Abstract
Individuals with struvite uroliths are susceptible to recurrent urinary tract infections (UTI), sepsis, and renal disease. Unfortunately, little is known about the host-specific factors that predispose to this disease. In order to develop a rodent model that can address this problem, we inoculated female Fischer 344 (F344), Lewis (LEW), Sprague-Dawley (SD), and Wistar (WIS) rats with a host-adapted strain of Ureaplasma parvum. Animals were necropsied at 2 weeks postinoculation; 100% of F344, 42% of SD, 10% of LEW, and 10% of WIS rats remained infected. Severe bladder lesions and struvite calculi were seen in 64% of F344 rats; in other rat strains, bladder lesions were mild or absent. F344 rats with struvite uroliths had the highest urinary levels of proinflammatory cytokines, such as GRO/KC, interleukin-1α (IL-1α), and IL-1β. F344 rats without struvite stones at necropsy had milder bladder lesions and significantly lower urinary levels of proinflammatory cytokines but a more prominent inflammatory response than did other rat strains. Based on our results, struvite stone formation is linked to a robust inflammatory response that does not resolve UTI but instead promotes damage to surrounding tissues.
Although struvite calculi constitute only 2 to 3% of stones that are analyzed, they create greater clinical complications, such as sepsis and renal disease (30), than any other stone type. Not all factors that contribute to struvite stone formation are known, but urinary tract infections (UTI) are usually a predisposing factor. Infections with urease-producing bacteria, such as Klebsiella, Proteus, Pseudomonas, and Ureaplasma, significantly increase the risk of struvite formation due to increases in urine pH and direct damage of the uroepithelium by ammonia (13, 29).
Host-specific factors also increase the risk of struvite formation during a UTI episode. Obvious examples of host-specific factors are anatomic anomalies of the urogenital tract that disrupt the capacity to void urine (13, 29). However, in most patients a predisposing factor cannot be identified, and the underlying cause of struvite stone formation remains unclear. Since UTI is a prerequisite for struvite stone formation, risk factors for UTI also need to be considered. In women, colonization of the vagina with potential uropathogens combined with impaired host defense mechanisms increases the risk of UTI (7, 32, 38). In addition, genetic factors have been postulated to play a role in susceptibility to UTI (37).
Although ureaplasmas are not the only urease-producing bacteria known to cause struvite calculi (11, 12, 14), they are uniquely considered opportunistic pathogens because they can be readily isolated from the lower urogenital tract of healthy humans as well as individuals with disease (26, 39). Epidemiological studies show that women have much higher Ureaplasma colonization rates (5) as well as higher rates of infection-induced calculi that are culture positive for Ureaplasma (19) than do men. Since most diseases caused by mollicutes are influenced by a variety of host and environmental factors (25, 31), it is reasonable to suggest that the host response to Ureaplasma-associated UTI plays a significant role in complicated UTI and struvite stone formation. Thus, this microorganism can be particularly useful in the development of an animal model of struvite stone formation that focuses on the host response factors that contribute to disease.
Although Ureaplasma is not a natural pathogen of rodents, experimental infection of the rodent urinary tract with Ureaplasma has been established by inoculation into the bladder and/or renal pelvis (10, 22, 23, 27, 28). Experimental infection in different rat strains produces a wide spectrum of disease ranging from mild inflammation to predominantly hyperplastic lesions of the bladder with varying degrees of pyelonephritis and urolithiasis (10, 22, 23, 27, 29). These studies were done under different conditions with different isolates of Ureaplasma; therefore, direct comparisons cannot be made. Whether differences observed in these studies represent true variability in host susceptibility to infection with Ureaplasma and/or struvite formation in the urinary tract, differences in ureaplasmal strain virulence, or experimental confounders is unclear. If such host susceptibility differences exist, then a rodent model of UTI could be exploited to determine which host-specific factors contribute to UTI and stone formation. Our intention was to establish a rodent model of U. parvum infection of the lower female urogenital tract. By using a standardized clone of U. parvum that was host adapted in the female rat, we were able to demonstrate that there are rat strain differences in susceptibility to colonization with Ureaplasma and subsequent struvite stone formation. Moreover, the development of struvite calculi is tightly linked to a specific robust inflammatory response that involves uroepithelial hyperplasia.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free Fischer 344 (F344), Lewis (LEW), Sprague-Dawley (SD), and Wistar (WIS) virgin female rats were purchased from a commercial vendor (Charles River, Indianapolis, IN). All animals ranged in weight from 178 to 200 g. Animal colonies were monitored and found free of the following pathogens: Sendai virus, H-1 virus, rat corona virus, sialodacroadenitis virus, reovirus type 3, Kilham rat virus, Hantaan virus, Mycoplasma pulmonis, respiratory and enteric bacterial pathogens, endoparasites, and ectoparasites. All animals were handled in accordance with procedures approved by the University of Florida Institutional Animal Care and Use Committee.
In order to maintain barrier conditions, all animals were handled within a laminar flow hood. Rats were housed in Microisolator (Lab Products, Inc., Maywood, NJ) cages in the same room under the same temperature and light conditions. Control animals were always handled before infected animals and housed in separate cages in order to prevent contamination with Ureaplasma. All food, water, and caging were autoclaved before use.
Female rats were anesthetized with a ketamine (Ketaset; Bristol Laboratories, Syracuse, NY)-xylazine (Rompun; Haver-Lockhart, Shawnee, Kans.) cocktail (100 mg ketamine plus 150 mg xylazine) administered intraperitoneally at a dosage of 0.1 ml/100 g of body weight. While anesthetized, each rat was specifically identified with an ear notch code for identification purposes. The perineal area was disinfected with 70% alcohol, and a 24-gauge, 3/4-in.-long intravenous catheter (Terumo Medical Corp., Elkton, MD) lubricated with sterile KY jelly was gently introduced into the urethra. One ml of inoculum containing sterile 10B broth or 109 CFU of U. parvum was slowly infused into the bladder through the catheter. In order to adjust for any potential variability in inocula, all rat strains used in the study were infected with the same inoculum at the same time. Using a block experiment design, all rat strains within the same treatment group were commingled with each other in order to adjust for any potential variables in housing conditions. All infection experiments were repeated three times. For each infection experiment, a minimum of two rats per rat strain were infected, and all rat strains were represented in each experiment.
Necropsy.
Rats were necropsied at 2 weeks postinfection. Prior to anesthesia, free-catch urine was collected for cytokine analysis. All rats were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight) and exsanguinated by transection of a femoral artery and vein. Blood was collected for the detection of humoral immune responses specific to Ureaplasma. Rats were cultured for the presence of Ureaplasma in the vagina, bladder, and kidneys.
Ureaplasma preparation and culture.
A clinical isolate of U. parvum was obtained from the urine of a woman at the time of a recurrent symptomatic UTI episode (protocol approved by University of Florida Institutional Review Board and in full compliance with Health Insurance Portability and Accountability Act regulations). One colony was selected and cultured in 10B broth. Species identification of U. parvum was confirmed through PCR (36). In order to host adapt U. parvum to the rat, six in vivo passages were performed in SD rats. For each passage, 109 CFU were inoculated into the bladder of two SD rats. Animals were euthanized, and vagina, bladder, and urine were cultured for Ureaplasma 1 week postinoculation. For each passage, the isolate was grown overnight and infused into the bladder of a new animal. After six consecutive in vivo passages, the final isolate was prepared as a working stock (in vitro passage 2), its speciation as U. parvum was confirmed by PCR (34), and it was designated strain 257-48.
For infection studies, 1 ml of the working stock was grown in 45 ml of 10B broth for 12 to 16 h at 37°C. The Ureaplasma culture was pelleted by centrifugation at 10,000 × g, 4°C, for 50 min. Due to the delicate nature of Ureaplasma, the pellet was resuspended in 15 to 20 ml of fresh 10B broth instead of saline, to give a final concentration of 109 CFU per ml. The CFU of each inoculum was confirmed by culture on A8 agar. Thus, each rat of each strain was inoculated with the same broth culture at each experimental time. For each experiment, a minimum of two rats per strain were infected, and all rat strains were represented in each experiment.
Cultures obtained from animals at necropsy were serially diluted 10-fold in 10B broth to 10−5. Cultures for CFU determination of inoculum were serially diluted 10-fold to 10−10. For CFU determination, 20 μl from each sample and its corresponding dilutions (up to 10−5 for animal cultures and up to 10−8 for inoculum cultures) were plated on A8 agar. Agar plates were incubated at 37°C in 5% CO2; broth cultures were incubated at 37°C in ambient air. Broth tubes were checked daily for a color change, and the reciprocal of the last dilution to show growth was deemed the color-changing unit. Agar cultures were incubated for at least 5 days before colonies were counted to determine CFU.
Detection of urinary cytokines.
Urine from control and infected rats was analyzed for the presence of cytokines with an antibody-immobilized bead immunoassay (Lincoplex kit; Linco Research, Inc., St. Charles, MO). The manufacturer's protocol was followed for the detection of the following cytokines and chemokines: granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, gamma interferon (IFN-γ), IL-18, GRO/KC (the rat analog for human IL-8), and tumor necrosis factor alpha (TNF-α). Briefly, a standard cocktail was serially diluted in order to develop a standard curve that ranged from 3.2 to 2,000 pg/ml. Concentrations less than 3.2 pg/ml cannot be detected. Urine samples were diluted in assay buffer to obtain a total volume of 60 μl per well and run in duplicate. Urine samples were combined with premixed anti-rat cytokine antibody-immobilized beads and incubated overnight at 2 to 8°C. Samples were then incubated with 25 μl of detection antibody cocktail for 2 h at room temperature. Streptavidin-phycoerythrin buffer (25 μl) was added to the sample mixture and allowed to incubate at room temperature for an additional 30 min. The beads were washed and resuspended in sheath fluid. Signal detection measurements of antibody-immobilized beads were performed with a Luminex 100 machine (Luminex Co., Austin, Tex.). Concentrations of cytokines were determined by the intensity of the signal that corresponded to the concentration on the standard curve. Cytokine concentrations were corrected to reflect cytokine concentrations prior to sample dilution.
Stone analysis.
Bladder calculi were submitted to a commercial laboratory and analyzed by integrated crystallography (Louis C. Herring and Co., Orlando, FL).
Histopathology.
One-half of the bladder was fixed in 10% buffered formalin for 18 to 24 h. After fixation in buffered formalin, tissues were trimmed and transected so that a cross-sectional view of the bladder wall would be present on each section. Tissues were processed routinely and stained with hematoxylin and eosin. In order to develop a lesion scoring system, the pathologist was aware of which samples came from control animals but was blinded as to identity of rat strain. After development of the lesion scoring system, all slides were recoded so that the pathologist was blinded to rat strain and experimental treatment (infection status). Five lesion categories were developed for assessing lesion scores. Inflammation density of tissues was scored on the following scale: 0 for no lesions, 1 for minimal, 2 for mild, 3 for moderate, and 4 for dense cellular infiltrates. The primary location of inflammation was scored as follows: 1 for subepithelial, 2 for mural, and 3 for transmural. The scoring system for total area affected was as follows: 1 for less than 10%, 2 for 10 to 50%, and 3 for greater than 50%. Scoring for cell types that comprised the inflammatory infiltrate was 1 for primarily mononuclear cells (lymphocytes, plasma cells and macrophages), 2 for mononuclear cells and neutrophils, and 3 for mononuclear cells, neutrophils, and fibrous infiltrates. Epithelial changes in bladder tissues were scored as follows: 0 for none; 1 for minimal hyperplasia, ulceration, or effacement of epithelium by inflammation; 2 for mild hyperplasia and rare dense inflammatory infiltrates; 3 for the same changes noted for a score of 2 but accompanied with marked erosion and/or ulceration of the epithelial surface.
ELISA for detection of antibodies specific for Ureaplasma.
A minimum of five rats per strain and per treatment group (control versus infected) were examined for the presence of anti-Ureaplasma-specific antibodies. An OptEIA enzyme-linked immunosorbent assay (ELISA) reagent kit B (BD Biosciences, San Diego, CA) along with biotin-conjugated monoclonal mouse anti-rat immunoglobulin M (IgM), IgG1, IgG2a, IgG2b, and IgG2c (BD Biosciences, San Diego, CA) were used for the detection of anti-Ureaplasma antibodies in rat sera. Ureaplasma antigen was prepared as previously described and used at a concentration of 20 μg of protein/ml (3). Wells designated as negative controls for each Ig subclass were incubated with plain diluent buffer. Rat sera from control and infected rats were serially diluted at a ratio of 1:2 (concentration range, 1:32 to 1:4,096) and incubated overnight at 4°C. For each rat sample, all Ig subclasses were detected on the same ELISA plate. Biotin-conjugated mouse anti-rat Ig subclass antibodies were prepared at a concentration of 2 μg/ml, and ELISA plates were incubated at 37°C for 4 h. Avidin-horseradish peroxidase conjugate was used at a dilution of 1:1,000 and applied to each well for 30 min. After washing, 3,3′,5,5′-tetramethylbenzidine substrate (BD Biosciences) was used to measure horseradish peroxidase activity, and the substrate reaction was stopped with BD Opt EIA stop solution. Absorbance values were measured at 450-nm wavelength with an ELX 808 Ultra microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
The cutoff value for a true positive sample was determined for each Ig subclass for each rat strain by averaging the absorbance values of the corresponding control sera. For each control animal, the most dilute serum sample that had an absorbance value greater than the background control was used as the cutoff dilution value for that particular Ig subclass of that rat strain. Therefore, if the absorbance values in infected sera did not exceed the cutoff value for the corresponding control, that sample was given a value of 0.
Data analysis.
Data from multiple experiments were grouped together in order to make statistical analysis possible. Wherever possible, data were analyzed by two-way analysis of variance. Fisher's multiple comparison was used when the analysis of variance indicated a significant difference among group means. An unpaired Student's t test was used for comparisons that were limited to two groups. Contingency table analysis was used for comparisons among rat strains involving nominal data (positive versus negative). Nonparametric data were analyzed by the Kruskall-Wallis test, Mann-Whitney rank test, or Spearman correlation. For all analyses, a probability of P ≤ 0.05 was considered significant.
RESULTS
Colonization with Ureaplasma.
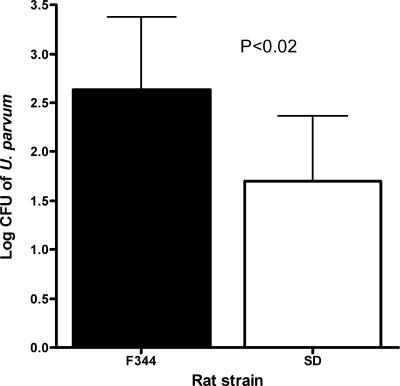
Ureaplasma was not isolated from any site from any control rat (data not shown). There were no significant differences in the frequency of colonization of the vagina and kidney among rat strains (Table 1). However, statistically significant differences were observed for the bladder (Table 1). F344 rats had a significantly higher rate of bladder colonization than other rat strains (P < 0.001). Further, F344 rats had significantly higher levels of U. parvum isolated from the bladder than SD rats at 2 weeks postinoculation (P < 0.02) (Fig. 1); WIS and LEW rats were excluded from the CFU analysis since only one animal per strain was culture positive in the bladder.
TABLE 1.
Animals culture positive for Ureaplasma in the vagina, bladder, and/or kidney at 2 weeks postinoculation with 109 CFU of U. parvum in the bladder
| Tissue site | No. of animals (%) culture positive for Ureaplasmaa
|
P valuea | |||
|---|---|---|---|---|---|
| F344 | SD | LEW | WIS | ||
| Vagina | 3/14 (21) | 3/12 (25) | 2/10 (20) | 1/10 (10) | NS |
| Bladder | 14/14 (100) | 5/12 (42) | 1/10 (10) | 1/10 (10) | 0.0001 |
| Kidney | 3/14 (21) | 1/12 (8) | 0/10 (0) | 0/10 (0) | NS |
| Infected at multiple sites | 5/14 (36) | 2/12 (17) | 1/10 (10) | 1/10 (10) | ND |
| Total (infected at any site) | 14/14 (100) | 7/12 (58) | 2/10 (20) | 2/10 (20) | 0.0002 |
Values were obtained by chi-square analysis. NS, not significant; ND, not done.
FIG. 1.
Isolation of U. parvum from bladder tissue of F344 (n = 15) and SD (n = 5) rats that were culture positive at 2 weeks postinoculation. Samples were collected from four separate experiments. Data were analyzed with Student's t test and are presented as the mean log CFU ± standard deviation.
Urinary calculi.
Calculi were not isolated from any control rats at any site. No experimentally infected SD or LEW rats developed any bladder or renal calculi. One WIS rat that was culture negative for Ureaplasma had a struvite calculus present in the bladder but had minimal signs of inflammation in the bladder (details discussed in “Histopathology,” below). There was no microscopic evidence of a nucleus or nidus of bacteria, cells, or inflammatory material, but the matrix did contain blood and protein. Nine of 14 F344 rats (64%) that were inoculated with Ureaplasma developed struvite calculi. Three of these nine calculi had no microscopic evidence of a nidus, but all nine stones had blood and protein in the matrix. The presence of struvite calculi in F344 rats was not statistically correlated with the log CFU of Ureaplasma recovered from the urine or bladder (data not shown).
Histopathology.
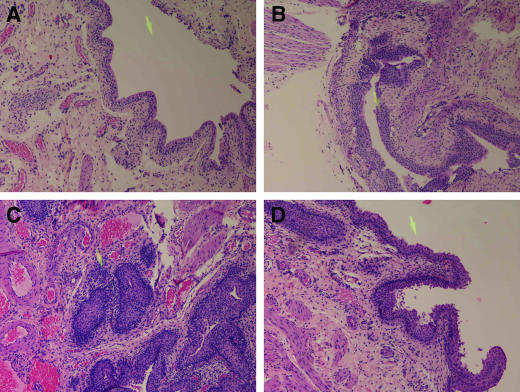
There were no detectable lesions in bladder tissue from control rats (Fig. 2). U. parvum-inoculated LEW, WIS, and SD rats did not show any qualitative or quantitative differences in bladder histopathology (see Fig. 3, below, for lesion score analysis). When present, lesions were limited primarily to the subepithelial layer of the bladder (Fig. 2b). In the bladder mucosa, the most consistent finding was spongiosis of epithelial cells, with some necrosis. In rare instances there was exfoliation of individual cells and a few foci of inflammatory cells rarely scattered throughout the epithelial layer. Further, this effect was more pronounced in the bladder tissue of SD rats that were culture positive for Ureaplasma (Fig. 2b). The inflammatory cell populations consisted of lymphocytes/plasma cells, macrophages, mast cells, and neutrophils. The lamina propria submucosa had mild to moderate vascular congestion and edema with minimal to mild inflammatory infiltrates, similar to what was present in the epithelial layer. The external muscularis layer and adventitia had similar changes as the lamina propria.
FIG. 2.
Rat bladder tissue obtained 2 weeks postinoculation. Bladders were inoculated with sterile medium (control) or 109 CFU of U. parvum (infected; panels B to D). All images are at a magnification of ×100, and the yellow arrow present on all figures is equivalent to 100 μm. (A) Bladder section from a control rat; (B) bladder section from a culture-positive SD rat; (C) bladder section from a culture-positive, struvite-positive F344 rat, with an arrow pointing to a dense infiltrate of inflammatory cells present within the subepithelial layer of the bladder; (D) bladder section from a culture-positive, struvite-negative F344 rat.
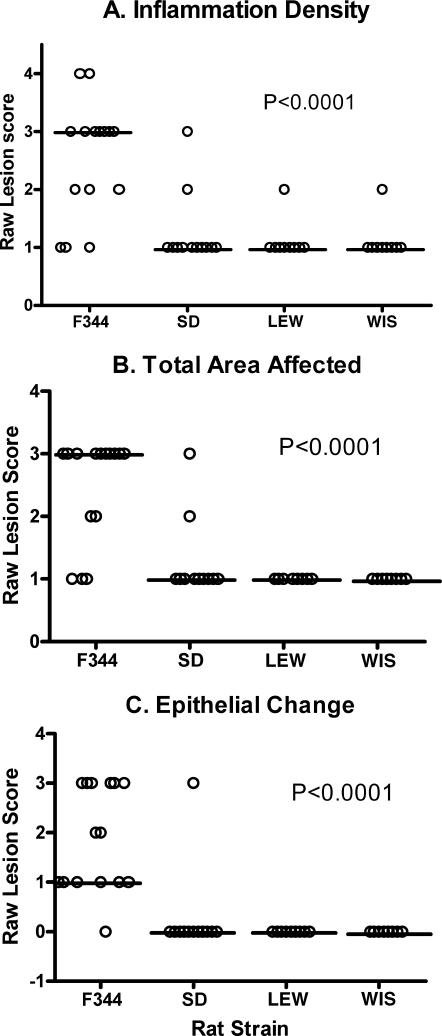
FIG. 3.
Lesion scores for bladder tissues obtained from U. parvum-inoculated rats: F344 (n = 15), SD (n = 12), LEW (n = 10), and WIS (n = 10). Horizontal bars represent the median value for each rat strain. Parameters for lesion scoring were as follows: density of inflammation (A), total area of the bladder affected (B), and degree of epithelial change (C). Samples were collected from four separate experiments. Lesion scores were analyzed with the Kruskall-Wallis test, and P values are presented within the corresponding graph.
Lesions in the F344 rat bladder tissue were more extensive and more severe than the other rat strains. In the nine F344 rats with struvite calculi, the primary location of inflammation extended into the transmural layers of the bladder (Fig. 2c). In struvite-positive F344 rats, the epithelium showed mild hyperplasia with varying degrees of marked erosion, ulceration, necrosis of some individual cells, and occasional cytoplasmic vacuolization and spongiosis. Widespread vascular congestion and diffuse edema were present in the submucosal layers as well as the external muscularis. Dense multifocal aggregates of lymphocytes/plasma cells, neutrophils, macrophages, and mast cells were common in the submucosa and also present in the external muscularis layers. In F344 rats without struvite calculi, the inflammation was less extensive and primarily localized within the mural layer of the bladder. Most notably, these animals had no to minimal epithelial hyperplasia, but ulceration and effacement of uroepithelium were still present. The inflammation in these animals was less extensive. A distinguishing feature was that fewer neutrophils and mast cells were present in the submucosal and external muscularis layers (Fig. 2d).
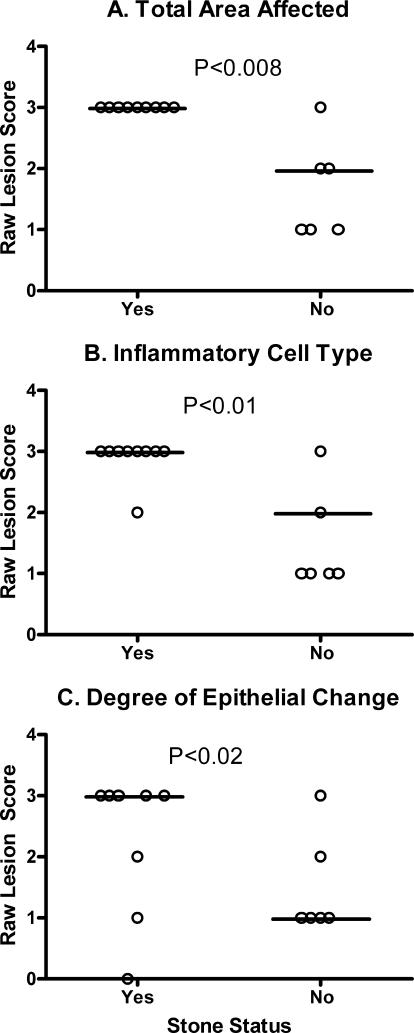
Lesion scores for location of inflammation and inflammatory infiltrate were not significantly different between rat strains (data not shown). However, bladder lesion scores (Fig. 3) were significantly higher in infected F344 rats than in the other rat strains for inflammation density (P < 0.0001), total area affected (P < 0.0001), and degree of epithelial change (P < 0.0003). Within the F344 infected group, animals with bladder stones had significantly higher lesion scores for total area affected (P < 0.008), inflammatory cell type (P < 0.01), and epithelial change (P < 0.02) (Fig. 4). Although not statistically significant, F344 rats with bladder stones tended to have a higher inflammation score and lesion location than their struvite negative counterparts.
FIG. 4.
Lesion scores for bladder tissues obtained from U. parvum-inoculated F344 rats. Horizontal bars represent the median value for each group. Animals were separated into two groups based on the presence of a struvite urolith (yes, n = 9; no, n = 5). Criteria were as follows: density of inflammation (A), total area of the bladder affected (B), and degree of epithelial change (C). Samples were collected from four separate experiments. Lesion scores were analyzed with the Mann-Whitney rank sum test, and P values are presented within the corresponding graph.
Urine cytokines in control animals.
There were no detectable levels (3.2 pg/ml or higher) of IFN-γ, IL-1β, IL-4, IL-6, IL-10, IL-12, IL-18, or TNF-α in the urine of control rats. Monocyte chemoattractant protein 1 (MCP-1), IL-1α, and GRO/KC were present in detectable levels in the urine of all strains, but there were no differences in levels among the strains (data not shown). An unexpected finding was a significantly higher level (P < 0.007) of GM-CSF in the urine of control SD rats (642 ± 442 pg/ml [mean ± standard deviation]). Although F344 rats also had detectable levels of GM-CSF (188 ± 147 pg/ml), this was significantly less than the levels present in SD rats (P < 0.007). GM-CSF was not detected in the urine of LEW or WIS rats.
Urine cytokines in infected animals.
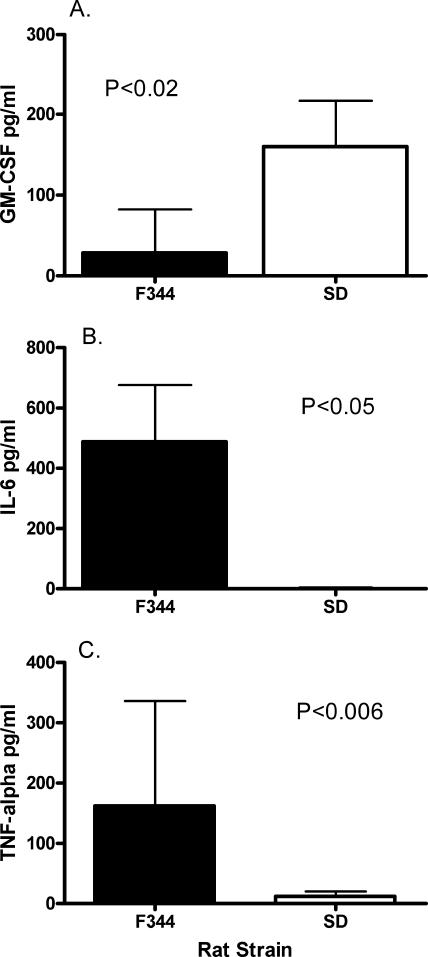
WIS and LEW rats were excluded from analysis because only one animal from each group was culture positive. Prior to analysis, the data from SD infected rats were stratified by urine culture results, i.e., culture positive versus culture negative. Because there were no differences in the levels of cytokines between these subgroups (data not shown), all of the samples within the SD inoculated group were combined for statistical analysis. Stratification was not necessary for F344 rats, since all were culture positive. When infected SD rats were compared to all infected F344 rats, SD rats had significantly higher levels of GM-CSF (P < 0.02) and significantly lower levels of both IL-6 (P < 0.05) and TNF-α (P < 0.006) than F344 rats (Fig. 5). Increased levels of IL-1β in the urine of F344 rats approached, but did not achieve, statistical significance (P < 0.06). Although GRO/KC, IFN-γ, IL-1α, IL-18, and MCP-1 were detected in urine, there were no significant differences in the levels among these rat strains (data not shown). IL-4, IL-10, and IL-12 were not detected in the urine of these animals.
FIG. 5.
Urinary cytokines GM-CSF (A), IL-6 (B), and TNF-α (C) that were present in U. parvum-inoculated rats. Urine was collected free catch at time of necropsy, and data are presented as the mean ± standard deviation from F344 (n = 11) and SD (n = 9) rats. Samples were collected from four separate experiments, and data were analyzed by using an unpaired Student's t test. P values for each analysis are presented within the corresponding graph.
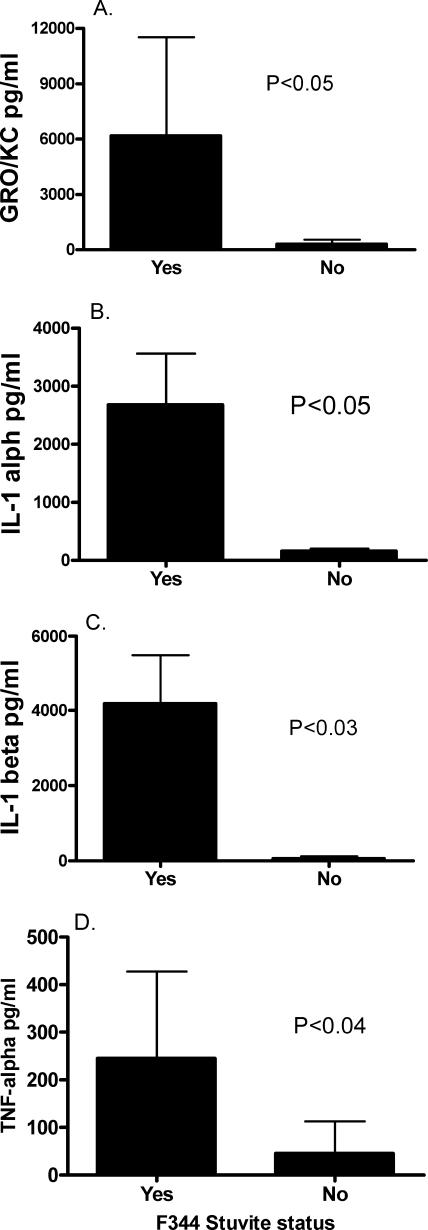
Since the presence of stones was associated with significant differences in bladder lesions of F344 rats, the urine cytokine levels in these subgroups were statistically analyzed. F344 rats with uroliths had significantly higher levels of GRO/KC (P < 0.05), IL-1α (P < 0.05), IL-1β (P < 0.03), and TNF-α (P < 0.04) than did stone-negative counterparts (Fig. 6).
FIG. 6.
Association of uroliths with increased urinary cytokines GRO/KC (A), IL-1α (B), IL-1β (C), and TNF-α (D) in U. parvum-inoculated F344 rats. Urine was collected free catch at time of necropsy from F344 rats, and data are presented as the mean ± standard deviation from rats with struvite calculi (n = 8) and rats without struvite calculi (n = 5). Samples were collected from four separate experiments, data were analyzed by using an unpaired Student's t test, and P values for each cytokine analysis are presented within the corresponding graph.
Correlations between bladder tissue lesion scores and urine cytokines from F344 rats were analyzed by the Spearman correlation test. There were no significant correlations between the inflammatory infiltrate, density of inflammation, or location of inflammation and levels of GRO/KC, IL-1α, or IL-1β (data not shown). However, there was a significant correlation between total area affected and urine GRO/KC (rs = 2.40; P < 0.02), IL-1α (rs = 2.33; P < 0.02), and IL-1β (rs = 2.83; P < 0.005). There was also a significant correlation between degree of epithelial change and urine GRO/KC (rs = 2.25; P < 0.02) and IL-1β (rs = 2.05; P < 0.04).
Correlations between bladder tissue lesion scores and urine GM-CSF in SD rats were also analyzed. There were no significant correlations between the levels of urine GM-CSF and any of the lesion-scoring parameters (inflammatory infiltrate, density of inflammation, location of inflammation, total lesion score, or degree of epithelial change) (data not shown).
Humoral immune response to U. parvum.
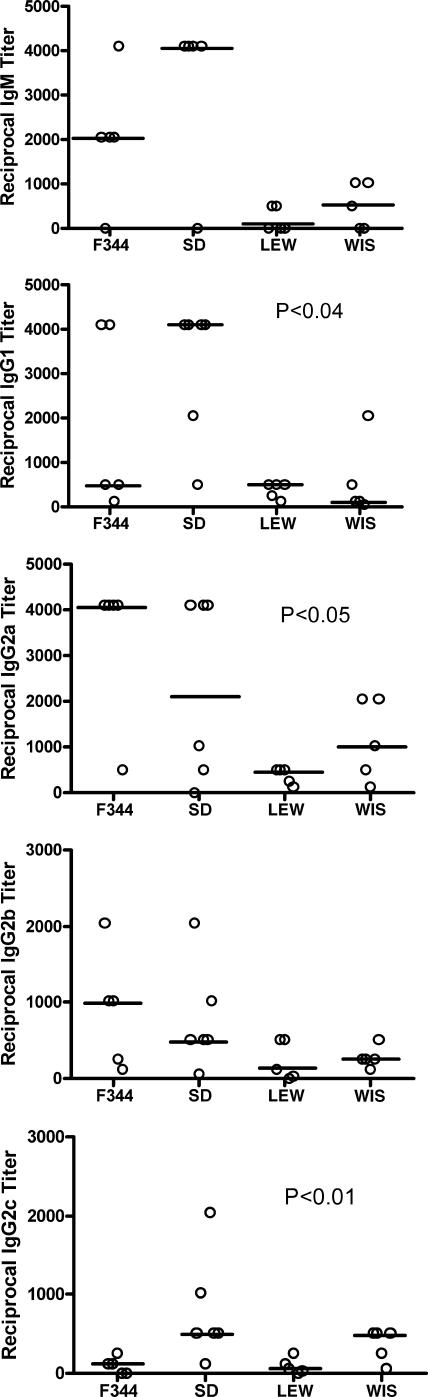
The relative distribution of anti-Ureaplasma Ig subclass titers in each rat strain is summarized in Fig. 7. Regardless of Ig subtype, LEW rats tended to have the lowest titers to U. parvum. F344 and SD rats tended to have the highest titers, but their responses varied with Ig subclass. For example, SD rats had the highest IgG1 (P < 0.04), but F344 rats had the highest anti-U. parvum IgG2a (P < 0.01) and IgG2b (P < 0.05) titers.
FIG. 7.
Immunoglobulin subclass responses to U. parvum infection: IgM, IgG1, IgG2a, IgG2b, and IgG2c responses in U. parvum-inoculated rats. Titer results are presented as the reciprocal of the last dilution with an absorbance value greater than each rat strain's corresponding control value. Horizontal bars represent the median value for each rat strain. Samples were collected from four separate experiments (n = 5 for each rat strain) and analyzed by using the Kruskall-Wallis test.
DISCUSSION
Animal models for Ureaplasma-induced struvite stone formation have primarily been used for identifying the conditions and components in urine that contribute to calculogenesis (9, 10, 22, 23), with little emphasis on determining host-pathogen relationships. Disease severity in most mycoplasmal infections is intimately linked to the host immune response, and tissue damage is usually due to an inappropriate immune response or inflammatory response that harms the host without adequate elimination of the infection (15, 18, 40). The rodent model of struvite stone formation described in this report provides a unique opportunity to determine the role of the host immune response in disease pathogenesis.
One caveat of experimental infection using a human pathogen in a rodent host is that both the host immune response and the colonization abilities of the microbe may be confounding factors in interpretation of the data. In order to limit this confounder in our study, a clinical isolate of U. parvum from a woman with symptomatic UTI was adapted in vivo in the SD rat. This standardized rat-adapted strain of U. parvum allowed us to demonstrate that rat strains differ in susceptibility to both UTI and struvite stone formation.
Although U. parvum was host adapted in the SD rat, it is particularly interesting that it was the F344 rat strain that was most susceptible to infection and struvite stone formation. In this model, microbial load does not appear to affect struvite stone formation, since F344 rats with or without struvites had equivalent numbers of U. parvum cultured from bladder tissue. What appears to be most critical to struvite stone formation in this rat strain is an exuberant inflammatory response that is also linked to uroepithelial hyperplasia. There were marked differences in bladder lesion severity from struvite-positive rats versus struvite-negative animals. Most notably, struvite-positive animals had more extensive inflammation that extended further into the submucosa and external muscularis layers of the bladder. Moreover, struvite-positive rats had significantly greater amounts of neutrophils and fibrous infiltrates that are consistent with an active inflammatory process. This inflammatory infiltrate also extended into the stuvite stone matrix and may have contributed to stone formation, since it was also present in the nuclei of most of the stones analyzed. The marked hyperplastic changes in the uroepithelum in F344 rats with uroliths are unlikely to be solely a response to mechanical irritation of the stone (6). If epithelial hyperplasia were induced by mechanical irritation alone, then the WIS rat with a struvite urolith should have displayed similar bladder pathology. Instead, this animal had mild changes that consisted only of scant multifocal foci of uroepithelial erosion. Therefore, we suggest that one key to urolith formation in the F344 rat is that the infection remains unresolved in the presence of an active inflammatory immune response (24).
The role of an overly exuberant inflammatory response in susceptibility to uroliths is further supported by the proinflammatory profile observed in urinary cytokines. F344 rats with uroliths had significantly greater amounts of GRO/KC, IL-1α, IL-1β, and TNF-α in their urine. This proinflammatory profile probably reflects a positive feedback loop through the stimulatory effects of IL-1 and TNF on IL-6 and GRO/KC secretion from epithelial cells (41). GRO/KC, which is the rat analog of IL-8 in humans, may be a critical component for the extensive venous congestion and epithelial hyperplasia present in the bladder tissue of F344 rats with uroliths. GRO/KC is a strong chemotactic agent for neutrophils (41), and struvite-positive rats had the highest density of neutrophils in the subepithelial layers of the bladder. GRO/KC has angiogenic activity and can serve as a tissue-remodeling factor (21). This activity is consistent with the extensive venous congestion observed in our rat model. GRO/KC is also capable of inducing hyperplasia in rat epithelial cells (36). Epithelial hyperplasia was a hallmark lesion in the bladder of F344 rats, and GRO/KC urine levels correlated with the degree of uroepithelial remodeling in these animals, thus suggesting that GRO/KC may be important in the pathogenesis of urolith damage to the bladder. In humans, IL-8 may play a role in the pathogenesis of renal scarring and vesicoureteral reflux (39). Therefore, it is even more intriguing that in our model the rat counterpart to IL-8 is linked to damage of the bladder epithelium and presence of stones.
Although SD rats were susceptible to infection with U. parvum, their incidence of infection was significantly less than for F344 rats. Over half of SD rats were able to clear U. parvum from the bladder. However, when infection was present, the observed bladder lesions were substantively less severe and extensive, the uroepithelium did not display hyperplastic change or marked erosion, and uroliths were not present. Importantly, unlike the intense proinflammatory response seen in F344 rats, most of the urinary cytokines in SD rats were barely detectable. An unexpected finding was the significantly elevated levels of GM-CSF in the urine of control as well as infected SD rats. The significance of this finding is unclear. Typically, GM-CSF enhances microbicidal activity, oxidative metabolism, phagocytic activity, and cytotoxicity in neutrophils and macrophages (8). Unlike LEW and WIS rats, 5 of 12 SD rats remained infected, and these rats exhibited signs that were similar to asymptomatic bacteriuria (ABU). Since elevated urinary GM-CSF was a unique feature in this rat strain, this cytokine may be signaling an underlying defect in their innate immune response, and this may be somewhat analogous to what occurs in patients with ABU that fail to develop more severe urinary complications (42).
Neither the LEW nor WIS rat strain had detectable levels of proinflammatory cytokines in urine. Only 20% of LEW and WIS inoculated rats were still colonized 2 weeks postinoculation, indicating that these strains were capable of clearance of U. parvum from the bladder. However, there was histological evidence of resolving inflammation in the uroepithelium and a humoral response to U. parvum, suggesting the microorganism was able to colonize the host long enough to induce a humoral immune response as well as a local inflammatory response prior to clearance.
Little is known about the mechanisms by which the host immune response may contribute to struvite urolith formation in humans. However, the inflammatory response of the urinary tract to injury and infection, along with the matrix components of struvite stones, suggest immune dysregulation contributes to stone formation. Patients with UTI have elevated levels of IL-6, IL-1α, IL-1β, and IL-8 in their urine (4, 17, 20, 32), and some of these cytokines also modulate epithelial differentiation and morphology (21). In addition, proinflammatory cytokines such as TNF-α and IL-6 also regulate expression of calprotectin by leukocytes and epithelial cells (16). Calprotectin is a primary protein constituent of struvite stones (1, 2) and also is recognized as a marker of inflammation and immune dysfunction (34, 35) because it is released by activated neutrophils and macrophages present at sites of inflammation (34, 35). Although we did not analyze F344 bladder tissue for the presence of calprotectin, both the histologic lesions and urine cytokine profile in these rats are similar to the profile observed in humans (4, 17, 20).
We have defined a rodent model that will permit us to address the early events of the innate immune system that facilitate clearance of a uropathogen from the lower urinary tract as well as the disruptions in the regulation of bladder inflammation that lead to struvite stone formation. Each rat strain described in our model system has specific strengths that are applicable to defining host-pathogen interactions in the urinary tract. For example, F344 rats will be particularly useful to study susceptibility to urolith formation and bladder damage, while SD rats will be amenable to study ABU in the absence of severe bladder damage. WIS and LEW rat strains can be used to elucidate the mechanisms required for successful clearance of a uropathogen from the bladder. In this study, our data support the concept that an exuberant host inflammatory response is associated with colonization and bladder damage. In addition, a critical factor in stone formation is the host local inflammatory response.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1 AI 45875 to M.B.B. L. Reyes was supported by a minority supplement from the National Institutes of Heath (RO1 AI45875).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Asakura, H., J. D. Selengut, W. H. Orme-Johnson, and S. P. Dretler. 1998. The effect of calprotectin on the nucleation and growth of struvite crystals as assayed by light microscopy in real-time. J. Urol. 159:1384-1389. [PubMed] [Google Scholar]
- 2.Bennett, J., S. P. Dretler, J. Selengut, and W. H. Orme-Johnson. 1994. Identification of the calcium-binding protein calgranulin in the matrix of struvite stones. J. Endourol. 8:95-98. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. B., G. H. Cassell, D. Taylor-Robinson, and M. C. Shepard. 1983. Measurement of antibody to Ureaplasma urealyticum by an enzyme-linked immunosorbent assay and detection of antibody responses in patients with nongonococcal urethritis. J. Clin. Microbiol. 17:288-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candela, J. V., E. Park, J. M. Gerspach, R. Davidoff, L. Stout, S. M. Levy, G. E. Leach, G. C. Bellman, and P. M. Lad. 1998. Evaluation of urinary IL-1α and IL-1β in gravid females and patients with bacterial cystitis and microscopic hematuria. Urol. Res. 26:175-180. [DOI] [PubMed] [Google Scholar]
- 5.Cassell, G., M. Brown, J. Younger, R. Blackwell, J. Davis, P. Marriott, and S. Stagno. 1983. Incidence of genital mycoplasmas in women at the time of diagnostic laparoscopy. Yale J. Biol. Med. 56:557-563. [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, S. M. 2002. Comparative pathology of proliferative lesions of the urinary bladder. Toxicol. Pathol. 30:663-671. [DOI] [PubMed] [Google Scholar]
- 7.Finer, G., and D. Landau. 2004. Pathogenesis of urinary tract infections with normal female anatomy. Lancet Infect. Dis. 4:631-635. [DOI] [PubMed] [Google Scholar]
- 8.Fleetwood, A. J., A. D. Cook, and J. A. Hamilton. 2005. Functions of granulocyte-macrophage colony-stimulating factor. Crit. Rev. Immunol. 25:405-428. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander, A. M., and A. I. Braude. 1974. Production of bladder stones by human T mycoplasmas. Nature 247:67-69. [DOI] [PubMed] [Google Scholar]
- 10.Grenabo, L., J. E. Brorson, H. Hedelin, and S. Pettersson. 1985. Concrement formation in the urinary bladder in rats inoculated with Ureaplasma urealyticum. Urol. Res. 13:195-198. [DOI] [PubMed] [Google Scholar]
- 11.Grenabo, L., G. Claes, H. Hedelin, and S. Pettersson. 1986. Rapidly recurrent renal calculi caused by Ureaplasma urealyticum: a case report. J. Urol. 135:995-997. [DOI] [PubMed] [Google Scholar]
- 12.Grenabo, L., H. Hedelin, and S. Pettersson. 1988. Urinary infection stones caused by Ureaplasma urealyticum: a review. Scand. J. Infect. Dis. Suppl. 53:46-49. [PubMed] [Google Scholar]
- 13.Hedelin, H. 2002. Uropathogens and urinary tract concretion formation and catheter encrustations. Int. J. Antimicrob. Agents 19:484-487. [DOI] [PubMed] [Google Scholar]
- 14.Hedelin, H., J. E. Brorson, L. Grenabo, and S. Pettersson. 1984. Ureaplasma urealyticum and upper urinary tract stones. Br. J. Urol. 56:244-249. [DOI] [PubMed] [Google Scholar]
- 15.Hickman-Davis, J. M. 2002. Role of innate immunity in respiratory mycoplasma infection. Front. Biosci. 7:d1347-d1355. [DOI] [PubMed] [Google Scholar]
- 16.Holt, J., M. K. Fagerhol, and I. Dale. 1983. Quantitation of a leukocyte protein (L1) in urine. Acta Paediatr. Scand. 72:615-616. [DOI] [PubMed] [Google Scholar]
- 17.Jantausch, B. A., R. O'Donnell, and B. L. Wiedermann. 2000. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr. Nephrol. 15:236-240. [DOI] [PubMed] [Google Scholar]
- 18.Jones, H. P., and J. W. Simecka. 2003. T lymphocyte responses are critical determinants in the pathogenesis and resistance to mycoplasma respiratory disease. Front. Biosci. 8:d930-d945. [DOI] [PubMed] [Google Scholar]
- 19.Kaya, S., O. Poyraz, G. Gokce, H. Kilicarslan, K. Kaya, and S. Ayan. 2003. Role of genital mycoplasmata and other bacteria in urolithiasis. Scand. J. Infect. Dis. 35:315-317. [DOI] [PubMed] [Google Scholar]
- 20.Ko, Y. C., N. Mukaida, S. Ishiyama, A. Tokue, T. Kawai, K. Matsushima, and T. Kasahara. 1993. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect. Immun. 61:1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch, A. E., P. J. Polverini, S. L. Kunkel, L. A. Harlow, L. A. DiPietro, V. M. Elner, S. G. Elner, and R. M. Strieter. 1992. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798-1801. [DOI] [PubMed] [Google Scholar]
- 22.Krieger, J., and G. E. Kenny. 1986. Evidence for pathogenicity of Ureaplasma urealyticum for the upper urinary tract derived from animal models. Pediatr. Infect. Dis. 5(6 Suppl.):S319-S321. [DOI] [PubMed] [Google Scholar]
- 23.Larsson, P. A., M. Cano, L. Grenabo, J. E. Brorson, H. Hedelin, S. Pettersson, and S. L. Johansson. 1989. Morphological lesions of the rat urinary tract induced by inoculation of mycoplasmas and other urinary tract pathogens. Urol. Int. 44:210-217. [DOI] [PubMed] [Google Scholar]
- 24.Mysorekar, I. U., M. A. Mulvey, S. J. Hultgren, and J. I. Gordon. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412-7419. [DOI] [PubMed] [Google Scholar]
- 25.Nichols, P. W., T. R. Schoeb, J. K. Davis, M. K. Davidson, and J. R. Lindsey. 1992. Pulmonary clearance of Mycoplasma pulmonis in rats with respiratory viral infections or of susceptible genotype. Lab. Anim. Sci. 42:454-457. [PubMed] [Google Scholar]
- 26.O'Leary, W. M. 1990. Ureaplasmas and human disease. Crit. Rev. Microbiol. 17:161-168. [DOI] [PubMed] [Google Scholar]
- 27.Pickering, W. J., and D. F. Birch. 1989. Bacteriologic and serologic findings in experimental pyelonephritis caused by Ureaplasma urealyticum. Infect. Immun. 57:1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering, W. J., D. F. Birch, and P. Kincaid-Smith. 1990. Biochemical and histologic findings in experimental pyelonephritis due to Ureaplasma urealyticum. Infect. Immun. 58:3401-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman, N. U., M. V. Meng, and M. L. Stoller. 2003. Infections and urinary stone disease. Curr. Pharm. Des. 9:975-981. [DOI] [PubMed] [Google Scholar]
- 30.Rodman, J. S. 1999. Struvite stones. Nephron 81(Suppl. 1):50-59. [DOI] [PubMed] [Google Scholar]
- 31.Schoeb, T. R., M. M. Juliana, P. W. Nichols, J. K. Davis, and J. R. Lindsey. 1993. Effects of viral and mycoplasmal infections, ammonia exposure, vitamin A deficiency, host age, and organism strain on adherence of Mycoplasma pulmonis in cultured rat tracheas. Lab. Anim. Sci. 43:417-424. [PubMed] [Google Scholar]
- 32.Sobel, J. D. 1997. Pathogenesis of urinary tract infection. Role of host defenses. Infect. Dis. Clin. North Am. 11:531-549. [DOI] [PubMed] [Google Scholar]
- 33.Stellrecht, K. A., A. M. Woron, N. G. Mishrik, and R. A. Venezia. 2004. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J. Clin. Microbiol. 42:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockley, R. A., I. Dale, S. L. Hill, and M. K. Fagerhol. 1984. Relationship of neutrophil cytoplasmic protein (L1) to acute and chronic lung disease. Scand. J. Clin. Lab. Investig. 44:629-634. [PubMed] [Google Scholar]
- 35.Striz, I., and I. Trebichavsky. 2004. Calprotectin: a pleiotropic molecule in acute and chronic inflammation. Physiol. Res. 53:245-253. [PubMed] [Google Scholar]
- 36.Suzuki, H., M. Mori, K. Seto, F. Shibata, S. Nagahashi, C. Kawaguchi, M. Suzuki, H. Matsui, K. Watanabe, S. Miura, and H. Ishii. 2000. Rat CXC chemokine GRO/CINC-1 paradoxically stimulates the growth of gastric epithelial cells. Aliment. Pharmacol. Ther. 14(Suppl. 1):94-100. [DOI] [PubMed] [Google Scholar]
- 37.Svanborg, C., G. Bergsten, H. Fischer, G. Godaly, M. Gustafsson, D. Karpman, A. C. Lundstedt, B. Ragnarsdottir, M. Svensson, and B. Wullt. 2006. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr. Opin. Microbiol. 9:33-39. [DOI] [PubMed] [Google Scholar]
- 38.Taylor-Robinson, D. 1984. Ureaplasmas as a cause of disease in man and animals: fact or fancy? Isr. J. Med. Sci. 20:843-847. [PubMed] [Google Scholar]
- 39.Uehling, D. T., D. B. Johnson, and W. J. Hopkins. 1999. The urinary tract response to entry of pathogens. World J. Urol. 17:351-358. [DOI] [PubMed] [Google Scholar]
- 40.Waites, K. B., and D. F. Talkington. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17:697-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, X., G. J. Dolecki, and J. B. Lefkowith. 1995. GRO chemokines: a transduction, integration, and amplification mechanism in acute renal inflammation. Am. J. Physiol. 269:F248-F256. [DOI] [PubMed] [Google Scholar]
- 42.Wullt, B., G. Bergsten, H. Fischer, G. Godaly, D. Karpman, I. Leijonhufvud, A. C. Lundstedt, P. Samuelsson, M. Samuelsson, M. L. Svensson, and C. Svanborg. 2003. The host response to urinary tract infection. Infect. Dis. Clin. North Am. 17:279-301. [DOI] [PubMed] [Google Scholar]