Abstract
Chromobacterium violaceum produces autoinducers, including homoserine lactones (HSLs), for genetic regulation. Among the seven HSLs derived from C. violaceum we evaluated, only C12-HSL stimulated the production of inflammatory cytokines in mammalian monocytic cell lines through the activation of the NF-κB signaling pathway besides their quorum-sensing role, like 3-oxo-C12-HSL from Pseudomonas aeruginosa.
Many bacteria sense their population density through a sophisticated cell-to-cell communication mechanism, and gene expression or their own growth rate is altered so that they can adapt to the surrounding environment. This type of gene regulation is known as quorum sensing (1, 19). The quorum-sensing system depends on diffusible signal molecules, termed autoinducers, which enable bacteria to monitor their own population density. This system controls some kinds of bacterial behavior, such as bioluminescence (8), swarming (5), biofilm formation (4), and the production of virulence determinants (6, 11).
The quorum-sensing system is considered to play a role in the pathogenesis of chronic respiratory infections due to Pseudomonas aeruginosa in patients with a compromised lower respiratory tract or a compromised systemic defense mechanism. In P. aeruginosa, two acyl-homoserine lactone-based systems, the las and rhl systems, have been described (10, 12, 14). Apart from its quorum-sensing function, the P. aeruginosa N-3-oxododecanoyl-homoserine lactone (3-oxo-C12-HSL) autoinducer has been suggested to modulate the immune responses of the infected host. In this context, it was demonstrated that 3-oxo-C12-HSL induces the production of interleukin-8 (IL-8), cyclooxygenase 2, and prostaglandin E2 in human fibroblasts and that it accelerates apoptosis in mammalian macrophages and neutrophils (15, 16, 18).
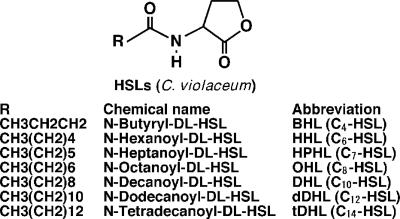
Chromobacterium violaceum, a gram-negative rod commonly found in soil and water, is an opportunistic pathogen, like P. aeruginosa (2, 3, 13). Although this bacterium was reported to produce several kinds of acyl-homoserine lactones (AHLs), including C4-, C6-, C7-, C8-, C10-, C12-, and C14-HSL, the involvement of HSLs in inflammatory host responses has not been evaluated (9, 17).
In this study, we examined the immunostimulatory function of C. violaceum-derived HSLs bearing a different carbon chain moiety and demonstrated that only C12-HSL exerted biological activities in mammalian cells similar to those exerted by the autoinducer of P. aeruginosa, 3-oxo-C12-HSL.
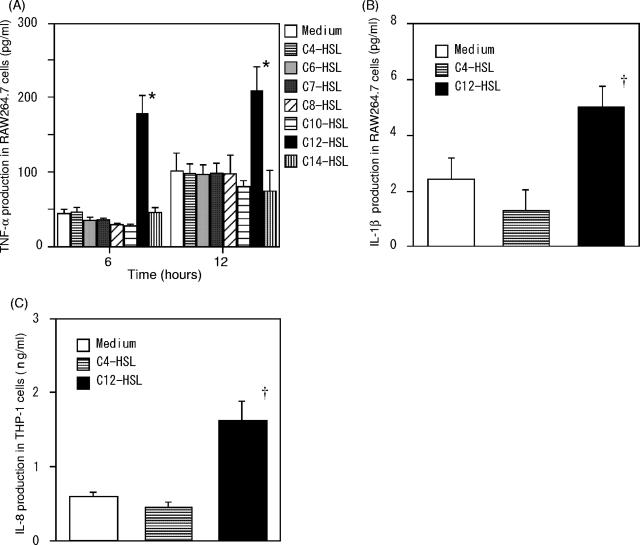
To examine the immunostimulatory function of C. violaceum, mouse macrophage-like RAW264.7 cells (kindly provided by K. Kawasaki and M. Nishijima, National Institute of Infectious Disease, Tokyo, Japan) were cultured in the presence of N-acyl HSLs bearing a different carbon chain moiety, i.e., C4-, C6-, C7-, C8-, C10-, C12-, and C14-HSL derived from C. violaceum (Fluka, Buchs SG, Switzerland) (Fig. 1), dissolved in dimethyl sulfoxide, at 37°C for 6 or 24 h in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. The concentration of tumor necrosis factor alpha (TNF-α), a representative of cytokines yielded by activated macrophages, was determined in the supernatants by enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 2A, only C12-HSL stimulated TNF-α production by RAW264.7 cells compared with all other groups, but no other HSLs induced TNF-α production, regardless of the incubation period, compared with medium alone. Similar results were achieved with RAW264.7 cells stimulated with the acyl-HSLs at an equal molar concentration (100 μM) (data not shown). Production of IL-1β by RAW264.7 cells was significantly higher when cells were treated with C12-HSL than with C4-HSL or medium alone (Fig. 2B). Similar results were achieved regarding IL-8 production by THP-1 cells, a human monocytic leukemia cell line (obtained from the Health Science Research Resources Bank, Osaka, Japan) maintained in RPMI 1640 medium with 10% heat-inactivated fetal calf serum and preincubated with 0.1 μM 22-oxyacalcitriol, an analogue of 1α,25-dihydroxy-vitamin D3 (Chugai Pharmaceutical, Tokyo, Japan) for 72 h before stimulation with AHLs (Fig. 2C). These results suggest that only C12-HSL of C. violaceum exerts immunostimulatory effects on mouse and human monocytic cells.
FIG. 1.
Chemical structure of autoinducers derived from C. violaceum.
FIG. 2.
C12-HSL derived from C. violaceum induces TNF-α and IL-1β production by RAW264.7 cells and IL-8 production by THP-1 cells. (A) RAW264.7 cells (5 × 105 cells/500 μl of cell culture medium in a 24-well plate) (Corning) were stimulated with 10 μg/ml AHLs. After stimulation at 37°C for 6 or 24 h, the culture supernatant was collected, and the concentration of TNF-α was measured using an ELISA kit (Biosource). (B) The experiment was similar to that described in panel A, but only C4-HSL and C12-HSL were used, and IL-1β in the supernatants was measured. (C) THP-1 cells (1 × 105 cells/200 μl of cell culture medium in a 96-well plate) (Falcon) were cultured in the presence of C4-HSL and C12-HSL as described in panel B, and the level of IL-8 in the supernatant was determined using an ELISA kit (BD Pharmingen). In all panels, results represent the means ± standard errors (n = 3 to ∼4 per data point); cells cultured in medium alone served as the control. A two-tailed Student t test was used for statistical comparison. *, P < 0.001 compared with all other groups; †, P < 0.05 compared with medium alone or C4-HSL.
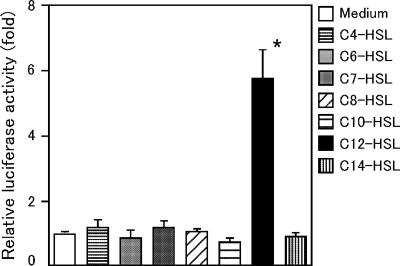
Following the cytokine analysis, we examined the activation of NF-κB, a key signaling molecule involved in inflammatory immune responses, using RAW/kB cells. These are stably transformed RAW264.7 cells that express luciferase in an NF-κB-dependent manner (7). RAW/kB cells were stimulated at 37°C for 6 h with C4-, C6-, C7-, C8-, C10-, C12-, and C14-HSLs, and luciferase activity was measured (Fig. 3). Incubation with C12-HSL significantly increased luciferase expression, whereas incubation with other HSLs did not influence the reporter gene expression. Similar results were achieved with RAW/kB cells stimulated with the acyl-HSLs at an equal molar concentration (100 μM) (data not shown).
FIG. 3.
C12-HSL derived from C. violaceum activates NF-κB. RAW/kB cells (4 × 104 cells/100 μl of cell culture medium in a 96-well plate) (Corning) were stimulated with 10 μg/ml AHLs or 1 μg/ml lipopolysaccharide (positive control) (data not shown). After stimulation at 37°C for 6 h, the cells were lysed in 25 μl of 5× cell lysis reagent (Promega Corp.), and then luciferase activity was measured using 5 μl of the lysate and 25 μl of luciferase assay substrate (Promega Corp.). Luminescence was quantified with a luminometer (Berthold Japan, Tokyo, Japan). Luciferase activity was normalized to the activity in the cells cultured without AHLs (medium alone) and presented as relative induction (n-fold). Results represent the means ± standard errors (n = 3 per data point). A two-tailed Student t test was used for statistical comparison. *, P < 0.001 compared with all other groups.
In this study, C12-HSL derived from C. violaceum stimulated the production of TNF-α and IL-1β in mouse RAW264.7 cells. It also induced the activation of NF-κB. Moreover, it induced the production of IL-8 in human THP-1 cells. These findings showed that C12-HSL induced the production of cytokines in mammalian monocytic cells. In this regard, several studies have demonstrated that 3-oxo-C12-HSL derived from P. aeruginosa exerts biological activities in mammalian cells. Taken together, these findings suggest that the carbon chain moiety of HSLs is important for the recognition of the autoinducer by the host cells. On the other hand, C8 acyl chain (octane), C8-OH (1-octanol), C12 acyl chain (dodecan), and C12-OH (1-dodecanol) (all chemicals were purchased from Sigma Aldrich) failed to activate NF-κB in the RAW/kB cells (data not shown). These results indicate that the acyl moiety alone is not sufficient to activate macrophages and that the whole structure of acyl-HSL is necessary for it to exert a biological activity. Furthermore, it is possible to infer that autoinducers derived from various bacteria are likely to exert biological activities in host cells. Moreover, by means of these autoinducers pathogenic microorganisms might alter the surrounding environment to survive and infect host tissues.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Camara, M., P. Williams, and A. Hardman. 2002. Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect. Dis. 2:667-676. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay, A., V. Kumar, N. Bhat, and P. Rao. 2002. Chromobacterium violaceum infection: a rare but frequently fatal disease. J. Pediatr. Surg. 37:108-110. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. H., L. C. Lin, C. E. Liu, and T. G. Young. 2003. Chromobacterium violaceum bacteremia: a case report. J. Microbiol. Immunol. Infect. 36:141-144. [PubMed] [Google Scholar]
- 4.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 5.Eberl, L., M. K. Winson, C. Sternberg, G. S. Stewart, G. Christiansen, S. R. Chhabra, B. Bycroft, P. Williams, S. Molin, and M. Givskov. 1996. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 20:127-136. [DOI] [PubMed] [Google Scholar]
- 6.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomi, K., K. Kawasaki, Y. Kawai, M. Shiozaki, and M. Nishijima. 2002. Toll-like receptor 4-MD-2 complex mediates the signal transduction induced by flavolipin, an amino acid-containing lipid unique to Flavobacterium meningosepticum. J. Immunol. 168:2939-2943. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 10.Middleton, B., H. C. Rodgers, M. Camara, A. J. Knox, P. Williams, and A. Hardman. 2002. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 207:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 12.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao, P. L., P. R. Hsueh, Y. C. Chang, C. Y. Lu, P. Y. Lee, C. Y. Lee, and L. M. Huang. 2002. Chromobacterium violaceum infection in children: a case of fatal septicemia with nasopharyngeal abscess and literature review. Pediatr. Infect. Dis. J. 21:707-709. [DOI] [PubMed] [Google Scholar]
- 14.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 15.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J. Immunol. 167:366-374. [DOI] [PubMed] [Google Scholar]
- 16.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636-2642. [DOI] [PubMed] [Google Scholar]
- 17.Swift, S., J. P. Throup, P. Williams, G. P. Salmond, and G. S. Stewart. 1996. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem. Sci. 21:214-219. [PubMed] [Google Scholar]
- 18.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]