Abstract
We have previously demonstrated that protection from allergic inflammation by Ascaris suum infection was characterized by a global increase in interleukin-10 (IL-10) and the development of protective CD4+/CD25+ T cells (L. Schopf, S. Luccioli, V. Bundoc, P. Justice, C. C. Chan, B. J. Wetzel, H. H. Norris, J. F. Urban, Jr., and A. Keane-Myers, Investig. Ophthalmol. Vis. Sci. 46:2772-2780, 2005). Here, we used A. suum pseudocoelomic fluid (PCF) in lieu of infection to define molecular mechanisms of allergic protection in a mouse model of allergic inflammation. Mice were sensitized with ragweed (RW) and PCF (RW/PCF), PCF alone, or RW alone and then challenged intratracheally, intranasally, and supraocularly with RW. Histological examination of the eyes and lungs, analysis of the bronchoalveolar lavage fluid (BALF), and characterization of ex vivo cytokine responses were performed to determine allergic inflammatory responses. RW/PCF-treated mice had suppressed allergic immune responses compared to mice given RW alone. To investigate whether IL-10 was involved in PCF-mediated allergic protection, similar experiments were performed using mice genetically deficient for IL-10. Persistent protection from allergic disease was observed in the absence of IL-10, indicating the primary mechanism of PCF protection is IL-10 independent. Ex vivo and in vitro analysis of PCF-treated dendritic cells (DC) demonstrated reduced activation receptor expression and cytokine production in response to either RW or lipopolysaccharide stimulation. These findings extend previous studies that showed infection with A. suum alters expression of allergic disease and suggest that PCF can contribute to this effect by interference with DC function.
Helminth infection is a potent activator of Th2 responses in the host (20) and is generally characterized by increased numbers of mast cells, eosinophils, and goblet cells as well as secretion of Th2 cytokines and immunoglobulin E (IgE) (36). Allergies are characterized by a similar Th2 profile in atopic individuals (19). In subjects from countries where helminth infection is endemic, there is a paradoxically lower incidence of allergic disease than in subjects from countries where the infections are less prevalent (34). This suggests that helminth infection may provide some protection from the development of allergic inflammation.
Epidemiologic studies examining the influence of helminth infection on allergic disease have demonstrated an inverse relationship between the longevity and magnitude of parasite infection and the development of the allergic response (3, 31). For example, asthmatic patients infected with Schistosoma mansoni were shown to have lower levels of the proallergic cytokines interleukin-4 (IL-4) and IL-5 than their noninfected counterparts when challenged with house dust mite antigen (4). Separate studies have suggested that parasites might directly suppress the allergic response, as anti-helminthic treatment of parasite-infected individuals correlates with increased allergen-specific cutaneous anaphylaxis and IgE antibody production (21, 32). The mechanism by which parasite-derived factors can inhibit allergic inflammation, however, remains speculative.
These studies explore the interrelationship between Ascaris infection and allergic disease. Ascaris infection affects over 1.4 billion people worldwide, and infection with this parasite has been implicated in altering the allergic response either positively by acting as an adjuvant to increase disease or negatively by acting to suppress the allergic response (5). We previously established, using a mouse model of allergic conjunctivitis, that acute helminth infection with A. suum leads to enhanced allergic disease (27). Conversely, these studies also demonstrated that chronic infection with A. suum provided protection from subsequent allergic exposure. Protection was characterized by a global increase in IL-10, which has been associated with CD4+/CD25+ T-regulatory cells. Transfer of CD4+/CD25+ T cells from mice chronically infected with A. suum to recipient mice sensitized with either ragweed (RW) and acute A. suum infection or RW alone resulted in a reduced response to subsequent RW challenge, suggesting that A. suum-induced CD4+/CD25+ T cells could directly reduce allergic disease to nonparasite allergens.
Infection with A. suum, as with most helminth infections, involves multiple stages of larval development in host organs during the migratory phase. The complex life cycle of the parasite complicates the therapeutic use of infective A. suum, as pulmonary fibrosis can develop in the lung tissue traversed by the migratory phase of the larvae (10, 16). In order to characterize the molecular basis of helminth modulation of allergic disease and to avoid the pathology associated with larval migration, we evaluated the activity of A. suum-derived products in a murine model of allergic inflammation. Pseudocoelomic fluid (PCF) was selected because it is a metabolically active fluid that is easily obtained from the body cavity of adult worms, and it contains a number of parasite antigens that induce antibody responses in several different mammalian species, including mice (15, 30). The main protein constituent of PCF is ABA-1, which represents the major IgE-binding component in patients with ascariasis (14, 25).
Recent studies have employed a variety of A. suum extracts to explore the relationship between infection and allergic inflammation; however, there has been considerable variation in both the preparation of the extracts and the inflammatory model used (17, 29). Souza et al. (28) compared Ascaris extracts from adult worms and eggs and found similar inhibitory profiles for allergic inflammation, suggesting that allergic inhibition is not parasite stage specific and likely involves numerous parasite antigens. In agreement with our chronic A. suum infection model (27), Paterson et al. found that an extract of Ascaris body fluid mixed with ovalbumin (OVA) (25) increased IL-10 production and the development T-regulatory cells. These Ascaris extract-induced T-regulatory cells were found to suppress delayed type hypersensitivity to nonparasite antigens (6, 25) but were not tested in type 1 allergic responses involving mucosal sites as examined here.
In the present studies, we sensitized mice with either PCF concurrent with the nonparasite antigens RW (RW/PCF) or OVA (OVA/PCF), PCF alone, RW alone, or OVA alone. Mice were subsequently challenged with the appropriate allergen (RW or OVA) and assessed for development of allergic conjunctivitis and/or asthma. PCF suppressed the development of allergic inflammation compared to control mice given allergen sensitization alone. Experiments using IL-10-deficient mice under similar conditions showed persistent protection from the development of allergy, indicating that PCF could protect in the absence of IL-10. Additional ex vivo and in vitro studies of PCF-treated dendritic cells (DC) demonstrated that PCF was able to suppress DC activation marker expression and cytokine production following lipopolysaccharide (LPS) stimulation. These findings suggest that PCF contains potent DC-inhibitory molecules that represent some, but not all, of the mechanisms of allergic suppression induced by infection with A. suum.
MATERIALS AND METHODS
Animals.
A/J mice were obtained from Harlan Laboratories (Indianapolis, IN), and BALB/c and C57BL/6 mice were obtained from the National Cancer Institute. Mice were housed and maintained in the Comparative Medicine Branch at a National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) animal facility (Rockville, MD). IL-10-deficient mice on a C57BL/6 background were obtained from the NIH Repository at Taconic (Germantown, NY) (11). The studies reported here conform to the principles for laboratory animal research outlined by the Animal Welfare Act (NIH/Department of Health and Human Services) guidelines for the experimental use of animals and were approved by the NIAID Animal Care and Use Committee. Each group contained 5 to 10 mice, and experiments were performed a minimum of three separate times.
PCF sensitization and RW challenge protocol.
On days 0 and 5, mice were sensitized systemically via a 200-μl intraperitoneal (i.p.) injection containing either 50 μg RW extract (Greer Laboratories, Lenoir, N.C.) (RW), 100 μg PCF (obtained from adult A. suum worms that tested negative for endotoxin) (PCF), 50 μg RW plus 100 μg PCF (RW/PCF), or phosphate-buffered saline (PBS) emulsified in an equal volume mixture with alum (Pierce Laboratories, Rockford, IL). The mice were subsequently challenged on days 14 and 15 with 1 mg RW extract in PBS dropwise in the eye (5 μl/eye) (18, 27). Control mice were challenged with PBS. For assessment of pulmonary inflammation, mice were also challenged with 50 μg of RW or PBS control intratracheally (i.t.) on day 14 and intranasally (i.n.) on day 15. Mice were sacrificed 72 h after the final challenge to evaluate conjunctival eosinophil infiltration, cellular inflammation in the lung, and cytokine levels in the sera and bronchoalveolar lavage fluid (BALF). In all cases, the mice were deeply anesthetized with an i.p. injection of ketamine (Fort Dodge Animal Health, Fort Dodge, Iowa)-xylazine (Phoenix Pharmaceuticals, St. Joseph, MO) (100 mg/kg of body weight and 10 mg/kg, respectively) and exsanguinated.
PCF sensitization and OVA challenge protocol.
On days 0 and 5, five to eight BALB/c mice per group were sensitized systemically via a 200-μl i.p. injection containing either 100 μg ovalbumin (OVA) (Sigma-Aldrich), 100 μg PCF (PCF), 100 μg OVA plus 100 μg PCF (OVA/PCF), or PBS emulsified in an equal-volume mixture with alum (Pierce Laboratories) to measure allergic lung inflammatory responses to a model nonpollen allergen. Mice were challenged intratracheally and intranasally using OVA (50 μg) and were exsanguinated after anesthesia 72 h after the final challenge to evaluate lung pathology and cellular inflammation.
BALF.
Immediately after exsanguination, lungs were cannulated with a 20-gauge intravenous catheter and gently washed once with 500 μl 1% fetal bovine serum (FBS) (HyClone, Logan, UT) in PBS (for cytokine analysis) or twice with 750 μl 1% FBS in PBS (for analysis of cellular infiltration). Samples for cytokine analysis were stored at −80°C. Samples for cellular analysis were prepared as a cytospin (Thermo-Shandon, Pittsburg, PA) for differential cellular analysis after staining with Kwik-diff (Thermo-Shandon), and a portion was used to determine total cell counts.
Histology.
To assess cellular infiltration in the conjunctiva and surrounding tissue, the eyes and lids were removed intact and immediately fixed in 10% neutral buffered formalin (EMD Chemicals, Gibbstown, NJ). Fixed tissues were sent to American Histolabs (Gaithersburg, MD) for slide preparation with paraffin embedding and staining with either Giemsa or hematoxylin and eosin for visualization of cellular inflammation. Quantitative cell counts were made on five 400× nonoverlapping fields per eyelid section and five sections per treatment group using slides that were masked to the reader (27). To assess pulmonary inflammation, lungs were lavaged for BALF and removed 72 h after the final allergen challenge. After removal, lungs were immediately placed in 10% neutral buffered formalin. Lung tissues were sent to Histoserv (Germantown, MD), embedded in paraffin, and stained with hematoxylin and eosin for visualization of cellular inflammation and periodic acid Schiff for visualization of mucus-containing goblet cells. Slides were masked to the reader, and a minimum of five lungs per group were scored with a ranking of 0 to 4 based on the level of peribronchial cuffing (PBC), perivascular cuffing (PVC), goblet cell hyperplasia, and interstitial inflammation. A score of 0 is normal lung with no inflammation or obvious increases in goblet cells in the bronchioles and alveolar spaces, 1 is a lung with minor PVC, 2 is a lung with moderate PVC and PBC cuffing, 3 is a lung with increased PVC and PBC with evidence of goblet cells in the smaller airways, and 4 is a lung with severe PVC and PBC, goblet cell hyperplasia, and interstitial inflammation (2).
Antigen-specific in vitro assays.
Spleens were removed, cells were made into single-cell suspensions, and red blood cells were lysed by treatment with ACK lysis buffer per the manufacturer's instructions (Biosource, Camarillo, CA). The remaining cells were then cultured in one of the following: medium alone as a negative control, medium and 2.5 μg/ml concanavalin A (Sigma) as a positive control, or medium and 50 μg/ml of RW extract. Medium used was composed of RPMI 1640 with Glutamax, gentamicin (10 mg/ml), 1 M HEPES, β-mercaptoethanol (Invitrogen, Carlsbad, CA), and 10% FBS (HyClone, Logan, UT). A total of 2 × 106 splenocytes/well were plated in a 24-well plate at a volume of 2 ml/well. Supernatants were harvested after 48 h in culture and immediately frozen at −80°C prior to analysis of cytokines
Quantitative measurement of cytokines by luminex technology.
Cytokines IL-4, IL-5, IL-13, and gamma interferon (IFN-γ) were measured using the Biosource Multiplex Assay kit (Biosource International, Camarillo, CA) per the manufacturer's instructions. All samples, including standards, were assayed in duplicate. Fluorescence was measured by using the Liquichip reader (QIAGEN, Valencia, CA).
BMDC assays.
Bone marrow dendritic cells (BMDC) were prepared from femur and tibia after removing the bone marrow cells by flushing with Hank's balanced salt solution supplemented with 1% HEPES (Invitrogen, Grand Island, NY). To induce DC development, 1 × 106 bone marrow cells/ml were cultured in complete RPMI 1640 medium (10% FCS, 10 mg/ml gentamicin, 0.1% β-mercaptoethanol, 1% HEPES in the presence of 40 ng/ml of murine recombinant granulocyte-macrophage colony-stimulating factor; PeproTech, Rocky Hill, NJ). On day 4, supernatants were gently removed and fresh medium was added together with 40 ng/ml granulocyte-macrophage colony-stimulating factor and 10 ng/ml of murine recombinant IL-4 (PeproTech) to the remaining semiadherent cells. The cells were harvested on day 6, seeded in 24-well plates, and stimulated with 100 μg/ml of PCF. After 6 h of stimulation with PCF, 20 ng/ml of LPS was added, and the cells were stimulated for additional 5 h. The cells and the supernatants were subsequently harvested for fluorescent-activated cell sorter (FACS) staining and cytokine analysis, respectively.
Ex vivo analysis of CD11c+ dendritic cells.
Mice were sensitized systemically via a 200-μl i.p. injection containing either 50 μg RW extract (Greer Laboratories) (RW), 100 μg PCF (PCF), 50 μg RW plus 100 μg PCF (RW/PCF), or PBS (PBS). All products and controls were emulsified 1:1 in an equal (100 μl)-volume mixture with alum (Pierce Laboratories). Mice were sacrificed at either 24 h or 6 days postsensitization, injected i.p. with 6 ml PBS, and gently lavaged. Additionally, a group of naïve mice was sacrificed, injected i.p. with 6 ml PBS, and lavaged as controls for cellular infiltration in response to alum alone. Peritoneal lavage cells were centrifuged (300 relative centrifugal force for 10 min); the resultant pellet was resuspended in 1 ml PBS with 2% FBS. A 100-μl aliquot of each sample for cellular analysis was prepared as a cytospin (Thermo-Shandon) for differential cellular analysis after staining with Kwik-diff (Thermo-Shandon), and an additional 50-μl portion was used to determine total cell counts. The remaining cells were stained for CD11c as well as the costimulatory markers CD86, CD40, and major histocompatibility complex (MHC) class II for analysis by FACS.
Flow-cytometric analysis (FACS).
Peritoneal lavage cells and BMDC were stained for CD11c as well as the costimulatory markers CD86, CD40, and MHC class II to determine cellular activation (clones HL-3, GL-1, HM40-3, and 39-10-8, respectively; BD Biosciences). FACS analysis was performed on CD11c+ gated cells to determine the expression of costimulatory markers on dendritic cells. FACS analysis was performed using a FACS Calibur (BDBiosciences) using CellQuest software. Data analysis was performed using FlowJo software 5.7.2 (Tree Star, Ashland, OR).
Data analysis.
Data are summarized as means ± standard errors. One-way analysis of variance with Tukey's multiple comparison posttest was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California). The statistical significance value was set at P < 0.05.
RESULTS
PCF administration significantly inhibits allergic conjunctivitis.
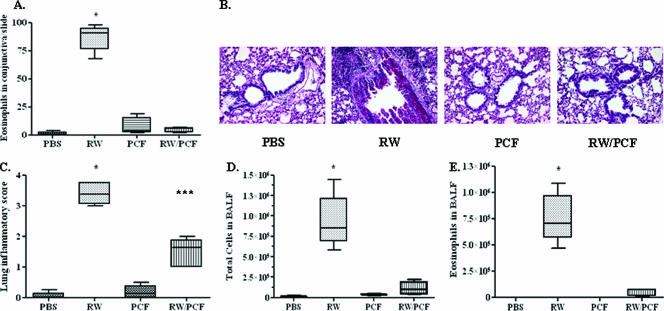
As has been previously reported (18, 27), systemic sensitization of mice with RW (RW group) results in significant increases in ocular inflammation in response to RW challenge in the eye compared with mice sham sensitized with PBS (PBS group) (P < 0.001) (Fig. 1A). Treatment of mice with PCF during the initial RW sensitization (RW/PCF) significantly reduced the number of conjunctival eosinophils compared with animals given RW alone (P < 0.001) (Fig. 1A). Administration of PCF alone did not significantly increase eosinophil numbers above PBS controls (P > 0.05).
FIG. 1.
Allergic conjunctival and pulmonary inflammation following RW sensitization and challenge. Five to eight A/J mice per treatment group were treated with PBS, PCF, RW, or RW and PCF. A. RW challenge in sensitized animals resulted in a significant infiltration of eosinophils into the conjunctiva (***, P < 0.001) compared with all other treatment groups). This allergen-induced eosinophil infiltration was reduced by PCF administration during RW sensitization (RW/PCF). B. Periodic acid-Schiff staining of representative lung tissue sections 72 h after allergen challenge. C. Lung sections were assigned an inflammatory lung score using a scale of 0 to 4, with 4 representing the score for a maximal inflammatory response in the lung. Mice given RW sensitization had a significant increase in lung inflammatory scores compared to those of mice sensitized with PBS or PCF treatment alone post-RW challenge (***, P < 0.001). Mice treated with PCF during RW sensitization had reduced lung inflammatory scores after RW challenge compared to animals given RW alone. D. Total cell numbers were counted from the BALF and demonstrated a significant increase in total cell numbers in the lung lumen in mice given RW sensitization (***, P < 0.001) compared with all other groups of mice. Mice treated with PCF during RW sensitization had significantly reduced cell numbers in the lung lumen after RW compared with animals given RW alone. E. Eosinophils were counted from the BALF as a measure of allergic inflammation. Mice given RW sensitization demonstrated a significant increase in eosinophil infiltrates in the BALF post-RW challenge (***, P < 0.001) compared to all other treatment groups. Mice treated with PCF during RW sensitization had significantly reduced eosinophil numbers in the BALF after RW challenge compared with animals given RW alone.
PCF administration significantly inhibits allergen-induced pulmonary eosinophilia.
PBS (Fig. 1B, far-left panel)- and PCF (Fig. 1B, right-center panel)-sensitized control mice had little response to RW challenge. Conversely, RW challenge in sensitized mice resulted in significant amounts of goblet cell hyperplasia, interstitial inflammation, and perivascular and peribronchiolar cuffing with an infiltration of lymphocytes and eosinophils. RW/PCF-treated mice had significant reductions in pulmonary inflammation compared with animals given RW alone (Fig. 1B, left-center panel), resulting in significantly increased lung inflammatory scores over what was observed in PBS-sensitized controls (P < 0.001) (Fig. 1C). Systemic administration of PCF during the initial RW sensitization significantly reduced overall airway pathology (Fig. 1B, far-right panel) compared with animals given RW alone (P < 0.05) (Fig. 1C).
Total cell numbers in the BALF were significantly increased after RW sensitization and challenge (P < 0.001) (Fig. 1D). Administration of PCF during RW sensitization, however, significantly reduced the total cellular number in the BALF after RW challenge compared to untreated RW-sensitized and -challenged mice (P < 0.001) (Fig. 1D). PCF administration alone did not alter the total cell number compared with PBS-treated control mice (P > 0.05). Eosinophil numbers were measured in the BALF as an indicator of allergic inflammatory responses in the lungs. The numbers of eosinophils were significantly increased in the BALF of the RW-sensitized and -challenged animals compared with PBS control mice (P < 0.001) (Fig. 1E). This number was significantly reduced in mice given systemic PCF during RW sensitization (P < 0.001) (Fig. 1E). PCF administration alone did not significantly increase the number of eosinophils in the BALF compared with PBS control animals (P > 0.05). These findings combined with the studies assessing the role of PCF in the prevention of allergic eye disease suggest that systemic administration of PCF may protect from allergic disease at multiple sites of allergen challenge.
PCF treatment decreases allergen-induced Th2 cytokine levels in in vitro antigen-specific recall assays and ex vivo in the BALF.
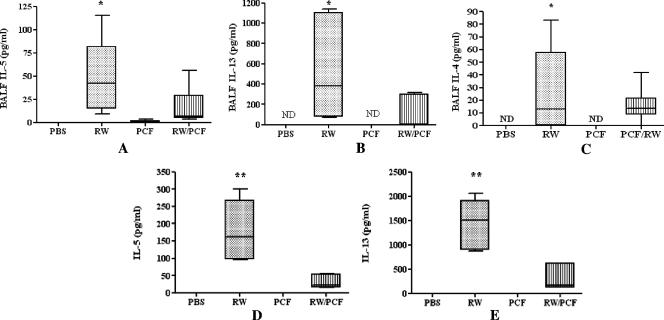
IL-4, IL-5, and IL-13 are potent inducers and potentiators of the allergic cascade. IL-5 has been shown to be involved in eosinophil maturation and activation (23), whereas IL-4 and IL-13 are important for smooth-muscle constriction, mucous production, and the B-cell immunoglobulin isotype switching to IgE (33). RW sensitization and challenge significantly increased the RW-induced production of the Th2-associated cytokines IL-5 (P < 0.01) (Fig. 2A) , IL-13 (P < 0.01) (Fig. 2B), and IL-4 (P < 0.05) (Fig. 2C) in the BALF compared with PCF alone and PBS-sensitized control mice. Exposure to PCF during RW sensitization significantly decreased the levels of both IL-5 (P < 0.05) (Fig. 2A) and IL-13 (P < 0.05) (Fig. 2B) but not IL-4 (P > 0.05) (Fig. 2C) after RW challenge compared with RW-sensitized and -challenged mice. Administration of PCF alone did not significantly increase the secretion of IL-5 (P > 0.05) (Fig. 2A), IL-13 (P > 0.05) (Fig. 2B), or IL-4 (P > 0.05) (Fig. 2C in the BALF compared with control mice treated with PBS.
FIG. 2.
Cytokines from the BALF (A to C) and antigen-specific recall assays (D and E) in response to RW challenge. The levels of the cytokines IL-5 (A), IL-13 (B), IL-4 (C), and IFN-γ (data not shown) were measured in the BALF. The level of the proallergic cytokines IL-5 (A), IL-13 (B), and IL-4 (C) were significantly higher (P < 0.05) in the BALF of RW-sensitized and -challenged mice than in control mice given PBS or PCF alone. Mice given PCF during RW sensitization had a significant reduction (P < 0.05) in the amount of IL-5 (A) and IL-13 (B) in the BALF compared with animals given RW sensitization alone. ND, not detected. (C) The level of IL-4 in the BALF from animals given PCF during RW sensitization was also reduced compared to that of animals sensitized with RW alone, but this reduction did not reach the level of significance. The level of the cytokines IL-5 (D) and IL-13 (E) were also assessed in antigen-specific recall assays in response to RW challenge and in response to RW challenge in vitro in splenocytes from RW-sensitized and -challenged mice compared with control mice given PBS or PCF alone (***, P < 0.001). Mice given PCF treatment prior to challenge had a significant reduction in the amount of both IL-5 (D) and IL-13 (E) in in vitro antigen-specific recall assays.
In vitro antigen-specific recall assays were performed on splenocytes taken from all four in vivo treatment groups (PBS, RW, PCF, and RW/PCF). Splenocyte cultures from mice in the RW-alone group produced significantly more IL-5 (P < 0.001) (Fig. 2D) and IL-13 (P < 0.001) (Fig. 2E) in response to RW stimulation in vitro than spleen cells from mice in the PCF-alone and PBS control groups. PCF treatment during RW sensitization in vivo suppressed the production of IL-5 (P < 0.001) (Fig. 2D) and IL-13 (P < 0.001) (Fig. 2E) in RW-induced spleen cell assays in vitro. In vivo treatment with PCF alone did not alter the in vitro production of either IL-5 or IL-13 above PBS control animals.
In order to determine if the decrease in Th2-associated cytokines was due to a simultaneous increase in Th2-blocking Th1 cytokines, the levels of IFN-γ were also assessed in the BALF and in vitro antigen-specific splenocyte recall assays. The levels of IFN-γ in response to RW challenge were below the level of detection for the multiplex assay in all cases. All groups of cells in the in vitro recall assays, however, did produce IFN-γ in response to the mitogen concanavalin A as a positive control (data not shown). Taken together, these results suggest that the decrease in Th2 cytokines was not due to a concurrent increase in Th1 cytokines.
PCF protects from allergic inflammation to a nonplant-derived allergen.
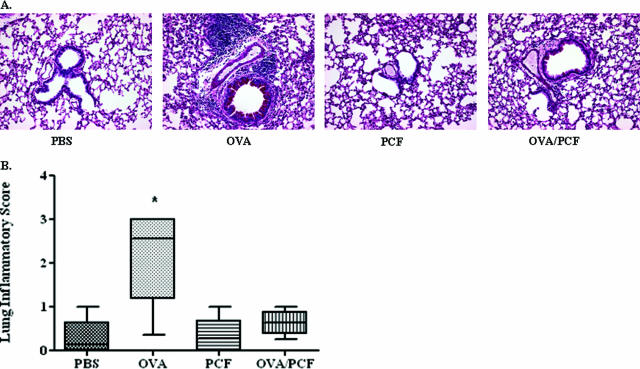
The immune-modulating activity of PCF was also supported by studies using a nonpollen-derived allergen (OVA). In these experiments, OVA sensitization and challenge (Fig. 3A, left-center panel) significantly increased lung inflammatory scores compared to animals either given PBS treatment (P < 0.001) (Fig. 3A, far-left panel, and B) or PCF alone (P < 0.001) (Fig. 3A, right center panel, and B). Cosensitization of OVA with PCF was found to significantly reduce allergic inflammation in the lung in response to OVA challenge (Fig. 3A, far-right panel) compared with mice given OVA sensitization and challenge alone (P < 0.001) (Fig. 3A, right-center panel, and B). These studies are in agreement with previous studies done using OVA in separate models of allergic disease (17, 25) and demonstrate the universality of PCF protection against a broad array of allergens.
FIG. 3.
Allergic pulmonary inflammation following OVA sensitization and challenge. Five to eight BALB/c mice per treatment group were sensitized with OVA to determine alterations of the allergic inflammatory response to a nonpollen allergen (OVA) post-PCF treatment. A. Periodic acid-Schiff staining of representative lung tissue sections 72 h after allergen challenge. B. OVA challenge in sensitized mice resulted in increased pulmonary inflammatory scores (**, P < 0.01) compared with all other treatment groups. Mice given PCF during OVA sensitization had a significant reduction of pulmonary inflammation compared to animals given OVA sensitization alone.
IL-10 does not appear to play a role in PCF-induced protection against allergic disease.
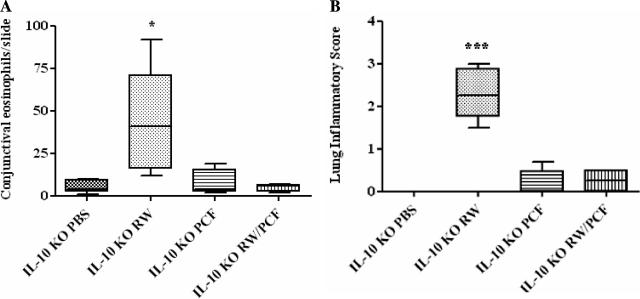
IL-10 was shown to be an important mediator of allergic protection in mice chronically infected with A. suum (27). Thus, IL-10-deficient mice were employed to determine if IL-10 was also mediating protection from allergic inflammation by PCF. Histological examination of the conjunctiva from IL-10-deficient animals treated with RW demonstrated significant eosinophilic infiltrates characteristic of allergic conjunctivitis compared with PBS-challenged control mice (P < 0.05) (Fig. 4A). IL-10-deficient mice that received treatment with PCF during the initial RW sensitization, however, had significantly fewer eosinophils migrating into the conjunctiva compared with control mice given RW alone (P < 0.01) (Fig. 4A) in a manner similar to that observed in wild-type mice. The number of eosinophils in the conjunctiva from mice given PCF sensitization alone was not significantly higher than the number of eosinophils observed in PBS control animals (P > 0.05) (Fig. 4A).
FIG. 4.
Allergic conjunctival and pulmonary inflammation following RW sensitization and challenge in IL-10-deficient mice. Five to eight IL-10-deficient mice were treated with PBS, RW, PCF, or RW and PCF. A. Five conjunctival sections per mouse per treatment group were counted to determine eosinophil infiltration 72 h postallergen challenge. RW sensitization and challenge in IL-10-deficient animals led to increased eosinophil infiltrates in the conjunctiva compared with all other groups (*, P < 0.05). Mice treated with PCF during RW sensitization had a reduced eosinophilic ocular infiltration after RW challenge compared with animals given RW alone. B. IL-10-deficient mice given RW sensitization had a significant increase in lung inflammatory scores compared to mice sensitized with PBS or PCF treatment alone post-RW challenge (***, P < 0.001). Mice treated with PCF during RW sensitization had a reduced lung inflammatory score after RW challenge compared with animals given RW alone.
Examination of pulmonary inflammation yielded results similar to those seen in the conjunctiva studies, with RW sensitization and challenge resulting in significant increases in lung inflammatory scores compared with PBS controls (P < 0.001) (Fig. 4B). Mice that received PCF alone or PCF combined with RW during the initial sensitization (RW/PCF) had significantly fewer pulmonary infiltrates compared with mice given RW alone (P < 0.001) (Fig. 4B). These pulmonary inflammatory responses suggest that PCF-induced protection from allergic disease at two distinct mucosal sites is not mediated by production of IL-10 and is unlike the IL-10-dependent protection observed in mice chronically infected with A. suum.
Exposure to PCF prevents antigen-specific activation of peritoneal and bone-marrow derived dendritic cells.
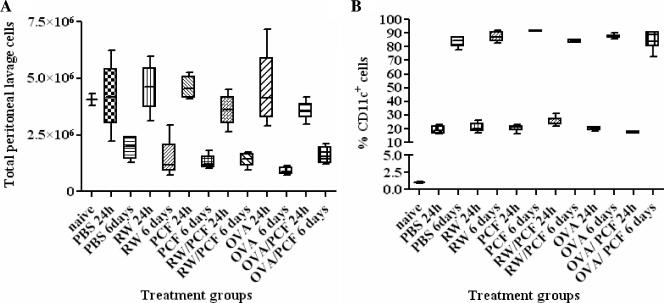
The induction of the allergic cascade requires the uptake and processing of the allergen by antigen-presenting cells (APC). Dendritic cells are highly potent, tissue-dwelling APC which have been shown to act as key sentinels and propagators of the allergic response (24). Therefore, we next assessed whether PCF could alter the allergic pathway in the initial antigen presentation stage by examining activation of CD11c+ cells removed ex vivo from the peritoneal cavity from naïve mice or at two time points (24 h and 6 days) after i.p. injection with PBS, PCF, RW, RW/PCF, OVA, or OVA/PCF (Fig. 5). Total cell numbers and the percentage of CD11c+ cells were quantitated for each treatment group. At 24 h after a single i.p. injection, the total number of lavage cells was not significantly different from that of naïve mice in any of the treatment groups (PBS, RW, PCF, RW/PCF, OVA, or OVA/PCF) (P > 0.05) (Fig. 5A). Examination of a cohort of mice 6 days after i.p. injection from the same treatment groups, however, revealed a significant decrease in total numbers of peritoneal lavage cells in all treatment groups than what was observed in naïve animals or from mice 24 h postperitoneal injection (P < 0.05) (Fig. 5A).
FIG. 5.
Ex vivo analysis of peritoneal lavage cells. A. Peritoneal lavage of mice 6 days after i.p. injection from PBS, RW, PCF, RW/PCF, OVA, or OVA/PCF treatment demonstrated a significant decrease in total numbers of peritoneal lavage cells than what was observed in naïve animals or from mice 24 h postperitoneal injection (P < 0.05). B. FACS analysis of lavage cells revealed significant increases in the percentage of CD11c+ DC cells in all groups of animals 24 h after i.p. injection compared with naïve control animals (P < 0.001) that was further increased in all groups at the 6-day post-i.p. time point (P < 0.001).
FACS analysis was performed on all treatment groups to determine the percentage of CD11c+ DC in the peritoneal lavage from naïve mice and in all the treatment groups 24 h and 6 days after i.p. injection. FACS analysis of lavage cells revealed significant increases in the percentage of CD11c+ DC cells in all groups of animals 24 h after i.p. injection compared with naïve control animals (P < 0.001) (Fig. 5B). The increase in percentage of CD11c+ cells in the peritoneum continued over time, as examination of lavage cells 6 days postsensitization demonstrated a significantly higher percentage of CD11c+ cells compared with animals 24 h postsensitization (P < 0.001) (Fig. 5B). Since the increase in percentage of CD11c+ cells at the two time points after i.p. injection was observed in the peritoneal lavage from control animals receiving PBS and alum injection alone, this finding suggests that the alum itself may be recruiting CD11c+ DC to the site of intraperitoneal injection.
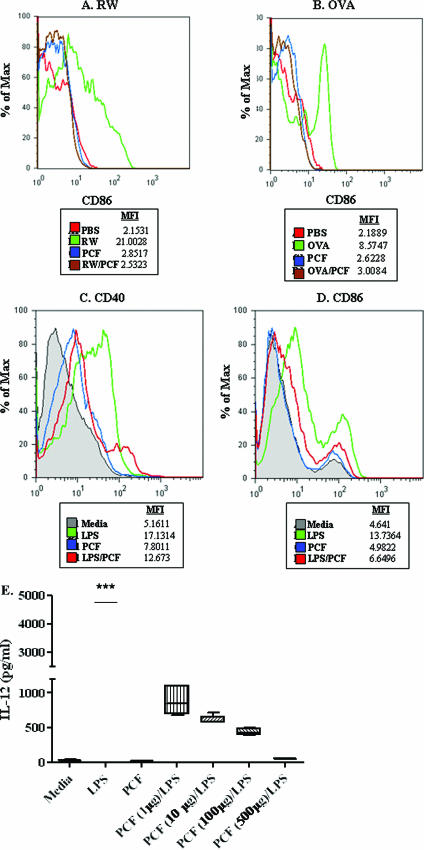
Next, the expression of the activation marker CD40, CD86, or MHC class II in CD11c+ cells was examined from naïve mice and animals 24 h and 6 days after a single peritoneal injection. Peritoneal lavage cells examined 24 h after i.p. injection did not have any significant increases in activation marker expression in any of the treatment groups over naïve controls (data not shown). Examination of cells lavaged 6 days after i.p. injection with RW (Fig. 6A, green) or OVA (Fig. 6B, green) led to increased expression of the costimulatory marker CD86 compared to intraperitoneal CD11c+ cells from mice that received sham sensitization with PBS (Fig. 6A and B, red). Intraperitoneal CD11c+ cells derived from RW/PCF-treated mice (Fig. 6A, brown) or OVA/PCF treated mice (Fig. 6B, brown) demonstrated reduced levels of CD86 compared to DC from mice in the RW alone group (Fig. 6A, green) or OVA alone group, respectively (Fig. 6B, green). The expression of MHC class II or CD40 on CD11c+ peritoneal lavage cells did not significantly change 6 days after i.p. injection in any of the treatment groups (data not shown), suggesting that the expression of these two activation markers may be regulated at different time points than the ones examined in these experiments.
FIG. 6.
Ex vivo and in vitro analysis of DC activation. CD11c+ cells removed ex vivo from the peritoneal cavity, spleens, and mediastinal LN after either i.p. sensitization with PCF, RW, or RW/PCF (A) or i.p. sensitization with PCF, OVA, or OVA/PCF (B). i.p. injection of either RW or OVA (A and B, respectively; green) led to increased expression of the costimulatory marker CD86 compared to intraperitoneal DC from mice that received sham sensitization with PBS (red) or PCF alone (blue). (A) Intraperitoneal DC derived from RW/PCF-treated mice (brown) showed reduced levels of CD86 compared to DC from mice in the RW alone group (green). (B) Similar reductions were seen in mice given OVA/PCF treatment (brown) compared with injection of OVA alone (green). Expression of LPS-induced CD40 (C) (green) and CD86 (D) (green) was dramatically reduced in BMDC in vitro after preexposure to PCF (D) (blue). (E) LPS-induced production of IL-12 was significantly inhibited in a dose-dependent manner when the BMDC were preexposed with increasing doses of PCF.
In order to determine if the increase in CD86 expression on CD11c+ DC was also occurring in the central or peripheral lymphatics, spleens and cervical lymph nodes (LN) were also removed from mice 6 days after peritoneal injection with antigen. However, CD11c+ cells removed from the spleens and LN 6 days after a single i.p. injection with either RW or OVA did not exhibit a similar increase in activation marker expression over mice given PBS alone, thus prohibiting an examination of PCF-induced suppression in the peripheral and central immune responses.
In addition to assessing peritoneal lavage CD11c+ DC, BMDC were also examined. Stimulation of BMDC with RW or OVA in vitro yielded only modest changes in activation marker expression (data not shown); LPS was used as the positive stimulus for activation. Exposure of BMDC to LPS significantly increased the expression of the activation markers CD40 (Fig. 6C, green) and CD86 (Fig. 6D, green) but not MHC class II (data not shown). The increase in expression of CD40 and CD86 (Fig. 6C and D, red) was significantly inhibited when the BMDC were pretreated with PCF for 6 h prior to LPS exposure.
LPS exposure in vitro also significantly increased the production of IL-12 from BMDC (Fig. 6E). This LPS-induced production of IL-12 was significantly inhibited in a dose-dependent manner when the BMDC were preexposed with increasing doses of PCF, suggesting that the PCF was preventing cytokine production in addition to activation (Fig. 6E).
DISCUSSION
A number of recent epidemiologic studies have examined the complex relationship between parasite infection and allergic disease (5, 35). Our group and others (19, 34) have evaluated the effect of parasite infection on allergic disease in mice. Previous studies have found increased tissue fibrosis in mice infected with A. suum eggs following the normal migration of third-stage larval migration through the lungs (26). Larva-induced tissue damage in the lungs confounds evaluation of the mucosal inflammatory responses to nonparasite allergens. We have, however, evaluated allergic responses in the conjunctiva of the eye of A. suum-infected mice as a paradigm of mucosal inflammation at sites where A. suum does not migrate (27).
Using allergic conjunctivitis as our measure of allergic inflammation, we previously found an inverse relationship between the frequency of parasite infection and the development of allergic inflammation. In particular, we found that chronic infection with A. suum resulted in a global increase in IL-10 (27). We further examined the role of parasitic infection on expression of allergic disease in the current study by using an A. suum antigen preparation from the pseudocoelomic fluid (PCF) of adult A. suum worms. PCF was used to identify parasite-derived products that contribute to the molecular mechanism of protection and also to prevent any confounding effects from A. suum larva-induced tissue damage during migration. PCF is a metabolically active fluid that maintains hemostatic pressure in the worm and provides precursor molecules for membrane and cuticular synthesis and heme-containing proteins for oxidative metabolism (15). Some of these molecules are recognized as antigens by infected rodents, rabbits (15), and pigs (30) and include ABA-1, the major IgE-binding antigen in patients that express resistance to ascariasis (25).
Administration of PCF into the peritoneum during the initial allergen (RW) sensitization significantly reduced eosinophil migration into the conjunctiva in response to allergen challenge, which is similar to what was observed when mice were chronically infected with A. suum. In addition, a single systemic administration of PCF was able to significantly reduce pulmonary eosinophilic inflammation and total lung pathology in response to challenge with RW.
The immune-modulating activity of PCF was also supported by studies using a nonpollen-derived allergen (OVA). A number of researchers have begun exploring the relationship between the coevolution of parasite infection and the development of allergic responses to common allergens (5, 19). A recent study by Bielory et al. (personal communication) has used epitope mapping technology to unmask conserved domains between a number of helminth-derived proteins and environmental allergens. These shared antigenic determinants could theoretically result in cross-reactivity between immune responses of A. suum and RW. Therefore, we conducted a second series of experiments employing OVA sensitization and challenge as a representative model nonplant allergen in lieu of RW. We and others have found OVA does not easily elicit a response in mouse conjunctiva without additional manipulation (13); therefore, only pulmonary inflammation was assessed in the OVA-induced allergy studies. The combination of OVA and PCF was able to significantly suppress pulmonary responses to challenge with OVA compared to mice sensitized and challenged with OVA alone. These findings suggest PCF is capable of suppressing the allergic response to a wide range of traditional and atypical allergens at multiple tissue sites.
Ascaris infection significantly increases Th2 cytokine responses in human and animal models (9, 22, 27). Conversely, PCF exposure during sensitization in RW-sensitized and -challenged animals significantly decreases allergen-induced Th2 cytokine responses in vivo. This decrease in Th2 cytokine production was likely not due to a concurrent increase in Th2-abrogating Th1 cytokines, as the levels of IFN-γ were not increased after PCF exposure compared to animals exposed to RW alone or PBS control.
Previous studies with an allergy protection model suggested that mice infected chronically with A. suum were protected from nonparasite allergen-induced disease through IL-10 (25). IL-10 does not appear to contribute to the mechanism of action of PCF, since mice genetically deficient in IL-10 that were exposed to PCF during the initial allergen exposure were protected from allergic challenge. Transforming growth factor β (TGF-β) is an additional regulatory-associated cytokine initially proposed to be protective in allergic disease (8, 24). However, studies in animals receiving PCF treatment (data not shown) did not detect increased TGF-β production after RW challenge. Recent studies by Boxall et al. demonstrated that TGF-β may contribute to airway remodeling in asthma (7), suggesting that TGF-β production may be detrimental rather than protective for allergic disease. These findings suggest that the role for TGF-β in allergic inflammation warrants further investigation.
Finally, ex vivo and in vitro studies were conducted to determine if PCF was affecting APC activation by examining DC activation marker expression. In these studies, the functional response of CD11c+ DC removed ex vivo from the peritoneal cavity, spleen, and LN of PCF-exposed mice post-RW and -OVA exposure was examined. These studies demonstrated that although the total number of peritoneal lavage cells decreased 6 days after peritoneal injection with antigen compared to either naïve mice or mice examined 24 h after i.p. injection, the percentage of CD11c+ cells increased dramatically. These findings suggest either that CD11c+ cells were propagating in situ or that additional DC were migrating to the site of antigen stimulation. In addition, examination of activation markers on the CD11c+ peritoneal lavage cells examined 6 days after antigen stimulation revealed that PCF suppressed the allergen-induced increased expression of the costimulatory molecule CD86 on CD11c+ DC in the peritoneum. Further in vitro studies using BMDC demonstrated that PCF inhibited LPS-induced expression of the activation markers CD40 and CD86. Preexposure of BMDC with PCF also suppressed LPS-induced production of IL-12 in a dose-dependent manner, suggesting that PCF was inhibiting cytokine production in addition to cellular activation. Since LPS is a potent stimulator of DC activation and cytokine production, this suppression was especially striking.
Whether PCF is inhibiting, down-regulating, or blocking Toll-like receptors (TLR), or directly suppressing ligand processing by the DC is currently under investigation. To date, there have been few clearly defined pathogen-activated molecular patterns detected in A. suum. Other helminth parasites such as Schistosoma express molecules that have been shown to act through TLR3, TLR4, TLR9, or TLR11 (1, 12). LPS-containing endotoxin is known as a potent activator of DC through TLR2 and TLR4 and is a possible contaminant of products derived from gastrointestinal parasites (24). Stimulation of these TLRs is known to initiate a signal transduction cascade through the adaptor molecule MyD88 or TIRAP and TRIF, leading to an increased transcription of NF-κB and the switching on of a number of immune response genes (26). The PCF used in these experiments, however, was determined by limulus assays to have endotoxin levels below the level of detection, and exposure to PCF alone did not effect DC activation. This suggests that PCF may not be activating directly through either TLR2 or TLR4, although it does not rule out a role for PCF in inhibiting those receptors. We are planning additional studies employing TLR-deficient mice to aid in the evaluation of TLR in our PCF-mediated protection model.
These studies, in total, suggest that PCF may prevent the development of allergic disease by inhibiting appropriate APC activation and initiation of the allergic cascade. The expression of products by A. suum could potentially limit local allergic reactions that contribute to a reduced host response to infection, since migrating larvae express antigens found in PCF (13, 28). We are currently conducting a number of experiments to verify these concepts and to better define the mechanisms of PCF-induced regulation of APCs, influence on T-regulatory cells, and suppression of allergic inflammation.
Acknowledgments
We thank Susan Pierce, Tom Wynn, and Marcus Hodges for their review of the manuscript and insightful suggestions. We also thank Brian Kelsall and Cecilia Johansson for their useful advice on dendritic cell assays.
Funding was provided by NIH/NIAID Intramural and USDA (CRIS 1265-32000-064-00D).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Aksoy, E., C. S. Zouain, F. Vanhoutte, J. Fontaine, N. Pavelka, N. Thieblemont, F. Willems, P. Ricciardi-Castagnoli, M. Goldman, M. Capron, B. Ryffel, and F. Trottein. 2005. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 280:277-283. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shami, A., R. Spolski, J. Kelly, A. Keane-Myers, and W. J. Leonard. 2005. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, M. I., B. S. Hoppe, M. Medeiros, Jr., and E. M. Carvalho. 2004. Schistosoma mansoni infection modulates the immune response against allergic and auto-immune diseases. Mem. Inst. Oswaldo Cruz 99:27-32. [DOI] [PubMed] [Google Scholar]
- 4.Araujo, M. I., A. A. Lopes, M. Medeiros, A. A. Cruz, L. Sousa-Atta, D. Sole, and E. M. Carvalho. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145-148. [DOI] [PubMed] [Google Scholar]
- 5.Arruda, L. K., and A. B. Santos. 2005. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr. Opin. Allergy Clin. Immunol. 5:399-402. [DOI] [PubMed] [Google Scholar]
- 6.Boitelle, A., C. Di Lorenzo, H. E. Scales, E. Devaney, M. W. Kennedy, P. Garside, and C. E. Lawrence. 2005. Contrasting effects of acute and chronic gastro-intestinal helminth infections on a heterologous immune response in a transgenic adoptive transfer model. Int. J. Parasitol. 35:765-775. [DOI] [PubMed] [Google Scholar]
- 7.Boxall, C., S. T. Holgate, and D. E. Davies. 2006. The contribution of transforming growth factor-beta and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur. Respir. J. 27:208-229. [DOI] [PubMed] [Google Scholar]
- 8.Capron, A., D. Dombrowicz, and M. Capron. 2004. Helminth infections and allergic diseases: from the Th2 paradigm to regulatory networks. Clin. Rev. Allergy Immunol. 26:25-34. [DOI] [PubMed] [Google Scholar]
- 9.Dawson, H. D., E. Beshah, S. Nishi, G. Solano-Aguilar, M. Morimoto, A. Zhao, K. B. Madden, T. K. Ledbetter, J. P. Dubey, T. Shea-Donohue, J. K. Lunney, and J. F. Urban, Jr. 2005. Localized multigene expression patterns support an evolving Th1/Th2-like paradigm in response to infections with Toxoplasma gondii and Ascaris suum. Infect. Immun. 73:1116-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyre, P., D. H. Nymeyer, B. M. McCraw, and T. R. Deline. 1976. Protection by acetylsalicylic acid and other agents in experimental acute interstitial pneumonia of calves. Vet. Rec. 98:64-66. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927-938. [PubMed] [Google Scholar]
- 12.Jenkins, S. J., J. P. Hewitson, S. Ferret-Bernard, and A. P. Mountford. 2005. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and -independent pathways. Int. Immunol. 17:1409-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn, M., N. P. Barney, R. M. Briggs, K. J. Bloch, and M. R. Allansmith. 1990. Penetrating the conjunctival barrier. The role of molecular weight. Investig. Ophthalmol. Vis. Sci. 31:258-261. [PubMed] [Google Scholar]
- 14.Kennedy, M. W., E. M. Fraser, and J. F. Christie. 1991. MHC class II (I-A) region control of the IgE antibody repertoire to the ABA-1 allergen of the nematode Ascaris. Immunology 72:577-579. [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, M. W., and F. Qureshi. 1986. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology 58:515-522. [PMC free article] [PubMed] [Google Scholar]
- 16.Liljegren, C. H., B. Aalbaek, O. L. Nielsen, and H. E. Jensen. 2003. Some new aspects of the pathology, pathogenesis, and aetiology of disseminated lung lesions in slaughter pigs. APMIS 111:531-538. [DOI] [PubMed] [Google Scholar]
- 17.Lima, C., A. Perini, M. L. Garcia, M. A. Martins, M. M. Teixeira, and M. S. Macedo. 2002. Eosinophilic inflammation and airway hyper-responsiveness are profoundly inhibited by a helminth (Ascaris suum) extract in a murine model of asthma. Clin. Exp. Allergy 32:1659-1666. [DOI] [PubMed] [Google Scholar]
- 18.Magone, M. T., S. M. Whitcup, A. Fukushima, C. C. Chan, P. B. Silver, and L. V. Rizzo. 2000. The role of IL-12 in the induction of late-phase cellular infiltration in a murine model of allergic conjunctivitis. J. Allergy Clin. Immunol. 105:299-308. [DOI] [PubMed] [Google Scholar]
- 19.Maizels, R. M. 2005. Infections and allergy - helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 17:656-661. [DOI] [PubMed] [Google Scholar]
- 20.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites-masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 21.McKay, D. M. 2006. The beneficial helminth parasite? Parasitology 132:1-12. [DOI] [PubMed] [Google Scholar]
- 22.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori, A., O. Kaminuma, K. Ogawa, A. Nakata, R. W. Egan, K. Akiyama, and H. Okudaira. 2000. Control of IL-5 production by human helper T cells as a treatment for eosinophilic inflammation: comparison of in vitro and in vivo effects between selective and nonselective cytokine synthesis inhibitors. J. Allergy Clin. Immunol. 106:S58-S64. [DOI] [PubMed] [Google Scholar]
- 24.O'Garra, A., L. Steinman, and K. Gijbels. 1997. CD4+ T-cell subsets in autoimmunity. Curr. Opin. Immunol. 9:872-883. [DOI] [PubMed] [Google Scholar]
- 25.Paterson, J. C., P. Garside, M. W. Kennedy, and C. E. Lawrence. 2002. Modulation of a heterologous immune response by the products of Ascaris suum. Infect. Immun. 70:6058-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce, E. J., C. M. Kane, and J. Sun. 2006. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem. Immunol. Allergy 90:82-90. [DOI] [PubMed] [Google Scholar]
- 27.Schopf, L., S. Luccioli, V. Bundoc, P. Justice, C. C. Chan, B. J. Wetzel, H. H. Norris, J. F. Urban, Jr., and A. Keane-Myers. 2005. Differential modulation of allergic eye disease by chronic and acute ascaris infection. Investig. Ophthalmol. Vis. Sci. 46:2772-2780. [DOI] [PubMed] [Google Scholar]
- 28.Souza, V. M., E. L. Faquim-Mauro, and M. S. Macedo. 2002. Extracts of Ascaris suum egg and adult worm share similar immunosuppressive properties. Braz. J. Med. Biol. Res. 35:81-89. [DOI] [PubMed] [Google Scholar]
- 29.Souza, V. M., J. F. Jacysyn, and M. S. Macedo. 2004. IL-4 and IL-10 are essential for immunosuppression induced by high molecular weight proteins from Ascaris suum. Cytokine 28:92-100. [DOI] [PubMed] [Google Scholar]
- 30.Urban, J. F., Jr., H. Alizadeh, and R. D. Romanowski. 1988. Ascaris suum: development of intestinal immunity to infective second-stage larvae in swine. Exp. Parasitol. 66:66-77. [DOI] [PubMed] [Google Scholar]
- 31.van den Biggelaar, A. H., C. Lopuhaa, R. van Ree, J. S. van der Zee, J. Jans, A. Hoek, B. Migombet, S. Borrmann, D. Luckner, P. G. Kremsner, and M. Yazdanbakhsh. 2001. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. Int. Arch. Allergy Immunol. 126:231-238. [DOI] [PubMed] [Google Scholar]
- 32.van den Biggelaar, A. H., L. C. Rodrigues, R. van Ree, J. S. van der Zee, Y. C. Hoeksma-Kruize, J. H. Souverijn, M. A. Missinou, S. Borrmann, P. G. Kremsner, and M. Yazdanbakhsh. 2004. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J. Infect. Dis. 189:892-900. [DOI] [PubMed] [Google Scholar]
- 33.Wills-Karp, M. 2004. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202:175-190. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, M. S., and R. M. Maizels. 2006. Regulatory T cells induced by parasites and the modulation of allergic responses. Chem. Immunol. Allergy 90:176-195. [DOI] [PubMed] [Google Scholar]
- 35.Yazdanbakhsh, M., P. G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490-494. [DOI] [PubMed] [Google Scholar]
- 36.Yazdanbakhsh, M., and L. C. Rodrigues. 2001. Allergy and the hygiene hypothesis: the Th1/Th2 counterregulation can not provide an explanation. Wien Klin. Wochenschr. 113:899-902. [PubMed] [Google Scholar]