A) Numbers of chlamydial IFU recovered from vaginal swabs at the indicated days after genital challenge. Each bar represents the mean ± SD for the animal group. *, significant difference in numbers of recovered chlamydial organisms from MHC-IIΔ/Δ mice compared to those of HLA-DR4 tg and C57BL/6 animals (P < 0.01, Kruskal-Wallis test). (B) Percentage of animals shedding Chlamydia after genital challenge. Significant differences were detected in the time required for resolution of infection between challenged MHC-IIΔ/Δ mice and all other experimental groups (P < 0.05, Kaplan-Meier test). Results are representative of two independent experiments.
Antichlamydial immune response in HLA-DR4 tg mice is comparable to that in conventional C57BL/6 mice.
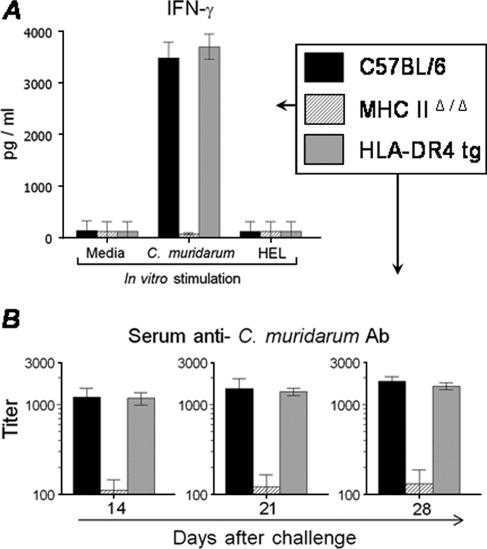
The cellular and humoral immune responses were analyzed in the HLA-DR4 tg, C57BL/6, and MHC-IIΔ/Δ animals after i.vag. challenge with 1,500 IFU of C. muridarum. On day 14 after challenge, splenocytes from challenged animals were stimulated in vitro with UV-inactivated C. muridarum and the production of IFN-γ and IL-4 was measured. As shown in Fig. 2A, splenocytes from C. muridarum-infected HLA-DR4 tg animals exhibited a high level (3,702 ± 242 pg/ml) of antigen-specific IFN-γ production, which is comparable to what was observed with splenocytes from similarly infected conventional C57BL/6 mice (3,482 ± 307 pg/ml). In contrast, splenocytes from MHC-IIΔ/Δ animals displayed only minimal amounts of IFN-γ production (68 ± 20 pg/ml). Mock-infected (PBS) animals and cells stimulated with medium alone or an unrelated antigen (HEL) did not exhibit antigen-specific IFN-γ production, suggesting the specificity of the immune response. There was no detectable IL-4 production in splenocytes from any animal group (data not shown).
FIG. 2.
Immune response to primary C. muridarum genital infection in HLA-DR tg mice. HLA-DR4 tg, MHC-IIΔ/Δ, and conventional C57BL/6 animals (five mice/group) were challenged i.vag with 1,500 IFU of C. muridarum, and cellular and humoral responses were analyzed at timed intervals. (A) Cytokine recall response. On day 14 after challenge, splenocytes were removed and cultured (106 cells/well) for 72 h with medium, C. muridarum, or the unrelated antigen HEL. IFN-γ production was measured by analyzing the culture supernatants by ELISA, and results are expressed as pg/ml of IFN-γ. (B) On days 14, 21, and 28, mice were bled and sera were analyzed for anti-C. muridarum total antibody (Ab) by ELISA. The 50% maximal binding was used to calculate titers. All results are expressed as means ± SD and are representative of two independent experiments.
The serum humoral response was measured on days 14, 21, and 28 after i.vag. C. muridarum challenge. As shown in Fig. 2B, HLA-DR4 tg mice displayed progressively increasing titers of anti-C. muridarum serum antibodies that were comparable to those of conventional C57BL/6 mice on days 14 (1,177 ± 182 and 1,213 ± 323, respectively), 21 (1,394 ± 130 and 1,514 ± 438, respectively), and 28 (1,620 ± 142 and 1,838 ± 217, respectively) after challenge. In contrast, MHC-IIΔ/Δ animals displayed severely reduced titers of serum antibody at each time point examined. Mock-infected (PBS) animals did not produce detectable titers of anti-C.muridarum antibodies, and none of the animal groups displayed serum antibodies against the unrelated antigen HEL (data not shown). Collectively, these results indicate that the antichlamydial immune response in HLA-DR4 tg animals was comparable to that in conventional C57BL/6 mice. Furthermore, the markedly reduced cellular and humoral responses against Chlamydia in MHC-IIΔ/Δ animals suggest indirectly that HLA-DR4 molecules present chlamydial antigens to T cells, leading to the generation of the measured antichlamydial responses.
Immune responses to CPAF following primary genital chlamydial challenge.
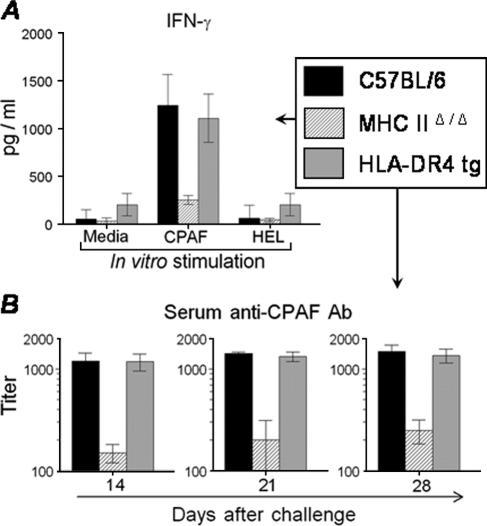
We examined whether human MHC-II molecules in HLA-DR4 tg mice present CPAF epitopes during an active genital chlamydial infection by comparing anti-CPAF immune responses in HLA-DR4 tg animals with those in C57BL/6 and MHC-IIΔ/Δ animals following genital C. muridarum challenge. On day 14 after challenge, splenocytes from mice were stimulated in vitro with CPAF, medium alone, or the unrelated antigen HEL. As shown in Fig. 3A, splenocytes from C. muridarum-infected HLA-DR4 tg animals exhibited a high level (1,106 ± 252 pg/ml) of antigen-specific IFN-γ production, comparable to that exhibited by splenocytes from similarly infected conventional C57BL/6 mice (1,242 ± 324 pg/ml). In contrast, splenocytes from MHC-IIΔ/Δ animals displayed only minimal amounts of IFN-γ production (250 ± 46 pg/ml). Cells treated with HEL or medium alone did not exhibit detectable IFN-γ production. There was no detectable IL-4 production in any culture supernatant (data not shown). These results indicate the induction via the HLA-DR4 molecules of a Th1-type anti-CPAF cellular immune response during genital C. muridarum infection.
FIG. 3.
CPAF-specific immune response after primary genital C. muridarum challenge. HLA-DR4 tg, C57BL/6, and MHC-IIΔ/Δ animals (five mice/group) were challenged i.vag with 1,500 IFU of C. muridarum, and CPAF-specific cellular and humoral responses were analyzed at timed intervals. (A) Cytokine recall response. On day 14 after challenge, splenocytes were removed and cultured (106 cells/well) for 72 h with medium, CPAF, or the unrelated antigen HEL. IFN-γ production was measured by analyzing the culture supernatants by ELISA, and results are expressed as pg/ml of IFN-γ. (B) On days 14, 21, and 28, mice were bled and sera were analyzed for anti-CPAF total antibody (Ab) by ELISA. The 50% maximal binding was used to calculate titers. All results are expressed as means ± SD and are representative of two independent experiments.
The humoral response against CPAF was also analyzed at various timed intervals in i.vag. C. muridarum-challenged HLA-DR4 tg versus C57BL/6 and MHC-IIΔ/Δ animals. As shown in Fig. 3B, HLA-DR4 tg mice displayed progressively increasing titers of anti-C. muridarum serum antibodies that were comparable to those for conventional C57BL/6 mice on days 14 (1,181 ± 228 and 1,201 ± 233, respectively), 21 (1,325 ± 140 and 1,416 ± 57, respectively), and 28 (1,352 ± 211 and 1,478 ± 245, respectively) after challenge. In contrast, MHC-IIΔ/Δ animals displayed severely reduced titers of serum antibody at each time point examined. At the same time points, there was no detectable CPAF-specific antibody response in mock-infected (PBS) animals and no antibody binding in plates coated with the unrelated antigen HEL. These results demonstrate the induction of a CPAF-specific humoral response via HLA-DR4 molecules after i.vag. C. muridarum challenge.
Intranasal vaccination with CPAF induces a robust immune response.
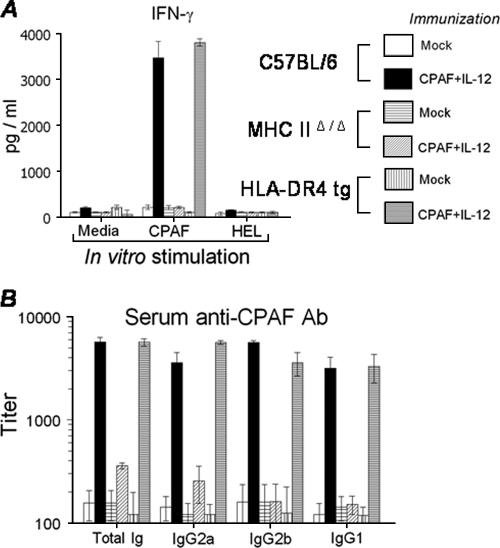
We have previously demonstrated that i.n. vaccination with CPAF plus IL-12 induces a robust Th1-type cellular and humoral immune response and leads to protective immunity against genital C. muridarum infection in conventional BALB/c mice (Murthy and Arulanandam, unpublished). The induction of immune responses at timed intervals after i.n. CPAF-plus-IL-12 immunization was examined in the present study using HLA-DR4 tg mice compared to similarly treated C57BL/6 and MHC-IIΔ/Δ mice. Animals were immunized with CPAF plus IL-12 on day 0 and boosted on days 14 and 28. On day 14 after primary immunization, splenocytes were stimulated in vitro with CPAF, medium alone, or HEL for 72 h and the production of IFN-γ and IL-4 was analyzed by ELISA. As shown in Fig. 4A, high levels of IFN-γ production were induced in splenocytes from CPAF-plus-IL-12-immunized HLA-DR4 tg mice (3,800 ± 77 pg/ml) and C57BL/6 mice (3,463 ± 363 pg/ml) but not MHC-IIΔ/Δ animals (25 ± 22 pg/ml) upon in vitro stimulation with CPAF. Splenocytes from mock-immunized (PBS) animals stimulated with CPAF or cells from any group stimulated with the unrelated antigen HEL did not display detectable IFN-γ production. There was minimal IL-4 production in each of the cell cultures (data not shown). Furthermore, splenocytes from mice receiving IL-12 alone did not exhibit CPAF-specific IFN-γ production (data not shown).
FIG. 4.
CPAF-specific immune response after intranasal CPAF-plus-IL-12 immunization. HLA-DR4 tg, C57BL/6, and MHC-IIΔ/Δ animals (five mice/group) were treated i.n. with CPAF plus IL-12 or PBS (mock) on days 0, 14, and 28, rested for 1 month, and then challenged i.vag with 1,500 IFU of C. muridarum. CPAF-specific cellular and humoral responses were analyzed at timed intervals. (A) Cytokine recall response. On day 14 after challenge, splenocytes were removed and cultured (106 cells/well) for 72 h with medium, CPAF, or the unrelated antigen HEL. IFN-γ production was measured by analyzing the culture supernatants by ELISA, and results are expressed as pg/ml of IFN-γ. (B) Ten days after the last booster, mice were bled and sera were analyzed for anti-CPAF antibody (Ab) by ELISA. The 50% maximal binding was used to calculate titers. All results are expressed as means ± SD and are representative of two independent experiments.
The induction of a CPAF-specific humoral response after immunization was examined 10 days after the last booster immunization. As shown in Fig. 4B, HLA-DR4 tg mice exhibited titers of anti-CPAF antibody comparable to those of similarly treated conventional C57BL/6 animals with high titers of total Ig (5,670 ± 465 and 5,560 ± 665, respectively), IgG2a (5,642 ± 253 and 3,572 ± 916, respectively), IgG2b (3,433 ± 873 and 5,400 ± 242, respectively), and IgG1 (3,299 ± 1,009 and 3,178 ± 880, respectively). In contrast, vaccinated MHC-IIΔ/Δ animals displayed severely reduced titers of serum antibody (anti-CPAF total Ig, 358 ± 23; IgG2a, 254 ± 100; IgG2b, 160 ± 75; and IgG1, 150 ± 30). Furthermore, the relative titers of anti-CPAF IgG2a were greater than those of IgG1 in immunized HLA-DR4 tg animals. Mock-immunized (PBS) animals displayed minimal CPAF-specific antibody responses. No binding was detected in ELISA plates coated with an unrelated antigen, HEL. These results demonstrate that intranasally administered CPAF is processed and presented on the HLA-DR4 molecules to induce Th1-type cellular and strong humoral responses in the HLA-DR4 tg animals.
Intranasal CPAF vaccination enhances resolution of genital chlamydial infection.
The resolution kinetics of a genital chlamydial infection was analyzed in CPAF-plus-IL-12-immunized HLA-DR4 tg, C57BL/6, and MHC-IIΔ/Δ mice. Animals were immunized i.n. with CPAF plus IL-12 or PBS on days 0, 14, and 28, rested for 1 month, and challenged i.vag. with C. muridarum (1,500 IFU). As shown in Fig. 5A, vaccinated HLA-DR4 tg and C57BL/6 mice displayed significantly reduced (<35%) vaginal chlamydial shedding as early as day 3 after challenge and at subsequent time points compared to corresponding mock-immunized (PBS) animals. Chlamydial numbers rapidly reduced in the vaccinated HLA-DR4 tg mice, and complete resolution of infection was attained in 67% of animals by day 15 and 100% of animals by day 18 after challenge (Fig. 5B). Vaccinated C57BL/6 animals exhibited kinetics comparable with those of 100% of animals completely resolving the infection by day 15 after challenge. Mock-immunized (PBS) HLA-DR4 tg and C57BL/6 animals completely resolved the infection between days 27 and 30 after challenge. In contrast, none of the MHC-IIΔ/Δ animals immunized with CPAF plus IL-12 or PBS had resolved the infection at day 30 after challenge (Fig. 5B) and all of the animals were still shedding a considerable number of organisms (198 ± 82 or 114 ± 29 IFU, respectively) at that point. Treatment with IL-12 alone did not appreciably affect bacterial clearance and was comparable to mock (PBS) immunization (data not shown). Collectively, these results demonstrate that (i) MHC class II molecules are important in the generation of protective immunity against genital chlamydial infection and that (ii) epitopes of CPAF presented on human HLA-DR4 molecules generate robust antichlamydial protective immunity, leading to accelerated resolution of genital chlamydial infection.
FIG. 5.
Resolution of genital C. muridarum challenge after intranasal CPAF-plus-IL-12 immunization. HLA-DR4 tg, C57BL/6, and MHC-IIΔ/Δ animals (five mice/group) were treated i.n. with CPAF plus IL-12 or PBS (mock) on days 0, 14, and 28, rested for 1 month, and then challenged i.vag with 1,500 IFU of C. muridarum. Vaginal chlamydial shedding was monitored at 3-day intervals. (A) Numbers of chlamydial IFU recovered from vaginal swabs at the indicated days after genital challenge. Each bar represents the mean ± SD for the animal group. *, significant difference between indicated groups (P < 0.01, Kruskal-Wallis test); ♣, significant difference in chlamydial recovery from CPAF-plus-IL-12-vaccinated or mock-immunized (PBS) MHC-IIΔ/Δ animals and all other groups (P < 0.01, Kruskal-Wallis test). (B) Percentage of animals shedding Chlamydia after genital challenge. Significant differences were detected in the time required for resolution of infection between challenged CPAF-plus-IL-12-vaccinated HLA-DR4 tg or C57BL/6 mice and all other experimental groups (P < 0.001, Kaplan-Meier test). Results are representative of two independent experiments.
DISCUSSION
We used HLA-DR4 tg mice to examine whether the presentation of CPAF epitopes on human MHC-II molecules can induce protective immunity against genital chlamydial challenge. Intranasal vaccination of HLA-DR4 tg mice with CPAF plus IL-12 elicited a Th1 cellular immune response and robust antibody production and significantly accelerated the resolution of genital C. muridarum infection, similar to what was observed for vaccinated conventional C57BL/6 animals. This protection was not evident in CPAF-plus-IL-12-immunized MHC-IIΔ/Δ animals. Results from this study suggest the presence of protective HLA-DR determinants on CPAF and support the translational value of this candidate vaccine antigen for humans.
To our knowledge, this is the first study using HLA-DR4 tg mice to examine immunity against chlamydial infections. HLA-DR4 tg mice were originally generated with HLA-DRA-IEa and HLA-DRB1*0401-IEβ chimeric genes and then backcrossed to MHC-IIΔ/Δ mice to eliminate any effect of endogenous MHC class II proteins (10). Therefore, all MHC class II-restricted responses in HLA-DR4 tg animals are induced via the human HLA-DR4 molecules. Chlamydia-infected HLA-DR4 tg mice developed a C. muridarum-specific Th1-type cellular response, as indicated by high IFN-γ production, and resolved the infection by approximately 30 days after challenge. The immune response and resolution kinetics in these mice were qualitatively and quantitatively comparable to those in similarly treated conventional C57BL/6 mice, in which immunity against primary genital C. muridarum infection has been extensively characterized (14). Chlamydial infections typically induce a Th1-type immune response in challenged C57BL/6 animals, and such responses, specifically IFN-γ, have been demonstrated to be important in the resolution of the infection (2, 14). Challenged MHC-IIΔ/Δ animals in the present study developed only minimal cellular and humoral Chlamydia-specific responses and failed to resolve the infection within the 30-day period monitored, indicating that protective immunity was induced primarily via the HLA-DR4 molecules in challenged HLA-DR4 tg animals. Collectively, these results suggest that HLA-DR4 tg mice are a good model for studying immune responses against genital chlamydial infections.
CPAF expression has been detected as early as 8 to 12 h after chlamydial challenge by Western blot analyses (4) and at approximately 30 h by immunofluorescence staining (28). We recently identified in situ CPAF expression in the genital tracts of C. muridarum-infected HLA-DR4 tg mice (Murthy and Arulanandam, unpublished). CPAF was detected in the columnar cells of the endocervix and endometrium of the genital tract of infected HLA DR4 tg mice at 4 days after challenge. These results are supported by our observations from in vitro experiments in which low levels of CPAF have been shown to degrade USF-1 and RFX-5, host transcription factors for MHC antigen expression (28), which may allow infected epithelial cells to evade immune detection. More recently, CPAF was also shown to degrade keratin-8, thus potentially allowing expansion of the chlamydial vacuole inside the host cell and also possibly prolonging survival of the infected cells (4). Therefore, neutralization of such CPAF activity in vivo could result in immune-mediated elimination of infected cells and/or prevention of chlamydial vacuolar expansion, thus leading to an abortive infection in such cells. In fact, we recently demonstrated that intranasal vaccination with CPAF and IL-12 induces a robust Th1-type CPAF-specific immune response and leads to significantly accelerated resolution of a genital chlamydial infection in conventional BALB/c animals (Murthy and Arulanandam, unpublished).
HLA-DR4 tg mice are a useful model for examining the immune responses against bacterial antigens in the context of human as opposed to murine MHC-II molecules. Intravaginal C. muridarum infection in HLA-DR4 tg mice induced a strong CPAF-specific Th1 cellular response and antibody production. These results are consistent with the findings of Sharma et al. (21) that Chlamydia-seropositive individuals and C. trachomatis-infected conventional BALB/c mice exhibit antibody responses against CPAF. Additionally, in our studies, similarly challenged MHC-IIΔ/Δ animals displayed only a minimal anti-CPAF immune response. Collectively, these results indicate that CPAF molecules are processed and presented to T cells during an active genital chlamydial infection and that such HLA-DR4 determinants are present within CPAF. An important implication of this finding is the possibility of inducing protective immunity in humans by using CPAF as a vaccine candidate.
Characterization of the immune responses to chlamydial vaccine candidate antigens has been widely pursued, both as a means to better understand the underlying T-cell-mediated immunity and to develop better strategies for vaccine development. However, such studies of humans are limited by difficulties in sampling and by technical difficulties in analyzing the ensuing T-cell response directly ex vivo without introducing culture artifacts. As a consequence, chlamydial vaccine candidates are routinely tested in murine models. The T-cell responses against these antigens occur in the context of murine MHC molecules, whose determinant binding characteristics differ from those of the corresponding human MHC alleles (6). Specifically, MHC molecules are highly polymorphic, and each allelic MHC product has a unique peptide binding motif that makes it specific for a unique peptide fragment of the antigen (7). Since individuals within a population that express different MHC alleles exhibit unique peptide binding properties, rules of determinant recognition established for one allele do not apply to another allele and determinant presentation data from one species cannot necessarily be extrapolated to another (7).
These constraints can be overcome by using mice that lack endogenous murine MHC class II molecules but express a human HLA allele (HLA-DR4 tg mice). The chimeric MHC molecules show the same peptide binding specificity as the HLA-DR4 molecules and are capable of presenting antigens to human T cells (10). In addition, since the cytoplasmic tail of the HLA-DR-IEα construct of the transgenic mice was derived from the murine MHC class II molecule, signaling and sorting of the MHC class II molecules via the cytoplasmic tail of the HLA-DR constructs can be assumed to be normal, without affecting the functioning of the antigen-presenting cells. The production of significant amounts of IFN-γ, but not IL-4 recall responses, by splenocytes of CPAF-immunized mice in this study indicates the induction of a Th1-biased anti-CPAF cellular immune response. Furthermore, CPAF-vaccinated HLA-DR4 tg mice, but not MHC-IIΔ/Δ animals, resolved the infection significantly earlier than the corresponding challenged mock-immunized animals. This clearly demonstrates that (i) CPAF-specific immune responses after vaccination induce protection against genital chlamydial challenge and, more importantly, (ii) CPAF contains protective determinants that are processed and presented by human HLA-DR4 molecules. This observation implies great importance for CPAF as a potential vaccine candidate for humans and warrants further mapping of protective CPAF epitopes that are restricted by human MHC class II alleles. In this regard, HLA-DR4 tg mice have been utilized to identify T-cell-reactive peptides against the outer surface protein (OspA) of Borrelia burgdorferi, the agent of Lyme disease (8). The immunodominant peptides identified using the HLA-DR4 tg mice were shown to generate immune responses similar to those generated in treatment-resistant Lyme-induced arthritis patients. These results reinforce the relevance of using HLA-DR4 tg mice to identify MHC-related epitopes that have relevance to human disease.
Our study indicates that HLA-DR4 tg mice that express the human HLA allele provide an appropriate model for studying immune responses against genital C. muridarum infection and constitute a viable platform for mapping candidate CD4+ T-cell antigens, such as CPAF, that may have translational value in humans.
Acknowledgments
This work was supported by National Institutes of Health grants AR048973-03 and SO6GM008194-24.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 3.Deane, K. H., R. M. Jecock, J. H. Pearce, and J. S. Gaston. 1997. Identification and characterization of a DR4-restricted T cell epitope within chlamydia heat shock protein 60. Clin. Exp. Immunol. 109:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, F., H. Su, Y. Huang, Y. Zhong, and G. Zhong. 2004. Cleavage of host keratin 8 by a chlamydia-secreted protease. Infect. Immun. 72:3863-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.el Asrar, A. M., M. H. Emarah, J. J. Van den Oord, K. Geboes, V. Desmet, and L. Missotten. 1989. Conjunctival epithelial cells infected with Chlamydia trachomatis express HLA-DR antigens. Br. J. Ophthalmol. 73:399-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhard, V. H. 1994. Structure of peptides associated with class I and class II MHC molecules. Annu. Rev. Immunol. 12:181-207. [DOI] [PubMed] [Google Scholar]
- 7.Forsthuber, T. G., C. L. Shive, W. Wienhold, K. de Graaf, E. G. Spack, R. Sublett, A. Melms, J. Kort, M. K. Racke, and R. Weissert. 2001. T cell epitopes of human myelin oligodendrocyte glycoprotein identified in HLA-DR4 (DRB1*0401) transgenic mice are encephalitogenic and are presented by human B cells. J. Immunol. 167:7119-7125. [DOI] [PubMed] [Google Scholar]
- 8.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 9.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed] [Google Scholar]
- 10.Ito, K., H. J. Bian, M. Molina, J. Han, J. Magram, E. Saar, C. Belunis, D. R. Bolin, R. Arceo, R. Campbell, F. Falcioni, D. Vidovic, J. Hammer, and Z. A. Nagy. 1996. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 183:2635-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly, K. A. 2003. Cellular immunity and Chlamydia genital infection: induction, recruitment, and effector mechanisms. Int. Rev. Immunol. 22:3-41. [DOI] [PubMed] [Google Scholar]
- 12.Madsen, L., N. Labrecque, J. Engberg, A. Dierich, A. Svejgaard, C. Benoist, D. Mathis, and L. Fugger. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA 96:10338-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori, M., P. G. Beatty, M. Graves, K. M. Boucher, and E. L. Milford. 1997. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program donor registry. Transplantation 64:1017-1027. [DOI] [PubMed] [Google Scholar]
- 14.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy, A. K., J. Sharma, J. J. Coalson, G. Zhong, and B. P. Arulanandam. 2004. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell. Immunol. 230:56-64. [DOI] [PubMed] [Google Scholar]
- 18.Nelson, D. E., D. P. Virok, H. Wood, C. Roshick, R. M. Johnson, W. M. Whitmire, D. D. Crane, O. Steele-Mortimer, L. Kari, G. McClarty, and H. D. Caldwell. 2005. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc. Natl. Acad. Sci. USA 102:10658-10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qvigstad, E., S. Digranes, and E. Thorsby. 1983. Antigen-specific proliferative human T-lymphocyte clones with specificity for Chlamydia trachomatis. Scand. J. Immunol. 18:291-297. [DOI] [PubMed] [Google Scholar]
- 20.Qvigstad, E., K. Skaug, and E. Thorsby. 1983. Proliferative human T cell responses to Chlamydia trachomatis in vitro. Acta Pathol. Microbiol. Immunol. Scand. Sect. C 91:203-209. [PubMed] [Google Scholar]
- 21.Sharma, J., A. M. Bosnic, J. M. Piper, and G. Zhong. 2004. Human antibody responses to a chlamydia-secreted protease factor. Infect. Immun. 72:7164-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma, J., F. Dong, M. Pirbhai, and G. Zhong. 2005. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect. Immun. 73:4414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, S., Y. Fan, R. C. Brunham, and X. Yang. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur. J. Immunol. 29:3782-3792. [DOI] [PubMed] [Google Scholar]
- 25.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 26.Westrom, L., and P. A. Mardh. 1983. Chlamydial salpingitis. Br. Med. Bull. 39:145-150. [DOI] [PubMed] [Google Scholar]
- 27.Zabay, J. M., J. Marco, J. Soler, L. Contu, L. Cappai, C. Carcassi, G. Gomez, J. M. Mulet, M. A. Munar, and C. Viader. 2005. Association of HLA-DRB3*0202 and serum IgG antibodies to Chlamydia pneumoniae with essential hypertension in a highly homogeneous population from Majorca (Balearic Islands, Spain). J. Hum. Hypertens. 19:615-622. [DOI] [PubMed] [Google Scholar]
- 28.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong, G. M., R. E. Reid, and R. C. Brunham. 1990. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect. Immun. 58:1450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]