Abstract
The var gene-encoded Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family is responsible for antigenic variation and sequestration of infected erythrocytes during malaria. We have previously grouped the 60 PfEMP1 variants of P. falciparum clone 3D7 into groups A and B/A (category A) and groups B, B/C, and C (category non-A). Expression of category A molecules is associated with severe malaria, and that of category non-A molecules is associated with uncomplicated malaria and asymptomatic infection. Here we assessed cross-reactivity among 60 different recombinant PfEMP1 domains derived from clone 3D7 by using a competition enzyme-linked immunosorbent assay and a pool of plasma from 63 malaria-exposed Tanzanian individuals. We conclude that naturally acquired antibodies are largely directed toward epitopes varying between different domains with a few, mainly category A, domains sharing cross-reactive antibody epitopes. Identification of groups of serological cross-reacting molecules is pivotal for the development of vaccines based on PfEMP1.
The Plasmodium falciparum variant surface antigens (VSA), including the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, play an important role in the pathogenesis of malaria (6, 12, 13, 16, 24, 25) by mediating adherence of infected erythrocytes to receptors on the vascular lining (33). This adherence enables the parasite to escape clearance in the spleen and can be detrimental to the human host by facilitating high parasite growth rates and unchecked and harmful inflammation (8). Individuals living in areas where P. falciparum is endemic acquire natural protective immunity from malaria over a period of several years by a gradual acquisition of specific immunoglobulin G (IgG) against different VSA (reviewed in references 7 and 12). PfEMP1 is the best-characterized VSA (40), and antibodies to these molecules have been associated with protection against malaria in both children (20) and pregnant women (29). PfEMP1 molecules are encoded by the var gene family, comprising 40 to 60 highly diverse genes per haploid genome (3, 35, 40). The genome of the clone 3D7 encodes 59 full-length var genes, which can be grouped into three major groups (A, B, and C) and two intermediate groups (B/A and B/C) on the basis of chromosomal location, direction of transcription with respect to chromosome telomeres, domain structure of the encoded proteins, and sequence similarities in coding and noncoding regions (15, 18). The extracellular and variable sequence of PfEMP1 comprises four different domain types: the N-terminal segment, the C2, the cysteine-rich interdomain region (CIDR), and the Duffy binding-like (DBL) domains (9, 40). The CIDR domains group as three (α, β, and γ) and the DBL domains as seven (α, β, γ, δ, ɛ, ζ, and x) distinct sequence classes (3, 36, 40). Groups A and B/A make up the largest PfEMP1s, with a 7- to 10-domain structure, which is different from the 4-domain-type structure predominant of groups B, B/C, and C (18).
Several studies have demonstrated that parasites causing severe P. falciparum malaria in young children who have little or no protective immunity tend to express VSA linked to severe malaria (VSASM) that are serologically distinct from those expressed by parasites causing uncomplicated and subclinical infection in older, more immune individuals (6, 24). The VSASM appear to be serologically more conserved and cross-reactive than VSA expressed during uncomplicated malaria infections (VSAUM) (25), consistent with the finding that immunity to severe malaria is acquired more rapidly than immunity to uncomplicated disease (28). We have previously established a link between expression of group A or B/A (here collectively named category A) PfEMP1 and the VSASM phenotype and a link between expression of group B, B/C, or C (here collectively named category non-A) PfEMP1 and the VSAUM phenotype (13). The serological diversity among parasites expressing VSASM is lower than that among parasites expressing VSAUM, possibly because of functional constraints (18), and this suggests that category A PfEMP1 molecules would be more likely to share cross-reactive antibody epitopes than molecules not belonging to this category.
The sequence similarity between different PfEMP1 domains varies, but it is generally limited and amino acid alignments will often be characterized by relatively short runs of conserved residues interrupted by much longer stretches of high sequence diversity (36, 41). Protective immunity against malaria could be acquired either as individuals build a broad repertoire of antibodies against polymorphic PfEMP1 epitopes or by slow acquisition of antibodies against conserved PfEMP1 epitopes. Previously, some reports have indicated that agglutinating VSA antibodies do not seem to be directed against conserved epitopes (22), while others have shown that some cross-reactivity must exist since a single P. falciparum infection is capable of inducing antibodies cross-reacting with VSA expressed on heterologous parasite isolates (10, 23). Additionally, antibodies reacting with linear conserved epitopes have been shown to be acquired by individuals living in areas where malaria is endemic (26, 38).
This is the first study addressing the question of whether groups or distinct sequence classes of PfEMP1 are more likely than others to share cross-reactive antibody epitopes.
MATERIALS AND METHODS
Protein expression.
A total of 60 (numbered 1 to 60) different recombinant protein domains representing 11 of the 14 group A and B/A; 20 of the 44 group B, C, and B/C PfEMP1; and VAR2CSA (PFL0030c) of clone 3D7 were cloned and expressed for competition enzyme-linked immunosorbent assay (ELISA). Domains and subtypes DBL (α, β, γ, δ, ɛ), C2, and CIDR (α, β, γ) were defined as described in reference 36, and specific primers for each recombinant protein domain were designed accordingly.
The DBL1α (PFB1055c, PFD1000c, PFD1235w, MAL6P1.316, PF08_0106), DBL3β (PFD1235w), C2 (PFD1235w), CIDR2β (PFD1235w), DBL2x (PFL0030c), DBL4ɛ (PFL0030c), DBL5ɛ (PFL0030c), and DBL6ɛ (PFL0030c) domains were PCR amplified from clone 3D7 genomic DNA and subcloned into the pGEX-4T1 vector (GE Healthcare, Hørsholm, Denmark). The NTS (PF11_0008, PF13_0003), DBL1α (PFA0005w, PFA0015c, PFA0765c, PFD0020c, PFD1015c, MAL6P1.4, PF07_0139, PF07_0050, MAL7P1.1, MAL7P1.56, PF08_0141, PF08_0142, PFI0005w, PFI1820w, PFI1830c, PF10_0406, PF11_0007, PF11_0008, PF11_0521, PFL0935c, PFL2665c, PF13_0003, PF13_0364), DBL1x (PFL0030c), CIDR1α (MAL7P1.55), CIDR1γ (PF08_0107) DBL2γ (PF11_0008), DBL3γ (PF13_0003), DBL4δ (PF13_0003), CIDR2β (PF13_0003), C2-1 and C2-2 (PF13_0003) domains were subcloned into the Gateway (Invitrogen, Taastrup, Denmark) pDEST-15 vector.
The recombinant domains were expressed as fusion proteins at the carboxy terminus of glutathione S-transferase (GST) from Schistosoma japonicum (34) in Escherichia coli and purified by affinity chromatography on glutathione Sepharose 4B (GE Healthcare).
The DBL1α (PFD0995c), DBL2β, and DBL5ɛ (MAL6P1.316) domains were subcloned into the pAcSecG2T baculovirus vector (BD Biosciences, Brøndby, Denmark) for production of GST-tagged proteins. The NTS, DBL2β-C2, DBL4γ, DBL4γ-DBL5δ, DBL5δ, CIDR1α (PFD1235w), DBL2δ (PF08_0107), DBL3x and CIDR2β (PF11_0008), and DBL2β and CIDR1γ (PF13_0003) domains were subcloned into the pBAD-TOPO vector (Invitrogen). For production of the carboxy-terminal V5 epitope and histidine-tagged protein, the inserts were excised by EcoRI and PmeI digestion and subsequently subcloned into the EcoRI and blunt-ended BglII sites of baculovirus transfer vector pAcGP67-A (BD Biosciences). Recombinant baculovirus was generated by cotransfection of the pAcSecG2T or pAcGP67-A construct and Bsu36I-linearized Bakpak6 baculovirus DNA (BD Biosciences Clontech) into insect Sf9 cells. Recombinant products were expressed by infection of insect High-Five cells with recombinant baculovirus. GST-tagged protein was purified as described above. His-tagged protein was purified from culture supernatants on a Co2+ metal chelate agarose column as previously described (13).
To test for systematic differences between serological recognition of bacterial and baculovirus-expressed polypeptides, antibody recognition of all proteins was compared by using the same pool of plasma as subsequently used for the competition ELISA. The mean optical densities (ODs) did not differ significantly between the proteins expressed in the two systems.
Plasma.
Plasma samples from 63 different malaria-asymptomatic Tanzanian donors (mean age, 11.3 years; standard deviation, 4.3 years) living in Mgome village in the Tanga region of Tanzania were pooled and used for the competition ELISA. This lowland village is characterized by an entomological inoculation rate ranging between 91 and 405 infectious bites/person/year (5). Prior to this study, informed consent was obtained from all individuals and/or their parents and ethical clearance was granted by the Ministry of Health and the Ethics Committee of the National Institute for Medical Research in Tanzania.
Competition ELISA.
The competition ELISA was carried out with the different protein domains expressed in E. coli or baculovirus. Blocking of the plasma pool was done with 4 μg/ml recombinant protein (x: recombinant proteins 1 to 60) for 2 h at room temperature. The preabsorbed pool was diluted 1:100, and 100 μl was added to duplicate wells of Maxisorp microtiter plates precoated with antigen (1 to 5 μg/ml) or GST as a control antigen (14). In addition to the preabsorbed pool (pPx), a nonabsorbed pool (nP) was included in each plate. Following 1 h of incubation at room temperature, plates were washed four times in phosphate-buffered saline (PBS)-Tx100 (PBS, 0.5 M NaCl, 1% Triton X-100, pH 7.4). Plates were incubated with peroxidase-conjugated rabbit anti-human IgG (Dako, Glostrup, Denmark) diluted 1:3,000 in PBS-Tx100 including 1% bovine serum albumin for 1 h and washed. Antibody reactivity was visualized by adding 100 μl/well o-phenylenediamine substrate (0.6%; Dako) diluted in distilled H2O with 0.05% (vol/vol) H2O2. Color reactions were stopped by the addition of 100 μl of 2.5 M H2SO4, and OD was measured at 492 nm. The percent reduction in antibody reactivity was calculated as follows: 100 − [(ODpPx/ODnP) × 100]. Most assays were performed twice, and the results were similar. Data from the last experiment or in case the assay only was performed once, the first and only experiment were assigned a white (0 to 50%), gray (>50 to 75%), or black (>75%) color according to the reduction in antibody reactivity and depicted in SigmaPlot 8.0 (Systat Software Inc., Point Richmond, CA). Proteins were defined as sharing cross-reactive antibody epitopes if the percent reduction in OD was greater than 50%.
Indirect ELISA.
To analyze whether CIDR domains were more frequently recognized by human antibodies compared to DBL1α domains, we tested the reactivity of plasma from 20 children aged 2 to 4 years and living in one of two villages, Mgome and Ubiri. Ubiri is an intermediate-altitude village with an estimated entomological inoculation rate of 1.8 to 34 infectious bites/person/year (5). DBL1α of PF11_0008, MAL6P1.316, PF08_0106, PF08_0142, PFI1820w, PFD0995c, PFL0935c, and PFB1055c; CIDR1α of PFD1235w; CIDR1γ of PF08_0107 and PF13_0003; and CIDR2β of PF13_0003 were cloned, expressed, and purified as described above. The ELISAs were optimized to give very similar ODs with a positive pool at different dilutions and the samples tested as described above.
RESULTS
Because of the limited availability of whole genome sequences from different P. falciparum genomes, our analysis was directed toward cross-reactivity among clone 3D7-derived PfEMP1 domains.
Protein expression and assay design.
We cloned and expressed 60 different recombinant protein domains representing 32 of the 59 full-length var genes in clone 3D7 and used these in a competition ELISA.
Prior to the competition ELISA analysis, a pool of plasma from 63 different Tanzanian donors (mean age, 11.3 ± 4.3 years) living in a village with a high malaria transmission intensity (19) was generated. The IgG reactivity of this pool was tested with each of the 60 recombinant domains as the coating antigen in an indirect ELISA previously optimized to give a high OD reading for each protein. The OD readings of the pool varied from a minimum OD of 0.5 (DBL1α of PF13_0003, PF11_0007, and PF07_0050), a median OD of 1.0 (PF11_0008 DBL3x, PF13_0003 DBL4δ and CIDR2β, and PFD1000c DBL1α), and a maximum OD of 4.0 (PFD1235w CIDR1α and PF13_0003 DBL2β).
Category A and non-A PfEMP1 and antibody cross-reactivity.
To detect antibody epitopes shared between PfEMP1 domains, the plasma pool was preincubated with each of the 60 recombinant domains and subsequently tested in a series of indirect ELISAs with the same 60 domains as the coating antigen. As a control experiment, the plasma pool was preincubated with GST and the ELISA reactivity to each of the recombinant domains was compared to the antibody reactivity before preincubation. For all 60 recombinant polypeptides, preincubation with GST exhibited a <25% reduction in OD, except for PF08_0142 DBL1α (32%) and PF13_-0003 CIDR1γ (26%). We therefore defined antibody cross-reactivity with a conservative cutoff of a 50% reduction in OD.
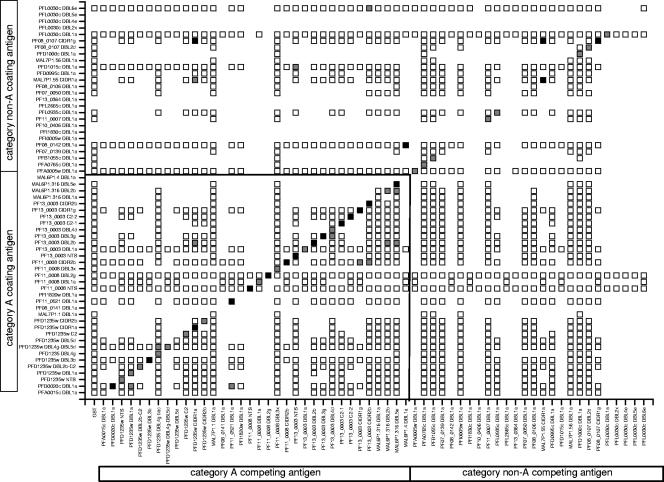
The 60 protein domains were sorted according to whether they appeared in category A or non-A PfEMP1 in a checkerboard diagram showing the inhibition of OD reactivity following preincubation with different domains (Fig. 1). Generally, there was only little evidence of serological cross-reactivity between domains, with only 11 (1%) of 1,245 heterologous protein combinations showing inhibition above the cutoff, whereas inhibition was seen in 37 (76%) of 49 assays when the competing and coating antigens were identical. Interestingly, category A domains (0.9%; 10 of 1,245 heterologous combinations) were more likely to inhibit ELISA reactivity than category non-A PfEMP1 domains (0.1%; 1 of 1,245 heterologous combinations) (Fig. 1). Of the 11 protein domains showing serological cross-reactivity, 4 were expressed in baculovirus and 7 were expressed in E. coli. A few domains were produced in both expression systems and shown to behave similarly in the competition ELISA experiments (data not shown).
FIG. 1.
Cross-reactive antibody epitopes shared among 60 PfEMP1 domains of the clone 3D7 genome. Reactivity for category A PfEMP1 domains (A and B/A according to reference 18) are on the left, and category non-A PfEMP1 domains (B, C, and B/C according to reference 18) are on the right. Responses with the same protein as the competition and coating antigen are on the diagonal. The reduction in antibody reactivity was calculated as described in Materials and Methods. White, <50% reduction; gray, 50 to 75% reduction; black, >75% reduction. Blanks with no square are protein combinations not tested.
Antibody cross-reactivity and PfEMP1 domain types.
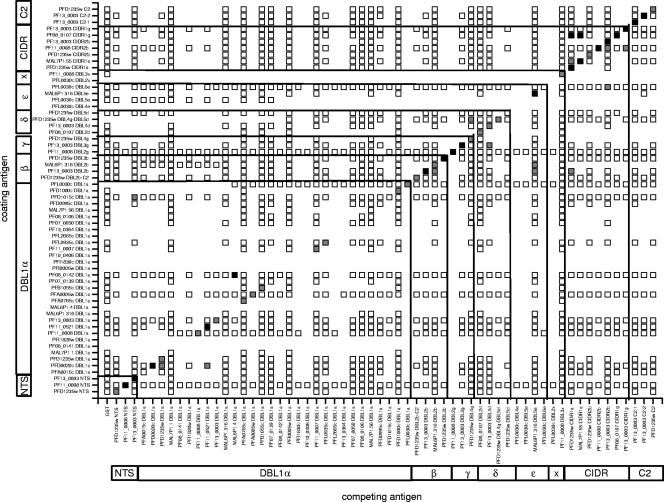
Since the different domain types (DBLα, -β, -γ, -δ, and -ɛ and CIDR) were not equally distributed between the two categories; we reanalyzed the data according to domain type (Fig. 2 and Table 1). Unexpectedly, DBL1α domains only rarely shared cross-reactive epitopes (1.0%; 3 of 305 heterologous combinations), indicating that antibodies to conserved amino acid sequences in DBL1α (such as LARSFADIG) were less frequent than expected. By contrast, 4 of 21 (19.0%) heterologous CIDR combinations showed inhibition in the competition assays (Table 1).
FIG. 2.
Cross-reactive antibody epitopes shared among 60 PfEMP1 domains of the clone 3D7 genome as described in the legend to Fig. 1. Reactivities are grouped according to domain type and subtype. Blanks with no square are protein combinations not tested.
TABLE 1.
Total numbers of different domain types encoded by the clone 3D7 genome and numbers of domain types tested and found to contain cross-reacting antibody epitopes
| Domain | No. of domains of following type:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NTS | α | β | γ | δ | ɛ | ζ | x | CIDR | C2 | |
| In clone 3D7 | 60 | 60 | 20 | 16 | 49 | 14 | 3 | 3 | 106 | 19 |
| Tested | 3 | 30 | 5 | 3 | 4 | 4 | 0 | 2 | 7 | 3 |
| Cross-reacting | 1 | 3 | 2 | 0 | 1 | 1 | 0 | 4 | 0 | |
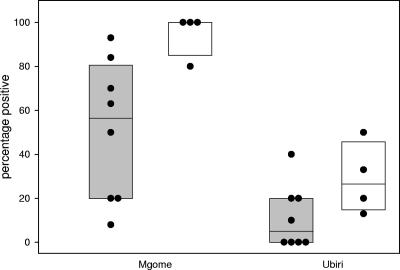
With eight different recombinant DBL1α and four different CIDR domains in an indirect ELISA, we found that plasma antibodies from 20 children aged 2 to 4 years more frequently recognized CIDR than DBL1α domains. Both domain types were more frequently recognized by plasma samples from a high malaria transmission intensity area, Mgome, compared to samples from Ubiri, a moderate malaria transmission intensity area of Tanzania (Fig. 3).
FIG. 3.
Percentages of children aged 2 to 4 years recognizing eight recombinant DBL1α and four different CIDR domains of clone 3D7 PfEMP1. Recognition of DBL1α (gray box plot) and CIDR (white box plot) domains was tested with plasma from 20 different children aged 2 to 4 years living in the villages of Mgome (high transmission) and Ubiri (moderate transmission). The 10th and 90th percentiles are shown. Dots show the percentages of samples positive in each individual protein ELISA.
Evidence of serological cross-reactivity was mainly found between similar domain types (CIDR versus CIDR or DBL versus DBL) and could often be explained by sequence similarity (Table 2). In four cases, inhibitory activity was present without any evidence of sequence similarity between the competing and coating antigens, indicating that conformational epitopes might contribute to cross-reactivity.
TABLE 2.
Sequence identities between PfEMP1 domainsa
| Competing antigen | Sequence identity with following coating antigen:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBL1α PFD0020c (A) | DBL1α PFD1015c (non-A) | DBL1α PF08_0142 (non-A) | DBL2β PF13_0003 (A) | DBL2β MAL6P1.316 (A) | DBL6ɛ PFL00030c (VAR2) | DBL3γ PF13_0003 (A) | CIDR1α PFD1235w (A) | CIDR1α MAL7P1.55 (non-A) | CIDR2β PFD1235w (A) | CIDR2β PF11_0008 (A) | CIDR1γ PF08_0107 (non-A) | C2 PFD1235w (A) | C2-1 PF13_0003 (A) | |
| NTS PF13_0003 (A) | NIb | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| DBL1α PFD1235w (A) | 100/197 (50) | 81/204 (39) | 92/195 (47), | 91/267 (34) | 83/268 (30) | 51/191 (26) | 70/307 (22) | NI | NI | NI | NI | NI | NI | NI |
| DBL1α PF11_0521 (A) | 105/190 (55) | 72/199 (36) | 80/195 (41) | 75/196 (38) | 68/204 (33) | 37/135 (27) | 51/205 (24) | NI | NI | NI | NI | NI | NI | NI |
| DBL1α MAL6P1.4 (A) | 89/194 (45) | 111/198 (56) | 125/205 (60) | 77/201 (38) | 57/151 (37) | 34/156 (21) | NI | NI | NI | NI | NI | NI | NI | NI |
| DBL2β MAL6P1.316 (A) | 77/204 (37) | 50/151 (33) | 65/206 (31) | 139/296 (46) | Identical | 69/254 (27) | 72/315 (22) | NI | NI | NI | NI | NI | NI | NI |
| DBL4δ PF13_0003 (A) | 33/136 (24) | NI | NI | 46/218 (21) | 50/222 (22) | 55/223 (24) | 113/292 (38) | NI | NI | 14/22 (63) | NI | NI | NI | NI |
| DBL5ɛ MAL6P1.316 (A) | 48/183 (26) | 48/191 (25) | 43/194 (22) | 59/189 (31) | 66/217 (30) | 89/271 (32) | 67/272 (24) | NI | NI | NI | NI | NI | NI | NI |
| CIDR1α PFD1235w (A) | NI | NI | NI | 15/28 (53) | NI | NI | NI | Identical | 93/327 (28) | 73/320 (22) | 89/352 (25) | 108/390 (27) | 26/76 (34) | NI |
| CIDR1α MAL7P1.55 (non-A) | NI | NI | NI | 42/199 (21) | NI | NI | NI | 93/327 (28) | Identical | 51/192 (26) | 65/239 (27) | 137/418 (32) | NI | NI |
| CIDR2β PF13_0003 (A) | NI | NI | NI | NI | NI | NI | NI | 69/321 (21) | 57/233 (24) | 132/329 (40) | 138/329 (41) | 44/164 (26) | NI | NI |
| CIDR1γ PF13_0003 (A) | NI | NI | NI | NI | NI | NI | NI | 94/384 (24) | 78/279 (27) | 85/279 (30) | 112/325 (34) | 32/79 (40) | NI | NI |
Values for domains sharing cross-reactive antibody epitopes are in boldface. The values shown are the number of identical residues/total number of residues (percent identity). The category of PfEMP1 (A or non-A) is shown in parentheses.
NI, no identity.
DISCUSSION
Results from previous work have shown that VSA associated with severe malaria are serologically more conserved than variants associated with uncomplicated or mild malaria (6, 24) and that expression of group A PfEMP1 confers a VSASM phenotype on parasites (13, 39). In this study, except for a few mainly category A domains, we found recombinant polypeptides with the primary sequence of clone 3D7 PfEMP1 domains rarely competing for antibodies present in plasma from individuals naturally exposed to P. falciparum. Among the different domain types, heterologous CIDR combinations showed inhibition more frequently in the competition assay and were more frequently recognized by individual plasma samples from children aged 2 to 4 years compared to DBL1α domains. This could be explained by a higher antigenicity and the presence of cross-reactive antibody epitopes in the CIDR domains. CIDR domains have previously been shown to induce cross-reactive antibodies in mice following immunization with plasmid DNA expressing CIDR domains (2, 11), and such antibodies have been suggested to modify disease progression in vaccine experiments where Aotus nancymai monkeys were challenged with a heterologous parasite line (21).
From a biological point of view, limited cross-reactivity among PfEMP1 molecules expressed from a single parasite genome would make sense. Individual parasites have the ability to swap expression among 50 to 60 different antigenically distinct PfEMP1 variants through a clonal antigen switching process which is not yet fully understood (4, 27, 35). By this process, P. falciparum is able to establish chronic monoclonal infections which are probably essential for parasite survival in areas with limited and highly seasonal transmission (37). If induction of cross-reactive PfEMP1 antibodies were a frequent event, this would limit the parasite's benefit of carrying many different var genes. The same applies to the initial phase of P. falciparum infections. Studies of var gene expression in parasites isolated just after liver release from individuals experimentally infected with strain NF54 (the parental strain of clone 3D7) suggest that different var genes are being transcribed by individual parasites and that many different PfEMP1 variants might be presented by the parasites establishing the asexual blood stage infection (17). This broad PfEMP1 expression could enhance parasite sequestration in hosts with different levels of preexisting immunity and extend the duration of infection. The presence of antibodies capable of cross-reacting with many different PfEMP1 molecules would reduce the chances of parasite survival even in hosts with little preexisting immunity.
Although we could not test for cross-reactivity between PfEMP1 molecules encoded by different genomes, such cross-reactivity clearly exists. By the age of 3 to 4 years, children in areas where malaria is highly endemic have acquired surface-reactive antibodies against most VSA variants (1). In the present study, antibodies could be detected against all of the recombinant protein domains tested despite its being very unlikely that any of the plasma donors had been exposed to parasites expressing PfEMP1 identical to those of clone 3D7.
The extracellular domain of the different PfEMP1 molecules within the clone 3D7 and other parasite genomes shows a high degree of primary sequence polymorphism. However, a number of conserved subfamilies, such as var1-3, have been described (15, 28, 30, 31). These var genes are present in many P. falciparum genomes, and within each subfamily stretches of high amino acid sequence similarity are found. Such PfEMP1 subfamilies are likely to induce cross-reactive antibodies, and it is conceivable that the global PfEMP1 repertoire is structured into distinct serologically cross-reactive types which could share functions such as binding identical receptors (e.g., CSA, CD36, and others) or being expressed at the same stage of the parasite life cycle (32). Thus, we would speculate cross-reactivity to be more frequent at the intergenomic level compared to the intragenomic level. Identification of groups of serologically cross-reacting PfEMP1 molecules is pivotal for the development of vaccines based on variant antigens that should induce broad protection.
Acknowledgments
We thank Lotte Bram and Susanne Pedersen for excellent technical assistance.
This study received financial support from the Danish Council for Health Science Research (grant 271-05-0427), a grant from the Foundation for the National Institute of Health through the Grand Challenges in Global Health initiative, and a grant from the Danish International Development Agency (104.DAN.8.L.312). A.T.R.J. is supported by the Howard Hughes Medical Institute. A.S., J.P.L., and P.M. are supported by the Gates Malaria Partnership.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Askjaer, N., C. Maxwell, W. Chambo, T. Staalsø, M. A. Nielsen, L. Hviid, C. Curtis, and T. G. Theander. 2001. Insecticide-treated bed nets reduce plasma antibody levels and limit the repertoire of antibodies to Plasmodium falciparum variant surface antigens. Clin. Diagn. Lab. Immunol. 8:1289-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch, D. I., B. Gamain, and L. H. Miller. 2003. DNA immunization with the cysteine-rich interdomain region 1 of the Plasmodium falciparum variant antigen elicits limited cross-reactive antibody responses. Infect. Immun. 71:4536-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. H. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 4.Biggs, B.-A., L. Gooze, K. Wycherley, W. Wollish, B. Southwell, J. H. Leech, and G. V. Brown. 1991. Antigenic variation in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 88:9171-9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bødker, R., J. Akida, D. Shayo, W. Kisinza, H. A. Msangeni, E. M. Pedersen, and S. W. Lindsay. 2003. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J. Med. Entomol. 40:706-717. [DOI] [PubMed] [Google Scholar]
- 6.Bull, P. C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B. S. Lowe, C. I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252-259. [DOI] [PubMed] [Google Scholar]
- 7.Bull, P. C., and K. Marsh. 2002. The role of antibodies to Plasmodium falciparum infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 10:55-58. [DOI] [PubMed] [Google Scholar]
- 8.Deitsch, K. W., and L. Hviid. 2004. Variant surface antigens, virulence genes and the pathogenesis of malaria. Trends Parasitol. 20:562-566. [DOI] [PubMed] [Google Scholar]
- 9.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. A. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giha, H. A., T. Staalsø, D. Dodoo, I. M. Elhassan, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 1999. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology 119:7-17. [DOI] [PubMed] [Google Scholar]
- 11.Gratepanche, S., B. Gamain, J. D. Smith, B. A. Robinson, A. Saul, and L. H. Miller. 2003. Induction of crossreactive antibodies against the Plasmodium falciparum variant protein. Proc. Natl. Acad. Sci. USA 100:13007-13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hviid, L., and T. Staalsø. 2004. Malaria immunity in infants: a special case of a general phenomenon? Trends Parasitol. 20:66-72. [DOI] [PubMed] [Google Scholar]
- 13.Jensen, A. T. R., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucciuini, A. Salanti, L. S. Vestergaard, J. P. Lusingu, R. Hermsen, R. Sauerwein, J. Christensen, M. A. Nielsen, L. Hviid, C. Sutherland, T. Staalsø, and T. G. Theander. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, A. T. R., H. D. Zornig, C. Buhmann, A. Salanti, K. A. Koram, E. M. Riley, T. G. Theander, L. Hviid, and T. Staalsø. 2003. Lack of gender-specific antibody recognition of products from domains of a var gene implicated in pregnancy-associated Plasmodium falciparum malaria. Infect. Immun. 71:4193-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraemer, S. M., and J. D. Smith. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527-1538. [DOI] [PubMed] [Google Scholar]
- 16.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 17.Lavstsen, T., P. Magistrado, C. C. Hermsen, A. Salanti, A. T. Jensen, L. Sauerwein, L. Hviid, T. G. Theander, and T. Staalsø. 2005. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malaria J. 4:21. [Online.] doi: 10.1186/1475-2875-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavstsen, T., A. Salanti, A. T. Jensen, D. E. Arnot, and T. G. Theander. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malaria J. 2:27. [Online.] doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusingu, J. P. A., L. S. Vestergaard, B. P. Mmbando, C. J. Drakeley, C. Jones, J. Akida, Z. X. Savaeli, A. Y. Kitua, M. M. Lemnge, and T. G. Theander. 2004. Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in northeastern Tanzania. Malaria J. 3:26. [Online.] doi: 10.1186/1475-2875-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusingu, J., A. Jensen, L. Vestergaard, D. Minja, M. Dalgaard, S. Gesase, B. Mmbando, A. Kitua, M. Lemnge, D. Cavanagh, L. Hviid, and T. Theander. 2006. Levels of plasma immunoglobulin G with specificity against the cysteine-rich interdomain regions of a semiconserved Plasmodium falciparum erythrocyte membrane protein 1, VAR4, predict protection against malarial anemia and febrile episodes. Infect. Immun. 74:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makobongo, M. O., B. Keegan, C. A. Long, and L. H. Miller. 2006. Immunization of Aotus monkeys with recombinant cysteine-rich interdomain region 1α protects against severe disease during Plasmodium falciparum Reinfection. J. Infect. Dis. 193:731-740. [DOI] [PubMed] [Google Scholar]
- 22.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, M. A., B. Grevstad, T. M. Elgadir, J. A. Kurtzhals, H. Giha, T. Staalsø, L. Hviid, and T. G. Theander. 2005. Differential induction of immunoglobulin G to Plasmodium falciparum variant surface antigens during the transmission season in Daraweesh, Sudan. J. Infect. Dis. 192:520-527. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen, M. A., T. Staalsø, J. A. L. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 168:3444-3450. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen, M. A., L. S. Vestergaard, J. Lusingu, J. A. L. Kurtzhals, H. A. Giha, B. Grevstad, B. Q. Goka, M. M. Lemnge, J. B. Jensen, B. D. Akanmori, T. G. Theander, T. Staalsø, and L. Hviid. 2004. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect. Immun. 72:3531-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oguariri, R. M., S. Borrmann, M. Q. Klinkert, P. G. Kremsner, and J. F. Kun. 2001. High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 69:7603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, and G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 29.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salanti, A., A. T. R. Jensen, H. D. Zornig, T. Staalsø, L. Joergensen, M. A. Nielsen, A. Khattab, D. E. Arnot, M. Q. Klinkert, L. Hviid, and T. G. Theander. 2002. A sub-family of common and highly conserved Plasmodium falciparum var genes. Mol. Biochem. Parasitol. 122:111-115. [DOI] [PubMed] [Google Scholar]
- 31.Salanti, A., T. Staalsø, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 32.Sharp, S., T. Lavstsen, Q. L. Fivelman, M. Saeed, L. McRobert, T. J. Templeton, A. T. Jensen, D. A. Baker, T. G. Theander, and C. J. Sutherland. 2006. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryot. Cell 5:1206-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman, I. W., S. Eda, and E. Winograd. 2003. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 5:897-909. [DOI] [PubMed] [Google Scholar]
- 34.Smith, D., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 35.Smith, J. D., C. E. Chitnis, A. G. Craig, D. J. Roberts, D. E. Hudsontaylor, D. S. Peterson, R. Pinches, C. I. Newbold, and L. H. Miller. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 37.Staalsø, T., A. A. Hamad, L. Hviid, I. M. Elhassan, D. E. Arnot, and T. G. Theander. 2002. In vivo switching between variant surface antigens in human Plasmodium falciparum infection. J. Infect. Dis. 186:719-722. [DOI] [PubMed] [Google Scholar]
- 38.Staalsø, T., E.A. Khalil, I. M. Elhassan, E. E. Zijlstra, A. M. Elhassan, H. A. Giha, T. G. Theander, and P. H. Jacobsen. 1998. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). Immunol. Lett. 60:121-126. [DOI] [PubMed] [Google Scholar]
- 39.Staalsø, T., M. A. Nielsen, L. S. Vestergaard, A. T. R. Jensen, T. G. Theander, and L. Hviid. 2003. In vitro selection of Plasmodium falciparum 3D7 for expression of variant surface antigens associated with severe malaria in African children. Parasite Immunol. 25:421-427. [DOI] [PubMed] [Google Scholar]
- 40.Su, X.-Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 41.Ward, C. P., G. T. Clottey, M. Dorris, D. D. Ji, and D. E. Arnot. 1999. Analysis of Plasmodium falciparum PfEMP-1/var genes suggests that recombination rearranges constrained sequences. Mol. Biochem. Parasitol. 102:167-177. [DOI] [PubMed] [Google Scholar]